Abstract
Chronic exposure to manganese (Mn) produces a spectrum of cognitive and behavioral deficits associated with a neurodegenerative disorder resembling Parkinson’s disease. The effects of high-dose exposure to Mn in occupational cohorts and in adult rodent models of the disease are well described but much less is known about the behavioral and neurochemical effects of Mn in the developing brain. We therefore exposed C57Bl/6 mice to Mn by intragastric gavage as juveniles, adults, or both, postulating that mice exposed as juveniles and then again as adults would exhibit greater neurological and neurochemical dysfunction than mice not preexposed as juveniles. Age- and sex-dependent vulnerability to changes in locomotor function was detected, with juvenile male mice displaying the greatest sensitivity, characterized by a selective increase in novelty-seeking and hyperactive behaviors. Adult male mice preexposed as juveniles had a decrease in total movement and novelty-seeking behavior, and no behavioral changes were detected in female mice. Striatal dopamine levels were increased in juvenile mice but were decreased in adult preexposed as juveniles. Levels of Mn, Fe, and Cu were determined by inductively coupled plasma-mass spectrometry, with the greatest accumulation of Mn detected in juvenile mice in the striatum, substantia nigra (SN), and cortex. Only modest changes in Fe and Cu were detected in Mn-treated mice, primarily in the SN. These results reveal that developing mice are more sensitive to Mn than adult animals and that Mn exposure during development enhances behavioral and neurochemical dysfunction relative to adult animals without juvenile exposure.
Keywords: manganese, development, behavior, neurochemistry, metals
Manganese (Mn) is an essential nutrient with a recommended daily intake of 2–5 mg/day for adults and 1–3 mg/day for children (National Research Council, 1989). Mn is necessary for homeostasis in the central nervous system (CNS) and is involved in the metabolism of proteins, carbohydrates, and lipids (Keen, 1984) through its role as a cofactor for multiple enzymes, including mitochondrial superoxide dismutase (Hearn et al., 2003) and glutamine synthetase (Takeda, 2003). Routes of exposure to Mn include inhalation in occupational settings, such as steel manufacturing, welding, and mining (Bader et al., 1999; Bowler et al., 2006; Josephs et al., 2005), and dietary intake through drinking water and soy-based infant formula (Krachler et al., 2000; Wasserman et al., 2006; Woolf et al., 2002), all of which lead to Mn accumulation in the brain.
Excessive exposure can cause Mn neurotoxicity or manganism, a neurodegenerative condition affecting cortical and basal ganglia structures, specifically the globus pallidus (Gp), striatum (St), and substantia nigra pars reticulata (SNpr) (Yamada et al., 1986). Early neuropsychological symptoms of the disorder include aggressiveness, anxiety, and decreased cognitive function (Mergler et al., 1994). These symptoms generally precede changes in motor function observed later in the progression of the disease, which are characterized by deficits that include dystonia, bradykinesia, rigidity, masked facial expression, and difficulty in walking backward (Wolters et al., 1989). Some of these clinical features are shared with Parkinson's disease (PD); however, dystonia, lack of resting tremor, and gait disturbances termed “cock walk” are more characteristic of manganism (Cersosimo and Koller, 2006). A variety of locomotor tests have been utilized to assess behavioral abnormalities in models of Mn neurotoxicity. Previous studies in aged mice exposed sc to Mn observed a decrease in horizontal movement (Dodd et al., 2005), whereas primates exposed to MnSO4 intervenously displayed gait dysfunction, rigidity, bradykinesia, and facial grimacing (Schneider et al., 2006), indicating extrapyramidal motor system dysfunction.
Mn neurotoxicity causes adverse motor symptoms similar to PD that are primarily attributed to loss of dopamine (DA) in the striatum and decreased output from gamma-aminobutyric acid neurons within the internal Gp but, unlike PD, dopaminergic neurons are largely spared (Perl and Olanow, 2007). Mn-induced decreases in striatal DA levels have been reported in rats (Dorman et al., 2000; Hirata et al., 2001), mice (Liu et al., 2006), rabbits (Mustafa and Chandra, 1971), and in nonhuman primates (Bird et al., 1984; Eriksson et al., 1987; Guilarte et al., 2008a; Neff et al., 1969)
Serotonergic neurotransmission is also a suspected target of Mn in the basal ganglia, although less data are available reporting effects on this system. Serotonin (5-HT) is a monoamine neurotransmitter that is involved in maintaining emotional stability in the CNS; however, modulation of the serotonergic neurons leads to lack of emotional stability resulting in anger, depression, sleeplessness, and loss of memory (Lesch et al., 1996), all early symptoms of manganism. 5-HT is metabolized to 5-hydroxyindoleacetic acid (5-HIAA) and was reported to be decreased in the Gp of primates exposed to aerosolized MnSO4 (Struve et al., 2007). Additionally, a decrease in 5-HT was identified in rats exposed to high-dietary Mn (Kimura et al., 1978). These studies suggest that serotonergic pathways are affected during Mn neurotoxicity, but the data from these adult models are difficult to extrapolate to developmental exposures.
Studies of Mn neurotoxicity in adult animals have examined metal accumulation in the brain, behavioral changes, and alterations in neurotransmitter levels, but it has not been previously investigated whether exposure early in life alters susceptibility to Mn during aging. We therefore exposed C57Bl/6 mice to Mn by intragastric gavage as juveniles, adults, or juveniles and again as adults and examined metal accumulation in multiple brain regions and serum as well as catecholamine and monoamine neurotransmitter levels and neurobehavioral parameters. The results indicate that developing mice are more sensitive than adult animals to Mn-induced changes in behavior and neurochemistry within the basal ganglia. Moreover, adult mice previously exposed to Mn as juveniles displayed a greater decrease in overall locomotor function and striatal catecholamines than naive adults exposed to Mn. This study demonstrates both that developing animals are highly susceptible to changes in behavior and striatal neurochemistry and effects of exposure persist during aging that render these brain regions more vulnerable to neurotoxic insult later in life. Consistent with this hypothesis, we also identified distinct patterns of glial activation and neuroinflammation that were strongly potentiated in adult animals preexposed as juveniles, discussed in detail in the companion article in this issue.
MATERIALS AND METHODS
Reagents.
All chemical reagents were obtained from Sigma Chemical Co. (St Louis, MO) unless otherwise stated. C57Bl/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME).
Animal exposure model.
Male and female C57Bl/6 mice were housed in microisolator cages (five animals per cage) and kept on 12-h light/dark cycles with access to laboratory chow and water ad libitum. Littermates from timed pregnant dams were paired in control and Mn-exposed groups and received 0.9% normal saline, 10 or 30 mg/kg MnCl2 by intragastric gavage daily during the following time periods: juvenile exposure, day 20–34 postnatal; adult exposure, from weeks 12 to 20; and juvenile and adult exposure, day 20–34 postnatal and weeks 12–20 (Supplementary Fig. 1). Animals were weighed prior to each gavage, and the amount of Mn delivered was adjusted accordingly. The amount of Mn delivered was adjusted for the molar concentration in the tetrahydrate form (MnCl2·4H2O) to achieve a precise dose of 10 or 30 mg/kg. Previous studies in our laboratory using this model administered 100 mg/kg Mn via intragastric gavage to adult female mice (Liu et al., 2006) for 8 weeks, but pilot studies in juvenile mice indicated a greater level of sensitivity to the neurological effects of Mn. We therefore revised the dosing paradigm downward to better test the hypothesis that low-dose juvenile exposure would potentiate later adult neurotoxicity upon subsequent exposure. All procedures were approved by the Institutional Animal care and Use Committee at Colorado State University and were performed under the supervision of veterinarians at the Laboratory Animal Resource facility.
Determination of catecholamines and monoamine neurotransmitters.
Brain samples were snap frozen in liquid nitrogen the day following the last day of saline or Mn gavage for each separate group (see Supplementary Fig. 1 for treatment schematic) and stored at −80°C until sample preparation. The samples were removed and kept on ice, weighed, and 300 μl of 0.2M perchloric acid containing 0.5 mg/ml of deoxyephinephrine (EPN) was applied per 10 mg of wet weight brain. Tissue samples were then disrupted by sonication and placed directly onto ice. Twenty microliter of sample was utilized for total protein concentration via the bicinchoninic acid protein assay. The tissue sample was then centrifuged at 14,000 × g for 10 min at 4°C. Aliquots of supernatant were loaded into glass high-performance liquid chromatography (HPLC) vials and analyzed within 8 h of sonication. Standards for all five compounds were processed in the same manner as the samples, and 1.0, 0.6, 0.3, and 0.1 mg/ml concentrations of standards concluded the runs for each day. The internal standard EPN was included in all runs in order to account for minute changes in detector sensitivity. The areas from the peaks in the samples were compared to those observed in the standards and values expressed as nanogram of neurotransmitter per milligram of total protein.
Levels of DA, 2-(3,4-dihydroxyphenyl)acetic acid (DOPAC), homovanillic acid, serotonin (5-HT), and 5-hydroxyindole acetic acid (5-HIAA) were determined by HPLC with electrochemical detection as described in previous studies from our laboratory (Liu et al., 2006), adapted from (Champney et al., 1992). Briefly, mobile phase consisted of 7% methanol, 0.946 g Na2HPO4, 2.8 g of citric acid, 18.6 mg of EDTA, and 20 mg of sodium octyl sulfate in 930 ml of Milli-Q water maintained at a pH of 4.6 and ran isocratically at a flow rate of 1.0 ml/min. An LED-6A electrochemical detector working electrode was set at + 0.67 V, recorder output set at 8 nA, response output at standard or 0.5 s, with negative polarity for the output, and polarity of electrode set at reduction. Experiments were performed through a Microsorb-MV C-18 reverse phase column with a pore size of 100 A, 5 mm size, and 25 cm in length (Varian, Walnut Creek, CA). Striatal tissues were processed from three to four separate animals from all treatment groups, and cerebral hemispheres were measured independently.
Neurobehavioral analysis.
Open-field activity parameters were determined using Versamax behavior chambers with an infrared beam grid detection array to assess animals’ movements in x-, y-, and z-planes. Activity was measured in the juvenile exposure group every other day for 2 weeks and for the adult exposure group once a week for 2 months during the period of oral gavage. Activity parameters were binned every minute for a total of 10 min and then analyzed using Versadat software (v. 4.00-127E; Accuscan Instruments, Inc., Columbus, OH). Analysis of juvenile animals compared 11–18 animals within each female and male treatment group, whereas 8–10 animals were assessed in adult treatment groups.
Levels of Mn, Cu, and Fe in brain tissue.
Brain regions were dissected following the last saline or Mn gavage for each separate group (see Supplementary Fig. 1 for treatment schematic) based upon stereologic coordinates using 1-mm brain blocks and stored at −80°C until sample preparation. Brain samples were weighed and transferred to acid-cleaned polypropylene tubes, 100 μl of nitric acid was applied to each sample and cooked for 2 h at 95°C to digest the tissue. Lipids were then digested with 50 μl of 30% hydrogen peroxide for 15 min at 80°C followed by 50 μl of hydrochloric acid incubation to complex the metals for 15 min at 80°C. Samples were then brought to a final volume of 1 ml using Milli-Q water. Analysis of the samples was performed by inductively coupled plasma-mass spectrometry (ICP-MS) on a Perkin-Elmer Elan DRC-II instrument (Perkin Elmer, Waltham, MA). Standards and blank tubes underwent the same digestion procedures as the samples. The concentration of each metal analyzed in brain samples is expressed as parts per million for each metal, based on wet weight of tissue. Brain tissue samples were processed from three separate animals from all treatment and exposure groups.
Statistical analysis.
Comparison of two means was performed by Student's t-test. Comparisons of three or more means was performed using one-way ANOVA followed by the Tukey-Kramer multiple comparison post hoc test using Prism software (v4.0c; Graphpad Software, Inc., San Diego, CA). Alpha level for all statistical analyses was set at p < 0.05.
RESULTS
Mn-Induced Locomotor Changes
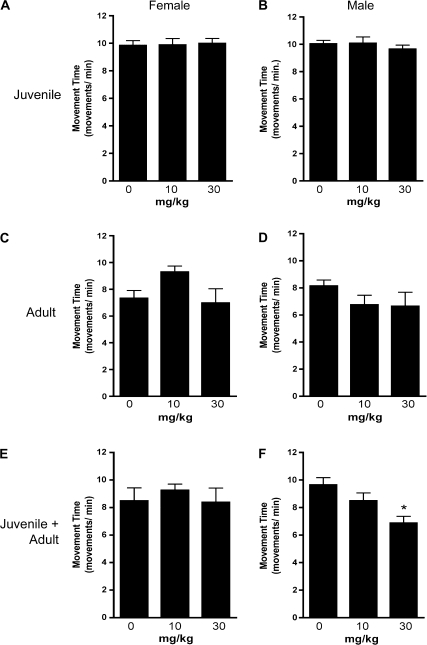
Locomotor function was determined by open-field activity measures to identify patterns of neurobehavioral dysfunction in developing and adult mice exposed to Mn. Time spent in the margin was decreased in juvenile males at both 10 and 30 mg/kg of MnCl2, whereas juvenile females displayed no change in margin time (Figs. 1A and 1B). In contrast, mice exposed only as adults displayed no change in margin time but male mice exposed to 10 and 30 mg/kg MnCl2 as juveniles and again as adults had a significant increase in time spent in the margin (Figs. 1E and 1F). Adult female mice displayed no change in margin time in any exposure group. The total number of movements was unchanged in female mice preexposed as juveniles (Fig. 2E) but was increased in male mice exposed to 30 mg/kg MnCl2 (Fig. 2F). The total number of movements for mice exposed only as juveniles (Figs. 2A and 2B) and only as adults (Figs. 2C and 2D) was unchanged at either dose of Mn. Total distance traveled and rearing movement were also assessed but no significant change was detected in any groups observed (Supplementary Figs. 2 and 3).
FIG. 1.
Developing and adult mice are differentially sensitive to Mn-induced effects on anxiety and novelty-seeking behavior. Time spent in the margin was recorded in open-field activity chambers and determined for each animal following exposure to Mn. Female juvenile mice had no significant change in margin time following exposure to 10 or 30 mg/Kg MnCl2 (A), whereas male juvenile mice (days 20–34) displayed a significant decrease in time spent in the margin at both 10 and 30 mg/Kg MnCl2 (B). (C) Female and (D) male mice exposed only as adults (weeks 12–20) were not vulnerable to Mn-induced neurobehavioral changes. (E) Female mice preexposed to Mn as juveniles and then again as adults (days 20–34 and weeks 12–20) had no change, whereas preexposed males (F) did have a significant increase in time spent in the margin at both 10 and 30 mg/kg MnCl2 compared to controls (*p < 0.05).
FIG. 2.
Developmental exposure to Mn increases sensitivity to locomotor dysfunction in adult male mice upon subsequent exposure. Total movement number was recorded in open-field activity chambers for each animal following exposure to Mn. No significant changes were detected in (A) female or (B) male juvenile (days 20–34) mice exposed to 10 and 30 mg/kg MnCl2 or in (C) female or (D) male mice exposed to MnCl2 only as adults (weeks 12–20). Likewise, there was no detectable change in total movement number in adult female mice preexposed as juveniles (E) but total movement number was decreased in adult male mice preexposed to 30 mg/kg MnCl2 as juveniles, relative to controls (days 20–34 and weeks 12–20) (F) (*p < 0.05).
Striatal Neurotransmitter Levels
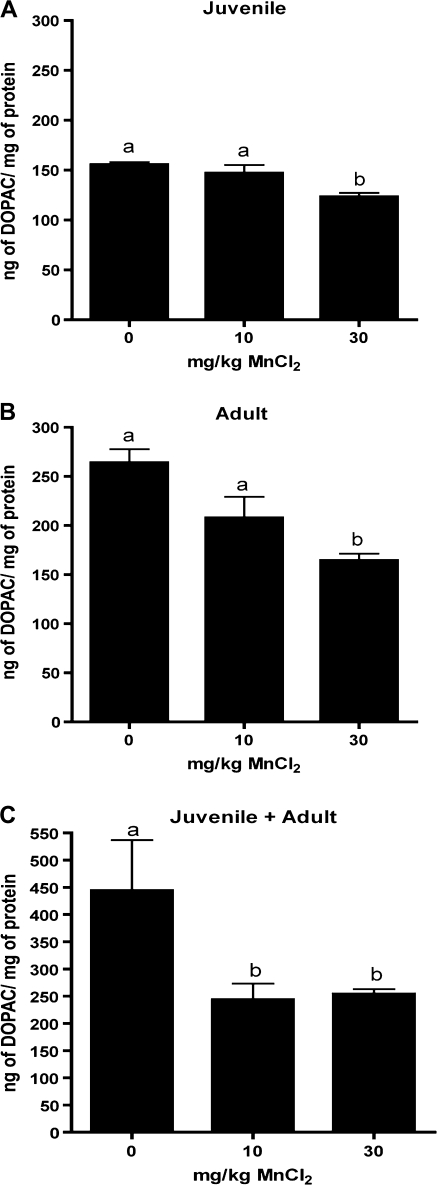
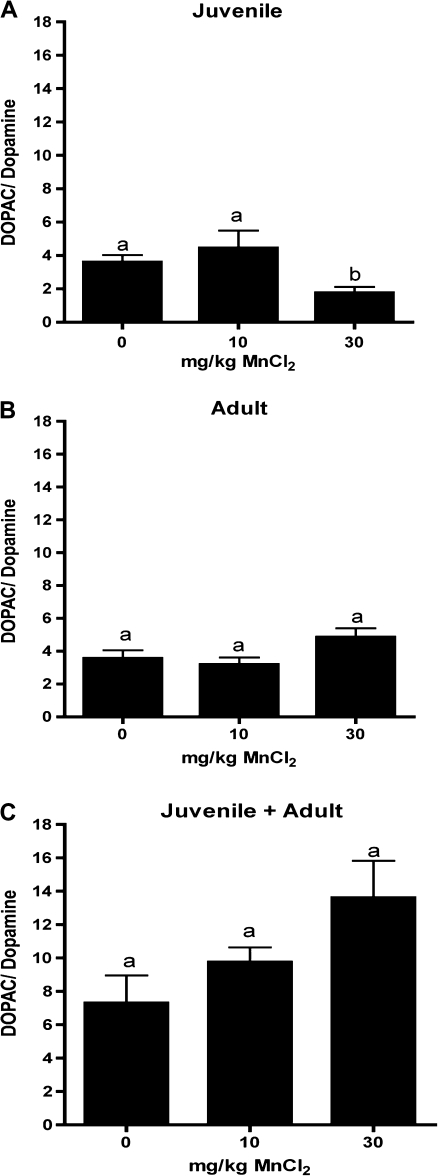
Levels of striatal catecholamines and monoamines were determined in each exposure group by HPLC using electrochemical detection. Juvenile mice exposed to 30 mg/kg MnCl2 had a significant increase in striatal DA compared to both the control and the 10 mg/kg MnCl2 groups (Fig. 3A). In mice exposed to Mn only as adults, DA was decreased in the 30 mg/kg MnCl2 group (Fig. 3B) but was decreased in both the 10 and the 30 mg/kg MnCl2 treatment groups in adult mice preexposed as juveniles (Fig. 3C). The DA metabolite, DOPAC, was decreased at 30 mg/kg MnCl2 in all three exposure groups but in adults preexposed to Mn as juveniles DOPAC levels were also decreased at 10 mg/kg MnCl2 (Figs. 4A–C). The ratio of DOPAC to DA, a general indicator of DA turnover, was increased in juvenile mice at 30 mg/kg MnCl2 (Fig. 5A) but was unchanged in any dose group in mice receiving Mn only as adults. The DOPAC/DA ratio was not different from controls in adult mice preexposed as juveniles, although an increasing trend was noted (Fig. 5C; p = 0.08). No significant change in 5-HT was identified in any of the animals exposed to Mn (Figs. 6A–C), and only the juvenile exposure group had a significant increase in the serotonin metabolite, 5-HIAA, in the 30-mg/kg dose group (Fig. 7A). No change was detected in 5-HIAA in other exposure groups (Figs. 7B and 7C).
FIG. 3.
Mn differentially modulates striatal DA levels in developing and adult mice. Juvenile mice (days 20–34) exposed to 10 and 30 mg/kg MnCl2 had a significant increase in striatal DA (A) but no significant change was detected in mice exposed only as adults (weeks 12–20) (B). DA levels were decreased in the striatum of adult mice preexposed to MnCl2 as juveniles (days 20–34 and weeks 12–20) (C) (*p < 0.05).
FIG. 4.
Mn differentially modulates striatal DOPAC levels in developing and adult mice. Juvenile (days 20–34) (A) and adult (weeks 12–20) (B) mice exposed to 30 mg/kg MnCl2 had significant decreases in striatal DOPAC levels. DOPAC levels were also decreased at both 10 and 30 mg/kg Mn in adult mice preexposed to MnCl2 as juveniles (days 20–34 and weeks 12–20) (C). Differing letters denote statistical significance (p < 0.05).
FIG. 5.
The DOPAC/DA ratio is selectively altered in juvenile mice following exposure to manganese. The ratio of DOPAC/DA was decreased in juvenile mice (days 20–34) exposed to 30 mg/kg MnCl2 (A) but no changes were detected in either naive adult mice exposed to 10 or 30 mg/kg MnCl2 (B) or in adult mice preexposed to 10 or 30 mg/kg MnCl2 as juveniles (C). Differing letters denote statistical significance (p < 0.05).
FIG. 6.
Striatal serotonin levels are unaffected by manganese in developing and adult mice. Mice were exposed to 10 and 30 mg/kg MnCl2 as (A) juveniles (days 20–34), (B) adults (weeks 12–20), and (C) juveniles and adults (days 20–34 and weeks 12–20). No changes in levels of striatal serotonin (d-hydroxytryptamine; 5-HT) were detected in any exposure group. Significance is denoted by differing letters (p > 0.05).
FIG. 7.
Striatal levels of the serotonin metabolite, 5-hydroxyindoleacetic acid, are decreased in juvenile mice exposed to manganese. Levels of 5-hydroxyindoleacetic acid (5-HIAA) were decreased in (A) juvenile mice (days 20–34) exposed to 30 mg/kg MnCl2 but no changes in 5-HIAA levels were detected in mice exposed to MnCl2 as (B) adults (weeks 12–20) or (C) juveniles and adults (days 20–34 and weeks 12–20). Differing letters denote statistical significance (p > 0.05).
Determination of Tissue Levels of Mn, Fe, and Cu in Various Brain Tissues
Levels of Mn, Fe, and Cu were analyzed by ICP-MS in multiple brain regions and serum to determine whether differences in uptake of Mn or perturbations in other transition metals associated with the alterations in behavioral and neurochemical parameters in Mn-exposed mice. In juvenile mice, there was an increase in Mn accumulation in the striatum (St) and substantia nigra (SN) at both 10 and 30 mg/kg MnCl2 (Table 1). There was also an increase in cortical Mn levels in the 30-mg/kg dose group. Mice exposed only as adults had an increase in Mn in the St at 10 mg/kg MnCl2 and a trend toward increase at 30 mg/kg in the St and SN (Table 1). Levels of Fe and Cu were also determined in the same brain regions and serum. A significant increase of Fe was observed in the SN of juvenile mice exposed to 30 mg/kg MnCl2 and in cortex at both 10 and 30 mg/kg MnCl2 (Table 2). Mice exposed to 30 mg/kg MnCl2 as adults had an increase of Fe only in the hypothalamus (Table 2). Although Cu levels were largely unaffected, some accumulation was detected in the SN of juvenile mice exposed to 10 and 30 mg/kg MnCl2 (Table 3). Interestingly, no change in serum Mn, Fe, or Cu was detected in any exposure group at the relatively low doses of Mn used in this study.
TABLE 1.
Manganese in Brain and Serum of Exposed Juvenile and Adult Mice (parts per million)
| Time of exposure | Treatment, mg/kg (MnCl2·4H2O) | Striatum | SN | Cortex | Hypothalamus | Serum |
| Juvenile | 0 | 0.26 ± 0.01 | 0.5 ± 0.07 | 0.48 ± 0.08 | 0.63 ± 0.12 | 0.004 ± 0.0003 |
| 10 | 0.49 ± 0.06a | 1.0 ± 0.65a | 0.47 ± 0.32 | 1.4 ± 0.17 | 0.009 ± 0.0009 | |
| 30 | 0.70 ± 0.01a | 2.5 ± 0.45a | 1.4 ± 0.13a | 1.1 ± 0.51 | 0.004 ± 0.0002 | |
| Adults | 0 | 0.69 ± 0.07 | 0.48 ± 0.18 | 0.45 ± 0.15 | 0.50 ± 0.21 | 0.006 ± 0.0007 |
| 10 | 2.1 ± 0.10a | 2.5 ± 0.11 | 3.1 ± 3.5 | 1.7 ± 0.36 | 0.008 ± 0.0008 | |
| 30 | 1.1 ± 0.12 | 5.6 ± 2.4 | 2.05 ± 0.43 | 1.5 ± 0.16 | 0.015 ± 0.0015 | |
| Juvenile and adult | 0 | 1.7 ± 0.19 | 1.3 ± 0.12 | 1.5 ± 0.15 | 1.5 ± 0.11 | 0.012 ± 0.0007 |
| 10 | 1.5 ± 0.05 | 1.8 ± 0.13 | 1.46 ± 0.06 | 1.4 ± 0.10 | 0.006 ± 0.0004 | |
| 30 | 1.7 ± 0.09 | 1.3 ± 0.15 | 1.42 ± 0.19 | 1.3 ± 0.20 | 0.012 ± 0.0008 |
Note. Data shown are means ± SEM (n ≥ 3).
Significance compared to controls within a specific brain region per exposure group is denoted by (p < 0.05).
TABLE 2.
Iron in Brain and Serum of Juvenile and Adult Mice Exposed to Manganese (parts per million)
| Time of exposure | Treatment, mg/kg (MnCl2·4H2O) | Striatum | SN | Cortex | Hypothalamus | Serum |
| Juvenile | 0 | 6.7 ± 1.4 | 10.1 ± 2.02 | 14.8 ± 9.6 | 17.2 ± 10.4 | 2.53 ± 0.32 |
| 10 | 8.05 ± 1.9 | 18.3 ± 12.8a | 10.7 ± 8.5 | 23.04 ± 8.87 | 3.32 ± 1.29 | |
| 30 | 9.3 ± 1.1 | 37.7 ± 1.23a | 32.1 ± 1.23a | 71.64 ± 90.6 | 4.79 ± 0.23 | |
| Adults | 0 | 26.7 ± 2.23 | 14.9 ± 6.3 | 13.1 ± 4.7 | 19.8 ± 10.1 | 3.15 ± 0.27 |
| 10 | 34.6 ± 1.32 | 41.2 ± 10.5 | 50.4 ± 14.1 | 28.7 ± 5.9 | 6.23 ± 0.62 | |
| 30 | 33.2 ± 1.57 | 43 ± 24 | 44.2 ± 3.35 | 41 ± 3.4a | 5.13 ± 0.35 | |
| Juvenile and adult | 0 | 26.6 ± 1.9 | 32.5 ± 0.35 | 23.3 ± 2.6 | 25.4 ± 2.28 | 2.91 ± 0.27 |
| 10 | 35.4 ± 1.2 | 34.5 ± 1.83 | 34.3 ± 1.18 | 31.0 ± 1.24 | 3.4 ± 0.20 | |
| 30 | 34.7 ± 0.43 | 24.9 ± 2.5 | 24.3 ± 2.6 | 20.9 ± 1.86 | 6.2 ± 0.56 |
Note. Data shown are means ± SEM (n ≥ 3).
Significance compared to controls within a specific brain region per exposure group is denoted by (p < 0.05).
TABLE 3.
Copper in Brain and Serum of Juvenile and Adult Mice Exposed to Manganese (parts per million)
| Time of exposure | Treatment, mg/kg (MnCl2·4H2O) | Striatum | SN | Cortex | Hypothalamus | Serum |
| Juvenile | 0 | 1.8 ± 0.07 | 2.0 ± 0.13 | 4.07 ± 0.68 | 4.1 ± 0.75 | 0.24 ± 0.004 |
| 10 | 1.9 ± 0.16 | 3.8 ± 0.93a | 2.8 ± 0.50 | 5.5 ± 0.54 | 0.22 ± 0.002 | |
| 30 | 2.5 ± 0.06 | 9.9b | 7.7 ± 1.23 | 3.04 ± 0.81 | 0.23 ± 0.003 | |
| Adults | 0 | 8.1 ± 0.91 | 4.1 ± 1.85 | 2.9 ± 0.82 | 5.5 ± 2.73 | 0.32 ± 0.009 |
| 10 | 10.1 ± 0.52 | 8.4 ± 0.46 | 13.5 ± 4.03 | 8.9 ± 2.4 | 0.34 ± 0.028 | |
| 30 | 9.5 ± 0.58 | 10.2 ± 1.58 | 13.2 ± 1.27a | 11.9 ± 1.47 | 0.34 ± 0.008 | |
| Juvenile and adult | 0 | 8.7 ± 0.72 | 8.0 ± 0.14 | 5.6 ± 0.52 | 7.6 ± 0.73 | 0.21 ± 0.015 |
| 10 | 9.6 ± 0.42 | 9.7 ± 0.52 | 7.08 ± 1.18 | 8.3 ± 0.52 | 0.26 ± 0.007 | |
| 30 | 10.1 ± 0.28 | 7.6 ± 1.03 | 5.86 ± 0.76 | 5.18 ± 0.35 | 0.25 ± 0.01 |
Note. Data shown are means ± SEM (n ≥ 3).
Significance compared to controls within a specific brain region per exposure group is denoted by (p < 0.05).
n equals 1 for this sample.
DISCUSSION
Manganism is primarily described as a disorder of the basal ganglia, with Mn accumulating preferentially in the St, Gp, and SNpr (Liu et al., 2006; Morello et al., 2008), although cortical structures are affected as well (Guilarte et al., 2008b). To better understand the phenotype of the disorder and the comparative sensitivity of the developing and adult brain to neurobehavioral and neurochemical deficits, mice were exposed to Mn both during development and adulthood and assessed for multiple indices of neurological dysfunction. The purpose of this treatment paradigm was to address a poorly studied aspect of Mn neurotoxicity, namely, how developmental exposure impacts neurological function during aging and whether neurotoxic exposures early in life can potentiate later susceptibility to disease.
Locomotor activity was notably decreased in male adult mice preexposed as juveniles (Fig. 2F), whereas no significant changes were observed in the absence of juvenile exposure (Fig. 2D), suggesting that this period of postnatal development may represent a window of sensitivity to Mn exposure. The age at which juvenile mice were exposed to Mn corresponds approximately to a prepubertal age in humans (Flurkey and Harrison, 2007). Female mice appeared refractory to the relatively low doses of Mn used in these studies, an interesting finding consistent with the known neuroprotective effects of estrogen (D'Astous et al., 2004; Tripanichkul et al., 2007). Decreased locomotor activity has also been described in Mn-exposed workers, nonhuman primate, and rodent models of Mn neurotoxicity (Dodd et al., 2005; Wolters et al., 1989), suggesting that low-dose exposure to Mn during critical developmental periods may potentiate susceptibility to Mn and possibly other environmental toxicants later in life.
Thigmotaxis, or margin time, was altered in mice exposed to Mn. This tendency to stay near the margin of the chamber is typically associated with either anxiety or a lack of novelty seeking in an animal model of PD (George et al., 2008). Male mice exposed only as juveniles to either 10 or 30 mg/kg MnCl2 displayed a decrease in margin time compared to controls (Fig. 1B), a sign of increase in novelty-seeking or hyperactivity behavior in juvenile male mice (Helms et al., 2008) and a symptom reported in children exposed to excessive levels of Mn through drinking water (Wasserman et al., 2006; Woolf et al., 2002). Although the present data support a correlation between Mn and hyperactivity, additional behavioral studies in animal models, as well as larger clinical epidemiology studies, will be required to better understand the mechanisms underlying observed increases in hyperactivity in the developing and adult brain. To our knowledge, this is the first report of locomotor effects from exposure to Mn between weaning and early adulthood.
The impact of early Mn exposure on striatal neurotransmitters was examined to better understand the basis for the observed neurobehavioral alterations in juvenile and adult mice. Striatal DA levels were decreased in mice exposed only as adults and in adults preexposed as juveniles but DA levels were decreased to a greater degree in the latter group and at a lower dose of Mn (10 mg/kg MnCl2), whereas DA was only decreased in naive adult mice at 30 mg/kg MnCl2 (Figs. 3B and 3C). These data demonstrate that developmental exposure to Mn increases vulnerability of the striatal system to DA loss during aging. Mice exposed to 30 mg/kg Mn as adults had a significant decrease in DOPAC, the DA metabolite, and similar to DA, the mice exposed as juveniles and adults had a decrease in DOPAC at both doses of Mn, further supporting that developmental exposure to Mn enhances susceptibility to alterations in striatal neurochemistry during aging. Loss of striatal DA due to Mn exposure in the adult brain is well established in other Mn toxicity models and is a pathological indicator of basal ganglia dysfunction (Liu et al., 2006).
Juvenile mice exposed to Mn showed an opposite effect from adult mice with respect to the striatal DA levels. A significant increase in striatal DA was detected at 30 mg/kg MnCl2 (Fig. 3A) and levels of DOPAC were decreased (Fig. 4A). The DOPAC/DA ratio was also decreased in juvenile mice exposed to 30 mg/kg MnCl2 but no significant changes were detected in either adult exposure groups (Fig. 5), suggesting a greater sensitivity of DA catabolism in the developing basal ganglia. Previous studies in neonatal mice exposed to Mn by oral gavage reported a similar increase in striatal DA but noted an increase in DOPAC (Cotzias et al., 1976; Dorman et al., 2000). The decreased ratio of DOPAC to DA in juvenile mice in the studies reported here (Fig. 5) may represent a decrease in overall DA turnover, most notably in adults sensitized by prior juvenile exposure.
Previous studies of Mn toxicity reported a decrease in the serotonin metabolite, 5-HIAA, in the pallidum of monkeys exposed to Mn (Golub et al., 2005; Struve et al., 2007), indicating that Mn affects serotonergic, as well as dopaminergic, pathways. In the studies reported here, juvenile mice exposed to 30 mg/kg of Mn were the only group that had a significant decrease in 5-HIAA (Fig. 7A). The finding of low levels of 5-HIAA in cerebral spinal fluid is a phenomenon found in children who suffer with attention deficit disorder (Miczek et al., 2002), which is consistent with the observed hyperactivity and loss of 5-HIAA in juvenile mice exposed to Mn in the present studies.
Accumulation of Mn was most prominent in juvenile mice, occurring in the cortex, St, and SN (Table 1), and in adult mice to a lesser degree in the St. Levels of Fe and Cu were essentially unchanged in adult animals but increased somewhat in the SN of juvenile mice exposed to 30 mg/kg MnCl2 (Tables 2 and 3), suggesting both that this brain region is particularly vulnerable in developing animals and that Mn accumulation seems to alter homeostatic regulation of other transition metals known to associate with oxidative damage (Uversky et al., 2001). These findings are consistent with previous studies in nonhuman primates exposed to Mn iv and by inhalation, and with rodent models using exposure via ingestion, that reported increased Mn levels in various brain regions (Dorman et al., 2000, 2006a; Guilarte et al., 2006; Struve et al., 2007). Interestingly, it appeared that Mn levels in several brain regions in control mice from the juvenile and adult exposure group were somewhat higher than Mn levels in the corresponding brain regions in naive adults (Table 1), due possibly to either stress resulting from juvenile gavage or to experimental variation between these two study groups. Because this is the first report of such findings, we cannot rule out either possibility and must rely on subsequent studies to confirm these findings. The variability in dose-related accumulation of Mn could also be explained in part by the robust elimination pharmacokinetics of Mn in rodent brain. It was previously reported that dietary Mn does not increase brain accumulation of inhaled Mn3O4 (Dorman et al., 2002), suggesting that once saturation is reached in brain tissue, further accumulation is difficult, particularly with chronic low-dose exposure. However, low-dose exposure to Mn at critical times during development might elicit other persistent changes, such as glial inflammatory activation, that amplifies the effects of even low doses of Mn upon subsequent exposures.
In conclusion, these studies indicate that the period of development in mice spanning weaning to early adulthood represents a critical window of sensitivity to the neurobehavioral effects of ingested Mn and that male mice are more severely affected than females. In adult mice, not only were preexposed animals more sensitive to Mn toxicity than naive mice not exposed early in life but this preexposure also resulted in greater effects on both dopaminergic and serotonergic neurochemical parameters in the striatum. Sex differences in susceptibility were also noted in adult animals, with only males displaying neurobehavior abnormalities. Additional studies will be required to determine the basis for these differences and to examine the possible neuroprotective role of estrogen in mitigating the neurotoxicity of Mn. Differences in accumulation of Mn and dysregulation of other transition metals, such as Fe and Cu, do not alone explain the observed potentiation of neurobehavioral deficits in adult mice preexposed to Mn as juveniles, suggesting that other cellular or molecular systems are affected that lead to persistent changes in the neurochemistry and function of the basal ganglia. Neuroinflammatory signaling in glial cells is a potential pathway affected by Mn exposure that could account for the persistence of the neurobehavioral deficits observed in these mice. Enhanced inflammatory activation of glia was noted in adult mice preexposed as juveniles that colocalized with increased expression of inducible nitric oxide synthase and nitrosative stress in neurons of the Gp and SNpr (see companion article, this issue). Thus, Mn exposure in juvenile mice appears to result in complex changes in both neurochemistry and behavior that enhance sensitivity to Mn and possibly other neurotoxicants during aging. The mechanism underlying these changes in neurobehavioral parameters is not entirely clear but may involve inflammatory responses of glial cells that persist into adulthood.
SUPPLEMENTARY DATA
Supplementary Figures 1–3 are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (ES012941 to R.B.T.).
Supplementary Material
References
- Bader M, Dietz MC, Ihrig A, Triebig G. Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int. Arch. Occup. Environ. Health. 1999;72:521–527. doi: 10.1007/s004200050410. [DOI] [PubMed] [Google Scholar]
- Bird ED, Anton AH, Bullock B. The effect of manganese inhalation on basal ganglia dopamine concentrations in rhesus monkey. Neurotoxicology. 1984;5(1):59–65. [PubMed] [Google Scholar]
- Bowler RM, Koller W, Schulz PE. Parkinsonism due to manganism in a welder: neurological and neuropsychological sequelae. Neurotoxicology. 2006;27:327–332. doi: 10.1016/j.neuro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. Neurotoxicology. 2006;27:340–346. doi: 10.1016/j.neuro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Champney TH, Hanneman WH, Nichols MA. gamma-Aminobutyric acid, catecholamine and indoleamine determinations from the same brain region by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. 1992;579:334–339. doi: 10.1016/0378-4347(92)80400-k. [DOI] [PubMed] [Google Scholar]
- Cotzias GC, Miller ST, Papavasiliou PS, Tang LC. Interactions between manganese and brain dopamine. Med. Clin. North Am. 1976;60:729–738. doi: 10.1016/s0025-7125(16)31856-9. [DOI] [PubMed] [Google Scholar]
- D'Astous M, Morissette M, Di Paolo T. Effect of estrogen receptor agonists treatment in MPTP mice: evidence of neuroprotection by an ER alpha agonist. Neuropharmacology. 2004;47:1180–1188. doi: 10.1016/j.neuropharm.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Dodd CA, Ward DL, Klein BG. Basal ganglia accumulation and motor assessment following manganese chloride exposure in the C57BL/6 mouse. Int. J. Toxicol. 2005;24:389–397. doi: 10.1080/10915810500366500. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Marshall MW, Parkinson CU, James RA, Wong BA. Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol. Sci. 2006a;92:201–210. doi: 10.1093/toxsci/kfj206. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Vitarella D, Byerly FL, Goetz J, Miller R. Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. J. Appl. Toxicol. 2000;20:179–187. doi: 10.1002/(sici)1099-1263(200005/06)20:3<179::aid-jat631>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Wong BA. Brain manganese concentrations in rats following manganese tetroxide inhalation are unaffected by dietary manganese intake. Neurotoxicology. 2002;23:185–195. doi: 10.1016/s0161-813x(01)00075-4. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Magiste K, Plantin LO, Fonnum F, Hedstrom KG, Theodorsson-Norheim E, Kristensson K, Stalberg E, Heilbronn E. Effects of manganese oxide on monkeys as revealed by a combined neurochemical, histological and neurophysiological evaluation. Arch. Toxicol. 1987;61:46–52. doi: 10.1007/BF00324547. [DOI] [PubMed] [Google Scholar]
- Flurkey KCJ, Harrison DE. The mouse in aging research. In: Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith A, editors. The Mouse in Biomedical Research. 2nd ed. Burlington, MA: American College Laboratory Animal Medicine, Elsevier; 2007. pp. 637–672. [Google Scholar]
- George S, van den Buuse M, San Mok S, Masters CL, Li QX, Culvenor JG. Alpha-synuclein transgenic mice exhibit reduced anxiety-like behaviour. Exp. Neurol. 2008;210:788–792. doi: 10.1016/j.expneurol.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow’s milk formula, soy formula, or soy formula with added manganese. Neurotoxicol. Teratol. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, McGlothan JL, Verina T, Zhou Y, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, et al. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J. Neurochem. 2008a;107:1236–1247. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J. Neurochem. 2008b;105:1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H-MRS and MRI study. Toxicol. Sci. 2006;94:351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Hearn AS, Stroupe ME, Cabelli DE, Ramilo CA, Luba JP, Tainer JA, Nick HS, Silverman DN. Catalytic and structural effects of amino acid substitution at histidine 30 in human manganese superoxide dismutase: insertion of valine C gamma into the substrate access channel. Biochemistry. 2003;42:2781–2789. doi: 10.1021/bi0266481. [DOI] [PubMed] [Google Scholar]
- Helms CM, Gubner NR, Wilhelm CJ, Mitchell SH, Grandy DK. D4 receptor deficiency in mice has limited effects on impulsivity and novelty seeking. Pharmacol. Biochem. Behav. 2008;90:387–393. doi: 10.1016/j.pbb.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Kiuchi K, Nagatsu T. Manganese mimics the action of 1-methyl-4-phenylpyridinium ion, a dopaminergic neurotoxin, in rat striatal tissue slices. Neurosci. Lett. 2001;311:53–56. doi: 10.1016/s0304-3940(01)02144-9. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Keen CL. Manganese. In: Frieden E, editor. Biochemical of the Essential Ultratrace Elements. New York: New York Plenum Press; 1984. pp. 89–132. [Google Scholar]
- Kimura M, Yagi N, Itokawa Y. Effect of subacute manganese feeding on serotonin metabolism in the rat. J. Toxicol. Environ. Health. 1978;4:701–707. doi: 10.1080/15287397809529692. [DOI] [PubMed] [Google Scholar]
- Krachler M, Domej W, Irgolic KJ. Concentrations of trace elements in osteoarthritic knee-joint effusions. Biol. Trace Elem. Res. 2000;75:253–263. doi: 10.1385/BTER:75:1-3:253. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol. Sci. 2006;91:521–531. doi: 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- Mergler D, Huel G, Bowler R, Iregren A, Belanger S, Baldwin M, Tardif R, Smargiassi A, Martin L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ. Res. 1994;64:151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF, De Almeida RM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl.) 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Morello M, Canini A, Mattioli P, Sorge RP, Alimonti A, Bocca B, Forte G, Martorana A, Bernardi G, Sancesario G. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats an electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology. 2008;29:60–72. doi: 10.1016/j.neuro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Mustafa SJ, Chandra SV. Levels of 5-hydroxytryptamine, dopamine and norepinephrine in whole brain of rabbits in chronic manganese toxicity. J. Neurochem. 1971;18:931–933. doi: 10.1111/j.1471-4159.1971.tb12022.x. [DOI] [PubMed] [Google Scholar]
- Neff NH, Barrett RE, Costa E. Selective depletion of caudate nucleus dopamine and serotonin during chronic manganese dioxide administration to squirrel monkeys. Experientia. 1969;25:1140–1141. doi: 10.1007/BF01900234. [DOI] [PubMed] [Google Scholar]
- National Research Council. Recommended dietary allowances. In: NRC, editor. Food and Nutrition Board. 10th ed. Washington, DC: National Academy Press; 1989. pp. 230–235. [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve MF, McManus BE, Wong BA, Dorman DC. Basal ganglia neurotransmitter concentrations in rhesus monkeys following subchronic manganese sulfate inhalation. Am. J. Ind. Med. 2007;50:772–778. doi: 10.1002/ajim.20489. [DOI] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res. Brain Res. Rev. 2003;41(1):79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Tripanichkul W, Sripanichkulchai K, Duce JA, Finkelstein DI. 17Beta-estradiol reduces nitrotyrosine immunoreactivity and increases SOD1 and SOD2 immunoreactivity in nigral neurons in male mice following MPTP insult. Brain Res. 2007;1164:24–31. doi: 10.1016/j.brainres.2007.05.076. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J. Biol. Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, et al. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters EC, Huang CC, Clark C, Peppard RF, Okada J, Chu NS, Adam MJ, Ruth TJ, Li D, Calne DB. Positron emission tomography in manganese intoxication. Ann. Neurol. 1989;26:647–651. doi: 10.1002/ana.410260510. [DOI] [PubMed] [Google Scholar]
- Woolf A, Wright R, Amarasiriwardena C, Bellinger D. A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 2002;110:613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. (Berl.) 1986;70:273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.