Abstract
Rodent models have been extensively utilized to identify putative human immunotoxicants; however, even when immunotoxicity is established, uncertainty remains whether the effects are predictive of human risk. Therefore, the objective of this study was to establish a polyclonal immunoglobulin M (IgM) antibody-forming cell (AFC) response model to directly characterize immunotoxicity in primary mouse or human B cells. CD40 ligand (CD40L) was selected to activate B cells because it effectively drives both primary human and mouse B cells in vitro to AFC in a physiologically relevant manner to mimic T-cell-dependent antibody responses in vivo. In this model, the IgM AFC response is induced by cell surface–expressed CD40L and promoted by recombinant cytokines. Reported here are the conditions required to induce IgM AFC responses using mouse splenic B cells or human peripheral blood B cells, allowing for species comparisons. Moreover, less than one order of magnitude difference was observed in the CD40L-induced B-cell AFC responses based on data from multiple donors. In addition to antibody production, proliferation and phenotypic changes characteristic of B-cell activation as well as the plasma cell phenotype were also significantly induced. Finally, two well-characterized immunotoxicants, arsenic and benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide, using the CD40L-induced IgM AFC response were compared in both mouse and human B cells. Collectively, an IgM AFC response model is described that can be applied to assess the sensitivity of antibody responses to modulation by xenobiotics using mouse as well as human primary B cells.
Keywords: immunotoxicology, antibody response, human B cell, in vitro model
Historically, immunotoxicology studies have been conducted in laboratory animals, with the most extensively utilized species being the mouse, in which the immune system has been well characterized. The T-cell-dependent anti-sheep erythrocyte (anti-sRBC) immunoglobulin M (IgM) antibody-forming cell (AFC) response, when combined with several other immune function assays, has been widely accepted as an exceptionally sensitive assay for identifying agents that alter immune competence. However, when assessing human risk associated with exposure to a given agent, significant uncertainties still arise from unknown interspecies differences that influence the sensitivity to, or mechanism of action produced by, xenobiotics, including immunotoxic agents. Hence, investigations conducted exclusively using in vitro and in vivo animal models may not always be predictive of toxicity in humans (Selgrade, 1999; Vos and Van Loveren, 1998). In light of the above, there are significant advantages in extending immunotoxicity evaluations in ways that can directly assess the effects of xenobiotics on immune function using human primary leukocytes.
T-cell-dependent antibody responses in vivo require multiple molecular signals. The initial signal occurs when T cells are activated upon engagement of the T-cell receptor (TCR) with antigen-derived peptides presented in major histocompatibility complex II on the surface of antigen-presenting cells. After ligation of TCR, CD40 ligand (CD40L), one of a large family of costimulatory molecules, is rapidly upregulated on the surface of T cells and triggers stimulatory signals in the B cell through binding to its receptor, CD40. It has been well established that the cognate interactions between CD40 on B cells and CD40L on T cells play an essential role in all stages of T-cell-dependent humoral immunity in vivo (Elgueta et al., 2009; van Kooten and Banchereau, 2000). The indispensable role of CD40L in T-cell-dependent humoral immunity in both humans and mice is evidenced by the severe deficiency that is observed in T-cell-dependent B-cell effector function in both species when functional CD40L is absent (Bishop and Hostager, 2003). Since its identification, CD40L has been employed to induce the differentiation of human primary B cells in vitro, as it most closely resembles the T-cell-dependent induction of effector B-cell function that occurs following T-cell–B-cell contact in vivo. In a limited number of previous studies, CD40L was found to drive differentiation of primary human B cells into antibody-secreting cells that secrete IgM, IgG, IgA, or IgE, dependent on the combination of cytokines (interleukin [IL]-2, IL-4, IL-6, IL-10, or IL-21) used to supplement the culture system (Armitage et al., 1993; Arpin et al., 1995; Ettinger et al., 2005; Rousset et al., 1992; Spriggs et al., 1992). Nevertheless, the application of CD40L in order to drive B-cell activation, proliferation, and/or differentiation in immunotoxicological assessments has, thus far, remained limited.
Both CD40L and CD40 are highly conserved between human and mouse, and human CD40L was found to be interchangeably bioactive in human and mouse B cells (Spriggs et al., 1992). The objective of this study is to describe a polyclonal B-cell IgM AFC response model that capitalizes on CD40L–CD40 interactions to induce an IgM AFC response using human peripheral blood B cells. Critical aspects of the assay system, including mode and magnitude of CD40L stimulation, key cytokines, and kinetics of the IgM response for mouse and human primary B cells, are discussed. Using the optimized model, extensive preliminary studies were also conducted to establish the magnitude of the inherent variability in background responsiveness (i.e., no treatment) of both CD19+ total and/or CD19+CD27− naive B cells from multiple human donors. The proliferative response and cell surface phenotypic changes induced by CD40L were also characterized, which not only serve as earlier markers of toxicity in addition to the functional IgM AFC response, but could also potentially identify mechanisms underlying the immunotoxic effect of xenobiotics exerted on B-cell effector function. Finally, the effects of arsenic and benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide (BPDE), two immunotoxicants identified by the mouse in vitro anti-sRBC AFC response (Burns et al., 1991; Kawabata and White, 1989), were determined using the CD40L-dependent model in primary mouse and human B cells.
MATERIALS AND METHODS
Chemicals.
Sodium (meta)arsenite was purchased from Sigma-Aldrich (St Louis, MO). BPDE was purchased from National Cancer Institute's Chemical Carcinogen Reference Standards Repository (Kansas City, MO).
Animals.
Virus-free, female C57BL/6 mice (6 weeks of age), were purchased from Charles River (Portage, MI). Mice were randomized, transferred to plastic cages containing sawdust bedding (five mice per cage), and quarantined for 1 week. Mice were provided food (Purina certified laboratory chow) and water ad libitum and were not used for experimentation until their body weight was 17–20 g. Animal holding rooms were kept at 21°C–24°C and 40–60% humidity with a 12-h light/dark cycle. Mice were used in accordance with guidelines set forth by the Michigan State University Institutional Animal Care and Use Committee.
Human leukocyte packs.
Human leukocyte packs were obtained commercially from anonymous donors (Gulf Coast Regional Blood Center, Houston, TX). All donors were routinely screened for HIV and hepatitis at the blood center.
Isolation of human and mouse B cells.
Human CD19+ total and/or CD19+CD27− naive B cells were isolated from peripheral blood mononuclear cells enriched from each leukocyte pack by density-gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ). Mouse B cells were isolated from spleens that were made into single-cell suspensions by passage through a 40-μm cell strainer (BD Biosciences, San Jose, CA). B cells were isolated from mouse spleen as opposed to peripheral blood due to insufficient numbers of B cells in mouse peripheral blood. Negative selection of human or mouse B cells was conducted using MACS Human B cell, Naive B cell, or Mouse B Cell Isolation Kit following the manufacturer's protocols (Miltenyi Biotec, Auburn, CA) and as described previously (Schneider et al., 2008). Typically, 3–4 × 107 B cells were obtained from each mouse spleen, and 3–6 × 107 CD19+CD27− naive B cells or 6–10 × 107 CD19+ total B cells were obtained from each human leukocyte pack. The purity of the isolated B cells was assessed using flow cytometry by enumerating the percentage of CD19+ or CD19+CD27− cells. In all cases, the purity of isolated B cells was ≥ 95%. Approximately 60% of isolated human peripheral blood CD19+ B cells were CD27− (naive) based on the donors assayed thus far, while ≥ 98% of mouse splenic B cells were CD27− (naive).
Cell culture.
The stably transfected mouse fibroblast line expressing human CD40L (CD40L-L cell) was a generous gift from Dr. David Sherr (Boston University School of Public Health). CD40L-L cells were maintained in Dulbecco's Modified Eagle's Medium (Invitrogen, Carlsbad, CA) supplemented with 10% bovine calf serum (BCS; HyClone, Logan, UT), 100 units/ml of penicillin, 100 μg/ml of streptomycin, 50μM of 2-mercaptoethanol, and 1× HT supplement (Invitrogen). Isolated human or mouse B cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated BCS, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 50μM of 2-mercaptoethanol. In all cases, cells were cultured at 37°C in 5% CO2.
CD40L-dependent in vitro polyclonal IgM AFC response.
CD40L-L cells were trypsinized, irradiated, and seeded into culture plates 1 day prior to experimentation. CD40L-L cells were routinely checked by flow cytometry to ensure high-level expression of human CD40L. A two-phase culture system was employed. In phase I, isolated human (96-well plate, 1.5 × 105 cell/culture) or mouse (48-well plate, 1.5 × 106 cell/culture) B cells were cocultured with irradiated CD40L-L cells in the presence or absence of 10 U/ml of recombinant human or mouse IL-2 (Roche Applied Science, Indianapolis, IN), 100 U/ml of IL-6 (human IL-6 from Roche Applied Science, mouse IL-6 from Jena Bioscience, Jena, Gemany), and 20 ng/ml of recombinant human or mouse IL-10 (Bender MedSystems, Burlingame, CA) for 3 (mouse) or 4 days (human). In experiments involving xenobiotic treatment, both mouse and human B cells were treated immediately prior to coculture with CD40L-L cells and ILs and were exposed to xenobiotics for the duration of the culture period (both phases, see below). The vehicle (VH) for BPDE was dimethylsulfoxide, while arsenic was directly dissolved in RPMI 1640 culture medium. In phase II, B cells were transferred to new culture plates without CD40L-L cells on day 3 or 4 and were cultured for an additional 3 days prior to being harvested for enzyme-linked immunospot (ELISPOT) analysis. Culture supernatants were harvested for ELISA. The number of viable cells was assessed with a Coulter particle counter (Beckman Coulter, Fullerton, CA) following lysis of dead cells by treatment with pronase (Calbiochem, San Diego, CA) as described previously (Schatz et al., 1993).
Antibodies, carboxyfluorescein succinimidyl ester labeling, and flow cytometry.
The following monoclonal antibodies and their isotype controls were purchased from BD Biosciences: purified anti-mouse CD16/CD32 (Fcγ III/II receptor), purified anti-mouse IgM, fluorescein isothiocyanate (FITC)-conjugated anti-human CD40L, allophyeocyanin (APC)-conjugated anti-human CD19, phycoerythrin (PE)-conjugated anti-human CD27, PE-conjugated anti-mouse CD19, PE-conjugated anti-mouse CD27, APC-conjugated anti-mouse CD138, PE-conjugated anti-human CD138, and FITC-conjugated anti-mouse IgM. The following monoclonal antibodies and their isotype controls were purchased from Biolegend (San Diego, CA): purified anti-human IgM, PE-Cy7–conjugated anti-human CD69, PE-conjugated anti-human CD86, PE-conjugated anti-mouse CD69, PE-Cy7–conjugated anti-mouse CD86, and FITC-conjugated anti-human IgM. In all flow cytometry experiments, Live/Dead Fixable Dead Cell Stain Kit (red or near-infrared dye; Invitrogen) was used to exclude dead cells per the manufacturer's protocol. Mouse B cells were incubated with anti-mouse CD16/CD32, and human B cells with human AB serum (Invitrogen) to block Fcγ receptors. Staining of surface markers was conducted in single-cell suspensions in 1× Hank's Balanced Salt Solution (Invitrogen) containing 1% bovine serum albumin (BSA; Calbiochem) and 0.1% sodium azide (Sigma-Aldrich), pH 7.6. For staining of intracellular IgM, cells were incubated with nonconjugated anti-mouse or human IgM to block surface IgM prior to permeablization of cells with Cytofix/Cytoperm solution or Perm/Wash buffer (BD Biosciences). Staining was conducted in Perm/Wash buffer (BD Biosciences). In all cases, cells were fixed with Cytofix buffer (BD Biosciences) and kept on ice in all steps. For studies of B-cell proliferation, isolated human or mouse B cells were incubated with 5μM of carboxyfluorescein succinimidyl ester (CFSE) (CellTrace CFSE Cell Proliferation Kit; Invitrogen) at 5 × 106 cell/ml following manufacturer's protocol. The CFSE-labeled cells were washed, adjusted to the desired cell density, and cocultured with CD40L-L cells in the presence of IL-2, IL-6, and IL-10. Cells were assessed on a FACSCalibur or a FACSCantoII cell analyzer (BD Biosciences) and analyzed using FlowJo (Tree Star, Ashland, OR) offline analysis software.
Enumeration of IgM-secreting cells by ELISPOT.
ELISPOT was performed as described previously (Lu et al., 2009). Briefly, ELISPOT wells were coated with purified anti-human (BD Biosciences) or mouse (Sigma-Aldrich) IgM antibody and blocked with 5% BSA. Harvested cells were washed and incubated in the ELISPOT wells for 16–20 h. Biotin-conjugated anti-human or mouse IgM antibody (Sigma-Aldrich) and streptavidin-horseradish peroxidase (Sigma-Aldrich) were sequentially added to the wells. The spots were developed with the aminoethylcarbazole staining kit (Sigma-Aldrich). Data were collected and analyzed using the CTL ImmunoSpot system (Cellular Technology Ltd, Shaker Heights, OH).
Statistical analysis.
Graphpad Prism 4.00 (Graphpad Software, San Diego, CA) was used for all statistical analysis. The mean ± SE was determined for each treatment group in the individual experiments. Homogeneous data were evaluated by one-way ANOVA, and Dunnett's two-tailed t-test was used to compare treatment groups with the VH control when significant differences were observed. IC50 values were calculated from nonlinear regression of data using the sigmoidal concentration response curve.
RESULTS
CD40L-Induced IgM AFC Responses in Primary Human and Mouse B Cells
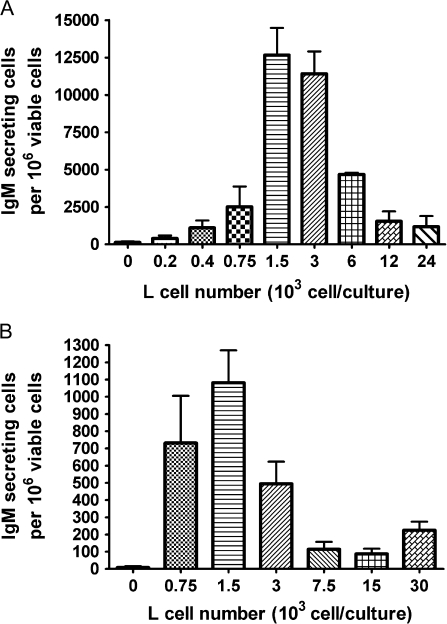
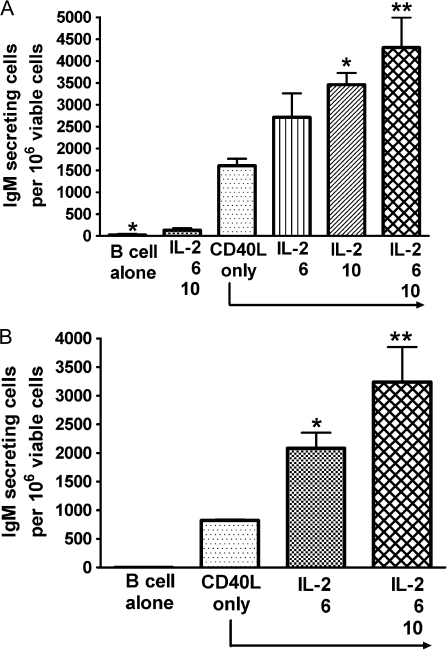
The initial series of studies aimed to establish and optimize the CD40L-induced IgM AFC responses in parallel for mouse and human primary B cells. The magnitude of the IgM AFC response was measured by enumerating IgM-secreting cells by ELISPOT. Due to the limited number of B cells that can be obtained from each human leukocyte pack, a 96-well format was adopted as the primary experimental platform for human B cells. Human CD40L was utilized to activate both mouse and human B cells since it is highly conserved between the two species (Spriggs et al., 1992). Cell surface–expressed CD40L was selected over recombinant CD40L as it is membrane bound and, hence, more closely mimics the CD40L expression by activated T cells. In preliminary studies, we also observed greater consistency in inducing IgM AFC responses using CD40L-L cells when compared to recombinant CD40L with both mouse and human B cells in our preliminary studies (data not shown). This CD40L-dependent model includes two culture phases, the first is in the presence of CD40L-L cells (4 days for human and 3 days for mouse), while the second is in the absence of CD40L-L cells (3 days for both). These assay conditions are based on prior findings from our laboratory, showing that when total human B cells were cultured with CD40L-L cells for the duration of the culture period, the IgM AFC response was significantly decreased (by ∼45%). This is also consistent with a previous finding that showed extended exposure to CD40L drives more B cells to become memory cells rather than antibody-secreting cells (Arpin et al., 1995). Preliminary kinetic studies suggested that, at least for human B cells, extension of either culture phase did not significantly increase the magnitude of the AFC response. Under our experimental conditions, 1.5–3 × 103 CD40L-L cell/culture induced the strongest IgM AFC response in human CD19+ total B cells, while 1.5 × 103 CD40L-L cell/culture led to the strongest response in CD19+CD27− naive B cells (Fig. 1). Different combinations of cytokines have been previously reported to induce antibody responses using human B cells (Armitage et al., 1993; Arpin et al., 1995; Ettinger et al., 2005; Kindler and Zubler, 1997). In our studies, recombinant human IL-2, IL-6, and IL-10, when combined with CD40L stimulation, induced a robust IgM response in both CD19+CD27− naive and CD19+ total human B cells (Fig. 2).
FIG. 1.
CD40L stimulation level on the magnitude of human B-cell IgM AFC response. (A) Human CD19+ total B cells (1.5 × 105 cell/culture) or (B) CD19+CD27− naive B cells (1.5 × 105 cell/culture) were cocultured with irradiated CD40L-L cells at indicated numbers per culture in the presence of recombinant human IL-2, IL-6, and IL-10 for 4 days and further cultured for an additional 3 days without CD40L-L cells. In both (A) and (B), cells were harvested on day 7 to enumerate IgM-secreting cells by ELISPOT. The cell number was determined by a particle counter, and the cell viability was assessed using a pronase activity assay. These data are representative of two separate experiments with at least four replicates per group. Data in panels (A) and (B) were obtained using total or naive B cells from two different donors in two separate experiments. The error bars indicate SE calculated for replicates of each treatment group.
FIG. 2.
Effect of cytokines on the magnitude of human B-cell IgM AFC response. (A) Human CD19+ total B cells (1.5 × 105 cell/culture) or (B) CD19+CD27− naive B cells (1.5 × 105 cell/culture) were cocultured with irradiated CD40L-L cells (3 × 103 cell/culture) for 4 days and further cultured for additional 3 days without CD40L-L cells. Cytokines were added as indicated to individual group. Cells were harvested on day 7 to enumerate IgM-secreting cells by ELISPOT. The cell number was determined using a particle counter, and the viability was assessed by a pronase activity assay. *p < 0.05, **p < 0.01, compared with CD40L-L cell–only group. These data are representative of two separate experiments (B cells from two donors) with at least four replicates per group. Data in panels (A) and (B) were obtained using total or naive B cells from two different donors in two separate experiments. The two donors were different from the donors from which data in Figure 1 were obtained. The error bars indicate SE calculated for replicates of each treatment group. The error bars indicate SE calculated for replicates of each treatment group.
The mouse CD40L-dependent model was optimized for IgM AFC responses using a similar approach (data not shown). Compared to CD19+ total or CD19+CD27− naive human B cells that require a B cell to CD40L-L cell ratio of 50:1 or 100:1, a ratio of 30:1 was required to induce the greatest magnitude of IgM AFC response from mouse B cells (data not shown). Although addition of IL-10 did not further enhance the magnitude of the mouse AFC response (data not shown), it was still included to ensure a valid comparison to human B cells.
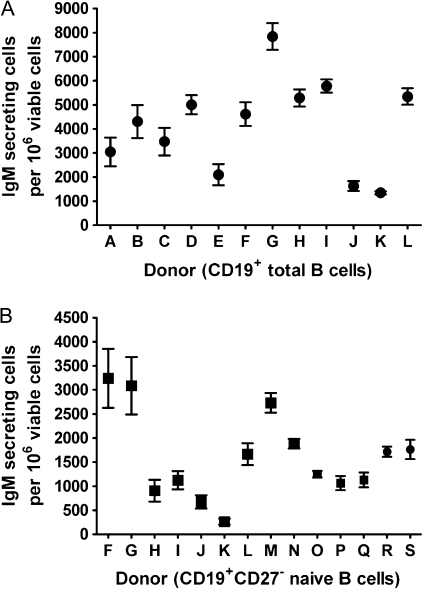
To determine the inherent variability in the responsiveness of human B cells to the CD40L-induced IgM AFC response, CD19+CD27− naive and CD19+ total human B cells from multiple human donors were assessed. To date, relatively consistent IgM AFC responses in CD19+CD27− naive B cells from 14 human donors and CD19+ total human B cells from 12 human donors have been observed using the optimized CD40L assay conditions (Fig. 3). When both CD19+CD27− naive and CD19+ total B cells from the same donor were assayed, the naive B cells yielded lower overall IgM AFC responses than observed using total B cells in all donors. This observation is consistent with previous studies in which naive B cells were found to be less responsive than memory B cells to CD40L-induced differentiation into antibody-secreting cells (Kindler and Zubler, 1997; Neron et al., 2005).
FIG. 3.
The magnitude of donor-to-donor variability in the CD40L-induced IgM AFC response. (A) Human CD19+ total B cells (1.5 × 105 cell/culture) or (B) CD19+CD27− naive B cells (1.5 × 105 cell/culture) were cocultured with irradiated CD40L-L cells (total B cells: 3 × 103 cell/culture; naive B cells: 3 × 103 cell/culture [donor F-L] or 1.5 × 103 cell/culture [donor M-S]) in the presence of recombinant human IL-2, IL-6, and IL-10 for 4 days and further cultured for additional 3 days without CD40L-L cells. Cells were harvested on day 7 to enumerate IgM-secreting cells by ELISPOT. The cell number was determined using a particle counter, and the viability was assessed by a pronase activity assay. CD19+ total B cells from 12 human donors and CD19+CD27− naive B cells from 14 human donors were assessed.
Characterization of CD40L-Induced Proliferation and Phenotypic Changes during the Plasmacytic Differentiation of Mouse B Cells and CD19+CD27− Naive Human B Cells
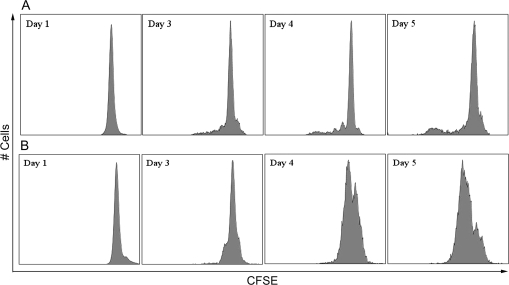
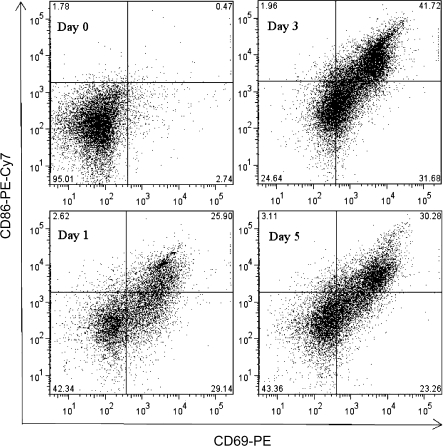
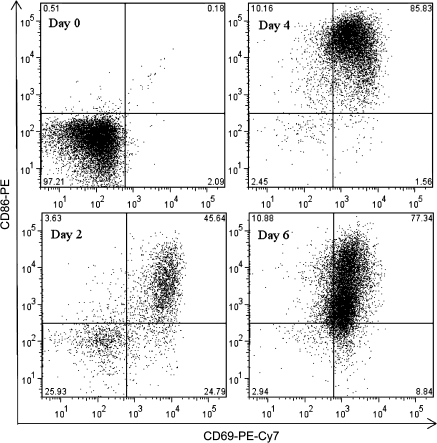
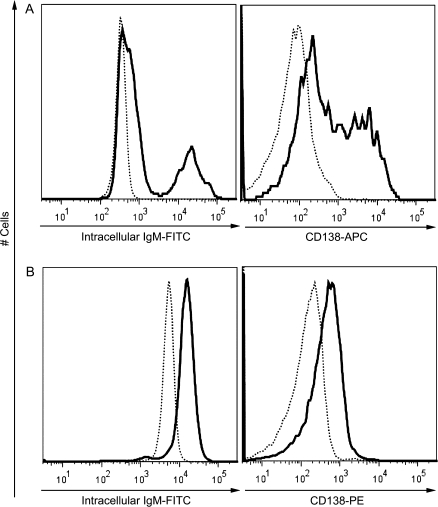
The plasmacytic differentiation of B cells into antibody-secreting cells is preceded by two critical events: activation and proliferation. Engagement of CD40 triggers the activation and proliferation of B cells in vitro (Banchereau et al., 1994). Kinetic studies were conducted using flow cytometry to investigate the proliferative responses and upregulation of activation markers of mouse and naive human B cells induced using the CD40L activation system. CFSE labeling was used to examine the proliferation of B cells. As illustrated in Figure 4, CD40L in combination with IL-2, IL-6, and IL-10 induced cell division of both mouse and naive human B cells, as indicated by the dilution of CFSE signal. A subpopulation of mouse B cells started cell division on day 3 and underwent further divisions between days 3 and 5. In contrast, the majority of human B cells had undergone cell division by day 5. Once activated, B cells upregulate the surface expression of various markers, including CD69 and B7.2/CD86. In the present study, a well-defined CD69+CD86+ subpopulation was induced from mouse or naive human B cells 3 or 2 days following CD40L stimulation, respectively (Figs. 5 and 6). A subpopulation of mouse B cells remained CD69−CD86− even 5 days after CD40L stimulation, consistent with the proliferation data (Fig. 5). In naive human B cells, greater than 40% of the entire cell population demonstrated a CD69+CD86+ phenotype on day 2 (Fig. 6). Four days after CD40L stimulation, the entire pool of viable human B cells was predominantly CD69+CD86+, indicating robust activation induced by CD40L. The plasmacytic differentiation of B cells could be further characterized by the occurrence of an antibody-secreting plasma cell phenotype, which comprises the upregulation of intracellular Ig and the surface expression of a plasma cell marker CD138 (syndecan-1) (Klein et al., 2003). As shown in Figure 7, both the expression of intracellular IgM and surface CD138 was upregulated in mouse or human cells on day 6 or day 7, respectively, indicating that an antibody-secreting plasma cell phenotype was induced by the CD40L model. Collectively, the data presented here suggest that utilizing the CD40L activation model, one may not only characterize the effect of xenobiotics on antibody production but can also dissect the stages of B-cell progression from initial activation to plasmacytic differentiation, to identify the critical period during which xenobiotic-mediated modulation occurs.
FIG. 4.
CD40L-induced proliferation of mouse and human B cells. (A) Mouse B cells or (B) human CD19+CD27− naive B cells were labeled with CFSE and then cocultured at 1.5 × 106 cell/culture (mouse) or 1.5 × 105 cell/culture (human) with irradiated CD40L-L cells (mouse: 5 × 104 cell/culture; human: 1.5 × 103 cell/culture) in the presence of recombinant mouse or human IL-2, IL-6, and IL-10 for up to 5 days, and CD40L stimulation was removed on day 3 (mouse) or day 4 (human). Cells were harvested at the indicated time points and assessed by flow cytometry. Dead cells were identified by staining with Live/Dead near-infrared (mouse) or red (human) Staining Kit and excluded from data analysis. These data are representative of two separate experiments (for humans, experiment used B cells from one individual donor) with three replicates per time point.
FIG. 5.
CD40L-induced activation of mouse B cells. Mouse B cells (1.5 × 106 cell/culture) were cocultured with irradiated CD40L-L cells (5 × 104 cell/culture) in the presence of recombinant mouse IL-2, IL-6, and IL-10 for up to 5 days, and CD40L stimulation was removed on day 3. Cells were harvested at the indicated time points and assessed by flow cytometry. Dead cells were identified by staining with Live/Dead near-infrared Staining Kit and excluded from data analysis. These data are representative of two separate experiments with three replicates per time point.
FIG. 6.
CD40L-induced activation of human B cells. Human CD19+CD27− naive B cells (1.5 × 105 cell/culture) were cocultured with irradiated CD40L-L cells (1.5 × 103 cell/culture) in the presence of recombinant human IL-2, IL-6, and IL-10 for up to 6 days, and CD40L stimulation was removed on day 4. Cells were harvested at indicated time points and assessed by flow cytometry. Dead cells were identified by staining with Live/Dead near-infrared Staining Kit and excluded from data analysis. These data are representative of two separate experiments (each experiment used B cells from one individual donor) with three replicates per time point.
FIG. 7.
CD40L-induced antibody-secreting plasma cell phenotype of mouse and human B cells. (A) Mouse B cells (1.5 × 106 cell/culture) or (B) human CD19+CD27− naive B cells (1.5 × 105 cell/culture) were cocultured with irradiated CD40L-L cells (mouse: 5 × 104 cell/culture; human: 1.5 × 103 cell/culture) in the presence of recombinant mouse or human IL-2, IL-6, and IL-10 for up to 5 days, and CD40L stimulation was removed on day 3 (mouse) or day 4 (human). Cells were harvested on day 6 (mouse) or day 7 (human) and assessed by flow cytometry. Dead cells were identified by staining with Live/Dead near-infrared Staining Kit and excluded from data analysis. These data are representative of two separate experiments (for humans, experiment used B cells from one individual donor) with three replicates per time point. Dotted lines represent the expression levels in nonstimulated naive B cells, while solid lines represent expression levels in stimulated B cells (day 6 for mouse and day 7 for human).
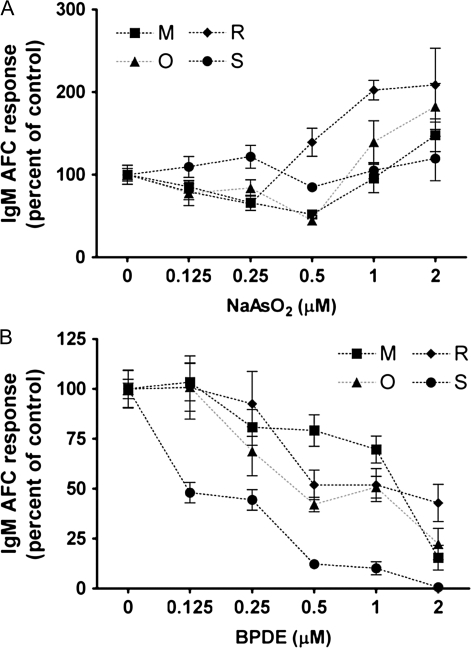
Suppression by Arsenic and BPDE of CD40L-Induced IgM AFC Response in Mouse and Human B Cells
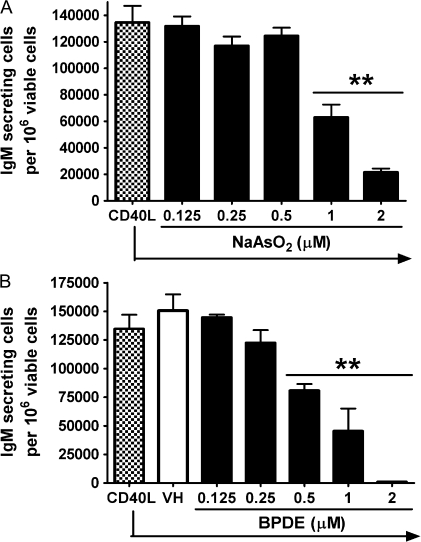
The immunosuppressive effects of arsenic and metabolites of benzo[a]pyrene have been widely studied in mice, and both suppressed the in vitro mouse anti-sRBC AFC response (Burchiel and Luster, 2001; Selgrade, 2007). Arsenic was first identified as a major contributor to the suppression of both the anti-sRBC antibody responses using gallium arsenide in vitro and in vivo in mice (Burns et al., 1991). BPDE was reported to be an ultimate metabolite of benzo[a]pyrene that directly caused immunosuppression (Kawabata and White, 1989). Therefore, a series of studies were conducted to determine if arsenic and/or BPDE similarly impair the CD40L-induced IgM AFC response using isolated mouse splenic B cells. The concentration range selected for both compounds were based on previous published studies in which suppression of the mouse anti-sRBC AFC response was observed (Burns et al., 1991; Kawabata and White, 1989). Mouse B cells were treated with arsenic or BPDE at various concentrations and then activated by CD40L in the presence of IL-2, IL-6, and IL-10. The total number of AFCs, which are defined as viable IgM-secreting cells per culture, was enumerated by ELISPOT. As illustrated in Figure 8, the frequency of IgM AFCs in the total pool of viable cells was significantly decreased by arsenic at 1 and 2μM and BPDE at concentrations between 0.5 and 2μM. The IC50 was calculated as 0.97μM for arsenic and as 0.55μM for BPDE for mouse B cells; 1μM of arsenic was also the lowest concentration found to suppress the in vitro mouse anti-sRBC AFC response (Burns et al., 1991).
FIG. 8.
Effect of arsenic and BPDE on the CD40L-induced IgM AFC response from mouse B cells. Mouse B cells (1.5 × 106 cell/culture) were treated with (A) NaAsO2 or (B) BPDE at indicated concentrations or VH, then cocultured with irradiated CD40L-L cells (5 × 104 cell/culture) in the presence of recombinant mouse IL-2, IL-6, and IL-10 for 3 days, and further cultured for additional 3 days without CD40L-L cells. Cells were harvested on day 6 to enumerate IgM-secreting cells by ELISPOT. Cell number was determined using a particle counter and the viability was assessed by a pronase activity assay. **p < 0.01, compared with CD40L group (arsenic) or VH-treated group (BPDE). These data are representative of two separate experiments with four replicates per treatment group. The error bars indicate SE calculated for replicates of each treatment group.
Very few studies to date have assessed the potential immunosuppressive effect exerted by arsenic or BPDE on the effector function of primary human leukocytes, including B cells. Following the establishment of the CD40L activation model, the effect of arsenic and BPDE on the IgM AFC response in human B cells induced by CD40L was investigated and also compared to mouse splenic B cells. Naive human B cells from 6 different donors were assessed for their sensitivity to the suppression of CD40L-induced IgM AFC response by arsenic and BPDE. The IgM AFC responses of naive B cells derived from two donors were not modulated by either arsenic or BPDE at all concentrations tested and the cells also demonstrated a highly proliferative phenotype (data not shown). It is unclear if the absence of responsiveness to xenobiotic modulation and proliferative phenotypes are related. In contrast, the IgM AFC responses of naive B cells derived from the other four donors were modulated by the treatment of arsenic and BPDE, as assessed by ELISPOT (Fig. 9). The overall profile of BPDE-mediated suppression of the IgM AFC responses from naive human B cells was similar to those observed in mouse B cells. When IC50 values were calculated for BPDE for each donor, a concentration range between 0.26 and 1.36μM was obtained based on data from four donors whose B cells demonstrated sensitivity to BPDE. In contrast, arsenic-mediated modulation of human IgM AFC response is quite different. Arsenic appeared to be modestly suppressive of the human AFC response at lower concentrations (0.25–0.5μM), but had no or an enhancing effect at concentrations above 1μM, the concentrations that significantly suppressed the mouse B cell IgM AFC response induced by CD40L activation. The lack of a concentration responsive relationship precluded the calculation of IC50 values for arsenic for human B cells.
FIG. 9.
Effect of arsenic and BPDE on the CD40L-induced IgM AFC response from human B cells. Human CD19+CD27− naive B cells (1.5 × 105 cell/culture) were treated with (A) NaAsO2 or (B) BPDE at indicated concentrations or VH and then cocultured with irradiated CD40L-L cells (1.5 × 103 cell/culture) in the presence of recombinant human IL-2, IL-6, and IL-10 for 4 days and further cultured for additional 3 days without CD40L-L cells. Cells were harvested on day 7 to enumerate IgM-secreting cells by ELISPOT. The cell number was determined using a particle counter, and the viability was assessed by a pronase activity assay. Data were normalized to the VH-treated group (100%) and presented as percent of control with six replicates per group. B cells from four human donors were assessed. The IgM AFC responses of B cells derived from two donors were not modulated by either arsenic or BPDE at all concentrations tested and cells demonstrated a highly proliferative phenotype. Data from these two donors were not included in this figure. The error bars indicate SE calculated for replicates of each treatment group.
DISCUSSION
Realization that the immune system is a sensitive target organ to modulation by a diverse collection of agents and environmental factors was a major driving force for the development of strategies to identify potential human immunotoxicants. Arguably, the most successful effort toward this end was the development of an immunotoxicology tiered testing approach that evolved from studies during the 1980s. The approach, which continues to be used worldwide, consists of well-defined immunological measurements and immune function assays that assess various aspects of immune competence (i.e., innate, humoral, and cell-mediated immunity) primarily in mice and rats (Luster et al., 1988). In spite of the wide adoption of the rodent-based immunotoxicology tiered approach, concerns persist whether studies conducted in rodent models reliably predict human hazard. With increasing development of biological therapeutics, testing of immune modulation and immunotoxicity has been extended in certain instances into nonhuman primate models; however, the approach still does not directly evaluate activity and toxicity in a human model. In the present study, we investigated the application of an assay system that directly assesses the capacity of a xenobiotic to alter effector function of freshly isolated human peripheral blood B cells.
Although used to investigate xenobiotic-mediated alteration of calcium signaling and growth suppression in B cells (Allan and Sherr, 2005; Allan et al., 2006; Karras et al., 1996), CD40L has not been utilized as an activation model to characterize xenobiotic-induced suppression of the B-cell AFC response. Lipopolysaccharide, a widely used polyclonal stimulator for mouse B cells, does not induce a significant antibody response in primary human B cells. In these studies, we explored the application of CD40L as an activation stimulus as it not only induces the differentiation of primary human and mouse B cells into antibody-secreting cells, it is also a physiologically relevant means to activate and differentiate B cells. An added advantage in using the CD40L system, as described here, is that the experimental conditions for activating and differentiating primary human and mouse B cells are remarkably similar, allowing for direct species comparison studies.
In the present investigation, we optimized the model system by characterizing a number of experimental conditions, which based on published literature, appeared to be the most critical toward induction of a strong IgM AFC response. The magnitude of CD40L stimulation is a critical factor that contributes to the outcome of B-cell activation in vitro (Neron et al., 2005). Extensive investigations were conducted to identify the optimal number of CD40L-L cells and duration of the coculture period that induces the strongest IgM AFC response in mouse and human B cells. For mouse, the optimal B cell:CD40L-L cell ratio was 30:1 and for human either 50:1 or 100:1. Moreover, we found that the peak day of IgM AFC response was modestly different between mouse and human B cells, day 6 versus day 7, respectively, with human B cells requiring 4 days of coculture with CD40L-L cells while mouse requiring only 3 days.
In addition to providing contact-dependent stimulation, activated CD4+ T cells also secrete cytokines to promote B-cell differentiation. Previously published studies have identified IL-2, IL-6, and IL-10 as critical T-cell–derived cytokines that promote IgM secretion (Arpin et al., 1995; Banchereau et al., 1994; Burdin et al., 1995). IL-4, which also drives B-cell differentiation, was not included in our study since IL-4, when combined with other B-cell stimuli, can induce isotype switching to IgE (Gascan et al., 1991; Splawski and Lipsky, 1994). Although it is certain that additional cytokines other than IL-2, IL-6, and IL-10 may further contribute to B-cell differentiation, there is significant functional overlap among various groups of cytokines due, at least in part, to shared intracellular signaling pathways. Therefore, although the assay system could be further refined, our results clearly show that IL-2, IL-6, and IL-10, when combined with CD40L stimulation, induced robust IgM AFC responses in both human and mouse B cells.
Donor-to-donor variability poses one of the most significant challenges in using human primary tissues as biological models, including in immunotoxicity evaluations. Based on our anticipation that significant inherent variability between donors would be observed with respect to the magnitude of the control IgM AFC response by CD40L in CD19+ total B cells and CD19+CD27− naive B cells, 12 and 14 donors, respectively, were assayed. In spite of the variations observed, the background responses in human CD19+ as well as in CD19+CD27− B cells were of a magnitude to allow assessments of biological activity and immunotoxic potential of xenobiotics. It is important to emphasize that variability between donors with respect to the magnitude of their control IgM AFC response becomes inconsequential since each donor also serves as their respective comparative control (i.e., CD40L stimulation in the presence versus absence of xenobiotic).
The most significant limitation in using human peripheral blood B cells experimentally is the number of B cells (especially the naive B cells) that can be obtained from each donor since typically B cells comprise approximately 3–5% of the circulating leukocytes. To circumvent this obstacle, a 96-well plate format was adapted for the human CD40L assay system, which allows at least 200 naive B-cell or 300 total B-cell replicates from one leukocyte pack. The assay system in its present form can be used to evaluate two to three agents at extensive concentration ranges using B cells from a single donor, which can be repeated in multiple donors to assess the sensitivity to any individual agent.
B-cell activation and proliferation are two critical steps prior to the plasmacytic differentiation. It has already been demonstrated that immunotoxic agents may disrupt early activation and/or the proliferation of B cells (Allan et al., 2006; Salas and Burchiel, 1998). Moreover, various immunosuppressants utilized therapeutically, such as rapamycin and mycophenolic acid, directly target lymphocyte proliferation. In the present study, we demonstrated that following coculture with CD40L-L cells in combination with appropriate cytokines, both proliferation and an activation phenotype (CD69+CD86+) were induced in primary mouse and naive human B cells. In light of that, in addition to measurements of IgM AFC responses, the CD40L assay system can also be used to assess the effects of xenobiotics on earlier events including B-cell activation and proliferation, for mechanistic studies. The use of primary B cells holds many advantages over immortalized B-cell lines, which are self-propagating and often possess constitutive activated or differentiated phenotypes independent of stimulation.
To further characterize the CD40L assay system, two well-characterized immunotoxicants shown to suppress the mouse anti-sRBC AFC response were investigated using primary mouse and human B cells. Specifically, arsenic and BPDE were demonstrated to suppress the in vitro mouse anti-sRBC AFC response, and it was suggested that their immunosuppressive effects involved alterations of B-cell function (Kawabata and White, 1989; Salas and Burchiel, 1998; Sikorski et al., 1991). Our results confirm that both arsenic and BPDE directly impair mouse B-cell function as assessed by the CD40L-induced IgM AFC response. Direct addition of BPDE also suppressed the CD40L-induced IgM AFC response in naive human B cells over a comparable concentration range. Interestingly, however, arsenic displayed a distinctly different profile of activity between mouse and human B cells. No effect was observed on the CD40L-induced IgM AFC response at lower doses, which in general was then increased at the highest arsenic concentrations due to a decrease in viable cells per culture. Collectively, this limited study demonstrates that although the mouse was predictive for BPDE-mediated humoral immune suppression in human B cells, the mouse was not predictive for arsenic-mediated modulation in human B cells. These studies emphasize the longstanding concern that the mouse immune system may not always serve as an accurate human surrogate. Moreover, the ability to readily obtain human peripheral blood cells represents a unique opportunity to directly investigate whether certain agents have the capability to suppress plasmacytic differentiation and/or the IgM AFC responses in human primary B cells.
Although evaluation of B-cell function was the primary focus of this investigation, one can envision that the assay system described here could be utilized in conjunction with several additional immune function parameters of T-cell and innate immunity. In doing so, human leukocytes could be the basis of a standardized assay battery, although not as extensive as the National Toxicology Program immunotoxicology tiered testing approach, to directly assess immunotoxicity in human leukocytes. In fact, an analysis of National Toxicology Program immunotoxicology historical data showed that when results from three different assays were used in combination, such as the mouse anti-sRBC IgM AFC response, immunophenotyping of leukocyte subpopulations by flow cytometry, and the natural killer cell activity assay, the results were remarkably predictive of immunotoxicity in the mouse as compared to the complete battery of assays (Luster et al., 1992). The importance of assay systems using primary human leukocytes that could serve as an adjunct to rodent-based screening is further supported by the differences in toxicity observed between mouse and human B cells to arsenic, which is one of the more extensively characterized immunotoxicants.
FUNDING
Dow Chemical Company and National Institutes of Health (R01 ES002520 and P42 ES004911) to N.E.K.
Acknowledgments
We thank Dr. David Sherr at Boston University School of Public Health for generously providing the mouse fibroblast line expressing human CD40L, Dr. Sandra O'Reilly for cell irradiation, Dr. Jessica Neal for assistance in preparing BPDE stock solution, Mr. Robert Crawford for providing technical assistance with flow cytometry, and Mrs. Kimberly Hambleton for administrative assistance in preparing and submitting the manuscript.
References
- Allan LL, Schlezinger JJ, Shansab M, Sherr DH. CYP1A1 in polycyclic aromatic hydrocarbon-induced B lymphocyte growth suppression. Biochem. Biophys. Res. Commun. 2006;342:227–235. doi: 10.1016/j.bbrc.2006.01.131. [DOI] [PubMed] [Google Scholar]
- Allan LL, Sherr DH. Constitutive activation and environmental chemical induction of the aryl hydrocarbon receptor/transcription factor in activated human B lymphocytes. Mol. Pharmacol. 2005;67:1740–1750. doi: 10.1124/mol.104.009100. [DOI] [PubMed] [Google Scholar]
- Armitage RJ, Macduff BM, Spriggs MK, Fanslow WC. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J. Immunol. 1993;150:3671–3680. [PubMed] [Google Scholar]
- Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu. Rev. Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 2003;14:297–309. doi: 10.1016/s1359-6101(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Burchiel SW, Luster MI. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin. Immunol. 2001;98:2–10. doi: 10.1006/clim.2000.4934. [DOI] [PubMed] [Google Scholar]
- Burdin N, Van Kooten C, Galibert L, Abrams JS, Wijdenes J, Banchereau J, Rousset F. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J. Immunol. 1995;154:2533–2544. [PubMed] [Google Scholar]
- Burns LA, Sikorski EE, Saady JJ, Munson AE. Evidence for arsenic as the immunosuppressive component of gallium arsenide. Toxicol. Appl. Pharmacol. 1991;110:157–169. doi: 10.1016/0041-008x(91)90298-s. [DOI] [PubMed] [Google Scholar]
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- Gascan H, Gauchat JF, Aversa G, Van Vlasselaer P, de Vries JE. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J. Immunol. 1991;147:8–13. [PubMed] [Google Scholar]
- Karras JG, Morris DL, Matulka RA, Kramer CM, Holsapple MP. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) elevates basal B-cell intracellular calcium concentration and suppresses surface Ig- but not CD40-induced antibody secretion. Toxicol. Appl. Pharmacol. 1996;137:275–284. doi: 10.1006/taap.1996.0081. [DOI] [PubMed] [Google Scholar]
- Kawabata TT, White KL., Jr Benzo(a)pyrene metabolism by murine spleen microsomes. Cancer Res. 1989;49:5816–5822. [PubMed] [Google Scholar]
- Kindler V, Zubler RH. Memory, but not naive, peripheral blood B lymphocytes differentiate into Ig-secreting cells after CD40 ligation and costimulation with IL-4 and the differentiation factors IL-2, IL-10, and IL-3. J. Immunol. 1997;159:2085–2090. [PubMed] [Google Scholar]
- Klein B, Tarte K, Jourdan M, Mathouk K, Moreaux J, Jourdan E, Legouffe E, De Vos J, Rossi JF. Survival and proliferation factors of normal and malignant plasma cells. Int. J. Hematol. 2003;78:106–113. doi: 10.1007/BF02983377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Kaplan BL, Ngaotepprutaram T, Kaminski NE. Suppression of T cell costimulator ICOS by Delta9-tetrahydrocannabinol. J. Leukoc. Biol. 2009;85:322–329. doi: 10.1189/jlb.0608390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster MI, Munson AE, Thomas PT, Holsapple MP, Fenters JD, White KL, Jr, Lauer LD, Germolec DR, Rosenthal GJ, Dean JH. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program's guidelines for immunotoxicity evaluation in mice. Fundam. Appl. Toxicol. 1988;10:2–19. doi: 10.1016/0272-0590(88)90247-3. [DOI] [PubMed] [Google Scholar]
- Luster MI, Portier C, Pait DG, White KL, Jr, Gennings C, Munson AE, Rosenthal GJ. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 1992;18:200–210. doi: 10.1016/0272-0590(92)90047-l. [DOI] [PubMed] [Google Scholar]
- Neron S, Racine C, Roy A, Guerin M. Differential responses of human B-lymphocyte subpopulations to graded levels of CD40-CD154 interaction. Immunology. 2005;116:454–463. doi: 10.1111/j.1365-2567.2005.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas VM, Burchiel SW. Apoptosis in Daudi human B cells in response to benzo[a]pyrene and benzo[a]pyrene-7,8-dihydrodiol. Toxicol. Appl. Pharmacol. 1998;151:367–376. doi: 10.1006/taap.1998.8455. [DOI] [PubMed] [Google Scholar]
- Schatz AR, Koh WS, Kaminski NE. Delta 9-tetrahydrocannabinol selectively inhibits T-cell dependent humoral immune responses through direct inhibition of accessory T-cell function. Immunopharmacology. 1993;26:129–137. doi: 10.1016/0162-3109(93)90005-b. [DOI] [PubMed] [Google Scholar]
- Schneider D, Manzan MA, Crawford RB, Chen W, Kaminski NE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated impairment of B cell differentiation involves dysregulation of paired box 5 (Pax5) isoform, Pax5a. J. Pharmacol. Exp. Ther. 2008;326:463–474. doi: 10.1124/jpet.108.139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selgrade MK. Use of immunotoxicity data in health risk assessments: Uncertainties and research to improve the process. Toxicology. 1999;133:59–72. doi: 10.1016/s0300-483x(99)00006-2. [DOI] [PubMed] [Google Scholar]
- Selgrade MK. Immunotoxicity: The risk is real. Toxicol. Sci. 2007;100:328–332. doi: 10.1093/toxsci/kfm244. [DOI] [PubMed] [Google Scholar]
- Sikorski EE, Burns LA, Stern ML, Luster MI, Munson AE. Splenic cell targets in gallium arsenide-induced suppression of the primary antibody response. Toxicol. Appl. Pharmacol. 1991;110:129–142. doi: 10.1016/0041-008x(91)90296-q. [DOI] [PubMed] [Google Scholar]
- Splawski JB, Lipsky PE. CD40-mediated regulation of human B-cell responses. Res. Immunol. 1994;145:226–234. doi: 10.1016/s0923-2494(94)80189-4. discussion 244–249. [DOI] [PubMed] [Google Scholar]
- Spriggs MK, Armitage RJ, Strockbine L, Clifford KN, Macduff BM, Sato TA, Maliszewski CR, Fanslow WC. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J. Exp. Med. 1992;176:1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten C, Banchereau J. CD40-CD40 ligand. J. Leukoc. Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Vos JG, Van Loveren H. Experimental studies on immunosuppression: How do they predict for man? Toxicology. 1998;129:13–26. doi: 10.1016/s0300-483x(98)00059-6. [DOI] [PubMed] [Google Scholar]