Abstract
We have previously shown that Greatwall kinase (Gwl) is required for M phase entry and maintenance in Xenopus egg extracts. Here, we demonstrate that Gwl plays a crucial role in a novel biochemical pathway that inactivates, specifically during M phase, “antimitotic” phosphatases directed against phosphorylations catalyzed by cyclin-dependent kinases (CDKs). A major component of this phosphatase activity is heterotrimeric PP2A containing the B55δ regulatory subunit. Gwl is activated during M phase by Cdk1/cyclin B (MPF), but once activated, Gwl promotes PP2A/B55δ inhibition with no further requirement for MPF. In the absence of Gwl, PP2A/B55δ remains active even when MPF levels are high. The removal of PP2A/B55δ corrects the inability of Gwl-depleted extracts to enter M phase. These findings support the hypothesis that M phase requires not only high levels of MPF function, but also the suppression, through a Gwl-dependent mechanism, of phosphatase(s) that would otherwise remove MPF-driven phosphorylations.
INTRODUCTION
The irreversible commitment to M phase is associated with the explosive activation of the key mitotic driver, Cdk1/cyclin B (M phase–promoting factor [MPF]; reviewed in Perry and Kornbluth, 2007). As a result, hundreds of proteins become phosphorylated during mitosis at the Ser-Pro or Thr-Pro motifs (hereafter S/TP sites, or CDK phosphosites) recognized by MPF and other cyclin-dependent kinases (CDKs; Dephoure et al., 2008). Because these phosphorylations are maintained during M phase but must be removed when cells exit mitosis, it has often been proposed that the phosphatase(s) targeting these sites must be turned off specifically during M phase. Failure to shut off such an “antimitotic” phosphatase would block the G2/M transition or prevent M phase maintenance by prematurely reversing the phosphorylations MPF adds to its substrates. One indication that such phosphatase inactivation might exist is the well-known effect of the phosphatase inhibitor okadaic acid (OA) in inducing precocious M phase in Xenopus oocytes and interphase extracts (e.g., Goris et al., 1989; Margolis et al., 2006). Additional support for this idea emerged from experiments in which the MPF regulator Cdc25 was labeled with 32P by in vitro CDK phosphorylation and then added to Xenopus extracts. Phosphates were removed from labeled Cdc25 more rapidly in interphase extracts than in cytostatic factor (CSF) extracts (derived from mature eggs in metaphase of meiosis II; Clarke et al., 1993; Lee et al., 1994; Margolis et al., 2006).
More recent evidence that an OA-sensitive phosphatase directed against CDK-driven phosphorylations is inhibited during M phase has emerged from analysis of the exit from meiosis II M phase that occurs when Ca2+ is added to CSF extracts to mimic fertilization. Ca2+ induces two waves of phosphatase activity directed against a variety of CDK phosphosites (Mochida and Hunt, 2007). The first wave involves the calcium-activated phosphatase calcineurin (see also Nishiyama et al., 2007). Of particular interest here, the second wave of dephosphorylation involves a phosphatase activity that, unlike calcineurin, is OA-sensitive. This second phosphatase activity is turned off during the next M phase (i.e., the first mitotic embryonic division) and is then turned on again after this mitosis. In contrast, calcineurin is not involved in mitosis; its role instead is specific for the release of CSF-arrested extracts from meiotic M phase.
We have found a novel cell cycle kinase called Greatwall (Gwl; also known as MAST-L), whose properties suggested that it might mediate the down-regulation of an antimitotic phosphatase, perhaps the second wave, OA-sensitive phosphatase just described. Gwl was originally identified in Drosophila; the major phenotype associated with null gwl mutations is cell cycle delay/arrest at the G2-to-M transition (Yu et al., 2004). We subsequently established that immunodepletion of the Gwl ortholog from Xenopus “cycling” egg extracts similarly blocks M phase entry. Gwl itself is active only during M phase, due largely to its phosphorylation at several sites by MPF. Addition of Gwl preactivated with these phosphorylations accelerates the G2-to-M transition in cycling extracts. Removal of Gwl from CSF extracts leads to an unusual cell cycle state we call “pseudomitotic exit” associated with the loss of MPF function. In contrast with normal M phase exit, cyclins remain undegraded after Gwl depletion; however, the Cdk1 kinase component of MPF is inactivated by inhibitory phosphorylations at Thr14 and Tyr15 (Yu et al., 2006; Zhao et al., 2008). Gwl thus influences the well-known autoregulatory loop governing the inhibitory phosphorylations of Cdk1, which are added by Myt1/Wee1 kinases and removed by Cdc25 phosphatase (Perry and Kornbluth, 2007).
The simple hypothesis that Gwl's major role is to help activate MPF as part of this autoregulatory loop cannot, however, explain several observations. For example, we found that the cell cycle effects of depleting Gwl or adding activated Gwl are substantially independent of known MPF regulators such as the kinases Plk1, MAPK, and Myt1/Wee1. Another unexpected finding was the ability of activated Gwl to promote certain phosphorylations of Cdc25 even when MPF activity was undetectable (Zhao et al., 2008). We thus began to consider the alternative hypothesis that Gwl's critical role is to block the action of a phosphatase directed against CDK phosphosites. If such a phosphatase were not inhibited, it would immediately remove phosphorylations added by MPF to many substrate proteins, including components of the autoregulatory loop. The loss of Gwl would thus eventually block or even reverse M phase, because MPF could not remain active when the autoregulatory loop is turned down, and also because MPF's downstream targets would be rapidly dephosphorylated. Among several findings in support of this hypothesis, addition of the phosphatase inhibitor OA overcomes the inability of Gwl-depleted interphase extracts to enter M phase (Zhao et al., 2008). We reasoned that Gwl would no longer be required if the antimitotic phosphatase it regulates was already turned off by OA.
In this article, we show that Gwl is a key negative regulator of PP2A associated with the B55δ regulatory subunit, which has very recently been shown by Mochida et al. (2009) to be major component of the second wave of OA-sensitive phosphatases turned on when Ca2+ is added to CSF extracts. Once Gwl is activated during the G2-to-M transition, its influence on PP2A/B55δ is independent of MPF. Defects in the ability of Gwl-depleted cycling extracts to enter M phase are corrected by the removal of PP2A/B55δ. Our results imply that Gwl is a critical mediator of a novel pathway leading to the inhibition of one or more phosphatases that can dephosphorylate CDK sites, and that this pathway plays a crucial role in M phase entry and maintenance.
MATERIALS AND METHODS
The preparation of CSF and cycling extracts from Xenopus eggs, the immunodepletion of these extracts with antibody against Gwl, the preparation of kinase dead and active wild-type Gwl as well as Cdk1, Cdk1-AF, and cyclin B1 from recombinant baculovirus constructs in Sf9 cells, and assays for histone H1 kinase activity have all been previously described (Yu et al., 2006; Zhao et al., 2008). The preparation of 32P-labeled CDK phosphosites and the assay for the dephosphorylation of these substrates in Xenopus extracts was according to Mochida and Hunt (2007).
Antibodies not previously described include: guinea pig antibodies against the catalytic C and B55α subunits of Xenopus PP2A (Maton et al., 2005; kindly provided by O. Haccard and C. Jessus, Université Pierre et Marie Curie, Paris, France), mouse mAb against the catalytic subunit of PP1 (BD Transduction Laboratories, San Jose, CA; catalogue no. 610373), rabbit antibody against phospho-Ser CDK substrates (Cell Signaling Technology, Beverly, MA; catalogue no. 2324), and mouse mAb 6F9 against the PP2A structural A subunit (Kremmer et al., 1997; Covance, Emeryville, CA; catalogue no. MRT-204R).
Rabbit antibody against the Xenopus B55δ subunit of PP2A and reconstituted, recombinant PP2A made from Xenopus A and C subunits and the rat B55δ subunit are described elsewhere (Mochida et al., 2009). As characterized in that report, the anti-B55δ antibody does not remove PP2A regulatory subunits of the B′ or B″ classes from Xenopus egg extracts.
RESULTS
Gwl Regulates an OA-sensitive Phosphatase Directed Against CDK Phosphosites
To test for a role of Gwl in phosphatase regulation, we assayed phosphatase activity in extracts immunodepleted for Gwl, using model substrates in which ∼25 amino acid peptides, each containing a single CDK phosphosite, were fused to a maltose-binding protein (MBP) tag. The fusion polypeptides were labeled by incubation with Cdk2-cyclin A in the presence of radioactive [γ-32P]ATP. Purified substrates were then added to egg extracts, and phosphatase activity was monitored by the release of radioactive orthophosphate.
As anticipated from a previous study using these same substrates (Mochida and Hunt, 2007), both untreated and mock-depleted control CSF (M phase) extracts displayed no measurable phosphatase activity against any tested peptide (with a single exception noted in the legend to Figure 1). In contrast, Gwl immunodepletion resulted in pseudomitotic exit and the immediate induction of phosphatase activity directed against four of the seven CDK phosphosites tested, although the efficiency of the dephosphorylation reaction varied with the substrate (Figure 1). The induced levels of phosphatase against the best substrates were routinely more than 20-fold higher than those in the controls, and the phosphatase activity was completely sensitive to the inhibitor OA. Because the phosphatase induced by Gwl depletion and the OA-sensitive phosphatase induced when CSF extracts are treated with Ca2+ share similar specificities against the same panel of substrates and similar sensitivities to various inhibitors (see below and Mochida and Hunt, 2007), these appear to be the same enzyme.
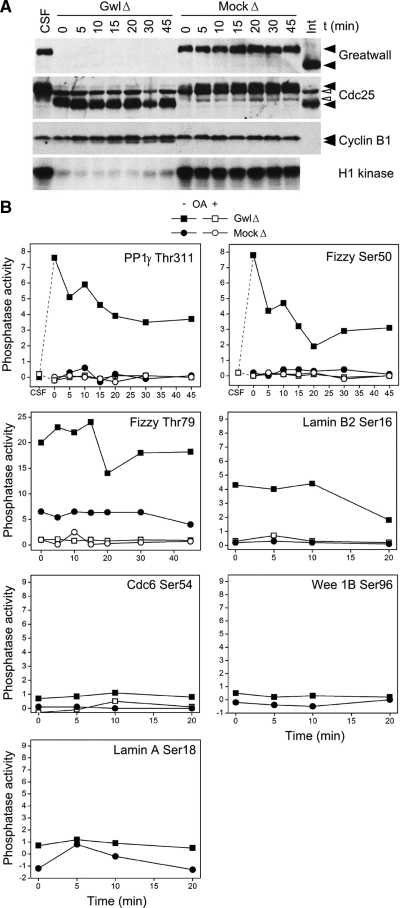
Figure 1.
Gwl depletion from CSF extracts induces OA-sensitive phosphatase(s) directed against CDK phosphosites. (A) Depletion of Gwl from CSF extracts causes pseudomitotic exit. Immunodepletion was performed as described (Yu et al., 2006) by incubating protein A beads coated with affinity-purified anti-Gwl for 1 h at 4°C and then removing the beads by centrifugation. The supernatant was then incubated at 22°C starting at t = 0. Mock-depleted (MockΔ) control extracts were treated with protein A beads alone. MockΔ and CSF extracts remain in M phase as demonstrated by M phase-specific phosphorylations of Gwl and Cdc25, as well as high histone H1 kinase activity (measuring CDK function). In contrast, Gwl-depleted (GwlΔ) extracts have low H1 kinase, and Cdc25 migrates at its interphase (Int) position. During this pseudomitotic exit, cyclin B1 remains undegraded, whereas inhibitory phosphorylations accumulate on Thr14 and Tyr15 of Cdk1 (Yu et al., 2006; see also Figure 2 below). The white arrowheads point to nonspecific background bands. (B) Induction of anti-CDK phosphatase activity after Gwl depletion. Three-microliter aliquots of the extracts shown in A were assayed for phosphatase activity as described (Mochida and Hunt, 2007), with or without the addition of 2.5 μM okadaic acid (OA). The substrates for this assay were MBP fusion proteins labeled in vitro with [γ-32P]ATP by Cdk2/cyclin A; the fusion proteins contained ∼25 residue regions surrounding known CDK targets (for details, see Mochida and Hunt, 2007). The y-axes of the graphs show the percentage of radioactive phosphate released from the input substrate. Dephosphorylations of substrates containing the sites Thr311 of the γ isoform of PP1, Ser50 and Thr79 of Fizzy/Cdc20, and Ser16 of Lamin B2 are strongly induced by Gwl depletion and are OA-sensitive. As previously noted (Mochida and Hunt, 2007), the Fizzy Thr79 phosphosite is dephosphorylated at a low rate during M phase (as seen in mock-depleted extracts), whereas dephosphorylation of the other three substrates is undetectable during M phase. The decay over time in the phosphatase activity of GwlΔ likely reflects the slow reacquisition of the extract's M phase characteristics due to the continued synthesis of Gwl and cyclins from endogenous mRNAs (Yu et al., 2006). The other assayed substrates shown in the figure (Ser54 of Cdc6, Ser96 of Wee1B, and Ser18 of Lamin A) are not obviously targeted by the phosphatase induced by Gwl depletion.
The effects of the immunodepletion seen in Figure 1 are specific to Gwl removal, because phosphatase induction is immediately reversed when these extracts are supplemented with active wild-type, but not kinase-dead, Gwl protein (Figure 2). Both of these Gwl proteins were expressed from baculovirus constructs and purified from infected insect cells treated with OA, so as to allow Gwl to accumulate MPF-driven phosphorylations needed for its activity (Yu et al., 2006; Zhao et al., 2008).
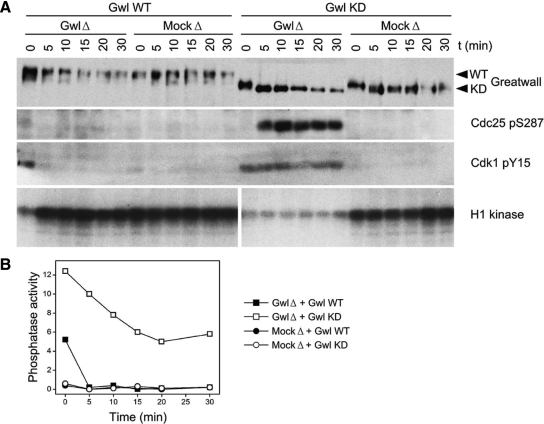
Figure 2.
Rescue of Gwl depletion from CSF extracts with exogenous Gwl protein. (A) Pseudomitotic exit caused by Gwl depletion (GwlΔ) of CSF extracts can be reversed by the addition of active wild-type (WT) but not kinase-dead (KD) Gwl protein, as judged by the interphase-specific phosphorylations of Cdc25 Ser287 (Stanford and Ruderman, 2005) and Cdk1 Tyr15 (Yu et al., 2006; Zhao et al., 2008), and the M phase-specific phosphorylation of histone H1 by CDKs. Mock-depleted (MockΔ) extracts remain in M phase regardless of the nature of the exogenously added protein. The exogenous proteins were purified from OA-treated Sf9 insect cells expressing baculovirus constructs (Zhao et al., 2008), and were added in threefold excess with respect to endogenous Gwl. Gwl proteins were added immediately after depletion at 4°C (t = 0), and the samples were then incubated at 22°C. (B) Phosphatase assays of the same samples shown in A, using the substrate containing the Thr311 PP1γ phosphosite. Phosphatase activity was consistently very low during M phase, but elevated during interphase (that is, in the GwlΔ samples supplemented with Gwl KD). The intermediate level of phosphatase activity observed in the GwlΔ sample supplemented with Gwl WT at t = 0 suggests that the exogenous kinase has not yet restored the extracts to M phase, in agreement with the extent of Cdc25 Ser287 and Cdk1 Tyr15 phosphorylations seen in A at t = 0.
Figure 3 provides additional evidence that Gwl helps turn off a phosphatase directed against CDK phosphosites (that is, an “anti-CDK phosphatase”). The addition of active wild-type (but not kinase-dead) Gwl to cycling Xenopus extracts at interphase causes both rapid inhibition of the anti-CDK phosphatase activity and the precocious entry of the extracts into M phase.
Figure 3.
Gwl addition to interphase cycling extracts inhibits anti-CDK phosphatase activity. (A) Cycling extracts at interphase were prepared as previously described (Yu et al., 2006; Zhao et al., 2008). At t = 0, the extracts were supplemented with active wild-type (Gwl WT) or kinase-dead Gwl (Gwl KD) and then incubated at 22°C. The series at the left is a control cycling extract without added Gwl. The exogenous proteins (tagged with the immunoglobulin Z domain, whose interactions with secondary antibodies account for ∼50% of the total signal on Western blots) were purified from OA-treated Sf9 insect cells expressing baculovirus constructs and were added in threefold excess with respect to endogenous (endo) Gwl, because Gwl WT prepared in this way has only about one-third the specific activity of the enzyme in M phase extracts (Zhao et al., 2008). Immunoblots were analyzed for Gwl, phosphorylation of Ser287 of Cdc25 (a marker for interphase (Int); Stanford and Ruderman, 2005), and cyclin B1. Gwl WT acquires more phosphorylations (including autophosphorylations) than Gwl KD (right and middle, respectively) in the OA-treated insect cells and in M phase Xenopus egg extracts; in the control, endo Gwl migrates during M phase roughly at the position of Gwl KD (arrowhead). Autoradiographs below show histone H1 kinase activity in the same samples. Addition of WT, but not KD, Gwl causes immediate M phase entry. (B) Phosphatase activity of the samples shown in A, measured using the PP1γ Thr311 substrate. Phosphatase levels are consistently greatly reduced during mitosis in the control and Gwl KD samples; this is also true of the precocious M phase caused by Gwl WT. Phosphatase levels during the first 10 min of incubation with Gwl WT are slightly elevated over M phase levels, probably because the exogenous Gwl and the interphase anti-CDK phosphatase compete to inactivate each other.
Gwl Does Not Control the Calcium-dependent Enzymes Calcineurin or CaMKII
The release from metaphase II arrest upon fertilization is normally triggered by a transient increase in intracellular Ca2+. The calcium spike turns on both the phosphatase calcineurin (see above) and Ca2+/calmodulin activated-kinase (CaMKII). Through a pathway involving the protein Erp1, CaMKII activation eventually leads to the destruction of cyclins and thus MPF inactivation (Liu and Maller, 2005; Rauh et al., 2005). One potential explanation for the results in Figure 1 is that Gwl depletion from CSF extracts simply recapitulates these Ca2+-triggered events. However, this is clearly not the case. Gwl depletion does not lead to cyclin destruction (Yu et al., 2006; Figure 1), so the loss of Gwl does not activate CaMKII. In addition, the calcineurin inhibitor cyclosporin A does not block the ability of Gwl depletion to cause pseudo M phase exit or to induce phosphatase against CDK phosphosites (Supplemental Figure S1). Moreover, all of the phosphatase activity induced by Gwl depletion (even against the Fizzy (Fzy) Ser50 site known to be targeted by calcineurin; Mochida and Hunt, 2007) is OA-sensitive (Figure 1), yet calcineurin is not itself inactivated by OA. These last two points indicate that Gwl depletion does not activate calcineurin.
Because Gwl itself is dephosphorylated and inactivated when CSF extracts exit metaphase II, we were also interested in understanding how Gwl is turned off during this process. One interesting possibility was that calcineurin or CaMKII might contribute to Gwl inactivation. To explore this idea, we tracked Gwl on Western blots during the first few minutes after Ca2+ addition to CSF extracts, the time window during which calcineurin and CaMKII are both activated (Mochida and Hunt, 2007; Nishiyama et al., 2007). Immediately upon Ca2+ addition, we observed a minor but reproducible change in Gwl's electrophoretic mobility that is consistent with the loss of one or more activating phosphates; complete Gwl dephosphorylation was achieved only later when the extracts had clearly exited M phase (Supplemental Figure S2, A and B). However, the addition of cyclosporin A did not prevent Gwl dephosphorylation or activation of the OA-sensitive phosphatase (Supplemental Figure S2, A and C). In addition, a CaMKII inhibitor blocked Gwl dephosphorylation (Supplemental Figure S2, B and D) even though this inhibitor has neither direct nor indirect effects on calcineurin (Nishiyama et al., 2007). These results in total indicate that calcineurin may partially dephosphorylate and inactivate Gwl upon CSF exit, but complete Gwl dephosphorylation and inactivation can nonetheless be achieved in the absence of calcineurin function when MPF is turned off through the CaMKII/Erp1/cyclin degradation pathway.
Once Activated, Gwl's Role in Phosphatase Inhibition Is Independent of MPF
It could be argued that the observed effects of Gwl in regulating a phosphatase directed against CDK phosphosites are simply an indirect reflection of cell cycle status. Gwl could be needed only to keep MPF activity high during M phase, and the phosphatase is turned off when MPF is turned on. The results of several experiments instead strongly argue the opposite: that once Gwl is activated, its suppression of the OA-sensitive phosphatase does not require MPF.
First, we investigated the events occurring during Gwl immunodepletion from CSF extracts. Our normal immunodepletion protocol involves incubating the extract for 60 min at 4°C with beads coupled with anti-Gwl antibodies; here, we interrupted the incubation at intermediate times, removed the beads with associated Gwl by centrifugation, and then examined the extracts at each time point by Western blotting and enzyme assays (Figure 4). The majority of Gwl was already removed after the first 10 min of incubation. Of particular interest, the phosphatase activity in the supernatant was already substantially induced at this time, even though H1 kinase activity (a measure of CDK function) remained high. Thus, Gwl depletion induces phosphatase activity before the loss of MPF activity.
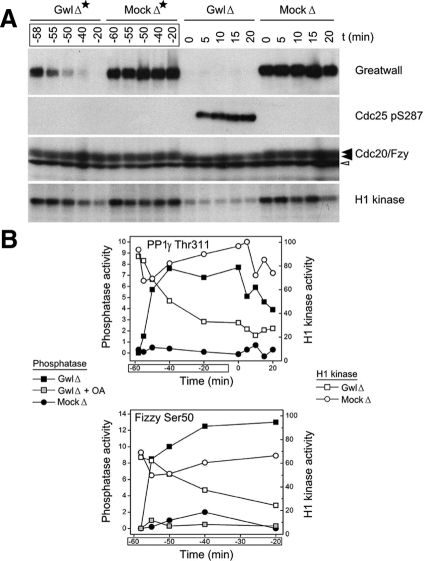
Figure 4.
When Gwl is depleted from CSF extracts, phosphatase activity is induced before MPF activity is lost. (A) Protein A beads coated with purified anti-Gwl antibodies, or control protein A beads, were added at t = −60 min to CSF extracts. To investigate the state of the extracts during the course of the immunodepletion, aliquots were taken at the indicated times (GwlΔ* and MockΔ*). At t = 0, extracts that had been immunodepleted for 1 h at 4°C were then incubated at 22°C and processed as in Figure 1 (i.e., GwlΔ* and GwlΔ indicate successive examinations of the same sample, the former during the immunodepletion at 4°C and the latter after transfer of the depleted samples to 22°C). (B) The samples in A were also analyzed for phosphatase activity against the CDK phosphosites at Thr311 of PP1γ (top) and Ser50 of Cdc20/Fizzy (bottom). The results are plotted on the same graph with quantitative data from phosphorimager scans of the H1 kinase assays shown in A. Note that the GwlΔ* samples from t = −55 min and t = −50 min reveal the induction of considerable phosphatase activity even though H1 kinase (i.e., CDK) activity remains at high, M phase-like levels similar to those in the mock depletion.
Second, we supplemented CSF extracts with constitutively active MPF (cyclin B plus Cdk1 containing T14A and Y15F mutations preventing its inhibitory phosphorylation) before Gwl immunodepletion (Figure 5). As measured by several mitotic markers, including elevated H1 kinase and the lack of interphase-specific phosphorylations on Cdc25, MPF levels remained sufficiently high so as to keep the extracts in a state with many characteristics of M phase. However, the anti-CDK phosphatase was activated to the same degree seen in control Gwl-depleted extracts (without added MPF) that exit M phase. In this experiment, therefore, removal of Gwl promoted phosphatase activation even though MPF activity remained high.
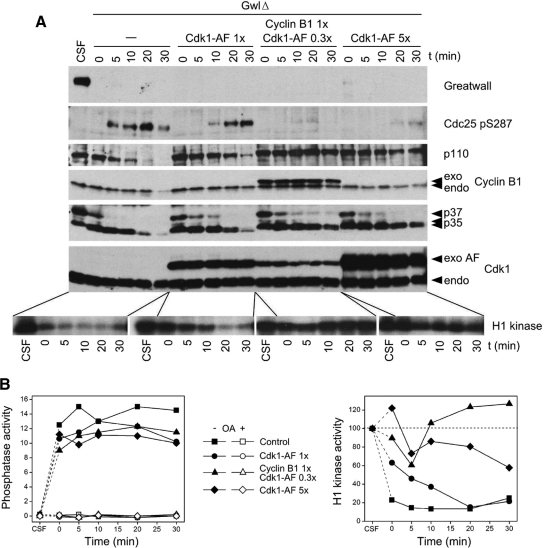
Figure 5.
Gwl depletion induces anti-CDK phosphatase activity even in the presence of constitutively active MPF. (A) CSF extracts were immunodepleted for Gwl as in Figure 1. At t = 0, the indicated proteins were added, and the extracts incubated at 22°C. The added proteins were synthesized from baculovirus constructs in Sf9 insect tissue culture cells treated with OA (Zhao et al., 2008). Cdk1-AF refers to constitutively active Cdk1 kinase with S14A and Y15F mutations that block inactivating phosphorylations at these sites; 0.3×, 1×, and 5×, to levels of exogenous protein compared with those of the corresponding endogenous protein. A mixture of cyclin B1 and Cdk1-AF is more effective than Cdk1-AF alone in maintaining M phase characteristics (such as the lack of phosphorylation of Ser287 on Cdc25 and high histone H1 kinase activity), likely because endogenous cyclin B is already bound to CDKs and exchanges only very slowly with exogenous Cdk1-AF (Kobayashi et al., 1994). p35, p37, and p110 indicate unknown proteins of these molecular weights revealed with an antibody directed against phospho-serine within (K/R)pSPX(K/R) motifs (Cell Signaling Technology; no. 2324). Constitutively active MPF or Cdk1 help retain M phase specific phosphorylations on p35 and p110, but not on p37, demonstrating differential sensitivities of these pS/TP phosphosites to variations in the ratio of CDKs and the anti-CDK phosphatase(s) that are induced when Gwl is depleted. (B) Graphs of the phosphatase activity (left; directed against Thr311 of PP1γ) and H1 kinase activity (right) of the samples shown in A. Note that in the samples supplemented with Cdk1-AF and cyclin B1 (i.e., constitutively active MPF) that Gwl depletion induces phosphatase activity even though H1 kinase levels are often even higher than those in CSF extracts.
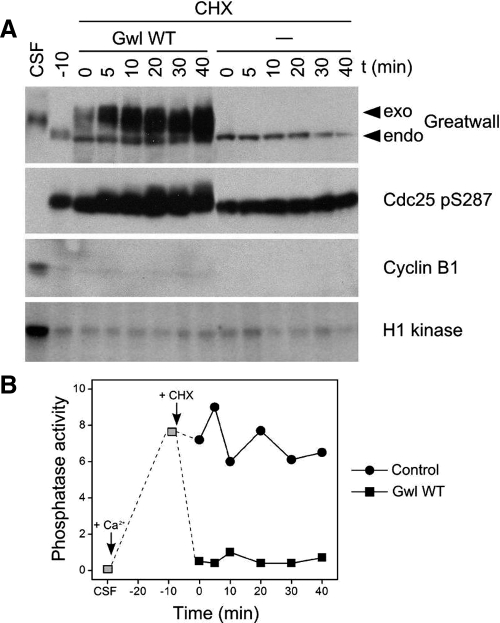
Third, we added Gwl to extracts devoid of mitotic cyclins and thus MPF; these were Ca2+-treated extracts that had just exited M phase and were then supplemented with the translation inhibitor cycloheximide to prevent new cyclin synthesis (Figure 6). The addition of Gwl inhibited the assayed phosphatase, even though H1 kinase activity was low and the extracts were demonstrably in interphase.
Figure 6.
Gwl inhibits anti-CDK phosphatase activity even in the near absence of CDK activity. (A) A CSF extract was treated with 0.3 mM Ca2+ and incubated for 20 min at 22°C to cause cyclin degradation and M phase exit. At that time (t = −10 min), 100 ng/μl cycloheximide (CHX) was added, and samples were incubated for 10 min at 22°C to prevent further protein synthesis. CDK (H1 kinase) activity is very low at this and subsequent times because of the lack of cyclins. At t = 0, the extract was split in two, and Gwl WT was added to one sample at about five times the concentration of the endogenous Gwl. (Our anti-Gwl antibody recognizes the most highly phosphorylated forms of Gwl less efficiently than other forms; see control extract in Figure 3.) Cdc25 in the Gwl-supplemented samples gradually acquires some M phase-like phosphorylations (compare with Figure 3) in spite of the lack of CDK activity. These phosphorylations are likely due to kinases like MAPK with CDK-like target specificities (Sheridan et al., 2008), coupled with the low phosphatase activity against these Cdc25 phosphosites. (B) Phosphatase assays of the samples shown in A, using substrate containing Thr311 of PP1γ. The addition of Gwl WT inhibits anti-CDK phosphatase either directly or indirectly.
These three experiments all establish conditions under which the activity of phosphatases directed against certain CDK phosphosites is dictated by the presence or absence of Gwl function, but not that of MPF. Once activated, Gwl's role in the control of this phosphatase must similarly be independent of other M phase–specific phosphorylations that are driven directly or indirectly by MPF. Because MPF can serve as an upstream activator of Gwl (Yu et al., 2006), the most straightforward conclusion is that Gwl acts downstream of MPF in regulating the presumptive antimitotic phosphatase. Our results thus point to Gwl as a key mediator that connects MPF activation during M phase with the inactivation of an OA-sensitive phosphatase that would otherwise dephosphorylate many MPF substrates.
We also note that Figures 5 and 6 allay an important concern about the reliability of phosphatase assays in undiluted Xenopus egg extracts. Ferrigno et al. (1993) have suggested that the high global levels of CDK-driven phosphorylations present during M phase (but not interphase) might competitively inhibit the ability of phosphatases in extracts to dephosphorylate exogenously supplied labeled substrates, leading to an inaccurate view of phosphatase regulation. However, our experiments indicate that the depletion or addition of Gwl can turn phosphatase activity on or off even if there are no accompanying changes in global cell cycle status.
Gwl Activation Leads to PP2A/B55δ Inactivation
Candidates for phosphatases regulated by Gwl include the major OA-sensitive enzymes PP1 and PP2A, as well as PPs 4–7. Half-maximal inhibition of the phosphatase measured in our assays of both interphase cycling extracts and Gwl-depleted CSF extracts occurs at OA concentrations of 200–300 nM and fostriecin concentrations of 7–20 μM (data not shown). These numbers provide only inexact guidance to the identity of the Gwl-regulated phosphatase, because the high concentrations of phosphatases in undiluted extracts dictate that inhibitors must be added in much higher amounts than published IC50 values for dilute solutions of purified enzymes. However, these results do exclude PP7 as a candidate (because the IC50 of OA on PP7 is greater than 1 μM) and further weakly indicate that the phosphatase we are assaying is unlikely to be PP1 or the less abundant PP5 (IC50 values of fostriecin for PP1 are 45–58 μM and for PP5 are 50–70 μM; Swingle et al., 2007); in addition, a previous study has suggested that half maximal inhibition of PP1 in undiluted Xenopus extracts requires ∼1 μM OA (Felix et al., 1990). The probable exclusion of PP1 is further supported by the failure of high concentrations (4 μM) of the PP1 inhibitors I-2 or PHI-1 (Eto et al., 1999) to affect the phosphatase activity on CDK sites (data not shown).
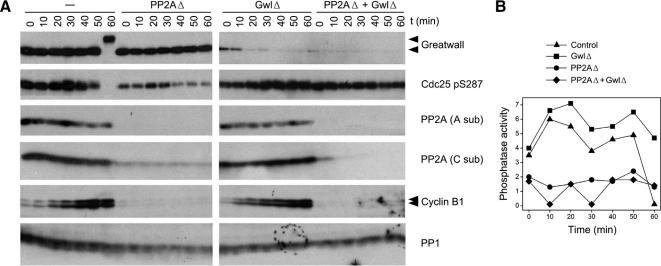
Because of the uncertainties associated with inhibitor studies in undiluted extracts, we sought to identify the phosphatase measured in our assays by the physical removal of candidate phosphatases. Given many precedents that PP2A is active against CDK phosphosites (see Discussion), we first immunodepleted cycling extracts with a mAb directed against PP2A's structural subunit (Kremmer et al., 1997). As seen in Figure 7, this procedure removes the large majority of PP2A while leaving PP1 substantially untouched. PPs 4, 5, and 6 are similarly retained in extracts immunodepleted with the same mAb (Mochida et al., 2009). The PP2A-depleted extracts have only ∼20–30% of the anti-CDK phosphatase activity that is present in interphase cycling extracts. This result equates the assayed phosphatase primarily with PP2A, although lesser contributions from other enzymes cannot be excluded.
Figure 7.
Immunodepletion of PP2A removes most of the phosphatase activity directed against CDK sites. (A) Interphase cycling extracts were immunodepleted at 4°C for Gwl, PP2A, or both enzymes as indicated. PP2A immunodepletion was performed with the 6F9 mAb against the structural A subunit (Kremmer et al., 1997; Covance; no. MRT-204R) coupled to protein G-Sepharose (Zymed, South San Francisco, CA). The control extract was mock-depleted for both Gwl and PP2A, the GwlΔ extract was mock-depleted for PP2A, and the PP2AΔ extract was mock-depleted for Gwl. The extracts were then incubated at 22°C, and samples were removed for immunoblotting at the indicated times. Antibodies used for the Western blot included the 6F9 antibody for the PP2A A subunit and antibodies against the catalytic (C) subunits of PP1 and PP2A previously described by (Maton et al., 2005); these blots show the specificity of the immunodepletion for PP2A but not PP1. All samples depleted for PP2A remain in interphase because cyclin B1 is degraded and fails to accumulate for unknown reasons (see text). (B) Phosphatase assays of the samples shown in A, using substrate containing Thr311 of PP1γ. Although the PP2A depleted samples are clearly in interphase, they have on average <30% of the phosphatase activity of the corresponding nonPP2A-depleted controls.
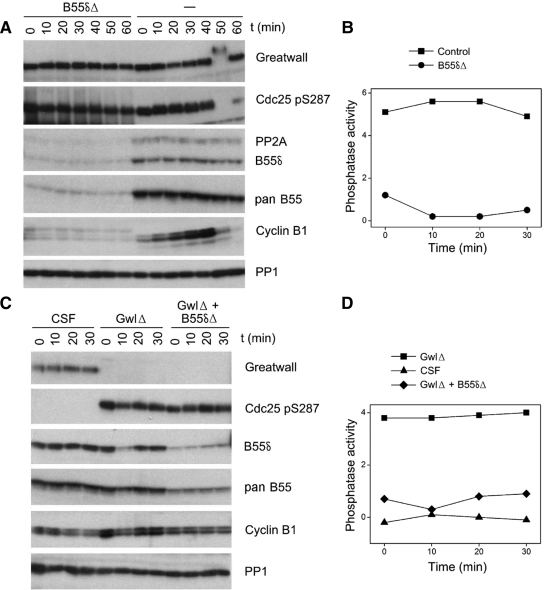
Classical PP2A holoenzymes are trimers consisting of a catalytic subunit (C), a structural subunit (A), and one of many possible regulatory subunits from the B (B55), B′ (B56), or B″ families (reviewed in Janssens et al., 2008). The best candidates for the enzyme measured in our assays are trimers with B55 family regulatory subunits, because of previous reports that in vitro and in vivo, such holoenzymes specifically dephosphorylate pS/TP sites phosphorylated by CDKs (Agostinis et al., 1992; Mayer-Jaekel et al., 1994). Although we were unable to remove all B55 subunits from Xenopus egg extracts with available pan-B55 antibodies, we were able to immunodeplete extracts for B55δ, a regulatory subunit recently shown to play a particularly important role in M phase entry (Mochida et al., 2009). (As discussed in several figure legends, we estimate that B55δ usually accounts for 70% or more of the total B55 subunits in egg extracts, although this proportion was sometimes as low as 25%.) B55δ immunodepletion from both interphase cycling extracts and Gwl-depleted CSF extracts can eliminate the large majority of the assayed phosphatase activity (Figure 8). PP2A with B55δ regulatory subunits (PP2A/B55δ) thus appears to be an important constituent of the OA sensitive, cell cycle–regulated phosphatase directed against CDK phosphosites.
Figure 8.
PP2A/B55δ is a major constituent of the phosphatase directed against CDK phosphosites. (A) Cycling extracts were either mock-depleted (−) or depleted for B55δ (B55δΔ) for 1 h at 4°C and then incubated at 22°C starting at t = 0. The B55δ-depleted extracts were unable to enter M phase because cyclins were immediately degraded (see Discussion). (B) Phosphatase assays of the first four timed aliquots of the samples shown in A, using substrate containing Thr311 of PP1γ. Note that although both the control and B55δ-depleted extracts were in interphase during these times, the large majority of measured phosphatase activity was removed in the latter samples. (C) CSF extracts were either successively depleted for Gwl and B55δ (GwlΔ + B55δΔ), depleted for Gwl and then mock-depleted for B55δ (GwlΔ) or double mock-depleted (CSF). (D) Phosphatase assays of the samples shown in C, using substrate containing Thr311 of PP1γ. The phosphatase activity induced by Gwl depletion was substantially removed by subsequent B55δ immunodepletion. As judged by comparing the Western blot signals obtained with B55δ and pan B55 antibodies from the B55δ-depleted samples, B55δ accounted for more than 80% of the total B55 population in the extracts used in A and B and ∼60% of the B55 molecules in the extracts used in C and D.
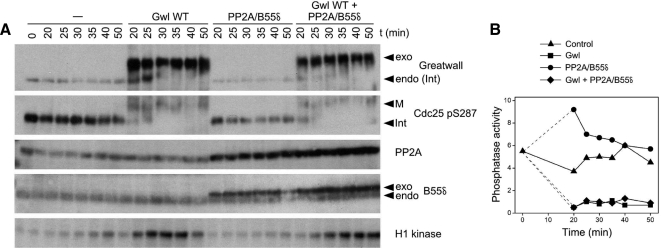
To determine whether Gwl promotes the inactivation of PP2A/B55δ, we supplemented interphase cycling extracts with both enzymes (Figure 9). The PP2A/B55δ holoenzyme used in this experiment was reconstituted from recombinant subunits; phosphatase assays (not shown) indicate that the activity of the purified holoenzyme is roughly equivalent to that of its endogenous counterpart if it is assumed (per Figure 8) that the large majority of phosphatase measured in extracts is contributed by PP2A/B55δ. The addition of active Gwl leads not only to the immediate inactivation of the endogenous phosphatase directed against CDK phosphosites, but also to the inactivation of exogenously supplemented PP2A/B55δ (Figure 9).
Figure 9.
Gwl blocks the activity of PP2A/B55δ. (A) Interphase cycling extracts were incubated for 20 min at 22°C and then supplemented with a fivefold excess of Gwl WT, a twofold excess of heterotrimeric PP2A containing the B55δ regulatory subunit, or both. Samples were processed for immunoblotting and H1 kinase assays. PP2A was detected with mAb against the A subunit and antibody against B55δ. (B) Phosphatase assays of the samples shown in A. In vitro experiments (not shown) indicate that the exogenous PP2A/B55δ contributes a level of anti-CDK phosphosite activity roughly equivalent to that of the endogenous phosphatase in the extract, consistent with the idea that the large majority of phosphatase assayed in extracts is contributed by PP2A associated with B55-type regulatory subunits. Note that Gwl WT suppresses the activity of both exogenous and endogenous phosphatases. The substrate used is that containing Thr311 of PP1γ.
Gwl's Cell Cycle Role Involves Inhibition of PP2A/B55δ
To test the idea that Gwl influences the cell cycle by negatively regulating PP2A/B55δ, we asked whether the removal of this phosphatase could help overcome the effects of removing Gwl. We thus depleted cycling extracts for Gwl alone or successively for Gwl and PP2A/B55δ (Figure 10). Control cycling extracts rapidly enter and then exit M phase (as judged by cyclin B degradation and the interphase-specific inhibitory phosphorylation on Ser287 of Cdc25) within 30 min. In contrast, and in accordance with Yu et al. (2006), Gwl-depleted cycling extracts do not enter M phase (cyclin B remains undegraded, and the Cdc25 Ser287 site remains phosphorylated). The failure of Gwl-depleted cycling extracts to enter M phase is substantially corrected by B55δ removal, because the double-depleted extracts lose almost all Ser287 signal and then degrade cyclin B on schedule. Therefore, the requirement for Gwl in the G2-to-M transition can be circumvented if PP2A/B55δ, alone among OA-sensitive phosphatases, is no longer present.
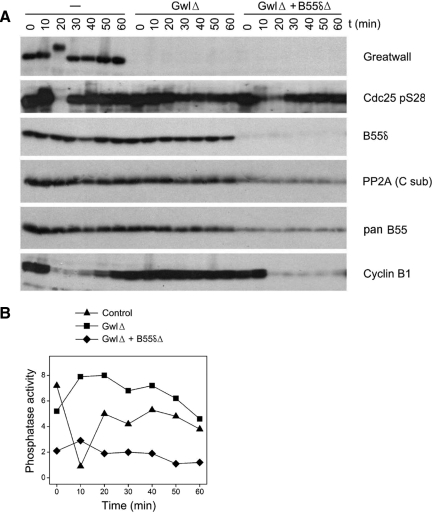
Figure 10.
B55δ removal rescues M phase entry defects in Gwl-depleted cycling extracts. (A) Cycling extracts were prepared as in Figures 3 and 8, except that the eggs were incubated an additional 30 min after calcium ionophore treatment but before crushing. Extracts were Gwl-depleted as indicated and then immediately afterward were either B55δ-depleted by three successive 30-min incubations at 4°C with protein G beads coupled to anti-B55δ (GwlΔ + B55δΔ) or mock-depleted with beads without the B55δ antibody (GwlΔ). Control extracts (−) were double mock-depleted. At t = 0, the extracts were incubated at 22°C. B55δ accounted for ∼70% of the B55-type subunits in these extracts, as estimated by comparing immunoblot signals with the B55δ antibody and a pan-B55 antibody. As judged by loss of the interphase-specific phosphorylation on Ser287 of Cdc25 and the degradation of cyclin B, control extracts and extracts depleted for Gwl and B55δ enter M phase at ∼20 min, whereas extracts depleted for Gwl alone do not enter M phase during the incubation. (B) Phosphatase assays of the samples shown in A; the substrate used is that containing Thr311 of PP1γ. Note that the phosphatase activity decreases in the control extract during M phase, whereas the activity is much reduced in the B55δ-depleted sample.
DISCUSSION
Phosphatase Down-Regulation During M Phase
Our results clearly show that PP2A/B55δ is one phosphatase that is inhibited by active Gwl, and that in agreement with another recent study (Mochida et al., 2009), PP2A/B55δ is a major component of the anti-CDK phosphatase. Because immunodepletion using the B55δ antibody did not quantitatively eliminate all the phosphatase activity, we cannot determine whether PP2A holoenzymes associated with other less abundant B55-type subunits (B55α, B55β, and B55γ) might also be targets of Gwl regulation and might also contribute to the anti-CDK phosphatase. We in fact regard these as likely possibilities, because 1) PP2A holoenzymes with B55α and B55β can block M phase entry in Xenopus extracts and oocytes (Lee et al., 1991; Iwashita et al., 1997); 2) in a rare extract in which B55δ was a minority of B55 subunits, immunodepletion of B55δ removed only a minority of the assayed phosphatase and also failed to block pseudomitotic exit (data not shown); and 3) Gwl regulates the cell cycle of Drosophila (Yu et al., 2004), whose genome encodes only a single B55 protein. Although we thus assume in the rest of the Discussion that PP2A holoenzymes with other B55 subunits are also down-regulated during M phase by a Gwl-mediated mechanism, it should be mentioned that these phosphatases are not functional equivalents because their substrate specificities are not identical (reviewed by Virshup and Shenolikar, 2009).
The argument that PP2A/B55 phosphatases are those most (if not exclusively) responsible for the dephosphorylation of the assayed CDK phosphosites agrees with several precedents. For example, in biochemical fractionations of mammalian and Xenopus extracts, phosphatase activities directed against a variety of CDK-phosphorylated sites copurified with PP2A, but completely purified away from the other major phosphatase, PP1 (Ferrigno et al., 1993; Che et al., 1998). In addition, and as mentioned previously, the types of PP2A holoenzymes previously reported to dephosphorylate various CDK phosphosites are those with B55-family regulatory subunits (Agostinis et al., 1992; Mayer-Jaekel et al., 1994). However, because roughly 20% of the phosphatase in our assays remains even after almost all of the PP2A A subunit has been removed (Figure 7), we cannot exclude that one or more other phosphatases might contribute to the anti-CDK activity.
Because our assays employ specific substrates that are largely targeted by PP2A/B55, we cannot ascertain whether Gwl might also promote the down-regulation of other phosphatases. Evidence that various OA-sensitive phosphatases are suppressed during M phase has been presented in the literature. For example, PP2A/B56δ (not B55δ) was described to dephosphorylate the Thr138 site needed for activation of Cdc25; this reaction occurs specifically during interphase and is shut down during M phase (Margolis et al., 2006). In addition, levels of PP1 have previously been reported to be significantly lower during M phase than during interphase (Walker et al., 1992); more recent evidence indicates that this mitotic down-regulation is likely achieved by M phase–specific phosphorylations that inhibit the PP1 catalytic subunit and activate the PP1 inhibitor I-1 (Wu et al., 2009). Many scenarios involving cross-talk in the regulation of various phosphatases can be imagined, so the possibility that Gwl might control (directly or indirectly) enzymes other than PP2A/B55(δ) will be an important question for future investigation.
Much remains to be learned about the spectrum of phosphosites targeted by Gwl-, or more generally, cell cycle–regulated phosphatases. The seven phosphosites followed in our assays (Figure 1) all share the canonical (S/T)PX(K/R) motif for CDKs, yet there are significant differences in the rates at which these sites are dephosphorylated in Xenopus egg extracts. These differences may in part reflect requirements of the phosphatase(s) for structural information beyond that in the immediate vicinity of the phospho-S or -T in order to recognize certain substrates. However, much variation exists in the sensitivity of CDK phosphoepitopes within intact proteins to the phosphatase(s) induced in Gwl-depleted extracts (e.g., Figure 5), so even within their normal contexts, some CDK sites are more recalcitrant than others to dephosphorylation.
Dephoure et al. (2008) have recently found by mass spectrometry that many phosphopeptides without canonical CDK sites accumulate during M phase in mammalian cells; these include peptides with either noncanonical proline-directed motifs, basophilic sites such as those recognized by Aurora A kinase, or acidophilic sites similar to those for Polo kinase. It is presently unclear whether the increases in these other types of phosphopeptides during M phase might also involve mitotic down-regulation of the phosphatases that target them.
The Roles of Gwl and PP2A/B55δ in Cell Cycle Control
Figure 11 presents our current working model for the function of Gwl and PP2A/B55δ in the G2/M transition. As MPF is activated through the autoregulatory loop, it phosphorylates and activates Gwl. Gwl then directly or indirectly helps inactivate phosphatases including PP2A/B55δ (and likely PP2A with other B55-type regulatory subunits). By preventing the premature dephosphorylation of CDK phosphosites on autoregulatory loop components, Gwl augments the explosive, spike-like activation of MPF. Furthermore, the suppression of PP2A/B55δ function protects the pS/TP sites on many MPF substrates from dephosphorylation. Our model thus characterizes Gwl as a promitotic kinase that suppresses antimitotic phosphatases including PP2A/B55δ.
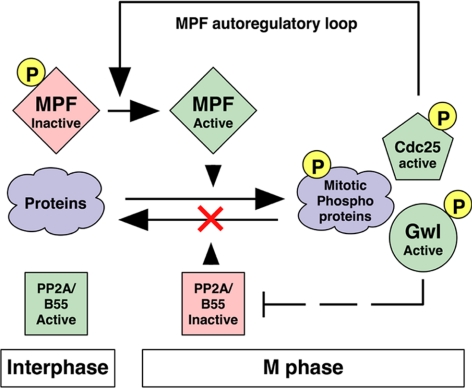
Figure 11.
A model for Gwl function in M phase. MPF phosphorylates many target proteins, including but not limited to Gwl and MPF autoregulatory loop components such as Cdc25 and (kinases [not shown] such as Plx1 and Myt1/Wee1). Phosphorylated, active Greatwall directly, or more likely indirectly, inactivates PP2A/B55δ, thus protecting MPF substrates from dephosphorylation. In this way, Greatwall can simultaneously influence the autoregulatory loop and also function outside of the loop. This scheme implies that M phase (both in mitosis and meiosis) requires feedback mechanisms that not only positively regulate MPF kinase, but also negatively regulate countering “antimitotic” phosphatases including PP2A/B55δ.
The model shown in Figure 11 accounts for the generally strong correlation between the activations of Gwl and MPF, the inactivation of phosphatase(s) directed against a subset of CDK phosphosites, and M phase itself. However, experimental manipulations can produce unusual conditions in which these factors do not act in concert. For example, in Figure 5 some extracts simultaneously display high levels of CDK and phosphatase activities. Such extracts are neither clearly in M phase nor in interphase, as different mitotic phosphosites are variously affected by particular ratios of the kinases and phosphatases that target them. During the review of this manuscript, another group reported results leading to a very similar view of the role of Greatwall in suppressing the activity of some form of PP2A during M phase (Vigneron et al., 2009). These researchers used a different approach to establish conditions under which both CDK and anti-CDK phosphatase activities are high: With a tour-de-force of immunodepletion, they concurrently removed the kinases Myt1, Wee1, and Greatwall from CSF-arrested extracts. In contrast with our findings in Figure 5, their triply-depleted extracts displayed properties more characteristic of interphase than M phase, as the majority of CDK phosphosites became dephosphorylated. These differing outcomes illustrate the instability of the system when mitotic kinases and antimitotic phosphatases compete with each other.
The results seen in Figure 10 indicate that the removal of a single phosphatase, PP2A/B55δ, is sufficient to overcome the failure of Gwl-depleted extracts to enter M phase. This finding is consistent with previous studies showing that suppression of PP2A (but not PP1) can cause precocious activation of MPF (Clarke et al., 1993; Maton et al., 2005) and with a recent demonstration that M phase entry is negatively regulated by PP2A/B55δ (Mochida et al., 2009). Our working model (Figure 11) presumes that during mitotic entry, Gwl can suppress PP2A/B55δ only after Gwl itself is turned on by MPF. We were thus surprised to find that the removal of Gwl from interphase extracts leads to a slight but consistent increase in anti-CDK phosphatase (Figures 7 and 10). This phenomenon suggests that a low level of Gwl activity might normally be present in interphase extracts devoid of MPF. Possibly, interphase kinases with target specificities similar to that of MPF (e.g., other CDKs or MAPK) might phosphorylate Gwl and potentiate this putative low-level activity. If so, one can envision that Gwl might actually participate in the triggering mechanism for M phase entry (i.e., Gwl might function upstream as well as downstream of MPF during the G2-to-M transition). We have occasionally seen evidence for a partial Gwl activation before MPF activation during Xenopus oocyte maturation (data not shown), but much more work will be required to evaluate the idea that Gwl is part of the mitotic trigger.
M phase exit requires not only cyclin degradation, but also the dephosphorylation of many mitotic phosphoproteins by OA-sensitive phosphatase(s) (Wu et al., 2009). Besides PP2A/B55δ, other likely contributors include PP2A holoenzymes associated with other B55-family regulatory subunits, but at this time we cannot in fact exclude the participation of any OA-sensitive enzyme. Unraveling the contributions of individual phosphatases to mitotic exit is extremely challenging, because only a small amount of phosphatase might suffice once the countering kinase (MPF) is inactivated.
Wu et al. (2009) have nonetheless recently ascribed an important role to PP1 in the global dephosphorylation of phosphoproteins during mitotic (but not meiotic) exit. This conclusion is difficult to reconcile with biochemical experiments in which phosphatase activity against various CDK phosphosites fractionated completely away from PP1 (Ferrigno et al., 1993; Che et al., 1998). One interesting potential explanation is that PP1 works indirectly on these phosphosites by helping to reverse Gwl action. Because Gwl is activated in large part by phosphorylations at several S/TP sites (Yu et al., 2006), we envision that at the end of M phase, Gwl itself is inactivated by phosphatases such as PP2A/B55δ directed against CDK phosphosites. However, Gwl is an AGC kinase, whose target specificities are very different from CDKs; for example, AGC enzymes strongly disfavor serines or threonines followed by proline (Zhu et al., 2005). Thus, the phosphorylations that Gwl adds to its substrates during M phase are likely to be removed at the end of mitosis by a different phosphatase, potentially a form of PP1.
A confusing, if peripheral, aspect of our results is that under particular conditions, the immunodepletion of PP2A A or B55δ subunits leads to the loss of cyclin B1 (Figures 7, 8, and 10). This confusion already exists in the literature; for example, OA induces cyclin degradation in CSF extracts or certain interphase extracts (Lorca et al., 1991), whereas OA treatment or PP2A depletion causes premature M phase entry in other types of interphase extracts or in immature oocytes (e.g., Maton et al., 2005; Zhao et al., 2008). When it occurs, cyclin degradation is probably due to blockage of PP2A's role in ensuring the stability and activity of Erp1, an inhibitor of the anaphase promoting complex (APC; Wu et al., 2007). The specific conditions leading to cyclin stability or degradation upon phosphatase removal are unclear but are likely to involve varying CDK levels. For example, the B55δ-depleted cycling extract shown in Figure 8 did not accumulate cyclin B1 and thus failed to enter M phase, whereas that shown in Figure 10 did both. The two extracts were prepared identically, except that in the latter, the eggs were incubated an additional 30 min after calcium ionophore treatment but before crushing, and thus this extract had higher initial cyclin levels than the former.
We do not yet know how Gwl promotes PP2A/B55δ inactivation. The most direct route would involve Gwl's phosphorylation of a phosphatase subunit; however, in vitro kinase assays have provided no indication that Gwl can target any component of the PP2A/B55δ preparation used in Figure 9 (data not shown). Another possibility for a relatively direct connection between Gwl and PP2A is suggested by the recent finding of Vigneron et al. (2009) that these molecules can be coimmunoprecipitated from mammalian cells overexpressing them, as well as from Xenopus CSF extracts. We have been unable to reproduce the latter results with confidence; the interaction could be very weak or involve only a small fraction of Gwl or PP2A. Furthermore, the functional significance of such an interaction is unclear: The binding of Gwl to PP2A could inactivate the phosphatase, but this binding could alternatively represent dephosphorylation of Gwl by PP2A.
At present, we instead favor the model that Gwl works indirectly through other regulators of PP2A. Several such regulators are already well known, including leucine carboxylmethyltransferase (LCMT1), PP2A methylesterase (PME-1), and the cis-trans prolyl isomerase PTPA (reviewed by Janssens et al., 2008). However, it is also likely that some PP2A regulators remain to be discovered, as suggested by recent findings that the small (114 residue) adenovirus protein E4orf4 specifically binds to and inhibits PP2A holoenzymes containing B55 family subunits and that expression of E4orf4 in tissue culture cells leads to G2/M cell cycle arrest characterized by high levels of Cdk1 activity (Li et al., 2009). It is conceivable that PP2A/B55 might be suppressed during M phase of normal cell divisions by an unknown, analogous inhibitor that is targeted and activated by Greatwall. We are currently investigating the pathway connecting Gwl with PP2A by looking both for potential Gwl substrates and for biochemical changes that contribute to PP2A/B55δ inactivation during M phase.
Supplementary Material
ACKNOWLEDGMENTS
We thank Olivier Haccard, Catherine Jessus, Eunah Chung (Harvard Medical School, Boston, MA), and Joan Ruderman (Harvard Medical School, Boston, MA) for generous gifts of antibodies and Tim Hunt and Jian Kuang for helpful discussions. This work was supported by National Institutes of Health Grant GM48430 to M.L.G. and by fellowships from EMBO and the Japan Society for the Promotion of Science to S.M.
Abbreviations used:
- CDK
cyclin-dependent kinase
- CSF
cytostatic factor
- Gwl
Greatwall kinase
- MPF
M phase–promoting factor (Cdk1/cyclin B)
- OA
okadaic acid.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-07-0643) on September 30, 2009.
REFERENCES
- Agostinis P., Derua R., Sarno S., Goris J., Merlevede W. Specificity of the polycation-stimulated (type-2A) and ATP, Mg-dependent (type-1) protein phosphatases toward substrates phosphorylated by p34Cdc2 kinase. Eur. J. Biochem. 1992;205:241–248. doi: 10.1111/j.1432-1033.1992.tb16774.x. [DOI] [PubMed] [Google Scholar]
- Che S., Wu W., Nelman-Gonzalez M., Stukenberg T., Clark R., Kuang J. A phosphatase activity in Xenopus oocyte extracts preferentially dephosphorylates the MPM-2 epitope. FEBS Lett. 1998;424:225–233. doi: 10.1016/s0014-5793(98)00158-6. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Hoffmann I., Draetta G., Karsenti E. Dephosphorylation of Cdc25-C by a type-2A protein phosphatase: specific regulation during the cell cycle in Xenopus egg extracts. Mol. Biol. Cell. 1993;4:397–411. doi: 10.1091/mbc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N., Zhou C., Villen J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. USA. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto M., Karginov A., Brautigan D. L. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry. 1999;38:16952–16957. doi: 10.1021/bi992030o. [DOI] [PubMed] [Google Scholar]
- Felix M. A., Cohen P., Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990;9:675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno P., Langan T. A., Cohen P. Protein phosphatase 2A1 is the major enzyme in vertebrate cell extracts that dephosphorylates several physiological substrates for cyclin-dependent protein kinases. Mol. Biol. Cell. 1993;4:669–677. doi: 10.1091/mbc.4.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris J., Hermann J., Hendrix P., Ozon R., Merlevede W. Okadaic acid, a specific protein phosphatase inhibitor, induces maturation and MPF formation in Xenopus laevis oocytes. FEBS Lett. 1989;245:91–94. doi: 10.1016/0014-5793(89)80198-x. [DOI] [PubMed] [Google Scholar]
- Iwashita J., Shima H., Nagao M., Sagata N. cDNA cloning of a novel B subunit of Xenopus protein phosphatase 2A and its biological activity in oocytes. Biochem. Biophys. Res. Commun. 1997;232:218–222. doi: 10.1006/bbrc.1997.6259. [DOI] [PubMed] [Google Scholar]
- Janssens V., Longin S., Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem. Sci. 2008;33:113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Stewart E., Poon R. Y., Hunt T. Cyclin A and cyclin B dissociate from p34cdc2 with half-times of 4 and 15 h, respectively, regardless of the phase of the cell cycle. J. Biol. Chem. 1994;269:29153–29160. [PubMed] [Google Scholar]
- Kremmer E., Ohst K., Kiefer J., Brewis N., Walter G. Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol. Cell. Biol. 1997;17:1692–1701. doi: 10.1128/mcb.17.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Solomon M. J., Mumby M. C., Kirschner M. W. INH, a negative regulator of MPF, is a form of protein phosphatase 2A. Cell. 1991;64:415–423. doi: 10.1016/0092-8674(91)90649-j. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Turck C., Kirschner M. W. Inhibition of Cdc2 activation by INH/PP2A. Mol. Biol. Cell. 1994;5:323–338. doi: 10.1091/mbc.5.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Brignole C., Marcellus R., Thirlwell S., Binda O., McQuoid M. J., Ashby D., Chan H., Zhang Z., Miron M. J., Pallas D. C., Branton P. E. The adenovirus E4orf4 protein induces G2/M arrest and cell death by blocking protein phosphatase 2A activity regulated by the B55 subunit. J. Virol. 2009;83:8340–8352. doi: 10.1128/JVI.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Maller J. L. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr. Biol. 2005;15:1458–1468. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Lorca T., Fesquet D., Zindy F., Le Bouffant F., Cerruti M., Brechot C., Devauchelle G., Doree M. An okadaic acid-sensitive phosphatase negatively controls the cyclin degradation pathway in amphibian eggs. Mol. Cell. Biol. 1991;11:1171–1175. doi: 10.1128/mcb.11.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis S. S., et al. Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell. 2006;127:759–773. doi: 10.1016/j.cell.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maton G., Lorca T., Girault J.-A., Ozon R., Jessus C. Differential regulation of Cdc2 and Aurora-A in Xenopus oocytes: a crucial role of phosphatase 2A. J. Cell Sci. 2005;118:2485–2494. doi: 10.1242/jcs.02370. [DOI] [PubMed] [Google Scholar]
- Mayer-Jaekel R.E., Ohkura H., Ferrigno P., Andjelkovic N., Shiomi K., Uemura T., Glover D.M., Hemmings B.A. Drosophila mutants in the 55 kDa regulatory subunit of protein phosphatase 2A show strongly reduced ability to dephosphorylate substrates of p34cdc2. J. Cell Sci. 1994;107(Pt 9):2609–2616. doi: 10.1242/jcs.107.9.2609. [DOI] [PubMed] [Google Scholar]
- Mochida S., Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- Mochida S., Ikeo S., Gannon J., Hunt T. Regulated activity of PP2A-B55delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28:2777–2785. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T., Yoshizaki N., Kishimoto T., Ohsumi K. Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature. 2007;449:341–345. doi: 10.1038/nature06136. [DOI] [PubMed] [Google Scholar]
- Perry J. A., Kornbluth S. Cdc25 and Wee1, analogous opposites? Cell Div. 2007;2:12. doi: 10.1186/1747-1028-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh N. R., Schmidt A., Bormann J., Nigg E. A., Mayer T. U. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- Sheridan D. L., Kong Y., Parker S. A., Dalby K. N., Turk B. E. Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. J. Biol. Chem. 2008;283:19511–19520. doi: 10.1074/jbc.M801074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford J. S., Ruderman J. V. Changes in regulatory phosphorylation of Cdc25C Ser287 and Wee1 Ser549 during normal cell cycle progression and checkpoint arrests. Mol. Biol. Cell. 2005;16:5749–5760. doi: 10.1091/mbc.E05-06-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingle M., Ni L., Honkanen R. E. Small-molecule inhibitors of Ser/Thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol. Biol. 2007;365:23–38. doi: 10.1385/1-59745-267-X:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron S., Brioudes E., Burgess A., Labbe J. C., Lorca T., Castro A. Greatwall maintains mitosis through regulation of PP2A. EMBO J. 2009;28:2786–2793. doi: 10.1038/emboj.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M., Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol. Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Walker D. H., DePaoli-Roach A. A., Maller J. L. Multiple roles for protein phosphatase 1 in regulating the Xenopus early embryonic cell cycle. Mol. Biol. Cell. 1992;3:687–698. doi: 10.1091/mbc.3.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Q., Guo J. Y., Tang W., Yang C. S., Freel C. D., Chen C., Nairn A. C., Kornbluth S. PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat. Cell Biol. 2009;11:644–651. doi: 10.1038/ncb1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., et al. A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr. Biol. 2007;17:213–224. doi: 10.1016/j.cub.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Fleming S. L., Williams B., Williams E. V., Li Z., Somma P., Rieder C. L., Goldberg M. L. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J. Cell Biol. 2004;164:487–492. doi: 10.1083/jcb.200310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Zhao Y., Li Z., Galas S., Goldberg M. L. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol. Cell. 2006;22:83–91. doi: 10.1016/j.molcel.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Haccard O., Wang R., Yu J., Kuang J., Jessus C., Goldberg M. L. Roles of Greatwall kinase in the regulation of Cdc25 phosphatase. Mol. Biol. Cell. 2008;19:1317–1327. doi: 10.1091/mbc.E07-11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Fujii K., Liu Y., Codrea V., Herrero J., Shaw S. A single pair of acidic residues in the kinase major groove mediates strong substrate preference for P-2 or P-5 arginine in the AGC, CAMK, and STE kinase families. J. Biol. Chem. 2005;280:36372–36379. doi: 10.1074/jbc.M505031200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.