Abstract
Directed cell migration requires the coordination of growth factor and cell adhesion signaling and is of fundamental importance during embryonic development, wound repair, and pathological conditions such as tumor metastasis. Herein, we demonstrate that the ArfGAP, paxillin-kinase-linker (PKL/GIT2), is tyrosine phosphorylated in response to platelet-derived growth factor (PDGF) stimulation, in an adhesion dependent manner and is necessary for directed cell migration. Using a combination of pharmacological inhibitors, knockout cells and kinase mutants, FAK, and Src family kinases were shown to mediate PDGF-dependent PKL tyrosine phosphorylation. In fibroblasts, expression of a PKL mutant lacking the principal tyrosine phosphorylation sites resulted in loss of wound-induced cell polarization as well as directional migration. PKL phosphorylation was necessary for PDGF-stimulated PKL binding to the focal adhesion protein paxillin and expression of paxillin or PKL mutants defective in their respective binding motifs recapitulated the polarization defects. RNA interference or expression of phosphorylation mutants of PKL resulted in disregulation of PDGF-stimulated Rac1 and PAK activities, reduction of Cdc42 and Erk signaling, as well as mislocalization of βPIX. Together these studies position PKL as an integral component of growth factor and cell adhesion cross-talk signaling, controlling the development of front–rear cell polarity and directional cell migration.
INTRODUCTION
Directed cell migration requires front–rear cell polarization and plays a fundamental role during development, the innate immune response and wound repair as well as in tumor cell metastasis (Lauffenburger and Horwitz, 1996; Ridley et al., 2003). Chemoattractant (e.g., growth factor) gradients in combination with extracellular matrix (ECM) cues provide the external stimuli necessary to establish and maintain the asymmetric shape of polarized cells, through the regulation of integrin-based cell adhesion and reorganization of the actin and microtubule cytoskeleton (Etienne-Manneville and Hall, 2002, 2008; Ridley et al., 2003). For example, coordinated signaling between PDGF receptors and integrins in fibroblasts and vascular cells facilitates directional cell migration during organogenesis, blood vessel patterning, and wound healing (Heldin and Westermark, 1999; Duchek et al., 2001; Nagel et al., 2004; Wood et al., 2006).
The nonreceptor tyrosine kinase FAK, in combination with the Src family kinases (SFKs), has emerged as a central effector, mediating cross-talk between activated growth factor receptor tyrosine kinases such as PDGF and engaged integrins (Hauck et al., 2000; Juliano, 2002). In turn, FAK/Src regulates focal adhesion turnover and cytoskeletal remodeling (Webb et al., 2004; Zaidel-Bar et al., 2007), through tyrosine phosphorylation of various key focal adhesion adapter proteins such as paxillin and p130Cas (Thomas and Brugge, 1997; Schlaepfer et al., 1999; Brown and Turner, 2004), as well as critical regulators of Rho GTPase signaling including the guanine nucleotide exchange factors (GEFs) PIX, Vav, DOCK180, and GTPase-activating proteins (GAPs) such as p190RhoGAP and ASAP1 (Brown et al., 1998; Rossman et al., 2005; Feng et al., 2006; Chang et al., 2007). Furthermore, morphology and migration assays indicate that adhesion and growth factor signaling through FAK/Src as well as the Erk/MAPK cascade are necessary for cell polarization during directed cell migration (Tilghman et al., 2005; Vicente-Manzanares et al., 2005; Owen et al., 2007; Platek et al., 2007).
Critical to the development of front–rear polarity is the activation of the small GTPase Cdc42 (Ridley et al., 2003), as well as the recruitment of active Rac1 to the cell's leading edge to promote localized activation of the actin polymerization machinery and the establishment of a dominant lamellipodium (Ridley et al., 2003). The p21-activated kinase PAK, an effector of both Cdc42 and Rac1, plays a critical role in coordinating these events via interaction with βPIX (Cau and Hall, 2005), which in turn can bind and recruit active Rac1 at the leading edge (ten Klooster et al., 2006). Importantly, the mechanism through which active βPIX is selectively restricted to the front of polarized cells has not been determined.
The focal adhesion scaffold protein, paxillin has emerged as an important nexus for the regulation of Rho family GTPase signaling (Brown and Turner, 2004; Deakin and Turner, 2008), through its ability to bind key regulators and effectors. In particular, the LD4 motif of paxillin, provides the docking site for a cassette of proteins including the Arf GAPs PKL/GIT2 or the closely related GIT1 (Premont et al., 1998, 2000), linked in turn to PIX, PAK, and Nck (Turner et al., 1999). Perturbation of the paxillin-PKL/GIT interaction results in the disregulation of Rac1 signaling and the formation of multiple random membrane protrusions, suggesting a key role in the spatial regulation of Rac1 activity (West et al., 2001). Gene ablation of PKL/GIT2 in mouse neutrophils results in defective chemotaxis toward G-protein–coupled receptor ligands, fMLP and C5a (Mazaki et al., 2006), and mutation of GIT in Caenorhabditis elegans exhibited defective gonad distal tip cell migration (Lucanic and Cheng, 2008). RNA interference (RNAi) of PKL/GIT2 in HeLa cells also resulted in aberrant cell spreading and focal adhesion dynamics through disregulation of Rac1 and Cdc42, respectively (Frank et al., 2006). Interestingly, PKL/GIT proteins are also substrates for FAK/Src and PKL tyrosine phosphorylation is necessary for Rac1-stimulated recruitment of the PKL-PIX-PAK-Nck complex to focal adhesions during cell spreading (Brown et al., 2005), whereas recent studies identify an important function for GIT1 in EGF-dependent vascular smooth-muscle cell migration (Yin et al., 2005). Herein we identify PKL/GIT2 as an integral component of growth factor–adhesion cross-talk controlling directed cell migration. We present evidence that PKL is tyrosine phosphorylated by FAK/Src in response to PDGF and that this posttranslational modification is critical for the regulation of Rac1 and Cdc42 GTPase activities, phospho-Erk signaling and thereby the development of front–rear cell polarity through its ability to interact with paxillin in focal adhesions and recruitment of βPIX and active PAK to the cell's leading edge.
MATERIALS AND METHODS
Reagents
The plasmids encoding pEGFP-C1 PKL wild type (WT), Y286/392/592F (3YF), paxillin-binding subdomain deletion (ΔPBS2), pEGFP-paxillin WT, and the ΔLD4 mutant have been described previously (West et al., 2001; Brown et al., 2005). Plasmids pKH3 HA-FAK WT and FAK kinase-dead (KD, K454R) were generously provided by Dr. Jun-Lin Guan (University of Michigan, Ann Arbor, MI). Super-FAK (K578/581E) has been described (Brown et al., 2005). pLXSH Src WT, the KD mutant (K295R), and constitutively active Src (Y527F) were kindly provided by Dr. Jonathan Cooper (Fred Hutchinson Cancer Research Center, Seattle, WA); pcDNA3 Myc-Rac1 WT was provided by Dr. Marc Symons (Feinstein Institute for Medical Research at North Shore-LIJ, Manhasset, NY); pGEX-2T PAK3 p21-binding domain (PBD) was provided by Dr. Richard Cerione (Cornell University, Ithaca, NY); pGEX-2T WASp CRIB was provided by Dr. Nathalie Lamarche-Vane (McGill University, Montreal, QC, Quebec); and pcDNA3-HA Erk was provided by Dr. J. Silvio Gutkind (NIH, Bethesda, MD). Antibodies anti-PKL/GIT1, anti-paxillin (clones 165), anti-FAK, and anti-Erk were obtained from BD Transduction Laboratories (Lexington, KY); anti-Src was generously provided by Dr. Sheila Thomas (Harvard Medical School, Boston, MA); anti-hemagglutinin (HA) monoclonal 12CA5 was from Covance (Berkeley, CA); anti-Src pY418 and anti-FAK pY397 were from BioSource (Camarillo, CA); anti-myc (9E10), anti-green fluorescent protein (GFP), and anti-αPAK (N-20) were from Santa Cruz Biotechnology (Santa Cruz, CA); anti-α-actinin was from Sigma (St. Louis, MO); IgG-purified anti-GFP was from Molecular Probes (Eugene, OR); anti-phosphotyrosine (clone 4G10) was from Upstate Biotechnology (Charlottesville, VA); and anti-PAK pS199/204 and anti-phospho-Erk1/2 were from Cell Signaling (Danvers, MA). PDGF-BB and the Src inhibitor PP2 were obtained from Sigma (St. Louis, MO).
Cell Culture and Mouse PKL RNAi Knockdown
Mouse embryonic fibroblasts (MEFs; Hagel et al., 2002) NIH 3T3 (ATCC, Manassas, VA), SYF−/−, and FAK−/− MEFs were cultured as previously described (Brown et al., 2005). Transient transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or FuGene HD (Roche, Indianapolis, IN) at a reagent-DNA ratio of 3:1 according to the manufacturer's instructions. SiRNA oligonucleotides were designed to target all mRNA splice isoforms of mouse PKL/GIT2 (5′-CAATGGTGCTAACTCTATATT-3′ and 5′-CAACTTCTTTCATCCTGAATT-3′). The control small interfering RNA (siRNA; 5′-AAACTCTATCTGCACGCTGAC-3′) was from Ambion (Austin, TX). PKL siRNA oligonucleotides were transfected into normal MEFs using Metafectene (Biontex-USA, San Diego, CA) according to the manufacturer's instructions. RNAi knockdown efficiency was determined by Western blotting with PKL/GIT1 antibody (BD Transduction Laboratories) and a PKL-specific antibody, kindly provided by Dr. Rick Horwitz (University of Virginia, Charlottesville, VA).
Immunoprecipitation and Immunoblotting
For endogenous PKL immunoprecipitation, cells were lysed in stringent lysis buffer (62.5 mM Tris-HCl, pH 6.8, 150 mM NaCl, 1% SDS, 10% glycerol, 20 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride [PMSF], 0.2 mM sodium vanadate [NaVO4]). For GFP-PKL or paxillin immunoprecipitation, cells were lysed in buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1% Triton X-100, 10% glycerol, 20 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF, and 0.2 mM NaVO4) or modified radioimmunoprecipitation buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 10% glycerol, 20 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF, and 0.2 mM NaVO4). Forty-eight hours after transfection, cells were serum-starved for 5 h followed by stimulation with 20 ng/ml PDGF-BB for the time course indicated. Immunoprecipitation and Western blotting analyses were performed as previously described (Brown et al., 2005). The quantification of Western blots was performed using NIH ImageJ software (http://rsb.info.nih.gov/ij/; NIH, Bethesda, MD).
Immunofluorescence and Microscopy Imaging
Cells were fixed and permeabilized with 3.7% paraformaldehyde and 0.5% Triton X-100 in PBS followed by procedures as previously described (Brown et al., 2005). Samples were incubated with primary antibodies in PBS containing 3% BSA for 2 h, rinsed four times, and reincubated with secondary antibodies at room temperature for 1 h. Nuclei were visualized by staining with Hoechst 33342 (1 μg/ml, Molecular Probes). Fluorescence imaging was performed on a Leica SP5 confocal microscope (Deerfield, IL) or Zeiss Axioskop microscope (Thornwood, NY) using a 63× oil immersion objective. Images were captured using a CCD camera (Hamamatsu Orca ER, Bridgewater, NJ) or SPOT camera and processed using ImageJ software.
Modified Boyden Chamber Assay
The modified Boyden chamber assays were performed as previously described (LaLonde et al., 2006). Briefly, the filter was coated on both sides with 5 μg/ml human fibronectin (Sigma) for 2 h at room temperature. For the chemotaxis assay, PDGF-BB (20 ng/ml)-containing medium was added to the lower chamber to establish the growth factor gradient. Alternatively, for chemokinesis analysis, PDGF-BB (20 ng/ml)-containing medium was added to both upper and lower chambers to establish a uniform growth factor concentration. Transfected cells (5 × 104) were plated in the upper chamber and cultured at 37°C, 5% CO2 for 5 h. Cells on the top side of the filters were removed by a cotton swab followed by fixation with 3.7% paraformaldehyde in PBS (pH 7.4) for 15 min. RNAi treated or transfected cells that migrated to the bottom side of the filter were counted from 15 randomly chosen fields for each filter. For the GFP-PKL–transfected cells, GFP expressing cells served as the control population.
Cell Polarity and Migration Analysis
Transfected cells or RNAi treated cells were seeded on fibronectin-coated (5 μg/ml) cover slips and cultured to confluency. Monolayers were scraped using a pipette tip and washed four times with prewarmed medium. Transfected cells at the wound edge were identified by immunofluorescence imaging. Time-lapse live-cell images were captured every 1 min for 60 min for membrane dynamics analysis or every 10 min for 6 h for evaluation of cell migration. A second set of cells was fixed and stained with rhodamine-phalloidin or Alexa633 phalloidin for F-actin, anti-giantin for Golgi, anti-βPIX (Chemicon, Temecula, CA) and Hoechst 33342 to visualize nuclei. Cells at the wound edge with Golgi polarized to the front-facing 120° sector were scored as positive for polarization.
RESULTS
Requirement of PKL in Directional Cell Migration and Cell Polarity
Endogenous PKL expression in MEFs was suppressed using siRNA, and scrape-wound assays were performed to assess the role of PKL/GIT2 in directional cell migration. Western blotting confirmed specific knockdown of PKL, but not its homologue GIT1 (Figure 1A). Time-lapse live-cell imaging, combined with cell centroid tracking, showed that PKL RNAi cells migrated less efficiently than control cells (Figure 1, B and C), exhibiting significantly lower velocity as well as reduced directional persistence (Figure 1C). A second PKL siRNA targeting a separate sequence produced comparable results (Supplemental Figure S1). The role of PKL in directional cell migration was further evaluated by modified Boyden chamber assays. Cell chemotaxis toward PDGF-BB, an important physiological regulator of directed cell migration during wound repair, was reduced by ∼75% in the PKL RNAi cells whereas chemokinesis analysis, which is a measurement of random cell migration, showed no significant difference to control cells (Figure 1D). These results indicate a role for PKL in growth factor–dependent directional cell migration, but not for random cell migration.
Figure 1.
PKL/GIT2 is required for directional cell migration and cell polarity. (A) PKL RNAi knockdown in MEFs. Normal MEFs were transfected with mouse-specific siRNA for PKL. At 60 h after transfection, cells were lysed and subjected to Western immunoblotting. Cell lysates were blotted for PKL, GIT1, and α-actinin. (B) Effect of PKL RNAi on cell migration in a wound-healing assay. Confluent control RNAi and PKL RNAi cells were scratched and recorded by Hoffman Modulation Contrast microscopy. Time-lapse images were captured every 10 min for 6 h. Representative images were taken at 10 min and 3 and 6 h after wounding. (C) Quantification of migration after wound healing. Migration of individual cells at the wound edge was determined by centroid tracking. Representative tracks of control RNAi versus PKL RNAi are shown. Cell migration velocity and directionality persistence were also calculated. Error bars, SEM from three independent experiments. (D) Modified Boyden chamber assays were performed on control versus PKL RNAi cells to evaluate chemotaxis and chemokinesis. Migration index of control cells was set at 100%. Error bars, SEM from five independent experiments. (E) Kymograph analysis of membrane protrusion. Control and PKL RNAi cells were plated for the scrape-wound healing assay, and images of cells at the wound edge were captured every minute for 1 h. Kymograph analysis of membrane protrusion was processed using NIH ImageJ software. (F) Role of PKL in cell polarization at the wound edge. Four hours after wounding, cells were fixed and stained with a Golgi marker (anti-Giantin, red), nucleus (Hoechst 33342, blue), and F-actin (Alexa633-phalloidin, gray). Percentage of cells exhibiting reoriented Golgi into the 120° sector facing the wound was quantified. Random distribution of Golgi is predicted to be ∼33% (red line). For statistical results, Student's t test, *p < 0.05, **p < 0.01. Scale bar, 10 μm.
Polarized membrane protrusion as well as the reorientation of the Golgi toward the leading edge is the major feature of directional cell migration (Kulkarni et al., 2000; Ridley et al., 2003). Examination of cells at the wound edge showed that control RNAi cells exhibited a typical polarized morphology with a broad lamellipodium facing into the wound, whereas PKL RNAi cells developed numerous small angular protrusions/retractions (Figure 1E, Movies S1 and S2). Kymograph analysis confirmed that control RNAi cells protruded persistently, whereas PKL knockdown cells formed unstable protrusions that retracted frequently (Figure 1E, Supplemental Figure S1). Furthermore, PKL RNAi cells showed defective Golgi reorientation at the wound edge compared with control cells (Figure 1F, Supplemental Figure S1). It is of note that at low cell density, control RNAi cells also developed stable protrusions with a broad single lamellipodium and exhibit persistent directional migration. In contrast, PKL knockdown cells exhibited unstable cell protrusive activity in multiple directions (unpublished observations). Thus PKL is required for the development of front–rear asymmetry necessary for directional cell migration.
PKL Is Tyrosine Phosphorylated in Response to PDGF in an Adhesion-dependent Manner
Previous studies in our laboratory have demonstrated that cell adhesion to fibronectin stimulates PKL tyrosine phosphorylation (Brown et al., 2005). To determine whether PKL is similarly regulated by soluble growth factors, we examined the PKL phosphotyrosine profile after PDGF-BB stimulation. As shown in Figure 2A, PKL (arrow, lower band) was transiently phosphorylated in response to PDGF-BB, reaching maximal phosphorylation at 5–10 min and gradually decreasing to basal levels by 30 min. The related protein GIT1 (top band) was also transiently tyrosine-phosphorylated consistent with previous reports (Yin et al., 2005). Additionally, immunoprecipitation and Western blotting for GFP-PKL WT (wild-type), transiently expressed in MEFs, showed a similar profile of PKL tyrosine phosphorylation in response to PDGF-BB (Figure 2B). Thus, GFP-PKL was utilized as a reporter for the subsequent biochemistry and cell biology studies.
Figure 2.
PKL is tyrosine-phosphorylated by Src and FAK in response to PDGF. (A) Endogenous PKL and GIT1 were precipitated from quiescent and PDGF (20 ng/ml)-stimulated MEFs and blotted with phosphotyrosine antibody (PY, clone 4G10). (B) MEFs were transiently transfected with GFP-PKL wild type (WT) and GFP-PKL Y286/392/592F (3YF). Exogenous GFP-PKL WT or 3YF was precipitated from quiescent and PDGF-stimulated cells using anti-GFP antibody and probed with anti-phosphotyrosine antibody 4G10 and reprobed with anti-GFP. (C) Both adherent and suspended MEFs, expressing GFP-PKL WT were stimulated with PDGF for 10 min. Cells were immunoprecipitated with anti-GFP followed by Western blotting with phosphotyrosine and GFP antibodies. (D) MEFs expressing GFP-PKL WT were incubated in the absence and presence of Src inhibitor (10 μM PP2) for 30 min. Quiescent and PDGF-stimulated cells were lysed and immunoprecipitated using GFP antibody. GFP precipitates were probed with phosphotyrosine antibody and reprobed with anti-GFP. (E) GFP-PKL WT was expressed alone or with Src (WT, KD K295R, or constitutively active Y527F) in SYF−/− MEFs, and PKL phosphorylation was evaluated. Lysates were blotted with the Src pY418 and pan Src antibodies to confirm increased Src kinase activity. (F) GFP-PKL WT was expressed alone or with FAK (WT, KD, Y397F, and superFAK) in FAK−/− MEFs. PKL tyrosine phosphorylation was evaluated by blotting the GFP precipitates with 4G10. Lysates were blotted with FAK pY397, and pan FAK antibodies to confirm FAK kinase activity. Data are representative of three independent experiments.
There is substantial evidence indicating that growth factor—induced signal transduction in adherent cell types is anchorage dependent, requiring cross-talk with integrin-dependent signaling pathways (Juliano, 2002), and indeed PDGF-induced PKL tyrosine phosphorylation required adhesion to the ECM (Figure 2C). We have previously identified tyrosine residues 286, 392, and 592 on PKL as the major phosphorylation sites in response to cell adhesion on fibronectin (Brown et al., 2005). Therefore, the phosphotyrosine profile of the GFP-PKL 3YF (Y286/392/592F) mutant was examined in PDGF-stimulated adherent MEFs. This mutant was not phosphorylated after PDGF treatment (Figure 2B) indicating that these tyrosine residues encompass the major PDGF-induced phosphorylation sites and further suggesting that PKL represents a point of convergence of growth factor and adhesion signaling.
FAK and Src Kinases Are Essential for PKL Tyrosine Phosphorylation
SFKs function downstream of adhesion-dependent PDGF signaling to phosphorylate various focal adhesion proteins and thereby regulate cytoskeleton organization and cell motility (Thomas and Brugge, 1997). To examine whether Src functions upstream of PKL, cells were treated with PDGF in the presence or absence of the Src inhibitor, PP2. GFP-PKL tyrosine phosphorylation in the presence of PP2 was significantly reduced but not completely abolished (Figure 2D, Supplemental Figure S2A). To further evaluate the role of Src activity, the PKL phosphorylation profile was examined in Src/Yes/Fyn null (SYF−/−) MEFs (Klinghoffer et al., 1999) stimulated with PDGF and was only marginally increased over the serum-starved level (Figure 2E, Supplemental Figure S2B). Reexpression of WT Src in the SYF−/− cells produced robust PDGF-dependent PKL phosphorylation, whereas cells expressing KD Src (K295R) only exhibited partial rescue, with a delayed peak of PKL tyrosine phosphorylation occurring at 10 min (Figure 2E, Supplemental Figure S2B). In addition, SYF−/− MEF reexpressing constitutively active Src (Y527F) demonstrated elevated PKL phosphorylation in unstimulated serum-starved cells that was further enhanced after the addition of PDGF (Figure 2E, Supplemental Figure S2B). These data suggest that Src kinase activity is required but not solely responsible for PKL tyrosine phosphorylation in response to PDGF. The increased phosphorylation of PKL after expression of the Src KD mutant further suggests that Src scaffold function (Schlaepfer et al., 1997) and/or other kinases are likely involved.
It is well-established that FAK cooperates with SFK to regulate focal adhesion protein tyrosine phosphorylation in response to cell–ECM adhesion and growth factor signaling (Hauck et al., 2000; Sieg et al., 2000; Moissoglu and Gelman 2003). FAK autophosphorylation at tyrosine 397 facilitates Src association and activation through a FAK pY397–Src SH2 interaction (Schlaepfer et al., 1999). Thus, we examined the potential role of FAK in PDGF-induced PKL tyrosine phosphorylation. GFP-PKL WT was transfected into FAK−/− MEFs and FAK−/− MEFs reexpressing FAK WT, FAK Y397F, or the FAK KD mutant (K454R). Immunoprecipitation and Western blotting showed that as with the SYF−/− cells, minimal PKL phosphorylation was detected in PDGF-stimulated FAK−/− MEF cells (Figure 2F, Supplemental Figure S2C), whereas reintroduction of FAK WT significantly increased PDGF-dependent PKL phosphorylation (Figure 2F, Supplemental Figure S2C). In contrast, PKL phosphorylation was lower in FAK−/− MEFs reexpressing the FAK Y397F and KD mutants (Figure 2F, Supplemental Figure S2C), whereas overexpression of “superactive FAK” (K578/581E; Brown et al., 2005) resulted in enhanced PKL tyrosine phosphorylation in serum-starved cells that was further enhanced by the addition of PDGF. The fact that FAK KD and the Y397F mutant induced modest increases in PKL phosphorylation (Figure 2F, Supplemental Figure S2C) suggests that FAK scaffold-dependent signaling (probably through Src), as well as FAK kinase activity are involved in PDGF-stimulated PKL tyrosine phosphorylation.
Src/FAK Phosphorylation of PKL Regulates Directional Cell Migration
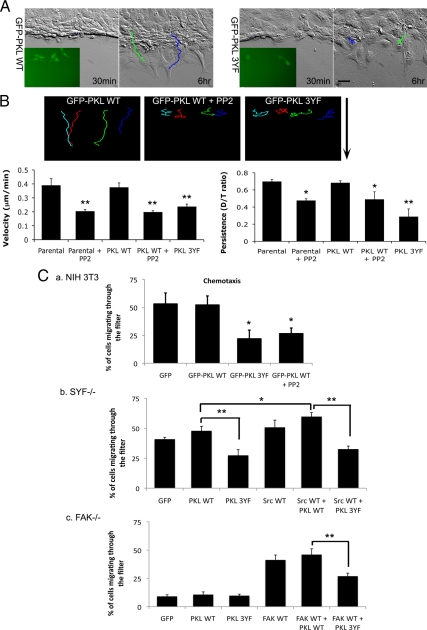
The role of PKL tyrosine phosphorylation in directed cell migration during wound healing was further analyzed using time-lapse microscopy and centroid tracking. GFP-PKL WT expressing MEF cells exhibited a cell migration profile similar to parental cells. In contrast, cells expressing the PKL 3YF mutant were defective in migration velocity and directional persistence (Figure 3, A and B, Movies S3 and S4). Importantly, inhibition of Src family kinases by PP2 treatment of GFP-PKL WT cells produced a comparable reduction cell migration rate and directional persistence (Figure 3B, Movie S5). As an additional assessment of directional cell migration, modified Boyden chamber assays were performed to evaluate the role of PKL phosphorylation in the regulation of cell chemotaxis toward PDGF. PKL WT-transfected NIH 3T3 cells exhibited migratory capacity comparable to GFP-transfected control cells, whereas PKL 3YF expressing cells were significantly defective in their migration (Figure 3Ca). As noted above, both Src and FAK function upstream of PDGF-induced PKL tyrosine phosphorylation. Consistent with activation of this signaling axis, PP2 treatment of normal MEFs or NIH 3T3 cells expressing PKL WT also resulted in impaired chemotaxis toward PDGF (Figure 3, B and Ca). However, the precise role for Src in PDGF-induced chemotaxis remains controversial and may be cell- and/or assay-specific (Klinghoffer et al., 1999; Shah and Vincent, 2005). Indeed, in our hands reintroduction of Src WT into SYF−/− MEFs resulted in a reproducible, albeit modest increase in chemotaxis (p = 0.0508; Figure 3Cb). Interestingly, reintroduction of Src WT along with GFP-PKL WT into the SYF−/− MEFs significantly (p < 0.05) rescued directional cell migration (Figure 3Cb), suggesting that the amount of endogenous PKL may be a limiting factor. In contrast, reintroduction of FAK alone into FAK−/− MEFs or in combination with PKL produced a similarly robust rescue in chemotaxis (Figure 3Cc). In contrast, Src or FAK reexpression together with the PKL 3YF mutant failed to rescue chemotaxis in either cell type (Figure 3C, b and c). Taken together, these data support a requirement for Src/FAK-induced PKL tyrosine phosphorylation in directed cell migration associated with wound healing and chemotaxis. Further studies will be required to determine whether FAK plays a more significant role than Src in this signaling axis.
Figure 3.
PKL tyrosine phosphorylation is essential for directed cell migration. (A) MEFs were transiently transfected with GFP-PKL WT or the 3YF mutant. Directional cell migration in a scrape-wound assay was analyzed. Initial identification of GFP-positive cells was performed by fluorescence microscopy. Subsequently, Hoffman Modulation Contrast time-lapse images were captured every 10 min for 6 h. Representative images of cell migration at 30 min and 6 h are presented. (B) Cell migration velocity and directionality persistence were quantified from at least three independent experiments (>12 cells for each mutant). Error bars, SEM. Student's t test, *p < 0.05; **p < 0.01. (C) Modified Boyden chamber assay for chemotaxis. (a) NIH 3T3, (b) SYF−/− MEFs, and (c) FAK−/− MEFs were transfected as indicated. Cells plated in the upper chamber (serum-free) were allowed to migrate for 5 h toward 20 ng/ml PDGF. Error bars, SEM from five independent experiments. Student's t test, *p < 0.05 compared with GFP control cells in panel a or as indicated in panels b and c.
PDGF Stimulates a PKL and Paxillin Interaction in a Tyrosine Phosphorylation-dependent Manner
PKL was originally identified as a binding partner for the paxillin LD4 motif (Turner et al., 1999). This interaction and thus the recruitment of PKL to focal adhesions were found to require adhesion-dependent PKL tyrosine phosphorylation (Brown et al., 2005). Whether this association is similarly regulated upon growth factor stimulation was determined by coimmunoprecipitation assays. Interestingly, the amount of GFP-PKL WT precipitated paxillin-mirrored PDGF-induced PKL phosphotyrosine content with maximal endogenous paxillin being precipitated by phosphorylated PKL after 5–10-min stimulation (Figure 4A). In striking contrast, little paxillin was detected in the GFP-PKL 3YF precipitates (Figure 4A). This result strongly indicates that PDGF-dependent PKL tyrosine phosphorylation is involved in the regulation of the paxillin-PKL association. To test if PDGF stimulated an interaction between endogenous paxillin and PKL, paxillin was immunoprecipitated from normal MEFs, and the blots were probed with PKL (GIT2)-specific or GIT1-specific antibodies. Both PKL and GIT1 transiently associated with the paxillin precipitates (Figure 4B), mirroring the PKL tyrosine phosphorylation profile in response to PDGF (Figure 4A). It is noteworthy that PKL interaction with paxillin was more transient than GIT1 in response to PDGF stimulation (Figure 4B). It suggests a distinct role of tyrosine phosphorylation in regulation of paxillin interaction with PKL.
Figure 4.

Tyrosine phosphorylation of PKL regulates its interaction with paxillin. (A) MEFs were transfected with GFP-PKL WT or GFP-PKL 3YF. Quiescent cells were stimulated with PDGF (20 ng/ml) at indicated time points, and exogenous PKL was precipitated with GFP antibody, followed by Western blotting for phosphotyrosine (4G10), GFP, paxillin (clone 165), and α-actinin antibodies. Lysates were blotted with paxillin antibody. (B) To demonstrate a growth factor-dependent interaction between endogenous PKL and paxillin, quiescent and PDGF-stimulated MEFs were precipitated with paxillin (clone 165) antibody followed by probing with anti-PKL or GIT1. Blotting for α-actinin served as a negative control. Lysates were blotted with pan-PKL/GIT1 and paxillin antibodies.
PKL Tyrosine Phosphorylation and Its Interaction with Paxillin Regulates the Polarized Morphology Associated with Directional Cell Migration
To evaluate the role of PKL tyrosine phosphorylation in the regulation of cell polarity during directional cell migration, Golgi reorientation was assessed using the scrape-wound assay (Gomes et al., 2005). GFP-PKL WT exhibited maximal reorientation of the Golgi apparatus toward the wound (p > 0.05) as with the nontransfected and GFP control cells (Figure 5). In contrast, cells expressing GFP-PKL 3YF failed to reorient their Golgi (Figure 5). Previous studies in our laboratory indicated that deletion of the PKL-binding paxillin LD4 motif also inhibits cell polarization and Golgi reorientation during the wound-healing process (West et al., 2001; Brown and Turner, 2004). Because PKL tyrosine phosphorylation plays an important role in regulating the PKL–paxillin interaction (Figure 4), a PKL ΔPBS2 mutant, with a deletion of the paxillin-binding subdomain was expressed to directly address the role of the PKL–paxillin interaction in the regulation of cell polarity. As shown in Figure 5, GFP-PKL ΔPBS2 cells exhibited a significant defect in Golgi reorientation after wounding, similar to cells expressing the GFP-paxillin ΔLD4 mutant (Figure 5). These data suggest that PKL tyrosine phosphorylation resulting in the stimulation of a PKL–paxillin interaction is required for cells to develop and/or maintain the front–rear polarized morphology and thus regulate directional migration.
Figure 5.
PKL phosphorylation and interaction with paxillin regulates Golgi reorientation in migrating cells. (A and B) MEFs expressing GFP-PKL WT, 3YF, ΔPBS2, GFP-paxillin WT, and ΔLD4 were cultured to confluency. Cells were scraped and cultured in complete media for 4 h. Cells were fixed and stained with Alexa633-phalloidin to label F-actin (gray) or anti-Giantin (red) for Golgi reorientation analysis. Cells with Golgi reorientation into the 120° sector facing the wound were scored as being polarized. Results are from three independent experiments, with at least 120 cells counted in each experiment. Error bar, SEM. Student's t test, *p < 0.05 compared with GFP-PKL WT cells (n = 5). Scale bar, 10 μm. Red line represents random orientation (33%).
PKL and Its Tyrosine Phosphorylation Differentially Regulates Rac1 and Cdc42 Activity
PDGF stimulates the activation of the small GTPases Rac1 and Cdc42 to regulate directional cell migration (Ridley et al., 2003). We therefore evaluated the role of PKL in the activation of both Rac1 and Cdc42 in PKL RNAi-treated MEFs by GST-pulldown assays using GST-PAK3 CRIB (for active Rac1) or GST-WASp CRIB (for active Cdc42) fusion proteins. Interestingly, RNAi depletion of PKL resulted in elevated basal activity of Rac1 in serum-starved cells, whereas the addition of PDGF further enhanced Rac1 activation (Figure 6A). In contrast, PKL RNAi inhibited PDGF-mediated Cdc42 activation, compared with control RNAi cells (Figure 6B).
Figure 6.
PKL and PKL tyrosine phosphorylation mediates Rac1 and Cdc42 activities. (A and B) Control RNAi and PKL RNAi cells were cultured in serum-free medium for 4 h. Cells were stimulated with 20 ng/ml PDGF (10 min) followed by lysis. Rac1 activity was determined by GST-PAK CRIB pulldown assay and blotting with Rac1 antibody (A). Cdc42 activity was determined by GST-WASp CRIB pulldown assay and blotting with Cdc42 antibody (B). (C and D) NIH 3T3 were cotransfected with myc-Rac1(C) or myc-Cdc42 (D) and GFP, GFP-PKL WT, or 3YF. Serum-starved and PDGF-stimulated (5, 10, 30, and 60 min) cells were lysed and incubated with GST-PAK CRIB or GST-WASp CRIB followed by Western blotting with myc (9E10) antibody to determine the effect on Rac1 or Cdc42 activation, respectively. Changes in Rac1 (A or C) or Cdc42 (B or D) activities were quantified relative to basal level (denoted as 1) in serum-starved RNAi control cells or GFP control cells (n = 3). Error bar, SEM. Student's t test, *p < 0.05.
To evaluate a potential link between PKL phosphorylation and Rac1/Cdc42 activity, GFP-PKL and myc-Rac1 or myc-Cdc42 were coexpressed in NIH 3T3. After PDGF stimulation, PKL WT expressing cells exhibited a transient increase in Rac1 activity from 5 to 10 min followed by a decrease at later time points. However, the active Rac1 peak was routinely lower than the maximal Rac1 levels in GFP control cells (Figure 6C). In contrast, cells expressing the nonphosphorylatable PKL 3YF mutant demonstrated elevated levels of Rac1 activity even when serum-starved (Figure 6C), whereas PDGF-stimulated Rac1 activity peaked at 10 min and stayed elevated at later time points (30–60 min; Figure 6C). Interestingly, the prolonged Rac1 activity of cells expressing PKL 3YF has also been observed after integrin activation in cells expressing the PKL ΔPBS2 or the paxillin ΔLD4 mutants, as well as in FAK knockdown fibroblasts (West et al., 2001; Tilghman et al., 2005). In contrast, Cdc42 activity, as with the PKL RNAi cells, was suppressed in PKL 3YF-expressing cells in response to PDGF compared with PKL WT (Figure 6D). Together these results identify a role for PKL and its tyrosine phosphorylation in the temporal regulation of Rac1 and Cdc42 activities downstream of PDGF receptor activation and further suggest that the defect in persistent migration and cell polarization of PKL RNAi and PKL 3YF cells is likely due to both disregulation of Rac1-mediated membrane protrusions/ruffling and Cdc42-guided cell polarization of the Golgi and the microtubule cytoskeleton (Cau and Hall, 2005).
PKL and Its Tyrosine Phosphorylation Regulates Erk Activity
Erk/MAPK activation downstream of growth factor and cell adhesion signaling plays a major role during directional cell migration via regulation of both focal adhesion turnover (Webb et al., 2004) and Golgi reorientation (Pullikuth and Catling, 2007). Furthermore, paxillin and GIT1 scaffold Erk signaling to regulate growth factor–stimulated epithelial cell morphogenesis and vascular smooth-muscle cell migration, respectively (Ishibe et al., 2003, 2004; Yin et al., 2005). To evaluate the potential role of PKL in mediating Erk activity, PDGF activation of Erk was examined in PKL RNAi cells. PDGF stimulation induced a transient increase of Erk activity in control cells as measured by the level of phospho-Erk, whereas PKL knockdown significantly suppressed the PDGF-induced phospho-Erk signals (Figure 7A). Because the PKL phosphorylation mutant significantly reduced both PDGF-induced chemotaxis and Golgi reorientation in scrape-wound assays (Figures 3 and 5), we examined the correlation between PKL tyrosine phosphorylation and Erk activity. Fibroblasts cotransfected with GFP, GFP-PKL, or GFP-PKL 3YF and HA-Erk were stimulated with PDGF. Compared with the GFP control, GFP-PKL WT cells demonstrated slightly elevated phospho-Erk levels that were not statistically significant. In contrast, the PKL 3YF mutant reduced PDGF-induced Erk phosphorylation at all time-points (Figure 7B), suggesting that PKL protein and its tyrosine phosphorylation are required for proper Erk activity that in turn facilitates Golgi reorientation (Bisel et al., 2008) as well as directional cell migration (Klemke et al., 1997; Pullikuth and Catling, 2007).
Figure 7.
PKL and PKL tyrosine phosphorylation regulates phospho-Erk signaling. (A) Serum starved control RNAi and PKL RNAi cells were stimulated with 20 ng/ml PDGF (5, 10, 30, and 60 min) followed by lysis. Lysates were blotted with phospho-Erk, pan-Erk, and PKL antibodies. (B) NIH 3T3 were cotransfected with HA-Erk and GFP, GFP-PKL WT, or 3YF. Serum-starved and PDGF-stimulated (5, 10, 30, and 60 min) cells were lysed and immunoprecipitated with anti-HA (12CA5) followed by Western blotting with phospho-Erk and pan Erk antibodies. Arrow indicates the position of the HA-Erk (lower band). The origin of the weaker upper band, also recognized by the Erk antibody in this precipitate is unknown. Lysates were blotted with GFP antibody to confirm the expression of GFP-PKL. Changes in pErk activities were quantified relative to basal level (denoted as 1) in serum-starved RNAi control cells or GFP control cells (n = 3). Error bar, SEM. Student's t test, *p < 0.05.
PKL Tyrosine Phosphorylation Is Required for Polarized βPIX Localization and Proper PAK Activity during Directed Cell Migration
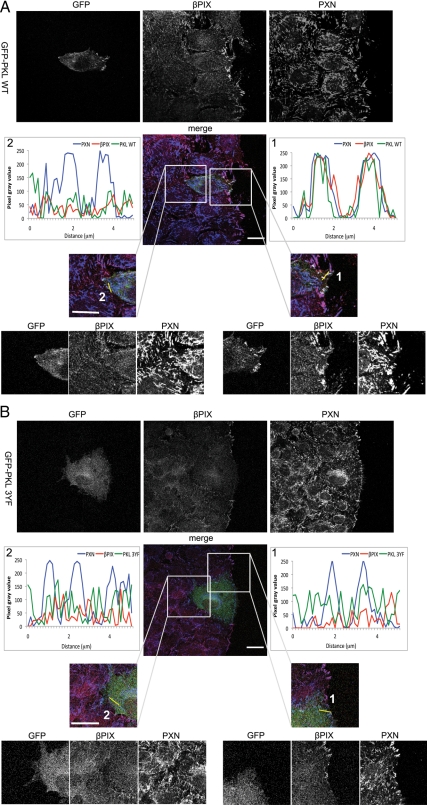
The asymmetric distribution of a βPIX and PAK complex to the leading edge and stimulation of PAK activity has been suggested to play a role in restricting local Rac1 activity to the leading edge in migrating cells to facilitate polarized actin cytoskeleton assembly (Cau and Hall, 2005; ten Klooster et al., 2006). Because the PKL-βPIX-PAK-Nck cassette is recruited by paxillin to focal adhesions in response to integrin engagement during cell spreading (Turner et al., 1999; Brown et al., 2002, 2005), we sought to determine whether PKL also regulates the redistribution βPIX during polarized cell migration. Using the scrape-wound assay, indirect immunofluorescence analysis was performed to examine the localization of GFP-PKL as well as endogenous βPIX and paxillin in leading-edge cells. In contrast to paxillin, which exhibited robust focal adhesion staining throughout the ventral surface, βPIX and GFP-PKL were asymmetrically enriched in the focal adhesions toward the front of the cells at the wound edge (90.0 ± 8.8%; Figure 8A). In contrast, expression of GFP-PKL 3YF, which is unable to bind paxillin and target to focal adhesions (Brown et al., 2005), resulted in a punctate distribution of endogenous βPIX in the cytosol, with substantially reduced localization of βPIX to the focal adhesions at the leading edge (24.1 ± 3.7%), whereas paxillin targeting to adhesions throughout the cell was not affected (Figure 8B, Supplemental Figure S3). Thus PKL tyrosine phosphorylation plays an important role in the polarized recruitment of βPIX to the leading edge of migrating cells.
Figure 8.
PKL tyrosine phosphorylation is required for polarized localization of βPIX to the leading edge. (A and B) NIH 3T3 cells transfected with GFP-PKL WT or 3YF (in green) were fixed and stained with βPIX (in red) and paxillin (in blue) 1 h after wounding. Enrichment of βPIX in focal adhesions toward the front of wound-polarized cells was evaluated in relation to paxillin adhesion staining and GFP-PKL WT (A) or GFP-PKL 3YF distribution (B). Scale bar, 10 μm.
Although the PKL interaction with paxillin facilitates the recruitment of the PKL-βPIX-PAK-Nck complex to adhesions, the role of PKL targeting and its tyrosine phosphorylation in the regulation of PAK serine/threonine kinase activity has not been formally tested. PAK kinase activity, as measured by phosphorylation within the N-terminal inhibitory domain using phospho-S199/204–specific PAK antibody (Cau and Hall, 2005), was transiently activated upon PDGF stimulation in control RNAi cells (Figure 9A, B), as previously reported (Bokoch, 2003). In contrast, PAK activity was disregulated in PKL RNAi cells, demonstrating an elevated basal level and prolonged phosphorylation in response to PDGF stimulation (Figure 9B). To further examine whether PKL tyrosine phosphorylation is involved in PAK modulation, we cotransfected GFP-PAK with PKL WT or the 3YF mutant. Interestingly, expression of the PKL phosphotyrosine mutant resulted in a similar disregulation in PAK activity after PDGF stimulation compared with PKL WT or GFP control cells (Figure 9C). Because of antibody limitations we were unable to determine the subcellular localization of the active PAK in either PDGF-stimulated cells or in the wounded monolayer. Nevertheless, these results indicate that PKL phosphorylation and its recruitment to focal adhesions is required for the temporal regulation of PAK activity.
Figure 9.
PKL and its tyrosine phosphorylation regulate PAK activity. (A and B) Control or PKL RNAi MEFs were stimulated with PDGF and blotted with PAK pS199/204 and pan PAK (N-20) antibodies. PKL RNAi knockdown was confirmed by blotting for PKL. Quantification of phospho-PAK in control and PKL RNAi cells is from three independent experiments. Error bar, SEM. Student's t test, *p < 0.05. (C) NIH 3T3 cells were cotransfected with GFP-PAK1 and GFP, GFP-PKL WT, or 3YF. Serum-starved and PDGF-stimulated cells (5, 10, 30, 60, and 120 min) were lysed and subject to immunoprecipitation by GFP antibody and Western blotting with PAK pS199/204 and GFP antibodies. The change in phospho-PAK levels was quantified relative to basal level (denoted as 1) in serum-starved GFP control cells (n = 2). Error bar, SEM.
DISCUSSION
Recent work in both mouse and worms has indicated an important role for PKL/GIT in migration-dependent physiological events such as development and the immune response (Mazaki et al., 2006; Lucanic and Cheng, 2008). However, the mechanism by which PKL/GIT coordinated these events was not determined. Our analysis of cell migration and morphology in PKL RNAi fibroblasts (Figure 1) indicated that their defective wound healing or chemotaxis toward a PDGF gradient was primarily the result of disregulated membrane protrusion activity, combined with defects in Golgi reorientation toward the wound edge and thus a loss of directional persistence of migration.
FAK and Src kinases play a critical role in coordinating cell morphology and motility upon cell adhesion and growth factor stimulation (Huveneers and Danen, 2009), and cells devoid of FAK or Src kinases are defective in front–rear polarity, Golgi reorientation, and directional cell migration (Klinghoffer et al., 1999; Sieg et al., 2000; Magdalena et al., 2003; Tilghman et al., 2005; Owen et al., 2007). Importantly, we determined that PKL is tyrosine-phosphorylated by Src/FAK in response to PDGF (Figure 2) and that disruption of this signaling axis, either through pharmacologic inhibition of their kinase activity, genetic ablation of Src/FAK, or the introduction of a nonphosphorylatable PKL 3YF mutant, produced comparable defects in cell persistence (Figure 3) and Golgi positioning (Figure 5), therefore indicating PKL as a major downstream effector of FAK/Src in coordinating polarized motility. Our results suggest that both the kinase activity and scaffold function of FAK and Src are involved in PDGF-induced PKL tyrosine phosphorylation. Future studies will be directed toward evaluating if there is a temporal hierarchy in Src- versus FAK-mediated phosphorylation of PKL and whether FAK and Src phosphorylate different PKL tyrosine residues to elicit distinct downstream effects.
PKL is recruited to focal adhesions during cell spreading via an interaction between its PBS2 domain and the LD4 motif of paxillin (Turner et al., 1999) and localization requires both PKL phosphorylation as well as activation of Cdc42/Rac1 (Brown et al., 2005). We have previously reported that expression of a paxillin LD4 mutant promotes random membrane protrusions (West et al., 2001) and prevents Golgi reorientation at the wound edge (Brown and Turner, 2004). Herein we demonstrate that PDGF also stimulates an association between PKL and paxillin (Figure 4) and show that expression of a PKL PBS2 mutant produces similar defects in Golgi repositioning. Thus tyrosine phosphorylation of PKL, downstream of Src/FAK activation, functions as a regulatory signaling axis for the development of front–rear cell polarity and directional cell migration through its interaction with paxillin. The mechanism by which PKL tyrosine phosphorylation contributes to its enhanced paxillin binding remains to be determined. One possibility is that phosphorylation, combined with PAK signaling (Brown et al., 2002) imparts a conformational change, thereby functionally unmasking the PKL PBS2 domain. Alternatively, phosphorylation of PKL enhances interactions with the SH2 domains of several scaffold proteins including Nck and Crk (Brown et al., 2005) that may in turn link PKL to other focal adhesion proteins, including paxillin or p130Cas (Brown and Turner, 2004; Smith et al., 2008), to stabilize PKL localization to adhesions. PAK-dependent phosphorylation within the paxillin LD4 motif at the leading edge may further regulate the PKL–paxillin interaction as previously reported for the related protein GIT1 (Nayal et al., 2006).
It is well established that tight control of the spatiotemporal activity of the small GTPases Cdc42 and Rac1 plays a critical role in regulation of cytoskeletal organization, cell shape change, and polarized cell migration (Hall, 1998; Bokoch, 2003; Ridley et al., 2003; Pankov et al., 2005). Cdc42 is locally activated both at the leading edge (Itoh et al., 2002; Nalbant et al., 2004), where it is required for restricting plasma membrane extension to the leading edge via a PAK-dependent process (Cau and Hall, 2005), and within the trans-Golgi network (Nalbant et al., 2004), where it controls repositioning of the Golgi/centrosome through activation of the Par6/aPKC complex (Nobes and Hall, 1999; Etienne-Manneville and Hall 2001). Temporal and spatial restriction of Rac1 activation to the leading edge (Kurokawa et al., 2004) is driven in part by integrin engagement with the ECM (Huveneers and Danen, 2009) and is necessary for activation of the actin polymerization machinery to drive stable membrane protrusion (Merlot and Firtel, 2003; Wittmann et al., 2003; Pankov et al., 2005). Both FAK/Src and paxillin have multiple connections to the regulation of these signaling pathways through both direct interactions with GEFs and GAPs as well as certain effector proteins (Brown and Turner, 2004; Playford and Schaller, 2004; Huveneers and Danen, 2009). For example, FAK and/or paxillin both associate with the p120RasGAP–p190RhoGAP complex, thereby controlling polarized cell migration by suppressing RhoA and indirectly promoting Rac1 activity at the leading edge (Tsubouchi et al., 2002; Tomar et al., 2009). Paxillin phosphorylation by Src/FAK can also bind a complex containing Crk, ELMO, and the atypical GEF, DOCK 180 complex to further activate Rac1 signaling (Cote and Vuori, 2007).
Paxillin, via interaction with phosphorylated PKL, also facilitates the localization of the Cdc42 and Rac1 GEF βPIX, as well as PAK in nonpolarized cells (Brown et al., 2005). However there are conflicting reports regarding whether the recruitment of the PKL/GIT-βPIX-PAK-Nck complex to adhesions promotes (Nayal et al., 2006) or terminates (Brown and Turner 2002; Nishiya et al., 2005) local Rac1 and PAK signaling. Importantly, βPIX becomes localized to adhesions at the leading edge of polarized fibroblasts, possibly via a Cdc42- and PAK-dependent event (Cau and Hall, 2005). Although the targeting partner for βPIX was unclear from this study, the localized recruitment of βPIX and PAK was found to be necessary for Rac1-dependent actin polymerization and membrane extension at the leading edge (Cau and Hall, 2005). Here we demonstrated that knockdown of PKL, disruption of the paxillin–PKL interaction or impairment of PKL tyrosine phosphorylation results in suppression of PDGF-stimulated Cdc42 activity and disregulation of both Rac1 and PAK activities (Figures 6 and 8). In addition, expression of the PKL 3YF phosphorylation mutant, presumably by functioning as a dominant negative, reduced the localized recruitment of βPIX to paxillin-rich adhesions at the leading edge of cells in the wounded monolayer. Combined with the propensity of PKL 3YF cells to exhibit nonpolarized membrane protrusion activity (Brown et al. et al., 2005; and Yu and Turner, data not shown), these results suggest that PKL recruitment to paxillin is both necessary for the polarized recruitment of βPIX, as well as the tight spatial and temporal control of PAK, Cdc42, and Rac1. They are also consistent with the proposed role for locally active PAK in the recruitment βPIX (Cau and Hall, 2005) to these adhesions as well as the requirement for active Cdc42/Rac1 and PAK in the targeting of the stable PKL-βPIX-PAK complex to paxillin (Brown et al., 2002; Schober et al., 2007; Zhang et al., 2008). In addition to its GEF activity, βPIX may also promote localized membrane protrusion via a direct interaction with active Rac1 (ten Klooster et al., 2006). Furthermore, the specificity of βPIX GEF activity toward Cdc42 versus Rac1 can be regulated through an interaction between the βPIX dimer and the scaffold protein 14-3-3 (Angrand et al., 2006; Chahdi and Sorokin, 2008). Paxillin and PKL/GIT also bind to 14-3-3 proteins via phosphorylation-dependent interactions (Angrand et al., 2006; Deakin et al., 2009). Thus local changes in the phosphorylation status of these proteins may further fine-tune the local Cdc42 and Rac1 signaling via βPIX.
As noted, PKL RNAi or disruption of PKL targeting to adhesions blocked Golgi reorientation toward the wound edge. The redirecting of the Golgi and microtubule organizing center/centrosome is required for directed secretion toward the leading edge to both maintain front–rear polarity and promote persistent migration (Etienne-Manneville, 2008). Our studies implicate PKL and its tyrosine phosphorylation at two levels. First the suppression of Cdc42 activity (Figure 6) likely blocks signaling to a key Cdc42 effector Par6, which through activation of aPKC drives microtubule assembly toward the leading edge (Etienne-Manneville, 2008). Second, growth factor–stimulated Erk activation was also inhibited after PKL knockdown or mutant expression (Figure 7). Active Erk, in addition to being required for focal adhesion turnover via modulation of myosin contractility (Klemke et al., 1997; Webb et al., 2004) and directional cell migration (Klemke et al., 1997; Matsubayashi et al., 2004), is necessary for Golgi condensation and reorientation through phosphorylation of the Golgi protein GRASP65 (Bisel et al., 2008; Yadav et al., 2009). It remains to be determined precisely how PKL mediates Erk activation, but FAK/Src kinases, as well as PAK via Raf activation are required for the spatiotemporal activity of Erk (Schlaepfer et al., 1999; Pulikuth and Catling, 2007). The PKL family member GIT1 interacts with Erk and facilitates its role in the regulation of cell migration (Yin et al., 2005); the ability of PKL to bind Erk has not been tested. FAK is also able to bind directly to PKL/GIT proteins (Zhao et al., 2000; Brown et al., 2005) and thus PKL/GIT1 may scaffold for the Src/FAK-dependent recruitment and activation of Erk at focal adhesions (Fincham et al., 2000). Interestingly, active Erk may subsequently be trafficked to the Golgi network via recycling endosomes (Pulikuth and Catling, 2007) to mediate its polarization, a process potentially regulated via the intrinsic Arf GAP activity of PKL/GIT proteins (Premont et al., 1998, 2000). Further studies will determine precisely how PKL and paxillin coordinate FAK/Src and PAK activity to control Erk activity and function during directed cell migration.
Finally, paxillin or FAK ablation in mice is embryonic lethal (Sieg et al., 2000; Hagel et al., 2002), and recent studies in vivo have shown that deletion of the PDGF receptor in mice or paxillin in Xenopus laevis resulted in impaired cell polarity and directional cell migration (Iioka et al., 2007; Pickett et al., 2008), giving rise to the developmental defect spina bifida and abnormal convergent extension, respectively (Iioka et al., 2007; Prasad and Montell, 2007; Llense and Martin-Blanco, 2008; Pickett et al., 2008). Furthermore, Rho family GTPase signaling, as well as PAK activity, was significantly disrupted in the absence of the PDGF receptor (Pickett et al., 2008). Additionally, a point mutation (S360N) in PKL was linked to human glioblastoma multiforme, a common, invasive and lethal brain tumor (Parsons et al., 2008). The coordination of intracellular signaling events to promote persistent migration is of paramount importance for organism development, homeostasis, and various pathologies. Thus, it will be important to further dissect the potential role of PKL and its binding partners, paxillin and βPIX-PAK, in the progression of these processes.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Turner laboratory for insightful discussion of the data. We also gratefully acknowledge Drs. Richard Cerione, Jon Cooper, Jun-Lin Guan, Rick Horwitz, S. Silvio Gutkind, Nathalie Lamarche-Vane, and Marc Symons for providing reagents and Ron Schmidt and Abby Racette for excellent technical assistance. This work was supported by National Institutes of Health Grants GM 047607 and HL 070244 to C.E.T. and American Heart Association Predoctoral Fellowship (0815776D) to J.A.Y.
Glossary
Abbreviations used:
- FAK
focal adhesion kinase
- PAK
p21-activated kinase
- PIX
PAK-interacting exchange factor
- PKL
paxillin-kinase-linker
- pTyr
tyrosine phosphorylation
- SFK
Src family kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-07-0548) on September 23, 2009.
REFERENCES
- Angrand P. O., et al. Transgenic mouse proteomics identifies new 14-3-3-associated proteins involved in cytoskeletal rearrangements and cell signaling. Mol. Cell Proteomics. 2006;5:2211–2227. doi: 10.1074/mcp.M600147-MCP200. [DOI] [PubMed] [Google Scholar]
- Bisel B., Wang Y., Wei J. H., Xiang Y., Tang D., Miron-Mendoza M., Yoshimura S., Nakamura N., Seemann J. ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65. J. Cell Biol. 2008;182:837–843. doi: 10.1083/jcb.200805045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M. Biology of the p21-activated kinases. Annu. Rev. Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Turner C. E. Paxillin: adapting to change. Physiol. Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Brown M. C., West K. A., Turner C. E. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol. Biol. Cell. 2002;13:1550–1565. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Cary L. A., Jamieson J. S., Cooper J. A., Turner C. E. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol. Biol. Cell. 2005;16:4316–4328. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. T., Andrade J., Radhakrishna H., Donaldson J. G., Cooper J. A., Randazzo P. A. ASAP1, a phospholipid-dependent Arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol. Cell Biol. 1998;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau J., Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- Chahdi A., Sorokin A. Protein kinase A-dependent phosphorylation modulates beta1Pix guanine nucleotide exchange factor activity through 14-3-3beta binding. Mol. Cell. Biol. 2008;28:1679–1687. doi: 10.1128/MCB.00898-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Lemmon C. A., Park D., Romer L. H. FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for β-PIX. Mol. Biol. Cell. 2007;18:253–264. doi: 10.1091/mbc.E06-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J. F., Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–393. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin N. O., Bass M. D., Warwood S., Schoelermann J., Mostafavi-Pour Z., Knight D., Ballestrem C., Humphries M. J. An integrin-alpha4-14-3-3zeta-paxillin ternary complex mediates localised Cdc42 activity and accelerates cell migration. J. Cell Sci. 2009;122:1654–1664. doi: 10.1242/jcs.049130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin N. O., Turner C. E. Paxillin comes of age. J. Cell Sci. 2008;121:2435–2444. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek P., Somogyi K., Jekely G., Beccari S., Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene. 2008;27:6970–6980. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Feng Q., Baird D., Peng X., Wang J., Ly T., Guan J. L., Cerione R. A. Cool-1 functions as an essential regulatory node for EGF receptor- and Src-mediated cell growth. Nat. Cell Biol. 2006;8:945–956. doi: 10.1038/ncb1453. [DOI] [PubMed] [Google Scholar]
- Fincham V. J., James M., Frame M. C., Winder S. J. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S. R., Adelstein M. R., Hansen S. H. GIT2 represses Crk- and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover. EMBO J. 2006;25:1848–1859. doi: 10.1038/sj.emboj.7601092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E. R., Jani S., Gundersen G. G. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Hagel M., George E. L., Kim A., Tamimi R., Opitz S. L., Turner C. E., Imamoto A., Thomas S. M. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol. Cell. Biol. 2002;22:901–915. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hauck C. R., Hsia D. A., Schlaepfer D. D. Focal adhesion kinase facilitates platelet-derived growth factor-BB-stimulated ERK2 activation required for chemotaxis migration of vascular smooth muscle cells. J. Biol. Chem. 2000;275:41092–41099. doi: 10.1074/jbc.M005450200. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Huveneers S., Danen E. H. Adhesion signaling-crosstalk between integrins, Src and Rho. J. Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- Iioka H., Iemura S., Natsume T., Kinoshita N. Wnt signalling regulates paxillin ubiquitination essential for mesodermal cell motility. Nat. Cell Biol. 2007;9:813–821. doi: 10.1038/ncb1607. [DOI] [PubMed] [Google Scholar]
- Ishibe S., Joly D., Zhu X., Cantley L. G. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol. Cell. 2003;12:1275–1285. doi: 10.1016/s1097-2765(03)00406-4. [DOI] [PubMed] [Google Scholar]
- Ishibe S., Joly D., Liu Z. X., Cantley L. G. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol. Cell. 2004;16:257–267. doi: 10.1016/j.molcel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Itoh R. E., Kurokawa K., Ohba Y., Yoshizaki H., Mochizuki N., Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 2002;22:6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- Klemke R. L., Cai S., Giannini A. L., Gallagher P. J., de Lanerolle P., Cheresh D. A. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinghoffer R. A., Sachsenmaier C., Cooper J. A., Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S. V., Gish G., van der Geer P., Henkemeyer M., Pawson T. Role of p120 Ras-GAP in directed cell movement. J. Cell Biol. 2000;149:457–470. doi: 10.1083/jcb.149.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K., Itoh R. E., Yoshizaki H., Nakamura Y. O., Matsuda M. Coactivation of Rac1 and Cdc42 at lamellipodia and membrane ruffles induced by epidermal growth factor. Mol. Biol. Cell. 2004;15:1003–1010. doi: 10.1091/mbc.E03-08-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLonde D. P., Grubinger M., Lamarche-Vane N., Turner C. E. CdGAP associates with actopaxin to regulate integrin-dependent changes in cell morphology and motility. Curr. Biol. 2006;16:1375–1385. doi: 10.1016/j.cub.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D. A., Horwitz A. F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Llense F., Martin-Blanco E. JNK signaling controls border cell cluster integrity and collective cell migration. Curr. Biol. 2008;18:538–544. doi: 10.1016/j.cub.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Lucanic M., Cheng H. A Rac/Cdc42–independent GIT/PIX/PAK signaling pathway mediates cell migration in C. elegans. PLoS Genet. 2008;4:e1000269. doi: 10.1371/journal.pgen.1000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdalena J., Millard T. H., Machesky L. M. Microtubule involvement in NIH 3T3 Golgi and MTOC polarity establishment. J. Cell Sci. 2003;116:743–756. doi: 10.1242/jcs.00288. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y., Ebisuya M., Honjoh S., Nishida E. ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr. Biol. 2004;14:731–735. doi: 10.1016/j.cub.2004.03.060. [DOI] [PubMed] [Google Scholar]
- Mazaki Y., et al. Neutrophil direction sensing and superoxide production linked by the GTPase-activating protein GIT2. Nat. Immunol. 2006;7:724–731. doi: 10.1038/ni1349. [DOI] [PubMed] [Google Scholar]
- Merlot S., Firtel R. A. Leading the way: Directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J. Cell Sci. 2003;116:3471–3478. doi: 10.1242/jcs.00703. [DOI] [PubMed] [Google Scholar]
- Moissoglu K., Gelman I. H. V-Src rescues actin-based cytoskeletal architecture and cell motility and induces enhanced anchorage independence during oncogenic transformation of focal adhesion kinase-null fibroblasts. J. Biol. Chem. 2003;278:47946–47959. doi: 10.1074/jbc.M302720200. [DOI] [PubMed] [Google Scholar]
- Nagel M., Tahinci E., Symes K., Winklbauer R. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 2004;131:2727–2736. doi: 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- Nalbant P., Hodgson L., Kraynov V., Toutchkine A., Hahn K. M. Activation of endogenous Cdc42 visualized in living cells. Science. 2004;305:1615–1619. doi: 10.1126/science.1100367. [DOI] [PubMed] [Google Scholar]
- Nayal A., Webb D. J., Brown C. M., Schaefer E. M., Vicente-Manzanares M., Horwitz A. R. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 2006;173:587–589. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiya N., Kiosses W. B., Han J., Ginsberg M. H. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat. Cell Biol. 2005;7:343–352. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen K. A., Pixley F. J., Thomas K. S., Vicente-Manzanares M., Ray B. J., Horwitz A. F., Parsons J. T., Beggs H. E., Stanley E. R., Bouton A. H. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J. Cell Biol. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R., Endo Y., Even-Ram S., Araki M., Clark K., Cukierman E., Matsumoto K., Yamada K. M. A Rac switch regulates random versus directionally persistent cell migration. J. Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. W., et al. An integrated genomic analysis of human glioblastoma mutiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett E. A., Olsen G. S., Tallquist M. D. Disruption of PDGFRα-initiated PI3K activation and migration of somite derivatives leads to spina bifida. Development. 2008;135:589–598. doi: 10.1242/dev.013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek A., Vassilev V. S., de Diesbach P., Tyteca D., Mettlen M., Courtoy P. J. Constitutive diffuse activation of phosphoinositide 3-kinase at the plasma membrane by v-Src suppresses the chemotactic response to PDGF by abrogating the polarity of PDGF receptor signaling. Exp. Cell Res. 2007;313:1090–1105. doi: 10.1016/j.yexcr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Playford M. P., Schaller M. D. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- Prasad M., Montell D. J. Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev. Cell. 2007;12:997–1005. doi: 10.1016/j.devcel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Premont R. T., Claing A., Vitale N., Freeman J. L., Pitcher J. A., Patton W. A., Moss J., Vaughan M., Lefkowitz R. J. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. USA. 1998;24:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont R. T., Claing A., Vitale N., Perry S. J., Lefkowitz R. J. The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing. J. Biol. Chem. 2000;275:22373–22380. doi: 10.1074/jbc.275.29.22373. [DOI] [PubMed] [Google Scholar]
- Pullikuth A. K., Catling A. D. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cell Signal. 2007;19:1621–1632. doi: 10.1016/j.cellsig.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rossman K. L., Der C. J., Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Broome M. A., Hunter T. Fibronectin-stimulated signaling from a Focal-Adhesion-Kinase-c-Src complex: involvement of the Grb2, p130Cas, and Nck adaptor proteins. Mol. Cell. Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D. D., Hauck C. R., Sieg D. J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Schober M., Raghavan S., Nikolova M., Polak L., Pasolli H. A., Beggs H. E., Reichardt L. F., Fuchs E. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 2007;176:667–680. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K., Vincent F. Divergent roles of c-Src in controlling platelet-derived growth factor-dependent signaling in fibroblasts. Mol. Biol. Cell. 2005;16:5418–5432. doi: 10.1091/mbc.E05-03-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg D. J., Hauck C. R., Ilic D., Klingbeil C. K., Schaefer E., Damsky C. H., Schlaepfer D. D. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Marra P., Marshall C. J. uPAR promotes formation of the p130Cas-Crk complex to activate Rac through DOCK180. J. Cell Biol. 2008;182:777–790. doi: 10.1083/jcb.200712050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Klooster J. P., Jaffer Z. M., Chernoff J., Hordijk P. L. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J. Cell Biol. 2006;172:759–769. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. M., Brugge J. S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tilghman R. W., Slack-Davis J. K., Sergina N., Martin K. H., Iwanicki M., Hershey E. D., Beggs H. E., Reichardt L. F., Parsons J. T. Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells. J. Cell Sci. 2005;118:2613–2623. doi: 10.1242/jcs.02380. [DOI] [PubMed] [Google Scholar]
- Tomar A., Lim S. T., Lim Y., Schlaepfer D. D. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J. Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi A., Sakakura J., Yagi R., Mazaki Y., Schaefer E., Yano H., Sabe H. Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration. J. Cell Biol. 2002;159:673–683. doi: 10.1083/jcb.200202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C. E., Brown M. C., Perrotta J. A., Riedy M. C., Nikolopoulos S. N., McDonald A. R., Bagrodia S., Thomas S., Leventhal P. S. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J. Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M., Webb D. J., Horwitz A. F. Cell migration at a glance. J. Cell Sci. 2005;118:4917–4919. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- West K. A., Zhang H., Brown M. C., Nikolopoulos S. N., Riedy M. C., Horwitz A. F., Turner C. E. The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL) J. Cell Biol. 2001;154:161–176. doi: 10.1083/jcb.200101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T., Bokoch G. M., Waterman-Storer C. M. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J. Cell Biol. 2003;161:845–851. doi: 10.1083/jcb.200303082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W., Faria C., Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J. Cell Biol. 2006;173:405–416. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Puri S., Linstedt A. D. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol. Biol. Cell. 2009;20:1728–1736. doi: 10.1091/mbc.E08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G., Zheng Q., Yan C., Berk B. C. GIT1 is a scaffold for ERK1/2 activation in focal adhesions. J. Biol. Chem. 2005;280:27705–27712. doi: 10.1074/jbc.M502271200. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R., Itzkovitz S., Ma'ayan A., Iyengar R., Geiger B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. M., Simmerman J. A., Guibao C. D., Zheng J. J. GIT1 paxillin-binding domain is a four-helix bundle, and it binds to both paxillin LD2 and LD4 motifs. J. Biol. Chem. 2008;283:18685–18693. doi: 10.1074/jbc.M801274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. S., Manser E., Loo T. H., Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell Biol. 2000;20:6354–6363. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.