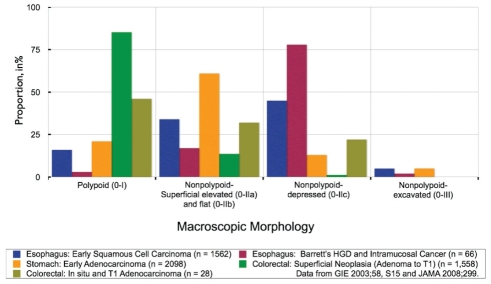
The detection, diagnosis and treatment of early cancers offers the best hope for the prevention and cure of gastrointestinal cancers – one of the leading causes of death worldwide (1). The detection of pre- or early cancer using white light endoscopy can be challenging because their morphology can be inconspicuous (ie, nonpolypoid; slightly elevated, flat, or slightly depressed]) and their colour can be minimally altered. Indeed, nonpolypoid neoplasms have been shown to be common and important in the esophagus, Barrett’s mucosa and stomach (Figure 1). Our recent prevalence study (2), highlighted their importance in the colon. We showed that nonpolypoid colorectal neoplasms (NP-CRNs) are relatively common and are potentially more dangerous than polypoid neoplasms of similar size because they have a higher risk of containing in situ or submucosal invasive carcinoma. Some nonpolypoid gastrointestinal neoplasms are fairly easy to detect and diagnose, whereas others can be quite difficult to visualize using white light illumination. The current technique and technology of image-enhanced endoscopy (IEE) is available to augment the detection, diagnosis and treatment of these subtle lesions.
Figure 1).
Distribution of superficial lesions according to morphological classification showing the relevance and importance of the nonpolypoid types in our endoscopic practice. Data regarding lesions of the upper gastrointestinal tract were derived from the Paris Classification (34). Data regarding lesions of the colon and rectum are reported from our unit (2). HGD High-grade dysplasia

Dr Tonya Kaltenbach is a gastroenterologist with the VA Healthcare System in Palo Alto, California, USA
There are two methods of IEE: dye-based and equipment-based (3). The objective of these two methods is to increase the contrast of structures, thus making the mucosal topography, morphology and borders of lesions viewable in finer detail. Used alone or in tandem, they may complement the white light examination as well each other (Figure 2). Detailed examination of the mucosa provides a cross-sectional view of the underlying pathology and facilitates discrimination between normal, non-neoplastic and neoplastic tissue. Taken together with size and morphology, and observation during submucosal injection, important information regarding the likelihood of submucosal invasion and whether the patient can undergo a safe and curative endoscopic resection is obtained.
Figure 2).
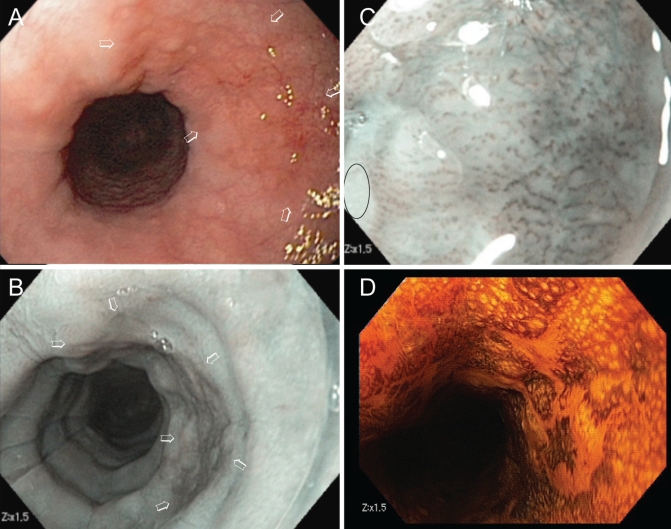
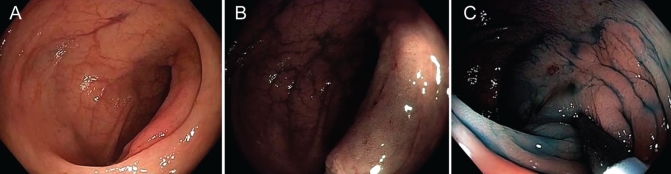
The benefit of the detection and diagnosis of pre- and early cancer of the gastrointestinal tract is significantly curtailed if safe and efficacious endoscopic treatment is unavailable. Image-enhanced endoscopy is an integral component of endoscopic diagnosis, which is critical for appropriate treatment decisions. A A red patch of mucosa detected in the mid-esophagus (arrows). B Narrow-band imaging showing a brown area with a sharp border compared with the surrounding mucosa, signifying the likelihood of high-grade dysplasia (arrows). C Abnormal intra-papillary capillary loop pattern seen under 1.5× digital magnification, consistent with the diagnosis. D The neoplasm remained unstained, while normal squamous epithelium stained brown after Lugol’s solution spray. Biopsy revealed high-grade dysplasia. Lesions that are diagnosed to have no potential for lymph node metastasis can be endoscopically resected (not shown), while a deeply invasive carcinoma should be biopsied and referred to surgery
The present article describes the techniques and applications of IEE and provides readers of the Journal with a resource to begin or to potentially improve on their use of IEE. For critical analysis of the literature, readers are directed toward a comprehensive review of the subject (3,4). We will present a description of the various techniques of IEE, and their specific preparations and properties. We will also include an outline of the supporting data regarding the use of IEE for the detection, diagnosis and therapy of a variety of nonpolypoid gastrointestinal neoplasms.
IEE TECHNIQUES
IEE can be accomplished using dyes or features of the newer endoscopic systems. Dye-based IEE includes the use of Lugol’s solution or diluted indigo carmine dye. Lugol’s solution, a vital dye, enhances contrast by staining glycogen granules brown in normal squamous mucosa; it does not stain inflammatory or neoplastic cells. Indigo carmine – a contrast dye – pools into the mucosal pits, grooves, erosions and depressions. It enhances surface topography by enabling visualization of the glands and pits, lesion borders and depth of the columnar mucosal lesions as compared with the surrounding normal tissue. Equipment-based techniques are performed by manipulating the light source (optical method) or the captured image by the CCD (electronic-based method). The processed images are then displayed as an IEE image. In general, equipment-based IEE methods are designed to enhance visualization of the microvessels of the mucosa, which are altered in abnormal tissue (5,6). The microvessels of neoplasms are typically enlarged, tortuous and increased in density (ie, more per unit area) in neoplastic tissues (Figure 3) (7). Details of the different techniques available in practice follow.
Figure 3).
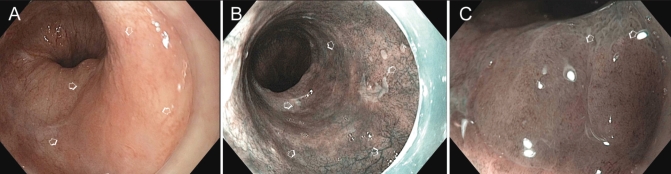
A A large patch of high-grade dysplasia in long-segment Barrett’s esophagus appeared slightly more reddish than the surrounding Barrett’s esophagus. B With narrow-band imaging, the patch appeared brown. C Comparison of the mucosal pattern of the area with high-grade dysplasia with the surrounding Barrett’s esophagus
Dye-based IEE methods
Lugol’s solution:
The esophageal and anal squamous mucosa are the most commonly targeted tissues of dye-based IEEs using Lugol’s solution. The dye is composed of a mixture of iodine and potassium iodide in water, which is used as a 2% solution in practice, made by mixing 8 mL of 5% Lugol’s solution (Humcon Co, USA) with 12 mL of sterile water immediately before use. Glycogen-rich cells of the normal, nonkeratinized squamous epithelium stain an intense green-brown for 5 min to 8 min. Low-glycogen epithelium harbouring inflammation, scars, dysplasia or carcinoma remain distinctly unstained (8). Ten millilitres to 20 mL is sprayed evenly over the targetted tissue through a specialized catheter sprayer (available through Olympus or Cook Medical, USA); excess Lugol’s solution that remains in the lumen of the esophagus or in the stomach is immediately suctioned after spraying. Because staining of normal tissue disappears quickly, abnormal areas require biopy or have their borders marked immediately after spraying. Importantly, Lugol’s solution may cause retrosternal pain, nausea, chemical esophagitis (rarely) and, if aspirated, chemical pneumonitis (9). Application of sodium thiosulfate solution may reverse Lugol’s staining and decrease its side effects, but is not commonly used in Western countries (10).
Indigo carmine dye:
Areas most commonly targeted using indigo carmine dye are the pits, grooves, erosions and depressions of the columnar mucosa. Indigo carmine is a blue, plant-based dye that is not absorbed by the mucosa but, instead, forms pools in areas of depth – thus highlighting the subtle pits, depressions and borders of abnormal tissue. The dye is used as a 0.2% solution made by mixing 5 mL of a 0.8% solution of indigo carmine (American Reagent Laboratories Inc, USA) with 15 mL of water. The dye is sprayed through the accessory channel of an endoscope coupled to a syringe reservoir. No adverse effects of indigo carmine have been reported. Transient, green-hued urine may occur when a substantial amount of indigo carmine has been sprayed onto the mucosa or injected into the submucosa.
Equipment-based IEE methods
Fujinon Intelligence Colour Enhancement:
The Fujinon Intelligence Colour Enhancement system (Fujinon Corporation, Japan) is an electronic-based endoscopic modality that targets the microvessels of the mucosa. This computerized spectral estimation technology arithmetically processes reflected photons into virtual endoscopic images according to a set of narrowed wavelengths. The system allows the user to select a combination of three wavelengths from which the processor uses a corresponding matrix to reconstitute an image (Figure 4). The Fujinon Endoscope 400 and 500 series endoscopes are operated by push buttons on the endoscope handle and are used with the 4400 video processor and light system, which is equipped with up to 10 variable setting functions. Certain wavelengths have been demonstrated to accentuate differences between esophageal and gastric mucosa at the gastroesophageal junction in early gastric cancer, as well as between normal and adenomatous colonic mucosa. Fujinon Intelligence Colour Enhancement has been described in the literature as virtual chromoendoscopy, computed virtual chromoendoscopy and spectral estimation technology.
Figure 4).
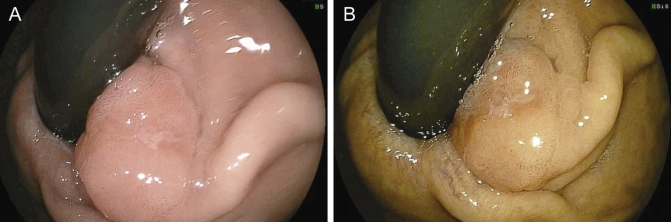
A 1.3 cm sessile gastroesophageal junction intramucosal adenocarcinoma viewed on retroflexion under white light (A) and Fujinon Intelligence Colour Enhancement (B) (Fujinon, Japan)
Narrow-band imaging:
Narrow-band imaging (NBI) targets the microvessels of the mucosa. It is an optical-based endoscopic method pioneered by Olympus, Japan. NBI uses optical techniques without the use of dyes to alter the light used to enhance mucosal surface imaging. The two NBI systems use specialized optical filters placed in front of a light source. NBI is based on the phenomenon in which the wavelength of light determines its depth of penetration into tissues. Blue light, with a short wavelength of 415 nm, penetrates the superficial epithelium while green light, with a longer wavelength of 540 nm, is used to visualize the submucosa. The red light wavelength that penetrates into the deeper tissue is omitted in the NBI mode. NBI exposes vascular patterns with high fidelity because hemoglobin absorbs blue light optimally; thus, mucosal vessels appear dark in colour. Narrowing the blue and green light also leads to a sharper visualization of the mucosa and submucosa. When the squamous cell lining of the esophagus or the columnar epithelium of the esophagus, stomach or colon become dysplastic or neoplastic, the microvessels change in configuration, density and size (Figure 5). At a standard magnification of 30×, the abnormal tissue appears brown in colour due to increased vessel density. The Olympus endoscope system series 180 or 260 are operated by push buttons on the endoscope handle.
Figure 5).
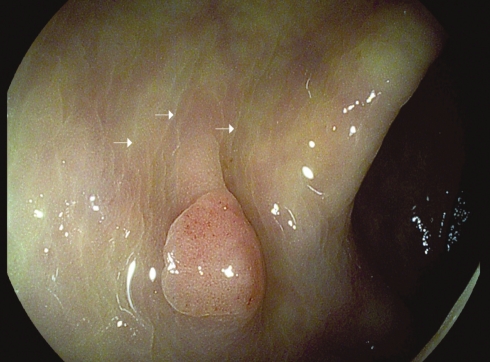
Flat tubular adenoma in the sigmoid colon viewed under white light (A), narrow-band imaging (B) and using diluted indigo carmine solution (C)
Pentax I-Scan:
The Pentax I-Scan (Pentax, Japan) is an electronic-based endoscopic system that targets the mucosal microvasculature of the gastrointestinal tract. It uses a computerized system to calculate a virtual image based on the three primary colour components to be separated. Each component is enhanced before it is combined with the others to reconstitute the final image (Figure 6). The Pentax EPK-i endoscope and processor system is operated with the use of push buttons on the endoscope handle. This endoscopic modality is currently the least studied.
Figure 6).
The innominate grooves (arrows) are interrupted by the presence of a small colon neoplasm
CLINICAL APPLICATION OF IEE
We typically initiate intestinal examination using standard high-resolution or high-definition white light, and apply techniques of IEE as an adjunct to further evaluate a focal area that appears abnormal on white-light imaging to detect, diagnose and treat neoplasms (Table 1). It is important that the mucosa be clean of debris or excess mucus, and is relatively still for successful application of IEE. Note, patients are prepared with one bottle of magnesium citrate (296 mL) taken with 1 L of water on the morning of the colonoscopy in addition to 4 L of poly-ethylene glycol solution taken the night before.
TABLE 1.
Tools for image-enhanced endoscopy
|
Esophagus
Early squamous cell carcinoma: Lugol’s solution and NBI in clinical practice:
In patients who undergo upper endoscopy for head and neck cancer, heavy alcohol use for more than 10 years and others at high risk for squamous cell carcinoma, we initiate the mucosal examination using a high-definition gastroscope (often equipped with a translucent distal attachment device) and then inspect with NBI endoscopy followed by Lugol’s chromoendoscopy if there are suspicious areas. Because hypopharyngeal squamous cells in situ and submucosal carcinoma appear similar to that in the esophagus, the same examination techniques using white light followed by equipment-based IEE has the potential to reveal early hypopharyngeal neoplasms.
In Japan, where squamous cell carcinoma has a higher prevalence than in Western countries, it is recommended that NBI be the primary light source during the examination phase of the orohypopharynx and esophagus because early squamous cell carcinoma appears as a distinct brown area in the normal whitish-green mucosa. It is notable that the Japanese use the red, green, blue sequential illumination endoscopy system equipped with optical magnification as opposed to the colour charge-coupled device system with digital magnification available in the United States. Thus, in our unit, we spray Lugol’s solution to the suspected area in the esophagus to further confirm our findings on standard endoscopy.
Supporting data:
Lugol’s solution has been useful in screening for squamous cell carcinoma of the esophagus in individuals at high risk, such as alcoholics, heavy tobacco users and patients with head and neck cancer (11,12). In a prospective multicenter French study of more than 1000 high-risk patients (13) who were screened with standard endoscopy, the application of Lugol’s solution increased the diagnostic yield of early cancer by 20%. Although the sensitivity for predicting neoplasm is high, the specificity of unstained areas with application of Lugols’s solution is low. For example, unstained lesions measuring 5 mm in size or greater were found in 26% of a cohort of more than 600 alcoholic men in Japan, with only 3% diagnosed with squamous cell carcinoma (14). The presence of multiple, irregularly shaped, unstained lesions, however, was strongly associated with esophageal squamous cell cancer, and 55% of such patients were found to harbour cancer. Lugol’s solution has also been shown to improve visualization of lesion borders, because tumour surface area was much larger when seen as unstained areas than with white-light endoscopy alone (15).
NBI can be useful to evaluate the morphological changes of intrapapillary capillary loops (IPCLs), which have been shown to be altered according to the depth of squamous cell carcinoma of the esophagus (16). NBI has been shown to be superior to white light endoscopy for the identification and evaluation of IPCLs (17). Preliminary results of a Japanese, multicentre, prospective, randomized controlled study (18) on the detection and diagnostic accuracy of superficial squamous cell carcinoma in the head and neck region, and the esophagus comparing NBI and conventional white light examination in a back-to-back endoscopy, showed a significantly higher detection rate and diagnostic accuracy with NBI.
Early Barrett’s cancer: Indigo carmine dye and NBI in clinical practice:
In our practice, we first inspect the esophageal epithelium of patients with Barrett’s esophagus using white light to assess for areas of redness, which reflect the increased vascularity. After a complete examination by white light, we use NBI along with selective application of indigo carmine dye to visualize whether the lesion is superficially elevated. It should be noted that this is not commonly practiced, but our observations are indicative of its potential (see Figure 3).
Supporting data:
Adenocarcinoma of the esophagus and the gastric cardia has increased in the Caucasian male population, which has a mean annual cancer incidence in patients with Barrett’s esophagus of approximately 1%. Pech et al (19) reported that most cases of high-grade dysplasia associated with Barrett’s esophagus are flat, reflecting the difficulty in its detection. The standard practice of random, four-quadrant biopsies using the Seattle endoscopic biopsy protocol (20) for Barrett’s surveillance has recently been challenged with the use of IEE to target surveillance biopsies. Evidence supporting the use of IEE in Barrett’s esophagus is, however, evolving; NBI has been studied in Barrett’s esophagus with mixed results (21).
Stomach
Early gastric cancer: Indigo carmine dye in clinical practice:
Because the prevalence of gastric cancer in our practice is low, we spray indigo carmine dye throughout the stomach in selected cases for which gastric cancer is a concern (ie, based on family history) or for margin delineation of an early gastric cancer during endoscopic resection.
Supporting data:
The use of indigo carmine is a standard part of diagnosis of early gastric cancer, and is used especially to delineate nonpolypoid early gastric cancer in endemic areas of the world. Following a complete examination of the stomach with white light endoscopy, diluted indigo carmine dye is sprayed throughout the stomach for a second-look examination to reveal subtle nonpolypoid lesions. We were not able to find comparative studies; however we do not have access to the Japanese language literature.
Colon
Neoplasia and early cancer in clinical practice:
We use high-resolution colonoscopes and begin the examination using white light to search for an abnormal patch of mucosa. The characteristics of NP-CRN include slightly red appearance, an altered or absent vascular network, localized friability or wall deformity. In addition, the absence of innominate grooves can point to or confirm the diagnosis of NP-CRN. We use the NBI modality and, if needed, apply indigo carmine dye to areas suspected of containing a neoplasm because it aids in the differentiation of non-neoplastic and neoplastic tissue, and is helpful in delineating the neoplastic lesion borders. While the use of equipment-based IEE is sufficient for this purpose in the majority of cases (perhaps more than 90%), the application of indigo carmine solution is still necessary to appreciate depressions. We routinely use one or both methods of IEE to obtain the endoscopic diagnosis before endoscopic resection to avoid the noncurative and potentially risky resection of invasive submucosal neoplasms.
Supporting data: Detection of colorectal neoplasia:
The application of IEE in high-risk groups, including inflammatory bowel disease and polyposis syndrome cohorts, has shown a higher yield in adenoma detection (22,23). Overall, IEE techniques such as those that use indigo carmine or optical/electronic technologies, have not improved adenoma detection rates in colorectal cancer screening populations (24,25).
Differentiation of non-neoplastic and neoplastic tissue:
Kudo et al (17) described the characteristic pit-pattern morphology of normal, non-neoplastic and neoplastic colon mucosa, using high-magnification colonoscopy and indigo carmine IEE. Matsuda et al (26) reported on the potential utility of pit-pattern analysis to distinguish non-neoplastic, mucosal and submucosal invasive neoplasms. Others in Western countries have reported overall high accuracy of high-definition NBI without magnification (27–30) and, most recently, significant superiority to high-definition white light endoscopy without magnification for real-time differentiation of non-neoplastic and neoplastic colonic polyps (31).
SUMMARY
IEE uses dyes and optical and electronic methods to enhance contrast and discrimination between normal, non-neoplastic and neoplastic mucosa. There are sufficient data supporting its use to potentially aid in the detection, diagnosis and treatment of esophageal, gastric and colorectal pre- or early cancer. As such, endoscopy provides only a preliminary diagnosis that can be used to guide the course of treatment – the final diagnosis will require multiple, cross-sectional, pathological examinations of the lesion. Despite its imperfections, endoscopic diagnosis is sufficiently accurate for current practice. Data support the standard use of Lugol’s solution for patients at high risk for squamous cell carcinoma of the esophagus, and the use of indigo carmine solution to diagnose and treat early gastric cancer. Other applications, such as the use of indigo carmine dye in the diagnosis and management of NP-CRN, have been described, particularly in high-risk populations. While the use of NBI to enhance the detection of adenoma during screening colonoscopy has not proven beneficial, there is an expanding body of literature regarding the use of NBI or other equipment-based IEEs for the diagnosis and management of nonpolypoid gastrointestinal neoplasms. Its role is likely to expand as the technology develops to look closer, see more and distinguish further. Several barriers to its widespread use include the lack of standardized training techniques, absence of an accepted mechanism for reimbursement, and perceptions of its cost and inefficiency. These barriers are perhaps a reflection of the development of a modality that is being perfected and the inherent limitation of the inability to evaluate based on multiple cross-sections that are available during pathological examination. As discussed above, it should not be viewed as a limitation. We emphasize that our successful detection, diagnosis and treatment of flat neoplasms could not have been accomplished without the use of IEE (2,32,33).
REFERENCES
- 1.2009. http://www.who.int/mediacentre/factsheets/fs297/en/index.html. (Version current at September 30, 2009).
- 2.Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, Matsui S, Friedland S. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–35. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 3.Kaltenbach T, Sano Y, Friedland S, Soetikno R. American Gastroenterological Association (AGA) Institute technology assessment on image-enhanced endoscopy. Gastroenterology. 2008;134:327–40. doi: 10.1053/j.gastro.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 4.Kaltenbach T, Soetikno R. American Society of Gastrointestinal Endoscopy. Endoscopic Learning Library; 2008. Image enhanced endoscopy – educational DVD. [Google Scholar]
- 5.Gono K, Yamazaki K, Doguchi N, et al. Endoscopic observation of tissue by narrowband illumination. Optic Rev. 2003;10:211–5. [Google Scholar]
- 6.Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–77. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 7.Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer. 2001;84:1354–62. doi: 10.1054/bjoc.2001.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori M, Adachi Y, Matsushima T, Matsuda H, Kuwano H, Sugimachi K. Lugol staining pattern and histology of esophageal lesions. Am J Gastroenterol. 1993;88:701–5. [PubMed] [Google Scholar]
- 9.Thuler FP, de Paulo GA, Ferrari AP. Chemical esophagitis after chromoendoscopy with Lugol’s solution for esophageal cancer: Case report. Gastrointest Endosc. 2004;59:925–6. doi: 10.1016/s0016-5107(04)00173-7. [DOI] [PubMed] [Google Scholar]
- 10.Kondo H, Fukuda H, Ono H, et al. Sodium thiosulfate solution spray for relief of irritation caused by Lugol’s stain in chromoendoscopy. Gastrointest Endosc. 2001;53:199–202. doi: 10.1067/mge.2001.110730. [DOI] [PubMed] [Google Scholar]
- 11.Muto M, Hironaka S, Nakane M, Boku N, Ohtsu A, Yoshida S. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc. 2002;56:517–21. doi: 10.1067/mge.2002.128104. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro U, Jr, Posner MC, Safatle-Ribeiro AV, Reynolds JC. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83:1174–85. [PubMed] [Google Scholar]
- 13.Dubuc J, Legoux JL, Winnock M, et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: A prospective study conducted in 62 French endoscopy centers. Endoscopy. 2006;38:690–5. doi: 10.1055/s-2006-925255. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama A, Ohmori T, Makuuchi H, et al. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer. 1995;76:928–34. doi: 10.1002/1097-0142(19950915)76:6<928::aid-cncr2820760604>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc. 2005;61:219–25. doi: 10.1016/s0016-5107(04)02756-7. [DOI] [PubMed] [Google Scholar]
- 16.Inoue H, Honda T, Nagai K, Kawano T, Yoshino K, Takeshita K. Ultra-high magnification endoscopic observation of of carcinoma in situ of the oesophagus. Dig Endosc. 1997;9:16–18. [Google Scholar]
- 17.Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288–95. doi: 10.1016/s0016-5107(03)02532-x. [DOI] [PubMed] [Google Scholar]
- 18.Muto M, Saito Y, Ohmori T, Kaise M, Inoue H, Ishikawa H. Multicenter prospective randomized controlled study on the detection and diagnosis of superficial squamous cell carcinoma by back-to-back endoscopic examination of narrow band imaging and white light observation. Gastrointest Endosc. 2007;65:AB110. (Abst) [Google Scholar]
- 19.Pech O, Gossner L, Manner H, et al. Prospective evaluation of the macroscopic types and location of early Barrett’s neoplasia in 380 lesions. Endoscopy. 2007;39:588–93. doi: 10.1055/s-2007-966363. [DOI] [PubMed] [Google Scholar]
- 20.Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology. 1993;105:40–50. doi: 10.1016/0016-5085(93)90008-z. [DOI] [PubMed] [Google Scholar]
- 21.Curvers WL, van den Broek FJ, Reitsma JB, Dekker E, Bergman JJ. Systematic review of narrow-band imaging for the detection and differentiation of abnormalities in the esophagus and stomach (with video) Gastrointest Endosc. 2009;69:307–17. doi: 10.1016/j.gie.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 22.Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880–8. doi: 10.1053/gast.2003.50146. [DOI] [PubMed] [Google Scholar]
- 23.East JE, Suzuki N, Stavrinidis M, Guenther T, Thomas HJ, Saunders BP. Narrow band imaging for colonoscopic surveillance in hereditary non-polyposis colorectal cancer. Gut. 2008;57:65–70. doi: 10.1136/gut.2007.128926. [DOI] [PubMed] [Google Scholar]
- 24.van den Broek FJ, Reitsma JB, Curvers WL, Fockens P, Dekker E. Systematic review of narrow-band imaging for the detection and differentiation of neoplastic and nonneoplastic lesions in the colon (with videos) Gastrointest Endosc. 2009;69:124–35. doi: 10.1016/j.gie.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 25.Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406–12. doi: 10.1136/gut.2007.137984. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008;103:2700–6. doi: 10.1111/j.1572-0241.2008.02190.x. [DOI] [PubMed] [Google Scholar]
- 27.Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174–81. doi: 10.1053/j.gastro.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Rastogi A, Pondugula K, Bansal A, et al. Recognition of surface mucosal and vascular patterns of colon polyps by using narrow-band imaging: interobserver and intraobserver agreement and prediction of polyp histology. Gastrointest Endosc. 2009;69:716–22. doi: 10.1016/j.gie.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 29.Sikka S, Ringold DA, Jonnalagadda S, Banerjee B. Comparison of white light and narrow band high definition images in predicting colon polyp histology, using standard colonoscopes without optical magnification. Endoscopy. 2008;40:818–22. doi: 10.1055/s-2008-1077437. [DOI] [PubMed] [Google Scholar]
- 30.Rogart JN, Jain D, Siddiqui UD, et al. Narrow-band imaging without high magnification to differentiate polyps during real-time colonoscopy: improvement with experience. Gastrointest Endosc. 2008;68:1136–45. doi: 10.1016/j.gie.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 31.Rastogi A, Keighley J, Singh V, et al. High Accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: A prospective study. Am J Gastroenterol. 2009 doi: 10.1038/ajg.2009.403. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Soetikno R, Friedland S, Kaltenbach T, Chayama K, Tanaka S. Nonpolypoid (flat and depressed) colorectal neoplasms. Gastroenterology. 2006;130:566–76. doi: 10.1053/j.gastro.2005.12.006. quiz 588–9. [DOI] [PubMed] [Google Scholar]
- 33.Kaltenbach T, Friedland S, Maheshwari A, et al. Short- and long-term outcomes of standardized EMR of nonpolypoid (flat and depressed) colorectal lesions > or = 1 cm (with video) Gastrointest Endosc. 2007;65:857–65. doi: 10.1016/j.gie.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 34.The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(Suppl 6):3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]