Abstract
Quantum dots (QDs), an important class of emerging nanomaterial, are widely anticipated to find application in many consumer and clinical products in the near future. Premarket regulatory scrutiny is, thus, an issue gaining considerable attention. Previous review papers have focused primarily on the toxicity of QDs. From the point of view of product regulation, however, parameters that determine exposure (e.g., dosage, transformation, transportation, and persistence) are just as important as inherent toxicity. We have structured our review paper according to regulatory risk assessment practices, in order to improve the utility of existing knowledge in a regulatory context. Herein, we summarize the state of academic knowledge on QDs pertaining not only to toxicity, but also their physicochemical properties, and their biological and environmental fate. We conclude this review with recommendations on how to tailor future research efforts to address the specific needs of regulators.
Keywords: ecotoxicology, toxicology, environmental fate, regulatory policy, risk assessment, nanoparticles
Quantum dots (QDs), an important class of emerging nanomaterial, are “among the most promising items in the nanotechnology toolbox” and are widely anticipated to eventually find application in a number of commercial consumer and clinical products (Azzazy et al., 2007). However, before QDs can enter the market, they will likely be subjected to some form of regulatory scrutiny.
The type of regulatory scrutiny QDs will face is currently unknown. Not a single jurisdiction in the world is presently mandating the creation of specific safety-related data for nanomaterials or has declared when and if such requirements can be expected (Pelley and Saner, 2009). At the same time, it is widely expected that nanomaterials, including QDs, will face particular regulatory scrutiny at some point in the near future.
The development of new regulatory requirements is an iterative process. Regulatory data requirements (such as new bioassays) can be a major impediment to innovation and will not be mandated lightly. Instead, such requirements will be developed once the existing scientific understanding suggests that regulators require more information to assess environmental, health, and safety (EHS) risks. In the absence of specific data requirements pertaining to EHS, regulators will have limited access to new information. They will thus find it difficult to arbitrate whether new regulatory measures will lead to overregulation (through an excessive emphasis on “precaution”) or if they are currently underregulating this class of products. The chicken-and-egg problem is best managed by maximizing the accessibility and utility of existing academic knowledge in the regulatory context—which is precisely what we set out to do.
Herein, we build on the seminal literature review on QDs previously published by Hardman (2006). Hardman's review predominantly focused on the toxicity of QDs, however. This is insufficient in the regulatory context, as regulators require knowledge of both the toxicity and the biological fate of substances and products (including the absorption, distribution, metabolism, and excretion (ADME) within a body, and the transportation and transformation within the natural environment). In other words, it is not only the toxicity but also the dosage, the likelihood of that dosage being administered, and the concentrations in the natural environment that matter from a risk perspective.
Aside from updating and reviewing data published since the 2006 review by Hardman, the main contribution of this paper is that it summarizes what has been reported on the biological fate of QDs in the academic literature to date. This approach improves the accessibility of current academic knowledge on QDs for risk assessors who are in the process of developing an approach to the regulation of nanomaterials.
In order to maximize accessibility, our paper is formatted according to the typical structure of a regulatory risk assessment, as depicted in Figure 1 above.
FIG. 1.
The typical structure of a regulatory risk assessment.
What are QDs?
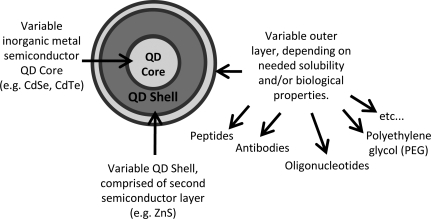
QDs, a heterogeneous class of engineered nanoparticles that are both semiconductors and fluorophores, are rapidly emerging as an important class of nanoparticles with numerous potential applications ranging from medicine to energy. In terms of their basic structure, QD are nanocrystals composed of a semiconductor core encased within a shell comprised of a second semiconductor material. A typical QD has a diameter in the range of 2–10 nm, which is comparable with the size of a large protein.
For biomedical applications, QDs are generally solubilized and require some form of biological “interfacing.” A number of strategies for solubilization and imparting biofunctionality have been devised; these have previously been reviewed by Michalet et al. (2005). A single QD contains a large number (10–100) of potential surface attachment groups, and therefore can readily be conjugated to biomolecules such as biotin, antibodies, oligonucleotides (DNA or RNA), or peptides (illustrated in Fig. 2, below). A standard nomenclature is generally utilized to describe the component parts of various QDs, as follows: Core/Shell-Conjugate. For example, a QD with a cadmium-selenium core and a zinc sulphide shell which has been biotin conjugated would be designated as CdSe/ZnS biotin.
FIG. 2.
Basic structure of a QD.
Key Applications of QDs
The properties of QDs make them potentially useful in a wide variety of settings, including electronics, computing, and various biomedical and clinical imaging applications.
In the field of electronics, researchers are looking to exploit both the semiconductor and luminescent properties of QDs in transistors, to build improved transistor capabilities. The luminescent properties of QDs are being explored for use in next generation versions of light-emitting diodes and diode lasers. QDs are also being explored for potential applications in the emerging field of quantum computing.
The many potential biomedical applications of QDs have been recently and extensively reviewed elsewhere (Azzazy et al., 2007; Delehanty et al., 2008; Hild et al., 2008; Medintz et al., 2008; Michalet et al., 2005; Jamieson et al., 2007; Li et al., 2007; Samia et al., 2006). Biomedical applications exploit the fluorescent properties of QDs, and particularly their advantages over traditional organic dyes, for both diagnostic and clinical applications. The in vitro biomedical and diagnostic applications of QDs include such techniques as the multicolor fluorescent labelling of cell surface molecules and cellular proteins in microscopy and other applications, detection of pathogens and toxins, DNA and RNA technologies, and fluorescence resonance energy transfer. QDs are also being explored for use in whole-body in vivo imaging of normal and tumor tissues. QDs may also find use in therapeutic applications such as targeted drug delivery, photodynamic therapy, and drug discovery.
QDs VIEWED THROUGH A REGULATORY LENS
Governments have traditionally regulated novel technologies on the basis of specific products and their intended uses (e.g., label claim), rather than on the basis of the technology itself. The specific commercial applications of QDs will therefore most likely dictate the approach to regulation, and the perspective of regulators is best served if QDs are classified in a way that is sensitive to the streaming of novel products into existing regulatory frameworks. For the sake of the discussion to follow, we break down the heterogeneous category of all QDs into subcategories based upon specific products or applications likely to be regulated in a similar fashion. We propose the following three regulatory classes of QDs. Note that the rudimentary classification scheme outlined below is intended to be sensitive to the perspective of regulators without presupposing the future emergence of nanotechnology-specific regulatory approaches. It would be premature to be more specific at this time because the regulatory system is currently under development and because terminologies and nuances vary internationally.
Class 1: Consumer products: QDs contained in consumer products, particularly electronics and quantum computing applications.
Class 2: Medical and imaging devices: QDs as in vitro diagnostic agents and as imaging devices used in the biomedical research setting.
Class 3: Pharmaceutical products: QDs as “nanomedicines” and in vivo diagnostic agents, that is, the use of QD in clinical imaging and drug delivery applications.
Closely tied to the regulation of pharmaceutical products is the regulation of food and food products. Any potential applications for QDs in the area of food and food packaging would likely be subject to a similar depth of regulatory attention, if not increased regulatory scrutiny, as compared with pharmaceuticals. Although we are not specifically aware of any research being conducted into the use of QDs in food or food packaging, a 2004 report reporting the use of QDs to specifically detect a strain of Escherichia coli known be a major cause of food borne illness (Su and Li, 2004) suggests that QDs, like other nanoparticles, may eventually find applications in this area.
Beyond the three regulatory product classes of QDs described above, one area in which the specific application of a product does not currently dictate the approach to regulation in a majority of jurisdictions is that of chemical substances. The level of regulatory oversight for chemical substances generally depends on a certain threshold concentration being released into the environment.
In the sections that follow, we will discuss the physicochemical properties, toxicity, and biological fate of QDs in sequence, according to the typical structure of a regulatory risk assessment, as illustrated above in Figure 1.
BASIC PHYSICOCHEMICAL PROPERTIES OF QDs
The health and safety properties of QDs will largely be dependent upon their basic physicochemical properties, such as (1) chemical composition (purity and chemical make-up), (2) shape and size (size may refer both to surface area and to size distribution), and (3) surface properties and solubility (surface reactivity, surface groups, inorganic or organic coatings, etc.). This component of risk assessment requires attention because the justification for novel data requirements hinges to a large extent on the unique behaviours exhibited by materials at the nanoscale.
Chemical Composition
In terms of their chemical composition, QDs are a highly heterogeneous group of products. QDs for biological applications, including those which are currently commercially available from companies such as Invitrogen, are most commonly comprised of cadmium and either selenium or tellurium (CdSe and CdTe QDs) and are frequently coated by a shell comprised of zinc sulphide (ZnS), but there are many other possible combinations.
Cadmium, selenium, and tellurium all have known toxicities in humans, including hepatic, renal, neurologic, and/or genetic toxicities (reviewed in Bertin and Averbeck, 2006; Taylor, 1996; Vinceti et al., 2001). For this reason, it will be important for regulators to know the exact chemical compositions of the both the core and shell structures of the QD.
Size and Shape
The size, or hydrodynamic diameter (HD), of QDs can be characterized by a variety of methods, including transmission electron microscopy (TEM), dynamic light scattering, high-solution atomic force microscopy and gel filtration chromatography. As will be discussed in further detail later, the HD may be predictive of whether or not a particular QD will be cleared from or retained in the body. As the HD of QDs will vary considerably depending upon the organic coating surrounding a QD core, it will be important to report the HD for any new QD formulation undergoing regulatory consideration.
Surface Properties and Solubility
QDs are not inherently water soluble—they are hydrophobic by nature. It is therefore necessary to solubilize QDs for applications in a biological environment by altering the surface properties of the QD. Solubilization can be accomplished in a number of ways, but the most common strategies are silanization and surface exchange with bifunctional molecules (i.e., molecules which possess both a hydrophobic side that can bind to the shell of the QD, and a hydrophilic side that can interact in an aqueous biological environment). For certain applications, it may also be desirable to impart certain functional properties upon the QDs. For example, if the QD is to be targeted to a particular cell structure, cell type, or tissue, then a targeting peptide or antibody may be attached to the surface of the QD. As will be described below, the surface composition and solubility properties of QDs can greatly affect the toxicity and biological fate of QDs. Regulators will therefore be interested in descriptions of the surface composition and measures of the solubility of individual QD preparations.
TOXICITY OF QDs
With notable exceptions, the vast majority of scientific papers reporting on the toxicity of QDs have limited their investigations to cytotoxic effects of QDs observed in short-term, cell culture-based assay systems, rather than addressing the question of how QDs might affect the overall growth, viability, and/or reproductive capacity of humans or other species. Interpretation of the body of evidence relating to the cytotoxic effects of QDs is further complicated as a result of the broad diversity of QDs being tested, as each individual type of QD possesses its own physicochemical properties and due to the diversity of test systems used. The dosage of QDs used and exposure times also vary widely in the literature. Furthermore, it is often unclear how the experimental dosages relate to concentrations associated with real-world commercial applications of QDs. It is therefore difficult to extrapolate the results of such studies in order to form any conclusions regarding the health and safety of QDs. Nonetheless, these studies may provide important insights that will be useful in guiding the eventual design of standardized toxicity tests and protocols.
A Summary of Studies Assessing the Toxicity of QDs
In 2006, Ron Hardman authored a comprehensive review regarding the toxicology of QDs and concluded that the toxicity of QDs was dependent upon their physicochemical properties as well as environmental factors (Hardman, 2006). In this seminal review paper, Hardman included a table summarizing the available literature concerning QD toxicity.
Below, in Table 1, we have adapted and extended Hardman's table to summarize studies that have been published following acceptance of the Hardman review paper in September 2005. We have attempted in this exercise to include all relevant studies up to December 2008. For the sake of completeness, the results of older studies, that is, those which were originally reported by Hardman in 2006, are also summarized below, in Table 2.
TABLE 1.
Summary of Quantum Dot Toxicity Studies Published from 2006 to 2008
| Quantum dot composition | Cell, tissue, or organism tested | Assay(s) used | [QD] used | Exposure time | Observed toxicity | Additional observations | References |
| CdTe, CdTe/CdS, CdTe/CdS/ZnS | K562 and HEK293T human cell lines | MTT viability assay | 0.2–3.0μM | 0–48 h | Cytotoxic: cells treated with CdTe and CdTe/CdS QDs were mostly nonviable by 48 h (for all concentrations tested). | ZnS shells may protect from release of cadmium ions and resulting cytotoxicity. | (Su et al., 2009) |
| *QDs were synthesized in aqueous solution. | Not cytotoxic: in contrast, cells treated with CdTeS/CdS/ZnS QD showed no cytotoxic effects up to 48 h (16μM at 24 h was also tested). | Authors postulate that residual organic solvents in nonaqueous QD preparations may have resulted in QD-independent cytotoxic effects in other reports. | |||||
| *QD size was not reported. | |||||||
| CdSe/ZnS-PEG (EviTag T1 490 QD). | Caco-2 (human colon carcinoma) cell line. | MTT viability assay | 0.84–105μM | 0–24 h | Commercially available QD demonstrated low cytotoxicity but induced cell detachment, suggesting possible toxicological effects in the gastrointestinal tract. | Acid treatment (simulated gastric fluid) increased the toxicity of PEG-coated QD, likely by inducing release of free Cd ions by QD degradation. | (Wang et al., 2008) |
| *Commercially available QD. | *In vitro model for small intestinal epithelium (i.e., the ingestion of QD). | Cell attachment assay | |||||
| CdSe | Primary rat hippocampal neuron cells in culture | MTT assay and DAPI staining were both used to assess viability | 1, 10, and 20nM | 24 h | Not cytotoxic: cells treated with 1nM QD for 24 h showed no decrease in cell viability. | Authors concluded that CdSe QD induced cell death of neurons in a dose-dependent manner. | (Tang et al., 2008) |
| *CdSe QD had a HD of 2.38 nm. | Cytotoxic: in contrast, cells treated with 10 and 20nM QD for 24 h showed decreases in cell viability on the order of 20 and 30%, respectively. | Authors also concluded that CdSe QD could induce dysregulation of cytoplasmic calcium levels in neurons. | |||||
| *QD were dialysed prior to use to remove free Cd ions. | |||||||
| CdTe; *red (6 nm), yellow (4 nm), and green (2 nm) variants tested. | HepG2 (human hepatoma) cell line | MTT viability assay | 0–100μM | 48 h | Cytotoxic: Concentrations causing a 50% reduction in MTT activity were 19.1, 4.8, and 3.0μM for red, yellow, and green QD, respectively. | Smaller QD appeared to be more cytotoxic than larger QD in this experimental system. | (Zhang et al., 2007) |
| *Preparations contained free Cd2+ ions. | Free cadmium was at least partially responsible for the observed QD cytotoxicity. | ||||||
| CdTe (red in color; 6 nm diameter) | Intravenous administration into rats | Functional, locomotion, and behavioral measurements; clinical chemistry; urinalysis; and histopathology | 2mM solution; 1 ml/kg administered (i.e., 2 μmol per kg body weight) | 0–24 h. *0, 0.5, 1, 2, 4, 24-h time points | A slight but significant reduction in body weight was observed in CdTe-treated rats (vs. vehicle controls), but few signs of overt toxicity were noted. | Based on an observed transient decrease in locomotor activity 2-h post-treatment, authors conclude that nervous system function may be affected by QD. | (Zhang et al., 2007) |
| CdSe/ZnS QD of two differing sizes and shapes: QD-565 (4.6 nm spheres) and QD-655 (6 × 12 nm ellipses) coated with PEG (neutral), PEG-amine (neg. charge), or polyacrylic acid (pos. charge). | Primary neonatal human epidermal keratinocytes (HEKs). | MTT viability assay was used to assess cytotoxicity. | 0, 0.2, 2.0, and 20nM | 24 and 48 h | Cytotoxicity: Pos. charged QD demonstrated the greatest cytotoxicity, with a 20nM concentration resulting in a significant loss of cell viability by 24 h (both sizes of QD). | Authors conclude that QDs with neutral surface coatings are significantly less toxic to skin cells (in some cases, actually nontoxic) than QDs with positively or negatively charged surface coatings. | (Ryman-Rasmussen et al., 2007) |
| *In vitro model for skin toxicity. | |||||||
| Low cytotoxicity: Treatment of HEKs with neg. charged QD (both sizes at 20nM) resulting in a significant loss of cell viability at 48 h only. | |||||||
| Authors also looked at release of inflammatory cytokines by HEK cells. | Very low cytotoxicity: in general, PEG-coated QD had no effect on cell viability, with the exception that treatment with 20nM PEG-QD-655 resulted in some loss of cell viability at 48 h. | Inflammatory response: only positively charged QDs significantly induced the release of cytokines (IL-1β, IL-6, IL-8). | |||||
| CdSe incorporated in PLA nanoparticles, coated with F-68 (nonionic), CTAB (neg. charge), or SDS (pos.charge). | HepG2 (human hepatoma) cell line | MTT viability assay | 0–400 ppm | 12–72 h | Cytotoxic: all QDs tested induced some loss in cell viability, with > 80% viability upon treatment with F-68 modified QDs. This was in contrast with treatment with CTAB modified CdSe QDs, where cell viability was found to be significantly decreased even at low concentrations (10, 20, and 50 ppm) and with incubations as short as 12 h. | Authors conclude that CdSe QDs modified with F-68 have “low cytotoxicity” based on observation of 80% or better cell viability upon QD treatment. | (Guo et al., 2007) |
| Authors conclude that surface modification with nonionic F-68 is less cytotoxic than modification with pos. charged CTAB. | |||||||
| *Size range was 159–266 nm, which is larger than the size specified in most definitions of nanoparticles. | |||||||
| CdSe/ZnS-Cys, CdTe-MPA, CdTe-Cys, CdTe-NAC | MCF-7 (human breast cancer) cell line | MTT and trypan blue cell viability assays | 10 μg/ml | 1–24 h | Cytotoxic: treatment of cells with all forms of CdTe QD resulted in significant cell death at both 1 and 24 h. | Authors conclude that CdTe QD are toxic and that CdSe/ZnS QD are nontoxic. Authors demonstrate release of free Cd ions by CdTe QD but not CdSe/ZnS QD. Authors conclude that CdTe QD induce cell death via both Cd ion dependent and independent (ROS) mechanisms. | (Cho et al., 2007) |
| Not cytotoxic: cells treated with CdSe/ZnS QD were mostly viable after 24 h of exposure. | |||||||
| CdSe/ZnS QD that were both PEG-coated and silanized | Human HSF-42 (skin fibroblast) and IMR-90 (lung fibroblast) cell lines | Cell proliferation, apoptosis, necrosis, and cell cycle distribution assays; microarray analysis | 0, 8, or 80nM (80nM = 40 mg/ml at M.W. of 500 kDa, or approx. 5 × 1010 QD per mm3). | 48 h | Not cytotoxic: did not see a decrease in cell numbers or increased apoptosis or necrosis in cells at 48 h (slight increase seen in skin but not lung fibroblasts). Evidence suggests only minimal impact on cell health and molecular response of QD exposed skin and lung cells. | QD were internalized by human skin and lung fibroblasts after 48 h of exposure. Gene expression of approx. 0.2% of genes was significantly different in QD-treated skin fibroblasts versus controls. | (Zhang et al., 2006) |
| CdSe/ZnS-peptide. | HEK 293T/17 (human embryonic kidney) and COS-1 (African green monkey kidney) cell lines | CellTiter 96-cell proliferation assay | 15–250nM | 1 h = “acute”; 24 h = “chronic” | Not Cytotoxic: cells treated with varying concentrations of QD for 1 h only demonstrated little or no toxicity. | Authors claim their results demonstrate differences between toxic effects of QD following acute (1 h) and chronic (24 h) exposure conditions. | (Delehanty et al., 2006) |
| *For targeted intracellular delivery. | Cytotoxic: cells treated with QD for 24 h showed significant cell death at higher QD concentrations (60–250nM). | Authors also point to cell type-dependent differences in QD-mediated cell toxicity. |
Note. PEG, polyethylene glycol.
TABLE 2.
Summary of Quantum Dot Toxicity Studies Published Prior to September 2005.
| QD composition | Cell, tissue, or organism tested | [QD] used | Exposure time | Observed toxicity | Reference |
| CdTe | PC12 (rat pheochronocytoma) and N9 (murine microglia) cell lines | 0.01–100 μg/ml | 2–24 h | Cytotoxic: 10 μg/ml | (Lovric et al., 2005) |
| CdSe/ZnS-DHLA QDs | B16F10 cells injected into mice | Injected 20,000–40,000 QD-treated cells into mice. | 4–6 h cell incubation time, mice sacrificed at 1–6 h | Not toxic: No toxicity observed in cells or mice. | (Voura et al., 2004) |
| CdSe/ZnS-MUA | Vero and HeLa cell lines; primary human hepatocytes | 0–0.4 mg/ml | 24 h | Cytotoxic: 0.2 mg/ml in Vero cells, 0.1 mg/ml in HeLa cells, 0.1 mg/ml in hepatocytes. | (Shiohara et al., 2004) |
| CdSe/ZnS-SSA | EL-4 cells (mouse lymphocytes) | 0.1–0.4 mg/ml | 0–24 h | Cytotoxic: 0.1 mg/ml altered cell growth; most cells nonviable at 0.4 mg/ml. | (Hoshino et al., 2004b) |
| CdSe/ZnS-SSA | EL-4 cells labelled with QDs and injected into mice | 0.1 mg/ml QDs per 5 × 107 cells | 2 h to 7 days | Not toxic: No toxicity observed in mice in vivo. | (Hoshino et al., 2004b) |
| CdSe/ZnS conjugates: NH2, OH, OH/COOH, H2/OH, MUA, COOH | WTK1 cells | 1–2μM | 12 h | Cytotoxic: 2μM QD-COOH induced DNA damage at 2 h. | (Hoshino et al., 2004a) |
| CdSe-MAA, TOPO QDs | Primary rat hepatocytes | 62.5–1000 μg/ml | 1–8 h | Cytotoxic: A concentration of 62.5 μg/ml was cytotoxic under oxidative/photolytic conditions. No toxicity observed on addition of ZnS cap to QDs. | (Derfus et al., 2004) |
| CdSe/ZnS | HeLa cells | 10 pmol QDs per 10,000 cells (approx. 10nM) | 10 days (cell culture) | Not cytotoxic: 10nM QD had minimal impact on cell survival. | (Chen and Gerion, 2004) |
| CdSe/ZnS amp-QDs, and mPEG QDs | Mice—QDs injected into tail vein | Injections of approx. 180nM, or 20 pmol/g animal weight | 15-min cell incubations, 1–133 days in vivo | Not toxic: No signs of localized necrosis at the sites of deposition. | (Ballou et al., 2004) |
| CdSe/ZnS-amphiphilic micelle | Mice—QDs injected into tail vein | 60μM/gram animal weight, 1μM and 20nM final [QD]. | Not indicated. | Mice showed no noticeable ill effects upon imaging. | (Larson et al., 2003) |
| CdSe/ZnS-DHLA | Dictyostelium discoideum and HeLa cells | 400–600nM | 45–60 min | No effects on cell growth noted. | (Jaiswal et al., 2003) |
| Avidin-conjugated CdSe/ZnS QDs | HeLa cells | 0.5–1.0μM | 15 min | No effect on cell growth or development noted. | (Jaiswal et al., 2003) |
| CdSe/ZnS-MUA QDs; QD-SSA complexes | Vero cells | 0.24 mg/ml | 2 h | Not cytotoxic: 0.4 mg/ml MUA/SSA-QD complexes did not affect the viability of Vero cells. | (Hanaki et al., 2003) |
| QD micelles: CdSe/ZnS QDs in (PEG-PE)and phosphati-dylcholine | Xenopus blastomeres | 5 × 109 QDs/cell (approx. 0.23 pmol/cell) | Days | Toxic: At a threshold of 5 × 109 QDs/cell, observed cell abnormalities, altered viability and motility. | (Dubertret et al., 2002) |
Note. PEG, polyethylene glycol.
Discussions in the literature relating to the potential toxic effects of nanotechnology applications often point to the fact that the bulk forms of nanomaterials, many of which have been in widespread use for many years, are themselves not toxic. As an example, consider the case of carbon nanotubes, which are nanoforms of carbon.
In the case of QDs, the situation is essentially reversed. The bulk forms of some of the component molecules of QDs—such as cadmium, selenium, and tellurium—are themselves known to be highly toxic. The question therefore becomes one of determining whether in nanoscale format (i.e., in the context of QDs) the toxicity of these substances can be eliminated, or at least drastically reduced.
In 2006, Hardman stated the following regarding the toxicity of QDs: “QD size, charge, concentration, outer coating bioactivity (capping material and functional groups), and oxidative, photolytic, and mechanical stability have each been implicated as determining factors in QD toxicity” (Hardman, 2006). Since 2006, a number of studies have provided further support for this statement. This suggests that the toxicity of QDs can, at least to some extent, be minimized through selection of an appropriate shell coating (Cho et al., 2007; Su et al., 2009), by modulating surface charge (Ryman-Rasmussen et al., 2007) or surface coating (Guo et al., 2007), by selecting a lower overall dosage of QDs (Tang et al., 2008), or by modulating the overall size of the QD (Zhang et al., 2007).
In a number of the toxicity studies summarized in Tables 1 and 2 below and particularly in earlier studies, the presence of free cadmium ions in the QD preparations interferes with extrapolation of the results regarding QD toxicity. This is a major methodological issue which we note is generally being addressed in more recent papers, either through the use of highly purified commercially available QDs or by dialysing QD preparations prior to their use in order to eliminate free cadmium ions. However, although minimizing the presence of free cadmium ions in QD preparations does seem to reduce the toxicity of QDs, this alone does not explain all of the QD toxicity reported in the literature.
Hardman (2006) stated that future research should attempt to evaluate the long-term toxic effects of QDs, as “QD-induced cytotoxicity was generally found in those studies that tended to be longer in nature” (Hardman, 2006). This advice has not been implemented—most of the recent studies (Table 1) relied heavily on the use of short-term in vitro assays, most notably the MTT (otherwise known as 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay of cell viability.
We conclude that the progress on the evaluation of the toxicity of QDs has only progressed marginally since Hardman's review. The most noteworthy changes from the perspective of product regulation are the advances made in QD coatings. Hardman's call for long-term toxicity studies—which likely would be echoed by regulators—remains unanswered by current academic research.
BIOLOGICAL FATE OF QDs
In this section, we will summarize the state of academic knowledge up to December 2008 concerning the biological fate (including ADME) of QDs.
For the purposes of the discussion to follow, we use the term “biological fate” to describe any number of the potential outcomes that may befall QDs. We have defined the term broadly, so as to encompass: (1) potential routes of human exposure to QDs; (2) the potential for the degradation of QDs into their component molecules; (3) the tendency of QDs to aggregate, which may affect their biological properties; (4) the question of whether, once exposure has occurred, QDs will accumulate in cells or tissues or whether they will be excreted into the surrounding environment; and finally (5) the question of what might happen to QDs following the excretion or release into the environment. These issues will each be described in detail in the sections that follow.
Potential Routes of Human Exposure to QDs
Central to a discussion of the toxicology and biological fate of QDs is the question of what potential sources of exposure might result in their uptake or absorption by humans. There are a finite number of potential means by which humans can theoretically become exposed to potentially toxic substances: (1) if airborne, substances could potentially be inhaled; (2) substances could be absorbed through the skin or eyes; (3) substances could be ingested; or (4) they could be injected by intravenous, subcutaneous or other injection methods. Below we discuss these four potential routes of exposure and detail and the state of current knowledge regarding the risk of human exposure to QDs through each mechanism.
Quantum Dot Aerosolization and Inhalation
Assessing human exposure to airborne nanomaterials represents a considerable challenge. As recently discussed by Maynard and Aitken (2007), considerations such as particle number, surface area, mass concentration and the basic physicochemical properties of QDs will likely need to be considered. The potential risks associated with the inhalation of QDs are rarely, if ever, discussed. Whether this is due to the fact that QDs do not readily become airborne or whether the potential for aerosolization has simply not yet been evaluated is unclear.
The greatest potential for aerosolization of QDs likely arises during the synthesis and manipulation phases of QD manufacturing, although we cannot rule out the possibility that future clinical applications of QDs could be formulated as aerosols. The conventional synthesis of QDs uses large volumes of high-boiling organic solvents at high temperatures into which aggressive and toxic chemicals must be quickly injected. The synthesis of QDs using microwave irradiation (Li et al., 2005) and chemical aerosol flow synthesis (Didenko and Suslick, 2005) have also been reported. As such, workplace exposure to aerosolized QDs is an area that will require careful consideration by regulators. We are not aware of any attempts in the literature to date which address the potential effects of inhaled QDs experimentally.
Absorption of QDs through the Skin and Eyes
The skin and eyes can both serve as portals of entry for localized or systemic human exposure to potentially toxic materials. Regulators will therefore want to know whether or not it is possible for QDs to enter the human body through dermal or ocular absorption, as well as the probability of exposure to QDs through these routes.
In an in vitro study, Ryman-Rasmussen et al. (2006) have reported that QDs are able to penetrate intact skin. According to their results, QDs with different core/shell shapes (spherical and ellipsoid) and sizes (4.6 nm in diameter and 12 × 6 nm) and with variably charged (neutral, anionic, or cationic) surface coatings were able to penetrate porcine skin within 24 h. In terms of the generalizability of these results to real-world setting, it is notable that the dosage of QDs administered in this study (62.5 pmol/cm2 of skin) was described as “occupationally relevant” by the authors, and that porcine skin is anatomically, physiologically, and biochemically similar to human skin (Ryman-Rasmussen et al., 2006). We are not aware of any in vivo studies on skin absorption nor any study on ocular absorption of QDs.
Ingestion of QDs
To date, the possible toxicity of ingested QDs has not been explored in any great detail. This may be due to the fact that at present, there is little likelihood of QD applications under active development being administered orally or incorporated into food products or food packaging. Notwithstanding this, regulators will need to understand the extent by which QDs could accidentally be ingested by people who work in the manufacturing industry, research laboratories, or diagnostic facilities. Alternatively, QDs could also be ingested by eating or drinking nanoparticle-contaminated food or water.
A recent report examined the possible toxic effects of ingested QDs, using Caco-2 cells as model for the epithelium of the small intestine. The authors of this report also examined the effect of low pH, simulating conditions that would be encountered in the human stomach, on CdSe QD cytotoxicity (Wang et al., 2008). Exposure of QD to low pH conditions in this report resulted in a loss of integrity of the QD structures, release of free cadmium ions, and therefore an increase in QD toxicity. This evidence demonstrates that the toxic effects of QDs could vary considerably depending upon the route of exposure.
The Direct Injection of QDs into Humans
For the vast majority, if not all, of the potential applications of QDs as in vivo nanodiagnostics and nanomedicines, QDs will likely be administered through direct injection into humans and animals. For applications such as sentinel lymph node mapping in animal models, QDs would be injected directly into tumor tissues, whereas intravenous injections are more likely in other clinical applications. A number of studies have looked at the ADME of injected QDs; these will be discussed in further depth below. For the purposes of conducting risk assessments, it will be important to specify the exact method of injection (intravenous, subcutaneous, etc.), as recent reports have suggested that the fate of QDs differs depending upon the mode of injection.
A Summary of Studies Assessing the Biological Fate of QDs
Increasingly, researchers have begun to address the question of what happens to QDs when they are administered in vivo. Do QDs accumulate in tissues and if so, do they preferentially become distributed in certain tissues versus others? Similarly, is it possible to specifically target QDs to particular tissues? This could be useful when using QDs as nanomedicines or in vivo imaging agents; it might, for example, be useful to specifically target tumor cells or affected lymph nodes in order to either diagnose or treat cancer patients. Once administered, are QDs eventually excreted, or do they tend to accumulate in tissues? What is their half-life following administration? These questions are all relevant to determining the ADME of QDs.
To provide a useful reference for regulators and researchers alike, the results of studies addressing the biological fate of administered QDs have been summarized in Table 3, below.
TABLE 3.
Summary of Studies Reporting the Biological Fate of QDs
| Quantum dot composition and (emitting wavelength) | Hydrodynamic diameter | Route of administration and (model organism) | Duration of exposure (and dose, if reported) | Fate of QD | Observations of in vivo toxicity | Reference |
| CdSeS/silica-hydroxyl (maximal emission at 570 nm) | 21.3 ± 2.0 nm | Intravenous injection (male mice) | 0–5 days (5 nmol per mouse) | - The plasma half-life of QD was 19.8 ± 3.2 h. | Authors noted a lack of toxic effects during the 5-day course of their experiment, but acknowledged that the long-term stability of the CdSeS/SiO2 QDs in vivo remained an unknown factor and that this is an area that will require further study. | (Chen et al., 2008) |
| - The clearance of QD was assessed at 57.3 ± 9.2 ml/h/kg. | ||||||
| - The liver and kidney were the main target organs for QD, but there accumulation was also noted in spleen and lung. The peak concentration of QD accumulation occurred 6-h postinjection (peak was 12 h in the lung). | ||||||
| - In this study, a fraction of free QD was excreted via urine as small molecules within 5 days. | ||||||
| - The majority of QDs bound to protein and aggregated into larger particles; these were metabolized in the liver and excreted via feces. | ||||||
| - QDs in the spleen, lung, and kidney were thoroughly eliminated within 48 h. | ||||||
| - After 5 days, 8.6% of the injected dose of aggregated QDs remained in the liver; it was difficult for this fraction to clear, indicating that clearance of QDs was incomplete. | ||||||
| - By 1-h postinjection, the QD were mostly cleared from the circulation. | ||||||
| - At the 1-h time point, QD conjugated to a lung-specific mAb accumulated primarily in the lung. QD not targeted to the lung accumulated primarily in the liver and spleen. | ||||||
| - By 24 h, lung-targeted QD had redistributed to liver and spleen, suggesting that they were being taken up by cells of the RE system. There was also an increase in radioactivity in the kidney, indicating excretion. | ||||||
| - Temporary inhibition of the RE system demonstrated involvement of RE cells in clearance of QD from lungs and redistribution to liver and spleen. | ||||||
| CdTe/ZnS-mAb (monoclonal antibody (mAb) targeted to lung). | Not reported | Intravenous injection (female mice) | 1 h, 24 h, 7 days, 19 days | - Long whole-body QD retention times were observed (> 2 weeks). | In this study, it was not possible to distinguish between any possible toxic effects of the QD themselves versus toxicity of the radiolabel, therefore no conclusions could be drawn regarding QD toxicity. | (Kennel et al., 2008) |
| *Radiolabeled with Te-125m | ||||||
| CdSeTe/ZnS-methoxy-PEG5000 (705 nm) | 18.5 nm | Intravenous injection—tail vein (mice) | 0–24 h, 1–28 days, and 6-month time points (40 pmol) | No significant excretion or metabolism of QDs was observed in the 28 days following dosing. QD were concentrated in the spleen, liver and kidneys. | Renal tissues, examined at 6 months by TEM, showed proximal tubular degeneration, indicating possible toxicity. Changes in mitochondria were particularly evident. | (Lin et al., 2008) |
| CdSe/ZnS | 16 nm | Injected subcutaneously—right anterior paw (mice) | 0–24 h | QD detected in lymph nodes within minutes (∼2.42% of total dose). Did not detect QD migration to liver, kidneys or spleen. Authors also found no evidence of QD excretion. Peak QD concentration in lymph nodes detected at 60 min; fourfold decrease seen by 24 h. | Toxicity was not assessed in this study. | (Robe et al., 2008) |
| CdSe/ZnS-Cys size series (515–574 nm). | 4.36–8.65 nm | Intravenous injection (rats and mice) | 0–4 h (100 μl of 3μM 99mTc-QD in mice; in rats 10 pmol/g animal weight) | - Radiolabeled QD with diameters of 4.36, 4.99, and 5.52 nm were found to be excreted into the bladder within 4 h. | Toxicity was not assessed in this study—authors argue that in vivo toxicity is less of an issue if QD are excreted. | (Choi et al., 2007) |
| *Zwitterionic surface charge (Cys) found to prevent serum protein absorption; this produced the highest solubility and smallest possible diameter. | ||||||
| - QD larger than 5.6 nm were never excreted, but instead were found to be trapped in the liver, lung, and spleen. | ||||||
| - The blood half-life of QD ranged from 48 min to 20 h, as the diameter of QD increased from 4.36 to 8.65 nm. | ||||||
| CdSe/ZnS-PEG (655 nm); and CdSeTe/ZnS-PEG (800 nm) | 22.6, 30.4, and 41.2 nm | Injection into human and mouse tumor models (mice) | 0–90 min. Animals kept for up to 2 years (5–25 μl of a preparation containing 25–100 pmol QD) | Injection of QD into tumors yielded rapid migration (within minutes) into adjacent sentinel lymph nodes. | Authors state that the toxicity of amp-coated CdSe/ZnS QD was minimal or nonexistent for over 2 years, as assessed by pathological examination of animals. | (Ballou et al., 2007) |
| CdSe/CdS-PEG (621 nm) | 37 nm | Intradermal injection—right dorsal flank (mice) | 0–24 h | Majority of QD remained at site of injection. Detected QD in regional lymph nodes within minutes. At 12–24 h, detected QD primarily in the liver (∼6% of total dose), lymph nodes (∼1%) and kidneys (∼0.5%). Also detected QD in spleen, hepatic lymph node and heart (heart may have been an artefact due to method of animal sacrifice). | Toxicity was not assessed in this study. | (Gopee et al., 2007) |
| CdTe/ZnS-PEG (commercially available QD705) | 13 nm | Intravenous injection via tail veins (mice) | Up to 28 days: 1, 4, 24 h, 3, 7, 14, and 28 days (40 pmol per mouse) | - Plasma kinetics revealed a clearance rate from the blood of 2.3 ml/h/kg. The plasma half-life was calculated at 18.5 h. | - Tissues were subject to pathological examination. This analysis revealed marked sinusoidal congestion and increased multinucleated giant cells in vascular areas of the spleen. Notably, liver and kidneys displayed no remarkable abnormalities. | (Yang et al., 2007) |
| - QD persisted in spleen, liver, and kidneys throughout the experimental period (up to 28 days). QD levels in liver and kidneys increased over time. | ||||||
| - QD were not detectable in feces and were present only at low levels in urine, indicating that essentially no excretion occurred in 28 days. | ||||||
| CdTe/ZnS-DOTA ± peptide (commercially available QD705) | ∼20 nm | Intravenous injection (tumor-bearing mice) | 1-, 5-, 18-, and 25-h time points (20 pmol per mouse) | - Peptide conjugated QD were specifically targeted to tumors. | Toxicity was not assessed in this study. | (Cai et al., 2007) |
| - For both peptide conjugated and unconjugated QD, a majority of the QD were found to localize to liver (100:1), spleen (40:1) and bone marrow (ratios represent tissue-to-muscle ratios). To a lesser extent, QD also localized to the kidneys (1:1 ratio) and lymph nodes. | ||||||
| CdSe/ZnS ± PEG (commercially available QD525 and QD800) | 21 and 12 nm | Intravenous injection (mice) | 0–15 min following injection, then 1.0, 4.5, 12, and 36 h (25 pmol per mouse) | - The circulation time of PEG-coated QD was 6 min (vs. 2 min for uncoated QD). | - Authors comment that no evidence of acute toxicity was observed during and following the experimental period. Authors also comment that their data “suggest” that the QD exhibited good stability in vivo, though they acknowledge that no formal serum stability studies were performed. | (Schipper et al., 2007) |
| - The major organ of uptake of QD was liver; QD also found in spleen. | ||||||
| - Localization of QD to liver and spleen was almost immediate (within 2 min). | ||||||
| - Found that the size of the QD had no effect on biodistribution, within the size range tested in this study. | ||||||
| - Authors found no evidence of clearance of QD from mice. | ||||||
| - PEG-coated QD also showed low-level uptake to bone. | ||||||
| CdTe/ZnS-mAb targeted to lung | Diameter not reported: molecular weight was 1–5 × 106 Da | Intravenous injection (mice) | 0–144 h | - QD bound to a lung-specific monoclonal antibody (mAb) were effectively targeted to the lung and remained in lung for up to 6 days. | Toxicity was not formally assessed in this study, but authors noted that no acute toxicity was observed. | (Woodward et al., 2007) |
| - QDs bound to a control mAb were found to migrate primarily to the spleen, liver, and kidneys. | ||||||
| - Authors observed that QD were cleared from the body to a limited extent, but that clearance was slow. | ||||||
| CdSe/ZnS-LM, CdSe/ZnS-BSA | 25, 80 nm | Intravenous injection (male Sprague-Dawley rats) | 10 days (5 nmol dose per rat) | - QD half-lives were etermined to be 39–59 min; QD were cleared from the plasma between 0.59 and 1.23 ml/min/kg. | Toxicity was not assessed in this study. | (Fischer et al., 2006) |
| - By 90 min, approx. 90% of the BSA-coated QD were found in the liver; other tissues (spleen, lymph nodes, bone marrow) also retained small amounts. | ||||||
| - There were distinct differences between the plasma clearance and tissue distribution of uncoated and BSA-coated QD. | ||||||
| - Authors could not detect QD in either feces or urine, and therefore concluded that the QD were not excreted. | ||||||
| InAs/ZnSe-DHLA-PEG | 8.7 nm | Injected both subcutaneously and intravenously (in both mice and rats) | Approximately 5 min | - QD were specifically engineered to have small diameters. | Toxicity was not assessed in this study. | (Zimmer et al., 2006) |
| - When injected subcutaneously, QD migrated to sentinel lymph nodes, as observed previously with larger QD, but also migrated further into the lymphatic system. | ||||||
| - QD injected intravenously were shown to extravasate from the vasculature (first demonstration of this point in the literature). | ||||||
| CdSe/ZnS-MAA-targeting peptides ± PEG (maximal emission spectra at both 550 and 625 nm) | In absence of peptide: 3.5 nm (green) or 5.5 nm (red). Diameter with peptide not reported, but size was approx. 190 kDa. | Intravenous injection into the tail vein (mice) | 5–20 min | - QD were specifically targeted to the circulatory systems of normal lungs and tumors using peptides. | Toxicity was not assessed in this study. | (Akerman et al., 2002) |
| - QD also accumulated in the liver and spleen, regardless of the peptide used for targeting. | ||||||
| - Adding PEG to the QD was shown to partially inhibit the nonspecific uptake of QD into the liver and spleen (suggesting the involvement of RE cells). |
Note. PEG, Polyethylene glycol.
Two basic methodologies have been used in the literature to examine the biological fate of QDs following in vivo administration in lab animals. The first methodology has taken advantage of the fluorescent properties of QDs; researchers determine the biodistribution of QDs following administration by tracking the fluorescent particles. In this regard, QDs offer a considerable advantage over many nanoparticles, in that their luminescent properties render them highly suitable for studies evaluating biological fate.
The second methodology involves generating radiolabeled versions of QDs (e.g., CdTe QDs containing radioactive Te-125m) and using these radioactive variants to track the biodistribution of QDs. One advantage of the radiolabeling methodology is that it allows for the derivation of quantitative data regarding fate. On the other hand, the tracking of radioactivity does not allow for distinguishing between QDs which remain active and those that become inactive, including those which have been degraded into their component molecules. In contrast, only intact QD particles should continue to fluoresce.
As with toxicity, a number of studies have concluded that the pharmacochemical properties of QDs including their size, solubility, aggregation and surface composition may influence the fate of the injected nanoparticles. For this reason, a concerted effort has been made in Table 3 below to report the specific properties of the QDs being assessed in each study, including their chemical composition, emitting wavelength, HD, and overall surface charge.
Of particular interest from a regulatory perspective, it has been suggested that the HD of QDs may influence whether or not they are excreted or will accumulate in tissues (Choi et al., 2007). For this reason, we have included a column to report the HD of the QDs under evaluation in each study.
We have also indicated the concentration, or dosage, of QDs that was utilized in each study. Because dose-response evaluations are a critical feature of the regulatory risk assessment process, we were pleased to note that authors are increasingly taking care to report the number of particles administered (nmol and pmol), rather than the overall mass. In some cases, authors have included observations of in vivo toxicity in their reports; these have been summarized in Table 3 under the column heading “Observations of in vivo toxicity.” Results of studies to date have yielded certain commonalities, but as yet there is no general agreement as to the fate of administered QDs. This will be discussed below under the sections on the accumulation and excretion of administered QDs.
Accumulation of Administered QDs in Organ Tissues
Based on the results of a number of studies looking at the biodistribution (and occasionally pharmacokinetics) of QDs administered in vivo, it is possible to reach a few tentative generalizations regarding the preference of QDs for accumulation in certain target organs.
First, administered QDs are generally completely—and likely also rapidly—cleared from the bloodstream. With respect to the timing of clearance of QDs from the bloodstream, estimates of the half-life of administered QDs in vivo vary from one report to another. Interestingly, one report noted that the blood half-life of a series of QDs varied considerably depending upon the HD of the QDs; aside from varying in terms of their size, the QDs in this series were otherwise physicochemically identical in terms of their composition (Choi et al., 2007). In this report alone, the blood half-life varied from 48 min to 20 h—a rather wide range. However, all of the reports in the literature were unanimous in concluding that QDs show a preference for deposition in organs and tissues and that they do not remain circulating in the bloodstream.
Second, QDs injected intravenously are more likely to accumulate in the liver and spleen. To a lesser extent, QDs injected in this fashion have also been detected in kidneys, lymph nodes, and bone marrow. A subset of the reports summarized in Table 3, for example the 2008 report by Kennel et al. (2008), have attributed the observed migration to the liver and spleen to clearance of QDs by phagocytic cells of the reticuloendothelial (RE) system, which suggests that QDs residing in organ tissues have already been internalized by cells.
When QDs are injected either subcutaneously (Robe et al., 2008; Zimmer et al., 2006), intradermally (Gopee et al., 2007), or directly into animal tumor tissues (Ballou et al., 2007), the pattern of organ deposition is different. QDs injected subcutaneously or into tumors seem to migrate to nearby lymph nodes and remain there. In the one study which looked at intradermal injection, QDs were found in liver, lymph nodes, and kidneys, but the vast majority of the QDs remained at the site of injection. The results of Zimmer et al. (2006) suggest that when the size, or HD, of QDs is above a certain threshold limit (in their study, they estimate this threshold to be approximately 10 nm), this may limit the ability of the QD to migrate further into the lymphatic system or to extravasate from the vasculature. Thus, a likely reason for the dependence upon mode of injection on final fate is that the scope of the migration of QDs in vivo is effectively limited by their size.
It is worth noting that there are no reports to date describing the migration of injected QDs into the brain. Whether this means that QDs are incapable of crossing the blood-brain barrier or whether they are simply cleared too quickly from the circulation by cells of the RE system is a question which, to the best of our knowledge, has not yet been assessed.
Excretion of Administered QDs
In their paper looking at the renal clearance of QDs, Choi et al. (2007) posit that an analysis of bodily fluids, including urine, bile and feces, should be a mandatory part of any human risk assessment following environmental exposure to nanoparticles. Provided that the initial exposure dose is known, such an analysis could help to estimate the total retained dose of nanoparticles.
To date, there have been a few studies in the literature which have looked at the excretion of QDs following their in vivo administration. Results to date have varied, and will be described in further detail below.
Lin et al. (2008) performed in-depth pharmacokinetic and toxicology studies in mice at time points of up to 6 months. According to their results, commercially available Qtracker 705 nontargeted QDs (QD705) injected intravenously into mice accumulated primarily within the liver, spleen, and kidney. The authors could find no evidence of excretion or metabolism of the QD705 nanoparticles within 28 days following dosing. Concerned by the persistence of the QDs, the authors examined the kidneys by TEM at 6-months postdosing, and observed significant renal toxicity in the dosed but not control mice. The “subtle but definitive” cytological changes noted in dosed mice consisted of proximal tubular degeneration, with pronounced changes in mitochondria in the proximal convoluted tubules. Based on these results, the authors caution that the in vivo administration of QD705 may be highly toxic.
In contrast, other studies have demonstrated efficient excretion of QDs by mice. For example, Chen et al. (2008) assessed the biodistribution and excretion paths of water soluble hydroxyl group-modified silica coated CdSeS QDs that were intravenously injected into mice. In contrast to the results described above (Lin et al., 2008), in this study the majority of injected QDs were cleared from mice, via both feces and urine, within 5 days following injection. Only a small amount (approximately 8.6%) of the injected QD dose was retained in the mouse beyond the 5-day time point (although the authors did comment that this remaining dose seemed to resist clearance from the liver, where they seemed to accumulate). Combined with the observed long-term stability of the silica coated CdSeS QDs, the results of Chen et al. seem to indicate few toxic effects linked to the in vivo administration of CdSeS QDs in mice.
From a safety and regulatory perspective, the 2007 report by Choi et al. (2007) opens an interesting avenue. This report demonstrated that, for CdSe/ZnS QDs with a zwitterionic charge and coated with cysteine, there appears to be a threshold HD (in this case less than approximately 5.5 nm) below which QDs are effectively cleared from the body through urine and bile. The authors are justifiably cautious about overinterpretation of their results, pointing out that measurements of diameter are inherently unreliable and therefore should not be substituted in lieu of rigorous testing for clearance from the body. However, these results suggest that it may be possible to optimize QDs for biological applications in such a way as to maximize their excretion from the body. Any toxic effects associated with QD administration to a patient would thereby be minimized. This does exclude, however, the potential of an environmental impact during the subsequent environmental fate.
However, the next logical question becomes: what would happen to QDs following their excretion from the human body? What would be the possible effects of QD accumulation in the environment following excretion? In this vein, the fledgling body of literature regarding the possible food chain transfer and bioaccumulation of QD is summarized below.
Food Chain Transfer and Bioaccumulation of QDs
In a November 2008 report by the UK Royal Commission on Environmental Pollution, the Commission noted that, with respect to nanomaterials, “there is a consensus that mechanisms of toxicity are poorly understood and that, with minor exceptions, appropriate ecological studies have not been undertaken, including studies that address food chain transfer and multigenerational effects” (Royal Commission on Environmental Pollution, 2008). It is therefore noteworthy that among the first reports to appear in the literature regarding the ecotoxicity and food chain transfer of nanoparticles are three publications pertaining to QDs (Bouldin et al., 2008; Gagne et al., 2008; Holbrook et al., 2008).
In one report, the authors examined the toxic effects of cadmium-telluride (CdTe) QDs on freshwater mussels (Gagne et al., 2008). This study concluded that uncoated (i.e., no shell structure) CdTe QDs were immunotoxic to freshwater mussels within 24 h, leading to oxidative stress (lipid peroxidation) in gills and genotoxicity (DNA damage) in the gills and digestive glands. The toxic effects of uncoated QDs are well documented in previous in vitro toxicity studies; this study supports the observed in vitro toxicity of uncoated CdTe QDs in an in vivo model of ecotoxicity.
Another report looked at the effects of commercially available cadmium-selenium QDs coated with a ZnS shell (Qdot 545 ITK Carboxyl QDs) on the freshwater alga Pseudokirchneriella subcapitata and the cladoceran Ceriodaphnia dubia (Bouldin et al., 2008). These model organisms were selected by the authors “because they are established model species in standard toxicological studies and ecological risk assessments,” and because they “provide a simple model for food chain transfer.” In this study, the authors found that aquatic organisms exposed to QDs were able to withstand concentrations of cadmium that were 500-fold or greater higher than was the case for bulk cadmium. This result is contrary to the widely held view that nanoforms of toxic materials (in this case, cadmium) are likely to have toxicological effects at lower concentrations due to their high surface area. Because in QDs, the cadmium is encapsulated by a shell substance, the nanoform of this substance appears to be overall less toxic than its bulk counterpart.
High concentrations of the coated QDs tested in this report were found to have lethal toxicological effects on freshwater algae: the median lethal concentration of QDs on P. subcapitata at 96 h was measured at 37.1 parts per billion (ppb). No lethality was found following 48 h of exposure of C. dubia to QDs at the highest concentrations tested (110 ppb), which suggested that toxic cadmium from the QD core was not bioavailable to the cladoceran species. One note of caution, however, is that this study found that QDs could be transferred up the food chain from dosed algae to C. dubia. Bioaccumulation effects could therefore theoretically result in potential exposure of higher order organisms to concentrations of QDs beyond what could be achieved in this experimental system.
A third report looked at the effects of QDs in an invertebrate rather than aquatic food chain, focusing on representative bacteria (E. coli), ciliate (Tetrahymena pyriformis), and rotifer (Brachionus calyciflorus) species (Holbrook et al., 2008). In this simplified invertebrate food web, the authors did not observe any significant bioconcentration or biomagnifications of QDs. This study utilized commercially available ellipsoid-shaped CdSe/ZnS QDs, and evaluated the effects of both carboxylated and biotinylated QDs. In this experimental system, there was no evidence of QD uptake by individual E. coli bacterial cells. Both carboxylated and biotinylated QDs could become attached to the exterior surface of aggregated E. coli cells, but there was no evidence of ingestion of these bacterial aggregates by the ciliates.
Despite the lack of trophic transfer from bacterial cells to ciliates, the authors did find evidence that QDs in aqueous media could bioconcentrate in ciliate species. Both the biotinylated and carboxylated QDs were taken up by the ciliates (although there were differences noted in the rate of uptake), and biotinylated QDs were furthermore found to be retained more than twice as long as carboxylated QDs. These results suggest that physicochemical properties of QDs, such as surface composition may modulate bioconcentration effects (Holbrook et al., 2008). Trophic transfer of QDs between the ciliates and rotifers was shown to occur, however the rotifers were able to eliminate the QDs. Quantum dot half-lives in rotifers ranged from 14.5 to 23.1 h and appeared to be independent of surface chemistry. This result suggested that although bioconcentration can occur in ciliate species, bioaccumulation resulting from ciliate predation would not be expected to occur in rotifers.
The three studies on the possible environmental effects described above are clearly a step in the right direction. These studies have shown the potential for bioaccumulation in aquatic species, but no evidence of bioaccumulation in an invertebrate food web. Further studies will be required in order to validate and expand upon these preliminary results.
Stability and Aggregation
One component of fate that we have not yet discussed is the potential for QDs to either degrade into their component molecules (i.e., their stability) or to become transformed by aggregation into higher order structures. In the section on toxicity above, we noted that the degradation of QDs and consequent release of free cadmium ions contributed to the overall toxicity of QD. In their report evaluating the biodistribution and metabolism of silica coated QDs, Chen et al. demonstrated that the aggregation state of QDs in vivo influenced their capacity to be excreted from the body, as well as the path by which QD were metabolized (Chen et al., 2008). From a regulatory perspective, the capacity for QDs to degrade or become transformed is therefore of great importance. Below, we will discuss factors which are known to influence the stability and aggregation potential of QDs.
Woodward et al. (2007) recently assessed the chemical stability of radiolabeled CdTe QDs in an aqueous environment. When uncapped CdTe core QD were suspended in aqueous buffer, approximately half of the radioactivity contained within the QDs was released into the environment within 3 days. In contrast, CdTe cores capped with a ZnS shell demonstrated vastly increased stability. In fact, CdTe/ZnS QDs remained stable in aqueous buffer for up to 36 days. This report suggests that the capping of QDs with ZnS significantly enhances their stability in aqueous media.
Researchers have also begun to explore the potential effects of pH on the overall stability of QDs. Chen et al. (2008) looked at the effect of pH (pH 4.8 vs. pH 7.4) on the stability of CdSeS/SiO2 QDs and found that these QD maintained their integrity for up to 5 days in both high and low pH buffers. In fact, they could not detect any leaching of free Cd ions from CdSeS/SiO2 QDs, suggesting that these dots were extremely stable in either pH environment. In contrast, Wang et al. assessed the stability of commercially available polyethylene glycol-coated CdSe/ZnS QDs and concluded that a low pH environment led to a loss of QD integrity and release of free cadmium ions (Wang et al., 2008). Thus the chemical composition of QDs appears to be one factor which influences the stability of QD in a low pH environment.
In a 2004 study, Derfus et al. (2004) demonstrated that exposure of CdSe QDs to air and ultraviolet light led to the degradation of the QD and the consequent release of free cadmium ions. This in turn increased the toxicity of QD that were exposed to air and UV light. The fact that air and UV light can destabilize QDs may not be of particular significance in the context of QDs administered to humans, but it could become a major factor when looking at the potential long-term effects of QDs released into the environment.
Another factor that will be of significant interest in terms of predicting the fate of QDs will be the tendency of the dots to aggregate into higher order structures. Several groups have observed the aggregation of QDs under a variety of conditions. For example, Zhang et al. (2007) recently assessed the stability of CdTe nanoparticles under cell culture conditions and observed the apparent agglomeration of red CdTe nanoparticles over time. The authors additionally noted that this aggregation of QDs was primarily extracellular. Another report looked at the tendency of CdTe QDs to aggregate when dissolved in aquarium water. In this study, it was observed that QD showed a clear tendency to aggregate in the particulate phase, whereas only approximately 15% of QDs were found in the dissolved phase (Gagne et al., 2008). This study suggested that QDs in an ecologically relevant aqueous environment may have a predisposition toward aggregation.
The surface chemistry of various QDs will likely affect their tendency to self-aggregate, and aggregated QDs may have very different health and environmental effects than nonaggregated particles. Research into the tendency of QDs to aggregate has been limited to date; going forward, it will be important to investigate the impacts of aggregation on the stability and biological effects of QDs.
A more complete understanding of both the stability and aggregation potential of QDs will be required in order to further elucidate both the biological and environmental fates of QDs.
FUTURE RESEARCH NEEDS FROM A REGULATORY PERSPECTIVE
At this early stage in the commercial development of QDs, the risk-relevant information available in the academic literature is still limited. Below we will discuss some lessons for regulators and researchers to keep in mind during the iterative process that may (or may not) lead to specific regulatory requirements for QD based products.
The Diversity of QD as a Product Class May Present a Substantial Regulatory Challenge
It is clear that the inherent toxicological potencies of various QDs differ significantly between various QD preparations. The composition of the core, shell, and surface coatings, as well as the overall size and shape of the QD may all impact upon the toxicological profile of different QD. As a result, some detailed and completed case-by-case risk assessments will need to be completed before any attempts at generalizing regulations across a specific group or the full spectrum of QDs might become possible. At this early stage, it is important that researchers continue to report on as many of the properties of the QD preparations that they are using in their studies as possible—this will greatly facilitate any future attempts at generalization. Regulators may want to think about incentives to promote this knowledge transfer. Because of the real possibility that QDs can become degraded, it will be additionally be necessary to report not only on the properties of the overall QD construct, but additionally its component molecules and concentrations. This will be particularly important in the case of QDs which are made up of substances like cadmium, selenium, and tellurium, which have known toxicological properties.
Where Possible, Studies of the Toxicity, and Biological Fate of QD Should Utilize Realistic Dosages
Researchers are making rapid progress in terms of understanding which factors (such as surface coatings and overall size) can be manipulated in order to reduce the overall toxicity of QDs and to improve the rate of their excretion from the human body. Ultimately, however, regulators will be interested in assessments of dose-response relationships. Admittedly, because QDs are as yet still at an early stage in terms of the development of commercial applications, it remains difficult to determine what might be realistic human and environmental exposure levels. However, it remains important to report on dosages and where possible, to utilize meaningful doses in all experimentation. Without an estimation of realistic dose levels to inform dose-response experimentation, it will not be possible for regulators to carry out a meaningful risk assessment.
Toxicity Data to Date are Insufficiently Standardized and based on too Few Endpoints
Research to date has been focused on in vitro assays of cytotoxicity. In vitro studies are very important and can serve as background information to inform the design of in vivo studies, but on their own they provide an insufficient basis for a complete risk assessment. Once the relationship between in vitro and in vivo assays of QDs is better understood, however, regulators may find great utility in rapid, cheap, and highly standardized in vitro assays. We should note that there is considerable pressure from European regulators to improve the utility of in vitro studies and the ability to extrapolate from in vitro to in vivo data in a regulatory context.
Cytotoxicity is an important starting point for beginning to understand the biological effects of QDs, but it is not sufficient as a sole endpoint. For applications of QDs as diagnostic or therapeutic tools, researchers are advised to carefully examine the existing regulatory requirements for pharmaceutical products. These requirements will provide important clues as to which data points regulators may require in order to complete a premarket regulatory risk assessment. The route of administration and the doses used will be key considerations in risk assessments. Data on the biological fate are also required (see below). Studies in the literature have thus far tended to focus on either toxicity or biological fate as endpoints. There would be great merit, moving forward, in designing experiments in such as way as to allow the simultaneous collection of data on both toxicity and biological fate.
Toxicity studies to date have been conducted on a variety of both human and nonhuman cells and cell lines, including the studies described above in the section on the food chain transfer and bioaccumulation of QDs. These data will be helpful in estimating the variance in susceptibilities across different species. Depending upon the location and quantities of QDs that may be found to be released into the environment and their environmental fate, regulators may require data on the toxicity of QDs on indicator species (e.g., water fleas are often used in this context) or other species that are particularly susceptible or exposed. Here, the existing regulatory requirements for the environmental assessment of pharmaceutical products and the assessment of so-called “new substances” under Toxic Substances Control Act in the United States and the Canadian Environmental Protection Act in Canada will provide important clues for researchers about which endpoints regulators may require in the assessment of QDs. The Organization for Economic Co-operation and Development, already an established leader for international testing protocols for new substances, is also leading international efforts in the standardization of regulatory protocols for nanomaterials.
Biological Fate Data are Insufficiently Standardized and based on too Few Endpoints
Our literature survey has shown that the degradation of QDs may be promoted by low pH, air, and ultraviolet light. The QD shell and surface coating may be critical in preventing or delaying this degradation process and, thus, the release of toxic substances such as free cadmium ions from the QD core. This fact will need to be taken into account in the design of studies on toxicity and biological fate. Experimental data suggests that uptake of QDs through the skin is a possible route of human exposure. To date, very little or nothing is known about the likelihood or possibility of QD entry through the eyes, nose or mouth, or via inhalation or ingestion.
Administration of QDs by intravenous injection in model animals has been shown to lead to accumulation of QDs in tissues, and primarily in the liver and spleen. The rate of QD accumulation in the human body will be of critical importance to regulators. Reports such as that by Choi et al. (2007), who reported that QDs below a certain threshold size limit may be efficiently excreted from the body whereas larger QD may accumulate, deserve a great deal of regulatory attention. This study in particular should be extended to examine alternate QD formulations, compositions, and shapes, to help facilitate any future generalizations regarding size thresholds in the regulatory context.
The results from a number of studies have indicated that the placement of molecules such as proteins onto the surface of QDs can greatly impact their pharmacokinetics and biodistribution. Yet, in a number of the in vitro studies summarized in Table 3, there have been no observations on whether animal serum proteins are adsorbed to the surface of the nanoparticles or whether the particles themselves are becoming aggregated. The lack of these observations makes it difficult to compare studies, to understand cause-effect relationships, and to link results from in vitro studies to those observed in vivo.
Research examining the environmental fate of QDs has just begun and interesting results have emerged. However, the existing data on food chain transport, bioaccumulation or biomagnification, and persistence in the natural environment are as yet insufficient to inform a complete environmental risk assessment, even for those products that have been tested. The extrapolation of the environmental risk assessments of one QD to other products is, as mentioned above, another step that will likely require additional data. We should note, however, that the quantity of environmental releases of QDs may eventually be found to be so limited that regulators may judge a complete environmental assessment to be a low priority.
FUNDING
Genome Canada through the Ontario Genomics Institute (grant number 2004-OGI-3-15); and McLaughlin Centre for Molecular Medicine supported A.S.D.
The McLaughlin-Rotman Centre for Global Health, Program on Ethics and Commercialization, is based at the University Health Network and University of Toronto and is primarily supported by Genome Canada through the Ontario Genomics Institute and the Ontario Research Fund, and the Bill and Melinda Gates Foundation. Other matching partners are listed at http://www.mrcglobal.org/.
Acknowledgments
The authors would like to acknowledge the helpful comments by three anonymous reviewers as well as the sources of funding described above. All errors and misconceptions remain our own.
References
- Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzazy HME, Mansour MMH, Kazmierczak SC. From diagnostics to therapy: Prospects of quantum dots. Clin. Biochem. 2007;40:917–927. doi: 10.1016/j.clinbiochem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Ballou B, Ernst LA, Andreko S, Harper T, Fitzpatrick JAJ, Waggoner AS, Bruchez MP. Sentinel lymph node imaging using quantum dots in mouse tumor models. Bioconjug. Chem. 2007;18:389–396. doi: 10.1021/bc060261j. [DOI] [PubMed] [Google Scholar]
- Ballou B, Lagerholm BC, Ernst LA, Bruchez MP, Waggoner AS. Noninvasive imaging of quantum dots in mice. Bioconjug. Chem. 2004;15:79–86. doi: 10.1021/bc034153y. [DOI] [PubMed] [Google Scholar]
- Bertin G, Averbeck D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review) Biochimie. 2006;88:1549–1559. doi: 10.1016/j.biochi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Bouldin JL, Ingle TM, Sengupta A, Alexander R, Hannigan RE, Buchanan RA. Aqueous Toxicity and food chain transfer of quantum dots in freshwater algae and ceriodaphnia dubia. Environ. Toxicol. Chem. 2008;1:1958–1963. doi: 10.1897/07-637.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Chen K, Li ZB, Gambhir SS, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J. Nucl. Med. 2007;48:1862. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- Chen F, Gerion D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett. 2004;4:1827–1832. [Google Scholar]
- Chen Z, Chen H, Meng H, Xing G, Gao X, Sun B, Shi X, Yuan H, Zhang C, Liu R, et al. Bio-distribution and metabolic paths of silica coated CdSeS quantum dots. Toxicol. Appl. Pharmacol. 2008;230:364–371. doi: 10.1016/j.taap.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Cho SJ, Maysinger D, Jain M, Roder B, Hackbarth S, Winnik FM. Long-term exposure to CdTe quantum dots causes functional impairments in live cells. Langmuir. 2007;23:1974–1980. doi: 10.1021/la060093j. [DOI] [PubMed] [Google Scholar]
- Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delehanty JB, Mattoussi H, Medintz IL. Delivering quantum dots into cells: Strategies, progress and remaining issues. Anal. Bioanal. Chem. 2008 doi: 10.1007/s00216-008-2410-4. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- Delehanty JB, Medintz IL, Pons T, Brunel FM, Dawson PE, Mattoussi H. Self-assembled quantum dot-peptide bioconjugates for selective intracellular delivery. Bioconjug. Chem. 2006;17:920–927. doi: 10.1021/bc060044i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didenko YT, Suslick KS. Chemical aerosol flow synthesis of semiconductor nanoparticles. J. Am. Chem. Soc. 2005;127:12196–12197. doi: 10.1021/ja054124t. [DOI] [PubMed] [Google Scholar]
- Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- Fischer HC, Liu L, Pang KS, Chan WCW. Pharmacokinetics of nanoscale quantum dots: In vivo distribution, sequestration, and clearance in the rat. Adv. Funct. Mater. 2006;16:1299. [Google Scholar]
- Gagne F, Auclair J, Turcotte P, Fournier M, Gagnon C, Sauve S, Blaise C. Ecotoxicity of CdTe quantum dots to freshwater mussels: Impacts on immune system, oxidative stress and genotoxicity. Aquat. Toxicol. 2008;86:333–340. doi: 10.1016/j.aquatox.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Gopee NV, Roberts DW, Webb P, Cozart CR, Siitonen PH, Warbritton AR, Yu WW, Colvin VL, Walker NJ, Howard PC. Migration of intradermally injected quantum dots to sentinel organs in mice. Toxicol. Sci. 2007;98:249. doi: 10.1093/toxsci/kfm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Liu W, Liang J, He Z, Xu H, Yang X. Probing the cytotoxicity of CdSe quantum dots with surface modification. Mater. Lett. 2007;61:1641–1644. [Google Scholar]
- Hanaki K, Momo A, Oku T, Komoto A, Maenosono S, Yamaguchi Y, Yamamoto K. Semiconductor quantum dot/albumin complex is a long-life and highly photostable endosome marker. Biochem. Biophys. Res. Commun. 2003;302:496–501. doi: 10.1016/s0006-291x(03)00211-0. [DOI] [PubMed] [Google Scholar]
- Hardman R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hild WA, Breunig M, Goepferich A. Quantum dots–Nano-sized probes for the exploration of cellular and intracellular targeting. Eur. J. Pharm. Biopharm. 2008;68:153–168. doi: 10.1016/j.ejpb.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Holbrook RD, Murphy KE, Morrow JB, Cole KD. Trophic transfer of nanoparticles in a simplified invertebrate food web. Nat. Nanotechnol. 2008;3:352–355. doi: 10.1038/nnano.2008.110. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Fujioka K, Oku T, Suga M, Sasaki YF, Ohta T, Yasuhara M, Suzuki K, Yamamoto K. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004a;4:2163–2170. [Google Scholar]
- Hoshino A, Hanaki K, Suzuki K, Yamamoto K. Applications of T-lymphoma labeled with fluorescent quantum dots to cell tracing markers in mouse body. Biochem. Biophys. Res. Commun. 2004b;314:46–53. doi: 10.1016/j.bbrc.2003.11.185. [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Biological applications of quantum dots. Biomaterials. 2007;28:4717–4732. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Kennel SJ, Woodward JD, Rondinone AJ, Wall J, Huang Y, Mirzadeh S. The fate of MAb-targeted Cd(125m)Te/ZnS nanoparticles in vivo. Nucl. Med. Biol. 2008;35:501–514. doi: 10.1016/j.nucmedbio.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, Webb WW. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science. 2003;300:1434–1436. doi: 10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]
- Li ZB, Cai W, Chen X. Semiconductor quantum dots for in vivo imaging. J. Nanosci. Nanotechnol. 2007;7:2567–2581. doi: 10.1166/jnn.2007.628. [DOI] [PubMed] [Google Scholar]
- Li L, Qian H, Ren J. Rapid synthesis of highly luminescent CdTe nanocrystals in the aqueous phase by microwave irradiation with controllable temperature. Chem. Commun. (Camb). 2005;4:528–530. doi: 10.1039/b412686f. [DOI] [PubMed] [Google Scholar]
- Lin P, Chen JW, Chang LW, Wu JP, Redding L, Chang H, Yeh TK, Yang CS, Tsai MH, Wang HJ, et al. Computational and ultrastructural toxicology of a nanoparticle, quantum Dot 705, in mice. Environ. Sci. Technol. 2008;42:6264–6270. doi: 10.1021/es800254a. [DOI] [PubMed] [Google Scholar]
- Lovric J, Bazzi HS, Cuie Y, Fortin GR, Winnik FM, Maysinger D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J. Mol. Med. 2005;83:377–385. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Aitken RJ. Assessing exposure to airborne nanomaterials: Current abilities and future requirements. Nanotoxicology. 2007;1:26–41. [Google Scholar]
- Medintz IL, Mattoussi H, Clapp AR. Potential clinical applications of quantum dots. Int. J. Nanomed. 2008;3:151. doi: 10.2147/ijn.s614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelley J, Saner M. International Approaches to the regulatory governance of nanotechnology. 2009 Regulation Paper, Regulatory Governance Initiative, Carleton University. Available from: http://www.carleton.ca/regulation/publications/Nanotechnology_Regulation_Paper_April2009.pdf. Accessed 7 July 2009. [Google Scholar]
- Robe A, Pic E, Lassalle HP, Bezdetnaya L, Guillemin F, Marchal F. Quantum dots in axillary lymph node mapping: Biodistribution study in healthy mice. BMC Cancer. 2008;8:111. doi: 10.1186/1471-2407-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal Commission on Environmental Pollution. Novel materials in the environment: The case of nanotechnology. 2008 Report available online from: http://www.rcept.org.uk/novel%20materials/Novel%20Materials%20report.pdfAccessed 21 January 2009. [Google Scholar]
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol. Sci. 2006;91:159–165. doi: 10.1093/toxsci/kfj122. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. J. Invest. Dermatol. 2007;127:143–153. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]
- Samia ACS, Dayal S, Burda C. Quantum dot-based energy transfer: Perspectives and potential for applications in photodynamic therapy. Photochem. Photobiol. 2006;82:617–625. doi: 10.1562/2005-05-11-IR-525. [DOI] [PubMed] [Google Scholar]
- Schipper ML, Cheng Z, Lee SW, Bentolila LA, Iyer G, Rao J, Chen X, Wu AM, Weiss S, Gambhir SS. microPET-based biodistribution of quantum dots in living mice. J. Nucl. Med. 2007;48:1511. doi: 10.2967/jnumed.107.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiohara A, Hoshino A, Hanaki K, Suzuki K, Yamamoto K. On the cyto-toxicity caused by quantum dots. Microbiol. Immunol. 2004;48:669–675. doi: 10.1111/j.1348-0421.2004.tb03478.x. [DOI] [PubMed] [Google Scholar]
- Su Y, He Y, Lu H, Sai L, Li Q, Li W, Wang L, Shen P, Huang Q, Fan C. The cytotoxicity of cadmium based, aqueous phase—Synthesized, quantum dots and its modulation by surface coating. Biomaterials. 2009;30:19–25. doi: 10.1016/j.biomaterials.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Su X, Li Y. Quantum dot biolabeling coupled with immunomagnetic separation for detection of Escherichia coli O157:H7. Anal. Chem. 2004;76:4806–4810. doi: 10.1021/ac049442+. [DOI] [PubMed] [Google Scholar]
- Tang M, Xing T, Zeng J, Wang H, Li C, Yin S, Yan D, Deng H, Liu J, Wang M, Chen J, Ruan DY. Unmodified CdSe quantum dots induce elevation of cytoplasmic calcium levels and impairment of functional properties of sodium channels in rat primary cultured hippocampal neurons. Environ. Health Perspect. 2008;116:915–922. doi: 10.1289/ehp.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. Biochemistry of tellurium. Biol. Trace Elem. Res. 1996;55:231–239. doi: 10.1007/BF02785282. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Wei ET, Malagoli C, Bergomi M, Vivoli G. Adverse health effects of selenium in humans. Rev. Environ. Health. 2001;16:233–251. doi: 10.1515/reveh.2001.16.4.233. [DOI] [PubMed] [Google Scholar]
- Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat. Med. 2004;10:993–998. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- Wang L, Nagesha DK, Selvarasah S, Dokmeci MR, Carrier RL. Toxicity of CdSe nanoparticles in Caco-2 cell cultures. J. Nanobiotechnol. 2008;6:11. doi: 10.1186/1477-3155-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JD, Kennel SJ, Mirzadeh S, Dai S, Wall JS, Richey T, Avenell J, Rondinone AJ. In vivo SPECT/CT imaging and biodistribution using radioactive Cd125 mTe/ZnS nanoparticles. Nanotechnology. 2007;18:175103. [Google Scholar]
- Yang RS, Chang LW, Wu JP, Tsai MH, Wang HJ, Kuo YC, Yeh TK, Yang CS, Lin P. Persistent tissue kinetics and redistribution of nanoparticles, quantum dot 705, in mice: ICP-MS quantitative assessment. Environ. Health Perspect. 2007;115:1339–1343. doi: 10.1289/ehp.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Stilwell JL, Gerion D, Ding L, Elboudwarej O, Cooke PA, Gray JW, Alivisatos AP, Chen FF. Cellular effect of high doses of silica-coated quantum dot profiled with high throughput gene expression analysis and high content cellomics measurements. Nano Lett. 2006;6:800–808. doi: 10.1021/nl0603350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen W, Zhang J, Liu J, Chen G, Pope C. In vitro and in vivo toxicity of CdTe nanoparticles. J. Nanosci. Nanotechnol. 2007;7:497–503. doi: 10.1166/jnn.2007.125. [DOI] [PubMed] [Google Scholar]
- Zimmer JP, Kim SW, Ohnishi S, Tanaka E, Frangioni JV, Bawendi MG. Size series of small indium arsenide-zinc selenide core-shell nanocrystals and their application to in vivo imaging. J. Am. Chem. Soc. 2006;128:2526–2527. doi: 10.1021/ja0579816. [DOI] [PMC free article] [PubMed] [Google Scholar]