Abstract
Chronic exposure to manganese (Mn) produces a neurodegenerative disorder affecting the basal ganglia characterized by reactive gliosis and expression of neuroinflammatory genes including inducible nitric oxide synthase (NOS2). Induction of NOS2 in glial cells causes overproduction of nitric oxide (NO) and injury to neurons that is associated with parkinsonian-like motor deficits. Inflammatory activation of glia is believed to be an early event in Mn neurotoxicity, but specific responses of microglia and astrocytes to Mn during development remain poorly understood. In this study, we investigated the effect of juvenile exposure to Mn on the activation of glia and production of NO in C57Bl/6J mice, postulating that developmental Mn exposure would lead to heightened sensitivity to gliosis and increased expression of NOS2 in adult mice exposed again later in life. Immunohistochemical analysis indicated that Mn exposure caused increased activation of both microglia and astrocytes in the striatum (St), globus pallidus (Gp), and substantia nigra pars reticulata (SNpr) of treated mice compared with controls. More robust activation of microglia was observed in juveniles, whereas astrogliosis was more prominent in adult mice preexposed during development. Co-immunofluorescence studies demonstrated increased expression of NOS2 in glia located in the Gp and SNpr. Additionally, greater increases in the level of 3-nitrotyrosine protein adducts were detected in dopamine- and cAMP-regulated phosphoprotein-32–positive neurons of the St of Mn-treated adult mice preexposed as juveniles. These data indicate that subchronic exposure to Mn during development leads to temporally distinct patterns of glial activation that result in elevated nitrosative stress in distinct populations of basal ganglia neurons.
Keywords: manganese, microglia, astrocyte, nitric oxide, neurotoxicity, development
Manganese (Mn) is an essential nutrient and a cofactor for multiple enzymes required for normal cellular function in the central nervous system (CNS), including glutamine synthetase and pyruvate decarboxylase (Hearn et al., 2003; Martinez-Hernandez et al., 1977; Takeda, 2003). However, increased exposure to Mn through both dietary and inhalation routes leads to neurotoxicity with subsequent damage to the extrapyramidal motor nuclei of the basal ganglia (Dorman et al., 2006; Olanow, 2004; Tran et al., 2002). In scenarios of sustained high exposure, this neurotoxicity can lead to a degenerative motor disorder, termed manganism, that presents with neurological symptoms resembling those of Parkinson's disease (PD; Yamada et al., 1986). It has been well described in adults exposed in occupational settings such as steel manufactures (Cowan et al., 2009) and welders (Bowler et al., 2006) that Mn toxicity progresses from cognitive and psychological deficits at lower exposures to irreversible neurodegeneration and motor dysfunction with higher exposures (Albin, 2000; Calne et al., 1994; Pal et al., 1999; Rodier, 1955).
Much less is known regarding the effects of Mn in the developing CNS, particularly with respect to the potential risk posed by exposures early in life that may predispose to later neurological injury. Varying reports indicate that children exposed to moderate levels of Mn in drinking water present with cognitive deficits and hyperactivity (Bouchard et al., 2007; Wasserman et al., 2006; Woolf et al., 2002). Concerns regarding dietary exposure are also raised based upon high levels of Mn in soy-based infant formula (Krachler et al., 2000) and by the elevation of brain Mn levels that occurs during chronic iron deficiency in rodent models (Garcia et al., 2007), also designated by World Health Organization as a major world health problem.
Mn toxicity leads to activation of both microglia and astrocytes that promotes neuronal injury (Henriksson and Tjalve, 2000; Verity, 1999), but the mechanisms underlying Mn-induced gliosis are not fully understood. Prior studies of Mn neuropathology have shown an altered interaction between neurons and glial cells that contributes to neuronal injury, in part due to increased production of inflammatory mediators such as prostaglandins, inflammatory cytokines, and nitric oxide (NO) by reactive glia (Gonzalez-Scarano and Baltuch, 1999). NO is produced from L-arginine by nitric oxide synthases (NOS1, 2, and 3), of which the inducible isoform (NOS2) is highly upregulated in activated astrocytes and microglia. NOS2 is not constitutively expressed but is rapidly induced by stimuli such as proinflammatory cytokines (Stuehr and Griffith, 1992). Enhanced expression of NOS2 is a prototypic inflammatory response of activated astrocytes, and previous studies from our laboratory have demonstrated that Mn strongly potentiates expression of NOS2 and production of NO in cytokine-primed astrocytes through a signaling mechanism involving soluble guanylyl cyclase (sGC), extracellular signal-regulated kinase (ERK), and the transcription factor nuclear factor-κB, leading to apoptosis in cocultured neurons in vitro and during Mn exposure in vivo (Liu et al., 2005, 2006; Moreno et al., 2008). Less is known regarding the role of microglial activation in Mn neurotoxicity or about interactions between microglia and astrocytes that may promote reactive astrogliosis. Microglia are not uniformly distributed in the brain and are enriched in several regions including the basal ganglia (Kim et al., 2000; Lawson et al., 1990). Activated microglia produce proinflammatory mediators such as NO, tumor necrosis factor-α, and interleukin-1β (Carreno-Muller et al., 2003; Giovannini et al., 2002; Wu et al., 2002), and specifically, Mn-induced activation in vitro has identified cyclooxgenase-2 as a key signaling pathway (Bae et al., 2006; Chang and Liu, 1999). However, the relative contributions of microglia and astrocytes to increased expression of NOS2 and induction of nitrosative stress in neurons following exposure to Mn in vivo are not well understood.
We therefore postulated that exposure to Mn during development would induce distinct patterns of microglial and astroglial reactivity that would sensitize nuclei of the basal ganglia to greater glial expression of NOS2 and induction of nitrosative stress upon subsequent adult exposures. To address this hypothesis, we exposed C57Bl/6J mice to Mn by intragastric gavage as juveniles, adults, or both and examined multiple indices of glial reactivity and neuropathology. These studies revealed a relatively greater sensitivity of the developing basal ganglia to Mn-induced activation of both microglia and astrocytes, which correlated with induction of NOS2 and increased nitration of neuronal proteins in the striatum (St), globus pallidus (Gp), and substantia nigra pars reticulata (SNpr). Early exposure to Mn increased glial reactivity and enhanced neuronal protein nitration following subsequent adult exposures, suggesting that early postnatal development represents a critical window of sensitivity to inflammatory activation of glia and induction of nitrosative stress in neurons.
MATERIALS AND METHODS
Reagents.
All chemical reagents were obtained from Sigma Chemical Co. (St Louis, MO) unless otherwise stated. C57Bl/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Primary antibodies for glial fibrillary acidic protein (GFAP) were from DakoCytomation (Carpinteria, CA) and Sigma. Antibodies for ionized Ca2+-binding adaptor molecule-1 (Iba-1) and NOS2 were from Wako Chemicals Inc. (Osaka, Japan) and BD Biosciences (San Jose, CA), respectively. Primary antibodies against 3-nitrotyrosine (3-NTyr) were from Upstate (Charlottesville, VA), dopamine- and cAMP-regulated phosphoprotein-32 (DARPP32) and tyrosine hydroxylase (TH) were from Chemicon (Burlington, MA), and major microtubule-associated protein 2 (MAP-2) was from Abcam (Cambridge, MA). Horseradish peroxidase–conjugated secondary antibodies and diaminobenzidine reagents were part of the Vectastain ABC kit from Vector Labs (Burlingame, CA). Secondary antibodies labeled with AlexaFluor-488 and -568 were from Invitrogen (Eugene, OR).
Animal exposure model.
C57Bl/6J mice were housed in microisolator cages (five animals per cage) and kept on 12-h light/dark cycles with access to laboratory chow and water ad libitum. Littermates from timed pregnant dams were paired in control and Mn-exposed groups and received 0.9% normal saline, 10 mg/kg MnCl2, or 30 mg/kg MnCl2 via intragastric gavage daily during the following time periods: juvenile exposure, postnatal days 20–34; adult exposure, from week 12 to week 20; and juvenile + adult exposure, postnatal days 20–34 and weeks 12–20 (Supplementary Fig. 1). Previous studies in our laboratory using this model administered 100 mg/kg Mn to adult female mice (Liu et al., 2006) for 8 weeks, but pilot studies in juvenile mice indicated a greater level of sensitivity to the neurological effects of Mn. We therefore revised the dosing paradigm downward to better test the hypothesis that low-dose juvenile exposure would potentiate later adult neurotoxicity upon subsequent exposure. Animals were weighed before each intragastric gavage and the amount of Mn delivered was adjusted accordingly. Additionally, the amount of Mn delivered was corrected for the molar concentration in the tetrahydrate form so as to achieve a precise dose of 10 or 30 mg/kg/day. All procedures were carried out under an institutional animal care and use committee–approved protocol at Colorado State University under the care of veterinary staff at the laboratory animal resources facility.
Immunohistochemistry and immunofluorescence.
Mice were anesthetized the day following the last day of saline or Mn gavage for each treatment group (see Supplementary Fig. 1) by inhalation of isofluorane and perfused intracardially with 4% paraformaldehyde in 0.1M NaKPO4 buffer (pH 7.4). Tissue was processed for immunohistochemical analysis as published previously by our laboratory (Liu et al., 2006; Moreno et al., 2008). Briefly, brains were collected and kept in 4% paraformaldehyde overnight and stored in 0.1M NaKPO4 buffer at 4°C. Paraffin-embedded 10-μm coronal serial sections were examined for protein expression in SNpr, Gp, and St by immunohistochemistry (IHC) using primary antibodies to GFAP (1:400) and Iba-1 (1:500). Sections were developed using horseradish peroxidase–conjugated secondary antibodies and diaminobenzidine reagents from the Vectastain ABC kit. For co-immunofluorescence studies, sections were incubated with anti-Iba1 (1:1000) or anti-GFAP (1:500) combined with anti-NOS2 (1:100) to examine gliosis. Antibiodies against MAP-2 (1:500) and DARPP-32 (1:300) were used in combination with anti-3-NTyr (1:100) to determine the levels of protein nitration in neurons. Specific protein epitopes were visualized with secondary antibodies labeled with AlexaFluor-488 or -568 and slides mounted in media containing 4′,6-diamidino-2-phenylindole dihydrochloride to identify cell nuclei. Images were acquired using either a Zeiss ×20 or ×40 air PlanApochromat objective or a ×63 oil objective. Nine individual brain sections were examined per treatment group, three for each parameter from three individual animals. Data from individual cells were summed for each section from six individual fields of view.
Pathological scoring.
Glial activation in juvenile and adult mice exposed to Mn was determined by pathological scoring based on expression of GFAP for astrocytes and Iba-1 for microglia in 10-μm serial sections through the St, Gp, and SNpr. IHC slides were imaged using a ×20 objective for analysis of astrocytes and ×40 objective for analysis of microglia, with six microscopic fields examined per treatment group with three animals per group. Bright-field images were acquired from serial sections in each brain region and independently examined by three researchers, including an anatomic veterinary pathologist, all of whom were blinded to the treatment groups. Images were evaluated for quantity and intensity of staining throughout the photomicrograph of the brain region examined. Treatment groups were assigned an IHC staining value based on comparison to a known control section from the same brain region. Two different scoring scales were used in this study. A scale of 1–5 was used for GFAP, and as baseline staining was always present in controls, this level of staining was assigned a value of “1.” Because higher total GFAP levels were noted in some treatment groups as well as increased intensity of GFAP-stained cells, increasing values were assigned for scoring those photomicrographs, with the highest and most intense images being scored as “5.” Due to the paucity of microglial staining in control sections from these brain regions, a score of “0” was given for the Iba-1 control samples and the most intensely stained photomicrographs were assigned a value of “3,” reflecting a more limited phenotypic change for this cell type.
Statistical analysis.
Comparison of two means was carried out by Student's t-test. Comparison of three or more means was carried out using one-way ANOVA followed by the Tukey-Kramer multiple comparison post hoc test using Prism software (v4.0c; GraphPad Software Inc., San Diego, CA). Pathological scoring data were analyzed by nonparametric one-way ANOVA and the Kruskal-Wallis post hoc test. For all experiments, alpha level for the analysis was set at p < 0.05.
RESULTS
Developmental Patterns of Mn-Induced Astrogliosis
Astroglial activation in juvenile and adult mice exposed to Mn was determined by pathological scoring based on expression of GFAP in the St, Gp, and SNpr, the three basal ganglia nuclei most vulnerable to Mn exposure (Josephs et al., 2005; Liu et al., 2006). There were no apparent changes in the staining intensity of TH in dopaminergic terminals or soma in any treatment group (Supplementary Fig. 2). A baseline level of GFAP expression was detected in each of the nuclei analyzed: St, Gp, and SNpr (Fig. 1). Mice treated only as juveniles showed a slight activation at 10 and 30 mg/kg MnCl2 in the St and Gp and a decrease in GFAP staining in the SNpr. In contrast to juveniles, mice treated only as adults had a significant increase in staining of GFAP in the SNpr at both exposure levels, 10 and 30 mg/kg MnCl2. Mice exposed as juveniles and adults had a significant increase in GFAP staining in the Gp at 30 mg/kg MnCl2 and SNpr at both 10 and 30 mg/kg MnCl2. No activation of astrocytes was observed in the St in any treatment group at these doses of Mn. Intense GFAP staining was detected in both the Gp and the SNpr of 30 mg/kg MnCl2–treated mice exposed as juveniles + adults, with both brain regions containing astrocytes undergoing hyperplasia (Fig. 1 and Table 1).
FIG. 1.
Differential exposure to Mn in juvenile and adult C57Bl/6J mice induces distinct patterns of astrogliosis in the basal ganglia. Mice were exposed to 0, 10, or 30 mg/kg MnCl2 by daily intragastric gavage as juveniles, adults, or both juveniles and adults. Multiple brain regions in the basal ganglia were assessed for activation of astroglia by immunohistochemical staining for GFAP including the St, Gp, and SNpr. Representative images of the SNpr are depicted for control mice and mice exposed to 30 mg/kg MnCl2 as (A) juveniles, (B) adults, and (C), both juveniles and adults. Scale bar = 10 μm.
TABLE 1.
Pathological Scoring of Astrogliosis in Specific Brain Regions of Mice Exposed to Mn: GFAPa
| Time of exposure | Treatment (MnCl2 in mg/kg) | Gp | St | SNpr |
| Juvenile | 0 | 3.5 ± 0.26 | 1 ± 0.33 | 3.5 ± 0.07 |
| 10 | 3.9 ± 0.07 | 1.8 ± 0.11 | 3.2 ± 0.06 | |
| 30 | 3.6 ± 0.11 | 1.4 ± 0.06 | 2.4 ± 0.04* | |
| Adult | 0 | 2.6 ± 0.19 | 0.3 ± 0.13 | 0.5 ± 0.16 |
| 10 | 3.5 ± 0.35 | 1.8 ± 0.08* | 4.1 ± 0.04* | |
| 30 | 3.2 ± 0.23 | 1.3 ± 0.05* | 3.4 ± 0.24* | |
| Juvenile + adult | 0 | 2.5 ± 0.12 | 1.3 ± 0.06 | 1.0 ± 0.0 |
| 10 | 2.1 ± 0.04 | 1.0 ± 0.02 | 1.8 ± 0.04* | |
| 30 | 3.9 ± 0.15* | 1.8 ± 0.07 | 2.8 ± 0.06* |
Note. Data shown are mean ± SEM (n ≥ 3). Significance compared with controls within a specific brain region (Gp, St, or SNpr) per exposure group is denoted by “*” (p < 0.05).
Normal astrocyte = 1; activated astrocyte = 5.
Developmental Patterns of Mn-Induced Microgliosis
Activation of microglia was similarly examined by pathological scoring in the St, Gp, and SNpr, based on the expression of Iba-1 and cellular morphology of microglia (Fig. 2). An increase in activation of Iba-1–positive microglia was observed only in juvenile mice and occurred at both 10 and 30 mg/kg MnCl2 (Table 2). Microglia in Mn-treated juvenile mice showed a retraction and thickening of cytoplasmic processes consistent with an activated amoeboid phenotype (Fig. 2). Juvenile animals exposed to 10 and 30 mg/kg MnCl2 had significantly higher pathology scores for Iba-1 staining than control animals in each brain region examined. Mice exposed only as adults had significantly increased Iba-1 staining at the highest exposure (30 mg/kg MnCl2) compared with controls. No Iba-1 staining was observed in adult mice pretreated as juveniles (Table 2).
FIG. 2.
Differential exposure to Mn in juvenile and adult C57Bl/6J mice induces distinct patterns of microgliosis in the basal ganglia. Mice were exposed to 0, 10, or 30 mg/kg MnCl2 by daily intragastric gavage as juveniles, adults, or both juveniles and adults. Multiple brain regions in the basal ganglia were assessed for activation of microglia by immunohistochemical staining for Iba-1 including the St, Gp, and SNpr. Representative images of the SNpr are depicted for control mice and mice exposed to 30 mg/kg MnCl2 as (A) juveniles, (B) adults, and (C) both juveniles and adults. Arrowheads indicate cell bodies. Scale bar = 10 μm.
TABLE 2.
Pathological Scoring of Microgliosis in Specific Brain Regions of Mice Exposed to Mn: IBA-1a
| Time of exposure | Treatment (MnCl2 in mg/kg) | Gp | St | SNpr |
| Juvenile | 0 | 0.33 ± 0.09 | 0.0 ± 0.0 | 0.67 ± 0.06 |
| 10 | 1.5 ± 0.06* | 1.3 ± 0.04* | 1.6 ± 0.06* | |
| 30 | 1.4 ± 0.08* | 1.2 ± 0.06* | 1.3 ± 0.13* | |
| Adult | 0 | 0.8 ± 0.13 | 0.8 ± 0.16 | 0.86 ± 0.05 |
| 10 | 1.5 ± 0.17 | 1.7 ± 0.08 | 0.6 ± 0.08 | |
| 30 | 2.8 ± 0.10* | 2.7 ± 0.1* | 2.2 ± 0.07* | |
| Juvenile + adult | 0 | 0.8 ± 0.08 | 0.5 ± 0.05 | 1.1 ± 0.03 |
| 10 | 0.6 ± 0.11 | 0.5 ± 0.11 | 1.1 ± 0.03 | |
| 30 | 0.5 ± 0.30 | 1.10 ± 0.09 | 1.7 ± 0.09 |
Note. Data shown are mean ± SEM (n ≥ 3). Significance compared with controls within a specific brain region (GP, ST, or SNpr) per exposure group is denoted by “*”(p < 0.05).
No microglia = 0; activated microglia = 3.
Mn Exposure Increases Expression of NOS2 in Astroglia
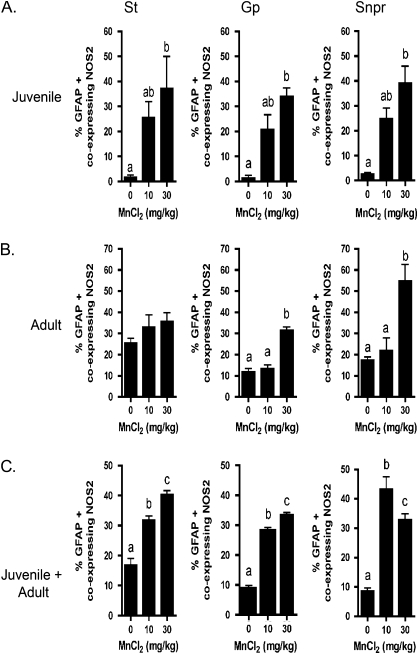
The expression of NOS2 was examined as a neuroinflammatory marker in activated astrocytes by co-immunofluorescence (Fig. 3). Expression of NOS2 was increased in GFAP-positive cells in Mn-treated animals compared with controls in the SNpr of each exposure group: juvenile, adult, and juvenile + adult, depicted in Figures 3A–C. Treatment with 30 mg/kg MnCl2 caused astrocyte hypertrophy and stress fiber formation in each exposure group (Figs. 3A–C). Quantification of the number of NOS2-positive astrocytes as a percentage of the total number of GFAP-positive cells indicated that NOS2 expression increased significantly in activated astrocytes in mice exposed as juveniles to 30 mg/kg MnCl2 in the St, Gp, and SNpr (Fig. 4A). Mice exposed to 30 mg/kg MnCl2 solely as adults had an increase in astrocytic NOS2 expression only in the Gp and SNpr but not in the St (Fig. 4B). Interestingly, mice exposed as both juveniles and adults had significantly increased NOS2 expression in the GFAP-positive cells at both 10 and 30 mg/kg MnCl2 (Fig. 4C), whereas mice exposed only as adults showed no change in astrocytic expression of NOS2 at 10 mg/kg MnCl2.
FIG. 3.
Activated astrocytes in C57Bl/6J mice express NOS2 following differential exposure to Mn as juveniles and adults. Astroglial expression of NOS2 was assessed via co-immunofluorescence in multiple regions of the basal ganglia from mice exposed to Mn. Representative images of the SNpr are presented from control mice and those treated with 30 mg/kg MnCl2 as (A) juveniles, (B) adults, and (C) both juveniles and adults. Images of GFAP and NOS2 expression are shown in green and red channels, respectively, and cell nuclei are highlighted by staining with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) in blue. Yellow areas in merged images indicate colocalization of NOS2 expression in GFAP-positive astrocytes in mice exposed to Mn. Scale bar = 10 μm.
FIG. 4.
Quantitative analysis of NOS2 expression in GFAP-positive astrocytes reveals regionally and developmentally distinct patterns of astroglial reactivity following exposure to Mn. The percent of GFAP-positive astrocytes expressing NOS2 was determined in mice exposed to 0, 10, or 30 mg/kg MnCl2 by daily intragastric gavage as (A) juveniles, (B) adults, and (C) both juveniles and adults in the St, Gp, and SNpr. Three serial sections were stained for each brain region per hemisphere from an average of three mice per treatment group. For quantitative cell counts, three microscopic fields were evaluated per brain region from each serial section, totaling n = 9 per treatment group. Data represent the mean percentage of GFAP-positive astrocytes expressing NOS2. Different letters denote significant differences between treatment groups, p < 0.05.
Mn Exposure Increases Expression of NOS2 in Microglia
Microglia were also examined for expression of NOS2 in the Gp, St, and SNpr of control and Mn-treated juvenile and adult mice. Representative images from the SNpr are presented in Figure 5 for each group of mice following exposure to 0 or 30 mg/kg MnCl2. Microglia in sections from control mice had a ramified phenotype with multiple fine processes characteristic of resting cells and were without evident expression of NOS2 (Figs. 5A–C). In contrast to microglia in control mice, Iba-1–positive cells in mice exposed to 30 mg/kg MnCl2 showed an activated phenotype characterized by a thickened, amoeboid appearance with fewer cytoplasmic processes and marked expression of NOS2. Quantification of the percent of total Iba-1–positive cells coexpressing NOS2 in each treatment group is presented in Figure 6. Mice exposed to Mn as juveniles had increased numbers of Iba-1–positive cells in the St at 30 mg/kg MnCl2 and in the Gp and SNpr at both 10 and 30 mg/kg MnCl2 (Fig. 6A). In mice exposed to Mn only as adults, the percent of Iba-1–positive cells coexpressing NOS2 was increased only in the SNpr at 30 mg/kg MnCl2 (Fig. 6B). In the absence of juvenile preexposure, adult exposure to Mn did not result in increased microglial expression of NOS2 at either 10 or 30 mg/kg MnCl2 in the St and Gp. Mice receiving Mn exposure as juveniles + adults showed an increase in NOS2 expression in Iba-1–positive cells at both 10 and 30 mg/kg MnCl2 in all three brain regions examined, the St, Gp, and SNpr (Fig. 6C).
FIG. 5.
Activated microglial cells in C57Bl/6J mice express NOS2 following differential exposure to Mn as juveniles and adults. Microglial expression of NOS2 was assessed via co-immunofluorescence in the St, Gp, and SNpr from mice exposed to Mn. Representative images of the SNpr are presented from control mice and those treated with 30 mg/kg MnCl2 as (A) juveniles, (B) adults, and (C) both juveniles and adults. Images of Iba-1 and NOS2 expression are shown in green and red channels, respectively, and cell nuclei were highlighted by staining with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) in blue. Merged images indicate colocalization of NOS2 expression in Iba-1–positive microglia in mice exposed to Mn. Scale bar = 10 μm.
FIG. 6.
Quantitative analysis of NOS2 expression in Iba-1–positive microglial cells reveals regionally and developmentally distinct patterns of microglial reactivity following exposure to Mn. The percentage of Iba-1–positive microglia expressing NOS2 was determined in mice exposed to 0, 10, or 30 mg/kg MnCl2 by daily intragastric gavage as (A) juveniles, (B) adults, and (C) both juveniles and adults in the St, Gp, and SNpr. Three serial sections were stained for each brain region per hemisphere from an average of three mice per treatment group. For quantitative cell counts, three microscopic fields were evaluated per brain region from each serial section, totaling n = 9 per treatment group. Data represent the mean percentage of Iba-1–positive microglia expressing NOS2. Different letters denote significant differences between treatment groups, p < 0.05.
Developmental Exposure to Mn Increases Adult Susceptibility to Neuronal Protein Nitration
To determine the effects of increased glial expression of NOS2 on neurons, formation of neuronal 3-NTyr protein adducts was evaluated by immunofluorescence as an indicator of NO production and peroxynitrite (ONOO−) formation, a measure of nitrosative stress and neuronal injury. Neurons were identified by staining with the general neuronal marker, MAP-2, and colocalization was determined by overlaying images of 3-NTyr and MAP-2. Figure 7 depicts representative images from the SNpr of each treatment group of mice at 0 and 30 mg/kg MnCl2. Only low levels of 3-NTyr adducts were detected in control juvenile or adult animals (Figs. 7A–C, 0 mg/kg MnCl2 panels), whereas exposure to 30 mg/kg MnCl2 resulted in large increases in immunofluorescence staining for 3-NTyr protein adducts in MAP-2–positive neurons in the SNpr, primarily in soma but also in the neuropil and dendrites (Figs. 7A–C, 30 mg/kg MnCl2 panels). Quantification of 3-NTyr protein levels in MAP-2–positive neurons was carried out by obtaining the ratio of fluorescence intensity of 3-NTyr to MAP-2 in all three exposure groups. Although some level of 3-NTyr staining was detected even in control sections, levels of 3-NTyr protein adducts were increased in the Gp and SNpr of juvenile mice exposed to 30 mg/kg MnCl2 (Fig. 8A), in the Gp of mice exposed only as adults to 30 mg/kg MnCl2 (Fig. 8B), and in the SNpr of mice exposed as both juveniles and adults (Fig. 8C). The regional pattern of neuronal 3-NTyr formation appeared to depend on the age at which the animals were exposed to Mn, as well as the time point at which analysis of protein nitration was carried out.
FIG. 7.
Mn exposure increases levels of 3-NTyr protein adducts in basal ganglia neurons. To detect modification of neuronal proteins by peroxynitrite (ONOO−) derived from increased production of NO by activated glia, serial sections from the SNpr of (A) juvenile, (B) adult, and (C) both juvenile and adult mice exposed to 0 and 30 mg/kg MnCl2 were stained with antibodies against the general neuronal marker MAP-2 (green) and 3-NTyr (red) and were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) to identify cell nuclei (blue). Representative images of 3-NTyr–modified proteins indicate colocalization of 3-NTyr adducts with both neuronal soma and dendrites in the SNpr of Mn-treated juvenile mice. Scale bar = 10 μm.
FIG. 8.
Quantitative analysis of 3-NTyr adducts indicates that modification of neuronal proteins by peroxynitrite correlates with regional patterns of glial activation and NOS2 expression. Specific brain regions were evaluated for colocalization of MAP-2 and 3-NTyr by immunofluorescence in control mice and those exposed to 30 mg/kg MnCl2. Graphs indicate quantification of levels of 3-NTyr protein adducts in neurons from St, Gp, and SNpr of mice exposed as (A) juveniles, (B) adults, and (C) juveniles and adults. Three serial sections were stained for each brain region per hemisphere from an average of three mice per treatment group. For quantitative cell counts, three microscopic fields were evaluated per brain region from each serial section, totaling n = 9 per treatment group. Data indicate increased protein nitration in the neurons of the Gp and SNpr. Different letters denote significant differences between treatments, p < 0.05.
Protein nitration was also examined in the St by co-immunofluorescence for 3-NTyr protein adducts and DARPP-32, a marker for striatal neurons expressing D1 and D2 dopamine receptors (Reiner et al., 1998, Fig. 9). Quantification of 3-NTyr protein levels in DARPP-32–positive neurons was carried out by obtaining the ratio of fluorescence intensity of 3-NTyr to DARPP-32 in all three exposure groups. No change in protein nitration was detected in DARPP-32–positive striatal neurons in either juvenile or adult mice exposed to 30 mg/kg MnCl2 (Figs. 9A and 9B), but increases in 3-NTyr adducts were evident in mice exposed to 30 mg/kg MnCl2 as both juveniles and adults (Fig. 9C).
FIG. 9.
Quantitative analysis of 3-NTyr adducts indicates that modification of specific striatal neuronal proteins by peroxynitrite correlates with regional patterns of glial activation and NOS2 expression. Striatal neurons were evaluated for colocalization of DARPP-32 and 3-NTyr by immunofluorescence in control mice and those exposed to 30 mg/kg MnCl2. Graphs indicate quantification of levels of 3-NTyr protein adducts in neurons from St of mice exposed as (A) juveniles, (B) adults, and (C) juveniles and adults. Three serial sections were stained for each brain region per hemisphere from an average of three mice per treatment group. For quantitative cell counts, three microscopic fields were evaluated per brain region from each serial section, totaling n = 9 per treatment group. Data indicate increased protein nitration in the neurons of the juvenile + adult exposure group. Different letters denote significant differences between treatments, p < 0.05.
DISCUSSION
Neuroinflammation is now recognized as an important determinant of neuronal injury in diverse neurodegenerative conditions, including Alzheimer's disease (AD), PD, and manganism. Within the basal ganglia, the St receives most of the input from motor neurons, whereas the Gp and SNpr are the two major output nuclei (Saka et al., 2002). In the present studies, it was found that both the St-pallidum and the substantia nigra had elevated Mn that correlated with alterations in locomotor function and changes in levels of striatal catecholamines (Moreno et al., 2009, this issue). Prior studies in our laboratory demonstrated that 100 mg/kg MnCl2 causes astrogliosis and increased expression of NOS2 that correlates with neuronal injury in the striatal-pallidal system in adult mice exposed via intragastric gavage (Liu et al., 2006). The present studies expanded upon this finding to examine the role of gliosis in developmental vulnerability to neuroinflammatory injury due to Mn using lower doses of Mn, 10 and 30 mg/kg, to assess the sensitivity of the developing mice to the neurological effects of Mn during a critical time period for striatal development in rodents (Soiza-Reilly and Azcurra, 2009).
Developmental exposure to Mn resulted in an increase in microglial activation in young mice and enhanced neuroinflammation and nitrosative stress in adult mice that were preexposed to Mn as juveniles. Greater inflammatory activation of glia was accompanied by increased expression of NOS2 and more extensive neuronal protein nitration throughout the basal ganglia. Increased protein nitrotyrosine adducts in DARPP-32–expressing striatal neurons in adult mice preexposed as juveniles (Fig. 9) is a particularly interesting finding that may help to explain some of the known behavioral and neurochemical deficits ensuing from chronic Mn exposure in the context of regional neuroinflammation.
Juvenile exposure to Mn caused an increase in microgliosis at both 10 and 30 mg/kg MnCl2 but not in mice exposed as both juveniles and adults, whereas increased activation of astrocytes was only detected in adult animals, suggesting a requirement for prior activation of microglia in promoting astroglial activation following Mn exposure (Tables 1 and 2). It should be noted that the basal levels of GFAP staining (Table 1) were scored as activated astrocytes that could be due to astrocytes being in a proliferative state during the initial juvenile exposure to Mn, increasing GFAP staining (Rice and Barone, 2000). It was previously reported that microglia activate before astrocytes during brain injury (Norton, 1999), and the data reported here seemed at first to be at odds with this model because mice exposed only as adults did have activated microglia at 30 mg/kg MnCl2 but the juvenile + adult exposure group did not. However, it is important to note the temporal nature of microglial activation, which occurs rapidly after stress and injury and then subsides, followed by astrocyte activation (Liberatore et al., 1999). Thus, it is likely that the initial stress response to Mn in adult animals without prior exposure as juveniles resulted in a robust microglial response, as well as detectable astrogliosis, whereas adult mice with previous exposure to Mn as juveniles were sensitized to exposure but had likely progressed beyond the initial phases of injury and microglial activation to a later-stage lesion with only activated astroglia remaining detectably increased. This phenomenon of adult mice preexposed as juveniles having astrogliosis but not microgliosis occurred specifically in both the SNpr and the Gp, indicating that these nuclei are highly susceptible to Mn neurotoxcity and are shown to accumulate the highest levels of Mn (data in companion article, this issue), which may help to explain the increase in gliosis, NOS2 expression, and protein nitration observed in these brain regions.
Multiple in vitro and in vivo studies identify NOS2 as a key inflammatory gene involved in Mn neurotoxicity (Bae et al., 2006; Liu et al., 2006; Moreno et al., 2008), but little is known about region- and cell-specific NOS2 expression in glia during developmental exposure to Mn. Increased NOS2 protein expression was detected in astrocytes in each of the three brain regions examined in juvenile mice exposed to 30 mg/kg MnCl2 but only in the Gp and SNpr of mice in the adult exposure group (Figs. 3 and 4). Unlike the juvenile and adult-only exposure groups, mice exposed as juveniles and again as adults had a significant increase in NOS2 expression in astrocytes in both the Mn treatment groups, indicating that prior exposure to Mn causes an increase in NOS2 expression in astrocytes at a lower dose of Mn, indicative of an enhanced neuroinflammatory response to secondary exposure. Expression of NOS2 was detected in greater numbers of astrocytes and microglia in the juvenile + adult exposure group than would be predicted by merely examining levels of GFAP and Iba-1 as phenotypic markers of activation, suggesting that expression of neuroinflammatory genes is greater in adult animals previously exposed as juveniles than in naive adults and that expression of NOS2 does not correlate precisely with increased levels of other phenotypic markers. Control levels of NOS2 expression appeared to be somewhat higher in microglia from naive adult animals than in those preexposed as juveniles (Fig. 6); however, these represent different treatment paradigms and the relative increase in the number of microglia expressing NOS2 was much greater in adults preexposed as juveniles.
Pathological scoring for microglial activation also revealed interesting differences in adult animals. Juvenile and adult mice showed an increased pathological score for microglial activation. Juvenile mice were sensitive at both 10 and 30 mg/kg MnCl2, whereas microglia were activated in adult mice only at 30 mg/kg MnCl2 (Table 2). However, the number of Iba-1–positive cells was extremely small in the SNpr of control adult mice (∼5–6 cells per section, per region) and of those Iba-1–positive cells, about 35–50% coexpressed NOS2 (Fig. 6B), somewhat enhancing the apparent microglial response in adult animals, relative to juveniles. The percent of Iba-1–positive cells coexpressing NOS2 was significantly increased with Mn exposure equally for 10 and 30 mg/kg MnCl2 in the juvenile + adult group, unlike the GFAP response, in which only the 30 mg/kg dose group expressed NOS2. These data indicate that microglia are highly sensitive to activation at lower dose of Mn (10 mg/kg) than astrocytes, suggesting that early activation of microglia may be required for subsequent and sustained activation of astrocytes in mice exposed as adults. This paradigm is consistent with previous studies reporting that microglia exposed to Mn secrete inflammatory cytokines (Chang and Liu, 1999; Filipov et al., 2005; Liu et al., 2009) that can promote activation of astrocytes with subsequent release of NO and prostaglandins (Hirsch et al., 1998; Moreno et al., 2008; Spranger et al., 1998). Thus, early activation of microglia may also help to explain the enhanced sensitivity to Mn in the basal ganglia and the magnification of subsequent astroglial inflammatory responses to additional exposures.
Glial cells are essential for the survival of neurons and play a vital role in the uptake of glutamate, glycine, and γ-aminobutyric acid, and in antioxidant defense, via astrocytic production of glutathione (Aschner, 1998; Aschner et al., 1994; Pekny and Nilsson, 2005). Deprecations in these and other trophic functions in reactive glia may act in concert with overproduction of inflammatory mediators to impair neuronal homeostasis. Excessive levels of NO produced by glia react with superoxide to form peroxynitrite, a potent neurotoxin (Huie and Padmaja, 1993; Koppenol et al., 1992) that nitrates protein tyrosine residues (Ischiropoulos, 2003) and is associated with neuronal injury in a number of neurological disorders, including ischemia, AD, and PD (Carbone et al., 2008; Hensley et al., 1998; Ma et al., 2007). Co-immunofluorescence studies (Figs. 7 and 8) indicated that juvenile mice were the most susceptible to neuronal 3-NTyr protein formation in the Gp and SNpr, whereas the SNpr was vulnerable in the preexposed adult group. These results give insight into the brain regions most susceptible to glial-induced injury to the neurons and that young mice were more vulnerable than adults preexposed as juveniles.
Striatal DARPP-32–positive neurons were also vulnerable to Mn-induced nitrosative stress, where 3-NTyr protein adducts in DARPP-32–expressing striatal neurons were increased in adult mice preexposed as juveniles (Fig. 9). Because these neurons project to both the Gp and the SNpr (Reiner et al., 1998), protein nitration and subsequent neuronal injury and dysfunction could disrupt pathways in the basal ganglia essential to the coordination of motor function. It has been suggested in related work that nitration of dopaminergic neurons may occur in late models of basal ganglia injury (Kuhn et al., 2004), which is perhaps why the increase of 3-NTyr expression in DARPP-32–positive neurons was only detected in adult mice preexposed as juveniles.
These studies demonstrate that mice exposed to moderate levels of Mn by intragastric gavage at a young age, approximately equivalent to a 2-year-old child during the initial day of the juvenile exposure (day 21) up to young adulthood on day 35 (Dobbing and Sands, 1979; Flurkey et al., 2007; Koop et al., 1986), are highly vulnerable to activation of microglia and astrocytes, expression of NOS2, and overall 3-NTyr expression. In addition, these data indicate that juvenile exposure to Mn enhances the susceptibility to a neuroinflammatory phenotype in adult mice upon re-exposure and leads to increased tyrosine nitration of DARPP-32–positive neurons in the striatum, as well as general neuronal protein nitration in the SNpr. Thus, early postnatal development appears to be a critical window of sensitivity to Mn exposure that may promote more severe glial responses and neuroinflammation in the basal ganglia upon subsequent exposure during aging.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (ES012941) to R.B.T.
Supplementary Material
Acknowledgments
The authors thank Dr David Carbone for his technical assistance and advice throughout the study.
References
- Albin RL. Basal ganglia neurotoxins. Neurol. Clin. 2000;18:665–680. doi: 10.1016/s0733-8619(05)70217-6. [DOI] [PubMed] [Google Scholar]
- Aschner M. Astrocytic functions and physiological reactions to injury: The potential to induce and/or exacerbate neuronal dysfunction—A forum position paper. Neurotoxicology. 1998;19:7–17. discussion 37–18. [PubMed] [Google Scholar]
- Aschner M, Mullaney KJ, Wagoner D, Lash LH, Kimelberg HK. Intracellular glutathione (GSH) levels modulate mercuric chloride (MC)- and methylmercuric chloride (MeHgCl)-induced amino acid release from neonatal rat primary astrocytes cultures. Brain Res. 1994;664:133–140. doi: 10.1016/0006-8993(94)91963-1. [DOI] [PubMed] [Google Scholar]
- Bae JH, Jang BC, Suh SI, Ha E, Baik HH, Kim SS, Lee MY, Shin DH. Manganese induces inducible nitric oxide synthase (iNOS) expression via activation of both MAP kinase and PI3K/Akt pathways in BV2 microglial cells. Neurosci. Lett. 2006;398:151–154. doi: 10.1016/j.neulet.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Environ. Health Perspect. 2007;115:122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Koller W, Schulz PE. Parkinsonism due to manganism in a welder: Neurological and neuropsychological sequelae. Neurotoxicology. 2006;27:327–332. doi: 10.1016/j.neuro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: Similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Moreno JA, Tjalkens RB. Nuclear factor kappa-B mediates selective induction of neuronal nitric oxide synthase in astrocytes during low-level inflammatory stimulation with MPTP. Brain Res. 2008;1217:1–9. doi: 10.1016/j.brainres.2008.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno-Muller E, Herrera AJ, de Pablos RM, Tomas-Camardiel M, Venero JL, Cano J, Machado A. Thrombin induces in vivo degeneration of nigral dopaminergic neurones along with the activation of microglia. J. Neurochem. 2003;84:1201–1214. doi: 10.1046/j.1471-4159.2003.01634.x. [DOI] [PubMed] [Google Scholar]
- Chang JY, Liu LZ. Manganese potentiates nitric oxide production by microglia. Brain Res. Mol. Brain Res. 1999;68:22–28. doi: 10.1016/s0169-328x(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Cowan DM, Fan Q, Zou Y, Shi X, Chen J, Aschner M, Rosenthal FS, Zheng W. Manganese exposure among smelting workers: blood manganese-iron ratio as a novel tool for manganese exposure assessment. Biomarkers. 2009;14:3–16. doi: 10.1080/13547500902730672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Marshall MW, Parkinson CU, James RA, Wong BA. Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol. Sci. 2006;92:201–210. doi: 10.1093/toxsci/kfj206. [DOI] [PubMed] [Google Scholar]
- Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol. Sci. 2005;84:139–148. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison DE. The mouse in aging research. In: Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith A, editors. In the Mouse in Biomedical Research. 2007. pp. 637–672. American College Laboratory Animal Medicine, Elsevier, Burlington, MA. [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol. Sci. 2007;95:205–214. doi: 10.1093/toxsci/kfl139. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Scali C, Prosperi C, Bellucci A, Vannucchi MG, Rosi S, Pepeu G, Casamenti F. Beta-amyloid-induced inflammation and cholinergic hypofunction in the rat brain in vivo: Involvement of the p38MAPK pathway. Neurobiol. Dis. 2002;11:257–274. doi: 10.1006/nbdi.2002.0538. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Hearn AS, Stroupe ME, Cabelli DE, Ramilo CA, Luba JP, Tainer JA, Nick HS, Silverman DN. Catalytic and structural effects of amino acid substitution at histidine 30 in human manganese superoxide dismutase: Insertion of valine C gamma into the substrate access channel. Biochemistry. 2003;42:2781–2789. doi: 10.1021/bi0266481. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Tjalve H. Manganese taken up into the CNS via the olfactory pathway in rats affects astrocytes. Toxicol. Sci. 2000;55:392–398. doi: 10.1093/toxsci/55.2.392. [DOI] [PubMed] [Google Scholar]
- Hensley K, Maidt ML, Yu Z, Sang H, Markesbery WR, Floyd RA. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J. Neurosci. 1998;18:8126–8132. doi: 10.1523/JNEUROSCI.18-20-08126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson's disease: A role in neurodegeneration? Ann. Neurol. 1998;44:S115–S120. doi: 10.1002/ana.410440717. [DOI] [PubMed] [Google Scholar]
- Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem. Biophys. Res. Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: Role of microglia. J. Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop M, Rilling G, Herrmann A, Kretschmann HJ. Volumetric development of the fetal telencephalon, cerebral cortex, diencephalon, and rhombencephalon including the cerebellum in man. Bibl. Anat. 1986;28:53–78. [PubMed] [Google Scholar]
- Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- Krachler M, Domej W, Irgolic KJ. Concentrations of trace elements in osteoarthritic knee-joint effusions. Biol. Trace Elem. Res. 2000;75:253–263. doi: 10.1385/BTER:75:1-3:253. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Sakowski SA, Sadidi M, Geddes TJ. Nitrotyrosine as a marker for peroxynitrite-induced neurotoxicity: The beginning or the end of the end of dopamine neurons? J. Neurochem. 2004;89:529–536. doi: 10.1111/j.1471-4159.2004.02346.x. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Liu M, Cai T, Zhao F, Zheng G, Wang Q, Chen Y, Huang C, Luo W, Chen J. Effect of microglia activation on dopaminergic neuronal injury induced by manganese, and its possible mechanism. Neurotox. Res. 2009;16:42–49. doi: 10.1007/s12640-009-9045-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Buffington JA, Tjalkens RB. NF-kappaB-dependent production of nitric oxide by astrocytes mediates apoptosis in differentiated PC12 neurons following exposure to manganese and cytokines. Brain Res. Mol. Brain Res. 2005;141:39–47. doi: 10.1016/j.molbrainres.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: The role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol. Sci. 2006;91:521–531. doi: 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- Ma TC, Mihm MJ, Bauer JA, Hoyt KR. Bioenergetic and oxidative effects of free 3-nitrotyrosine in culture: Selective vulnerability of dopaminergic neurons and increased sensitivity of non-dopaminergic neurons to dopamine oxidation. J. Neurochem. 2007;103:131–144. doi: 10.1111/j.1471-4159.2007.04735.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: Glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Moreno JA, Sullivan KA, Carbone DL, Hanneman WH, Tjalkens RB. Manganese potentiates nuclear factor-kappaB-dependent expression of nitric oxide synthase 2 in astrocytes by activating soluble guanylate cyclase and extracellular responsive kinase signaling pathways. J. Neurosci. Res. 2008;86:2028–2038. doi: 10.1002/jnr.21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Yeomans EC, Streifel KM, Sullivan KA, Brattin B, Taylor R, Tjalkens RB. Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicol. Sci. 2009 doi: 10.1093/toxsci/kfp220. Advance Access published on October 7, 2009; doi:10.1093/toxsci/kfp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton WT. Cell reactions following acute brain injury: A review. Neurochem. Res. 1999;24:213–218. doi: 10.1023/a:1022505903312. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson's disease. Ann. N.Y. Acad. Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: A review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perera M, Paullus R, Medina L. Immunohistochemical localization of DARPP32 in striatal projection neurons and striatal interneurons in pigeons. J. Chem. Neuroanat. 1998;16:17–33. doi: 10.1016/s0891-0618(98)00056-8. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000;108(Suppl. 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier J. Manganese poisoning in Moroccan miners. Br. J. Ind. Med. 1955;12:21–35. doi: 10.1136/oem.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka E, Iadarola M, Fitzgerald DJ, Graybiel AM. Local circuit neurons in the striatum regulate neural and behavioral responses to dopaminergic stimulation. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9004–9009. doi: 10.1073/pnas.132212499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiza-Reilly M, Azcurra JM. Developmental striatal critical period of activity-dependent plasticity is also a window of susceptibility for haloperidol induced adult motor alterations. Neurotoxicol. Teratol. 2009;31:191–197. doi: 10.1016/j.ntt.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Spranger M, Schwab S, Desiderato S, Bonmann E, Krieger D, Fandrey J. Manganese augments nitric oxide synthesis in murine astrocytes: A new pathogenetic mechanism in manganism? Exp. Neurol. 1998;149:277–283. doi: 10.1006/exnr.1997.6666. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Griffith OW. Mammalian nitric oxide synthases. Adv. Enzymol. Relat. Areas Mol. Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res. Brain Res. Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Tran TT, Chowanadisai W, Crinella FM, Chicz-DeMet A, Lonnerdal B. Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status. Neurotoxicology. 2002;23:635–643. doi: 10.1016/s0161-813x(02)00091-8. [DOI] [PubMed] [Google Scholar]
- Verity MA. Manganese neurotoxicity: A mechanistic hypothesis. Neurotoxicology. 1999;20:489–497. [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, et al. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf A, Wright R, Amarasiriwardena C, Bellinger D. A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 2002;110:613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: A neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 1986;70:273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.