Abstract
Wnt signaling pathways regulate proliferation, motility, and survival in a variety of human cell types. Dickkopf-1 (Dkk-1) is a secreted Wnt antagonist that has been proposed to regulate tissue homeostasis in the intestine. In this report, we show that Dkk-1 is secreted by intestinal epithelial cells after wounding and that it inhibits cell migration by attenuating the directional orientation of migrating epithelial cells. Dkk-1 exposure induced mislocalized activation of Cdc42 in migrating cells, which coincided with a displacement of the polarity protein Par6 from the leading edge. Consequently, the relocation of the microtubule organizing center and the Golgi apparatus in the direction of migration was significantly and persistently inhibited in the presence of Dkk-1. Small interfering RNA-induced down-regulation of Dkk-1 confirmed that extracellular exposure to Dkk-1 was required for this effect. Together, these data demonstrate a novel role of Dkk-1 in the regulation of directional polarization of migrating intestinal epithelial cells, which contributes to the effect of Dkk-1 on wound closure in vivo.
INTRODUCTION
In the intestine, a single layer of epithelial cells separates the luminal content from underlying tissues. Epithelial cells are constantly being replaced by a cycle of stem cell proliferation at the bottom of intestinal crypts, migration of cells toward the surface along the crypt–surface axis, and apoptosis of cells at the luminal interface (reviewed in Dignass, 2001; de Santa Barbara et al., 2003). Restoration of the epithelial barrier after injury, for example, after mechanical wounding or under inflammatory conditions such as intestinal inflammatory diseases, is facilitated by rapid restitution of the epithelial monolayer. This process is characterized by the directional migration and resealing of the epithelial cell sheet and it does not require changes in cell proliferation (Silen and Ito, 1985).
We identified Dickkopf-1 (Dkk-1) as a potential regulator of epithelial restitution based on gene expression changes in migrating model intestinal epithelial cells. Dkk-1 is a secreted glycoprotein that has been demonstrated to act as a potent inhibitor of the canonical Wnt/β-catenin signaling pathway (Glinka et al., 1998; Fedi et al., 1999). Dkk-1 competitively binds to the low-density lipoprotein receptor-related protein (LRP) family of cell surface receptors, which results in the degradation of cytosolic β-catenin and the silencing of T cell factor (TCF)-mediated gene transcription (Bafico et al., 2001; Semenov et al., 2001). It has been shown that Dkk-1 plays a crucial role in many biological processes, ranging from the induction of anterior mesoderm formation and head development during embryogenesis to bone formation and bone mass regulation in adult organisms (reviewed in Niehrs, 2006). However, relatively little is known about the importance of Dkk-1 in epithelial homeostasis. Previous reports indicate that epigenetic silencing of secreted Wnt inhibitors, including Dkk-1, is a common occurrence in inflammatory bowel disease and colorectal cancer (Gonzalez-Sancho et al., 2005; Aguilera et al., 2006; Dhir et al., 2008). Conversely, adenoviral overexpression of Dkk-1 in adult mice inhibits intestinal epithelial cell proliferation and leads to severe tissue destruction in ileum and colon (Pinto et al., 2003; Kuhnert et al., 2004). In addition, Dkk-1 has been identified as an important mediator of inflammation and is induced by proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interferon-γ (Gollob et al., 2005; Diarra et al., 2007).
In this report, we provide evidence that Dkk-1 at physiological concentrations inhibits the restitution of small epithelial wounds without altering proliferation. We observed that the Wnt inhibitor is rapidly secreted from migrating epithelial cells and inhibits cell migration by attenuating the directional polarization of leading edge cells. Directional orientation of migrating cells is a tightly regulated, multistep process that is initiated by the localized activation of Cdc42 at the front of the cell (Stowers et al., 1995; Nobes and Hall, 1999; Etienne-Manneville and Hall, 2001). This, in turn, leads to the recruitment and activation of a polarity complex containing Par6 and protein kinase C (PKC)ζ at the leading edge (Etienne-Manneville and Hall, 2001, 2003). Consistent with this model, we observed that Dkk-1 induced an increased, mislocalized activation of Cdc42 and the displacement of Par6 from the front of the migrating cell sheet. Furthermore, we saw an aberrant distribution of the microtubule organizing center (MTOC) and the Golgi apparatus in the presence of extracellular Dkk-1.
In light of these observations, we propose a previously unknown role of Dkk-1 in the regulation of epithelial restitution. Rather than altering cell proliferation, low concentrations of secreted Dkk-1 influence resealing of the epithelial monolayer by inhibiting cellular orientation, thereby attenuating cell migration.
MATERIALS AND METHODS
Cells
Human intestinal epithelial Caco-2 cells, as well as mouse 3T6 fibroblasts, were grown in DMEM medium supplemented with 10% fetal calf serum and 1% antibiotics. Intestinal epithelial cell (IEC)-6 primary rat intestinal epithelial cells were grown in DMEM with 5% fetal calf serum, 2 mM glutamine, and 0.1 U/ml insulin. Cells were maintained in a humidified incubator with 5% CO2.
Reagents
Primary antibodies were purchased from the following companies: Dkk-1 (Novus Biologicals, Littleton, CO), PKCζ/λ pT410/403 (Cell Signaling Technology, Danvers, MA), M30 (Invitrogen, Carlsbad, CA), GM130, Rac-1 (BD Biosciences, San Jose, CA), γ-tubulin, Par6 (Abcam, Cambridge, MA), Cdc42, PKCζ (Santa Cruz Biotechnology, Santa Cruz, CA), and β-Actin, β-catenin (Sigma-Aldrich, St. Louis, MO). In some experiments, an inhibitory Dkk-1 antibody at a concentration of 20 μg/ml (R&D Systems, Minneapolis, MN) was added to cells. Human recombinant Dkk-1 (rDkk-1; R&D Systems) and mouse rDkk-1 were used in the experiments. Recombinant murine Dkk-1 carrying the Strep-tag (Schmidt and Skerra, 2007) was prepared by refolding of inclusion bodies expressed in Escherichia coli, followed by purification via streptavidin affinity chromatography and gel filtration. Based on preliminary studies, rDkk-1 was used at a concentration of 100 ng/ml, unless indicated otherwise. Medium or mouse rDkk-1 protein buffer (100 mM Tris-HCl, 0.5 M NaCl, 1 mM EDTA, and 0.1 mM lauryl maltoside) at appropriate dilutions were used as control. Dkk-1 small interfering RNA (siRNA) was obtained from Santa Cruz Biotechnology and transfected using TransIT-siQUEST (Mirus Bio, Madison, WI). The green fluorescent protein (GFP)-Wiskott-Aldrich syndrome protein (WASP)-GTPase binding domain (GBD) construct was produced as described previously (Kim et al., 2000) and transfected into cells with Lipofectamine 2000 (Invitrogen) by using standard protocols. An empty enhanced (e)GFP-encoding plasmid was used as control. Only cells with a low to intermediate expression level were regarded for analysis, because overexpression of the WASP-GBD construct causes cell death. For quantitative analysis, areas of fluorescent signals in flattened z-stacks of 10-μm thickness were measured using ImageJ software (National Institutes of Health, Bethesda, MD). Leading edge or lateral membrane association was assumed when the signal was within 5 μm of the cell border.
Wounding of Epithelial Monolayers
Before all functional assays, postconfluent cells were starved overnight in serum-reduced Opti-MEM medium (Invitrogen). For Western blot and microarray analysis, confluent monolayers grown on tissue culture plastic were scraped 10 times horizontally and vertically by using a 20-μl plastic pipette tip attached to low suction to induce migration in the majority of cells. For immunofluorescence staining and functional assays, cells grown on collagen-coated glass coverslips or tissue culture plastic, respectively, received one linear wound. Wounded monolayers were washed once with phosphate-buffered saline (PBS) to remove detached cells and debris, and incubated with the appropriate stimuli in medium. For cell migration experiments, the rate of migration was determined by measuring the entire wound area immediately after wounding and at the indicated time points, and by normalizing to the control condition.
Western Blot
Cells were scraped into radioimmunoprecipitation assay lysis buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, and 50 mM Tris, pH 8.0) containing protease and phosphatase inhibitors (Sigma-Aldrich), sonicated, and cleared by centrifugation. Protein concentration was determined using a bicinchoninic acid protein assay, and samples were boiled in SDS sample buffer with 50 mM dithiothreitol. Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis (PAGE), and transferred onto nitrocellulose membranes. Membranes were blocked for 1 h with 5% (wt/vol) dry milk or bovine serum albumin (BSA) in Tris-buffered saline containing 0.1% Tween 20, and incubated with primary antibodies in blocking buffer overnight at 4°C. Antibodies were detected using horseradish peroxidase (HRP)-linked secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) and chemoluminescent substrate (Denville Scientific, Metuchen, NJ). All bands were normalized to actin loading control, or GTPase input in the Cdc42/Rac-1 pull-down experiments. In PKC activity assays, total PKC levels were assessed as an additional internal control.
Immunofluorescence and Live Cell Microscopy
Cells grown on coverslips were fixed/permeabilized with either 100% methanol or ethanol at −20°C for 20 min or in 4% (wt/vol) paraformaldehyde for 10 min followed by 0.5% (vol/vol) Triton X-100 for 5 min. Cells were then blocked with 3% (wt/vol) BSA for 1 h and incubated with primary antibodies overnight at 4°C. After incubation with fluorophore-labeled secondary antibodies (Invitrogen) for 1 h, nuclei were stained with ToPro-3 iodide (Invitrogen), and coverslips were mounted in p-phenylene. Images were taken on an LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY) with Plan-NEOFLUAR 100×/1.3 oil, 40×/1.3 oil, and 10×/0.3 dry objectives, by using software supplied by the vendor. For live cell microscopy, medium was changed to CO2-independent medium (Invitrogen), and images acquired on an Axiovert 200M inverted microscope (Carl Zeiss) with a Plan-NEOFLUAR 20×/0.5 dry objective. The recording area was equilibrated to 37°C before each experiment using a heated cabinet and heated stage (Brook Industries, Lake Villa, IL). An AxioCam MRc5 camera (Carl Zeiss) and Axiovision 4.6 software supplied by the vendor were used to record movies, which were postprocessed using ImageJ software. Cells were tracked using MetaMorph (Molecular Devices, Sunnyvale, CA), by following multiple representative nuclei at the leading edge of the monolayer. For calcium release studies, confluent cells were pre-incubated with rDkk-1 (100 ng/ml) or buffer for 1 h, washed with Hanks' buffer, and loaded with 2 μM fluo-4 acetoxymethyl ester (Invitrogen) or dimethyl sulfoxide (DMSO) control, according to the supplier's recommendations. Cells were then wounded in situ, and movies recorded immediately after wounding.
Assessment of Directional Orientation, Proliferation, and Apoptosis
To determine the directional orientation of epithelial cells after wounding of confluent monolayers, MTOC and Golgi apparatus were visualized essentially as described previously (Etienne-Manneville and Hall, 2001). In brief, cells were prepared for microscopy using anti-γ-tubulin (MTOC) and anti-GM130 (Golgi) antibodies, with nuclear counterstain using ToPro-3 iodide. MTOC and Golgi within a 90° angle facing the wound edge were considered correctly oriented. At least 100 MTOC and Golgi stacks were assessed per experiment and data point. Only cells within 150 μm of the wound edge were included in the analysis. For quantitative analysis, only the orientation of the MTOC was considered, except for experiments with IEC-6 cells. In the same experiments, proliferation and apoptosis were assessed by counting noninterphase and pyknotic nuclei, respectively. A minimum of 40 images per condition from three or more independent experiments were analyzed. In some experiments, zVAD-fmk (Promega, Madison, WI) at a concentration of 20 μM or DMSO carrier was added to monolayers to inhibit caspase activation. Proliferation was additionally assessed in a 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay, by using a Click-iT EdU Alexa Fluor 488 cell proliferation assay kit (Invitrogen). Scratch-wounded cells were treated with rDkk-1 (100 ng/ml) or control overnight before addition of 10 μM EdU for 2 h. At least 1000 nuclei were analyzed per experiment and data point.
Dkk-1 Protein Enzyme-linked Immunosorbent Assay (ELISA)
To assess Dkk-1 secretion by epithelial cells, cleared supernatants were analyzed by enzyme-linked immunosorbant assay using mouse anti-Dkk-1 primary and biotinylated goat anti-Dkk-1 secondary antibody (both from R&D Systems). Recombinant Dkk-1 was used as standard. Streptavidin-HRP was used to detect antibody complexes.
Cdc42/Rac-1 Pull-Down Assay
Cells were washed and scraped into MLB lysis buffer (Millipore, Billerica, MA) containing protease and phosphatase inhibitors. Then, 900 μg of protein per sample was incubated with 20 μg of PAK1 PBD agarose beads (Millipore) for 1 h at 4°C. Beads were washed twice with lysis buffer and boiled in SDS sample buffer with 50 mM dithiothreitol. Samples were then subjected to SDS-PAGE and blotted with an anti-Cdc42 or anti-Rac-1 antibody. Ten micrograms of total protein per sample was loaded as input control.
Statistics
All experiments were performed at least three times. Dunnett's or Bonferroni's post test following one-way analysis of variance, or two-tailed Student's t test were used to analyze the data. p < 0.05 was considered statistically significant. Results are displayed as mean ± SEM.
Online Supplemental Video Files
Videos of migrating Caco-2 cells as described under Immunofluorescence and Live Cell Microscopy have been submitted for online publication. Movies of control (Supplemental Figure 3video1.mov) and rDkk-1 (100 ng/ml)–treated cells (Supplemental Figure 3video2.mov) were captured at 2 images/minute for 5 h and are shown at 7 frames/s. Representative movies of intracellular calcium release (control: supplvideo3.mov; rDkk-1: supplvideo4.mov) were captured at 60 images/min for 30 s and are shown at 7 frames/s. Calcium release in these videos is displayed in white pseudocolor.
RESULTS
Dkk-1 Is Secreted from Intestinal Epithelial Cells after Wounding
To identify potential new regulators of IEC migration, we performed a microarray analysis on confluent cells and cells that were scratch-wounded multiple times to induce cell migration. The Wnt inhibitor Dkk-1 was found to be one of the most significantly up-regulated genes in migrating cells (3.8 ± 1.2-fold of stationary control; p < 0.05). Because Dkk-1 has been shown to regulate Wnt/β-catenin signaling, and conversely, Dkk-1 is induced by β-catenin/TCF (Niida et al., 2004; Gonzalez-Sancho et al., 2005), we first investigated the kinetics of Dkk-1 expression and secretion by using Caco-2 model IECs. We observed that wounding of epithelial monolayers returned the cells to a nonquiescent state with active β-catenin signaling (Figure 1A). Migrating cells with increasing distance from the leading edge, indicated as position 1 through 4 in the figure, exhibited a gradient of nuclear β-catenin accumulation, with a significantly stronger β-catenin signal in cells proximal to the wound. Consequently, Dkk-1 protein expression was rapidly induced after wounding, with peak expression occurring at 24 h (3.0 ± 0.4-fold of 0 h; p < 0.01) (Figure 1B). By analyzing supernatants from cells under stationary (nonwounded) and migrating (scratch-wounded) conditions, we confirmed that increased Dkk-1 protein expression is reflected in an enhanced secretion of the molecule from epithelial cells (Figure 1C). We found that Dkk-1 was constitutively secreted by Caco-2 cells at an average rate of 421pg/106 cells/h. Furthermore, we observed that Dkk-1 release was markedly increased after wounding, with an average secretion rate of 680 pg/106 cells/h. A statistically significant difference in the concentration of Dkk-1 in the supernatant was seen at the 24-h time point (resting, 43.3 ± 8.8 ng/ml; migrating, 85.1 ± 10.0 ng/ml; p < 0.05), coinciding with a peak in protein expression.
Figure 1.
Migrating IECs return to a nonquiescent state with active β-catenin signaling and increased Dkk-1 expression. (A) Caco-2 cells migrating for 1 h were stained for β-catenin (green). Nuclei are shown in blue. Bar, 50 μm. Cells at position 1 through 4 with increasing distance from the leading edge (indicated in the image on the right) were analyzed for nuclear β-catenin signal intensity. The graph is representative of three independent experiments. *p < 0.01 versus position 1; †p < 0.01 versus position 2. (B) Kinetics of Dkk-1 protein expression in migrating Caco-2 cells were determined by Western blot analysis. A statistically significant increase was observed at 24 h (*p < 0.01 vs. 0 h). (C) Dkk-1 secretion was assessed by ELISA of supernatants from stationary or migrating cells. The protein was preferentially secreted from migrating cells, with a significant difference at 24 h (**p < 0.05 vs. 0 h).
Dkk-1 Inhibits Epithelial Restitution without Altering Proliferation
Previous reports have shown that Dkk-1 inhibits cell migration and proliferation in vitro and in vivo (Pinto et al., 2003; Qin et al., 2007; Wang et al., 2008). Thus, to investigate whether Dkk-1 inhibits the restitution of intestinal epithelial cells, we used an in vitro cell culture model, consisting of Caco-2 epithelial cells and rDkk-1. We observed that in the presence of rDkk-1 (20 and 100 ng/ml), wound closure was significantly inhibited after 24 h (62.0 ± 5.4 and 46.4 ± 11.0% of control) and 48 h (75.3 ± 3.2 and 57.1 ± 9.1% of control; p < 0.01) (Figure 2A and Table 1). This effect was reverted by addition of an inhibitory Dkk-1 antibody (24 h; 98.6 ± 3.7% of control). Intriguingly, we observed that treatment of Caco-2 cells with anti-Dkk-1 antibody for 48 h increased the migration rate of these cells (139.4 ± 5.4% of control; p < 0.05), suggesting that in the absence of inhibitory antibody, accumulation of secreted Dkk-1 acts to inhibit the migration of epithelial cells. To confirm that this effect is mediated by secreted Dkk-1 and not the intracellular pool of the protein, we down-regulated Dkk-1 expression and release in Caco-2 cells by using siRNA (Figure 2B). Under these conditions, we observed that addition of exogenous rDkk-1 significantly inhibited the migration of both scramble siRNA (45.1 ± 9.1%) and Dkk-1 siRNA (52.1 ± 11.2% of control; p < 0.01) treated cells after 24 h. In addition, consistent with previously published data (Qin et al., 2007), Dkk-1 knock-down alone increased cell migration (133.7 ± 5.1% of control; p < 0.05). To investigate if Dkk-1 also affects cell migration in nontransformed cells, the experiment was repeated using primary rat IEC-6 (Figure 2C). In agreement with our results from Caco-2 cells, we observed a significant inhibition of wound closure after 24 h when cells were treated with rDkk-1 (73.6 ± 0.04% of control; p < 0.01). This effect was not caused by reduced cell proliferation in the time frame of our study, as suggested by previous publications (Pinto et al., 2003; Wang et al., 2008), because no difference in the number of EdU-positive cells was observed after treatment with Dkk-1 (Figure 2D). This was additionally confirmed by Ki67 staining, which revealed no changes in the number non-G0 cells in the presence of Dkk-1 (data not shown). Furthermore, the number of mitotic cells at the wound edge after 24 h was not reduced in the presence of increasing concentrations of Dkk-1 (Figure 2E). These observations suggest that another mechanism is responsible for the attenuated wound closure in this system.
Figure 2.
Exogenous Dkk-1 inhibits the migration of intestinal epithelial cells without affecting proliferation. (A) Scratch-wounded Caco-2 monolayers were treated with buffer, rDkk-1, or rDkk-1 (100 ng/ml) + anti-Dkk-1 antibody (20 μg/ml). Vertical bars represent the position of the leading edge. Bar, 200 μm. *p < 0.01 versus control. (B) Knockdown of Dkk-1 in Caco-2 cells after 48 h was confirmed by Western blot. Addition of rDkk-1 to cells treated with the indicated siRNA significantly inhibited cell migration (*p < 0.01; **p < 0.05 vs. control). (C) Migrating primary IEC-6 cells were treated with buffer or rDkk-1 (100 ng/ml), and wound closure was assessed after 24 h (*p < 0.01 vs. control). (D) EdU incorporation (green) in migrating Caco-2 cells treated with rDkk-1 or buffer. Nuclei are shown in blue. No difference in the number of EdU-positive cells was seen at the leading edge and in the confluent monolayer. Bar, 50 μm. (E) Noninterphase nuclei were counted in migrating cells treated with increasing concentrations of rDkk-1. No difference to untreated control was observed.
Table 1.
Distancea of migration (in micrometers) of Caco-2 cells in the presence of buffer, rDkk-1, or rDkk-1 + anti-Dkk-1 antibody
| Control | rDkk-1(20 ng/ml) | rDkk-1(100 ng/ml) | rDkk-1(100 ng/ml) +anti-Dkk-1(20 μ g/ml) | |
|---|---|---|---|---|
| 24 h | 181.6 ± 10.8 | 124.4 ± 6.6* | 106.2 ± 7.4* | 162.4 ± 9.0 |
| 48 h | 329.6 ± 13.3 | 250.1 ± 11.7* | 197.2 ± 11.9* | 345.2 ± 14.8 |
* p < 0.01 versus control.
aDistances were measured at 10 random points per well, with five wells per condition. Data are representative of three independent experiments. Migration was significantly inhibited by Dkk-1 after 24 and 48 h.
Dkk-1 Attenuates Directional Cell Movement in Migrating Epithelial Cells
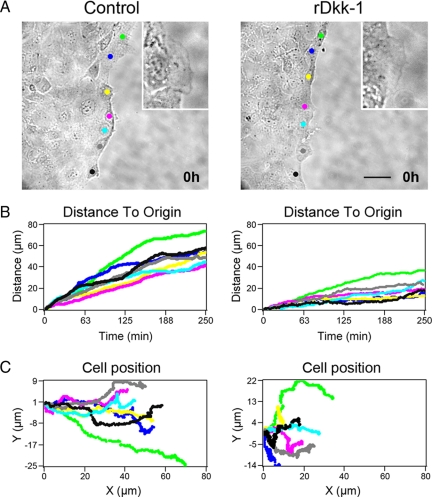
To analyze the kinetics of inhibition of IEC migration by Dkk-1, we performed live cell microscopy on migrating Caco-2 cells (Figure 3and Supplemental Video files). Cells were examined for a time period of 5 h, to elucidate whether inhibition of migration by Dkk-1 occurs early after wounding. Interestingly, cells treated with rDkk-1 exhibited prominent lamellipodial protrusions comparable with those seen in control monolayers (Figure 3A). Despite the prominence of lamellipodia observed in early time points in both conditions, computer-assisted tracking of cells at wound edges by following the movement of cell nuclei revealed greatly reduced net forward propulsion of the epithelial sheet in the presence of rDkk-1 (Figure 3B). Movement rates were fairly constant over the observation period, indicating that Dkk-mediated inhibition of migration is an early, sustained effect. Furthermore, computer analysis of cell movement revealed that treatment with rDkk-1 resulted in randomized migration of cells along the leading edge in contrast to control cells (Figure 3C and Supplemental Figure 1).
Figure 3.
Inhibition of migration by Dkk-1 is an early, sustained effect. (A) Migrating Caco-2 cells treated with buffer or rDkk-1 (100 ng/ml) were analyzed by live cell microscopy for 5 h. Still images are taken from Supplemental Figure 3video1.mov (control) and Supplemental Figure 3video2.mov (rDkk-1). Colored dots indicate the initial position of cells analyzed in B and C. Inset, magnified image of lamellipodia observed after 1 h. Bar, 50 μm. (B) Kinetics of migration of the same cells over 250 min. (C) Trace of the movement of multiple cells along the leading edge. Graphs are representative of three independent experiments.
Dkk-1 Inhibits Polarized Localization of Cdc42-GTP and Par6 in Migrating Intestinal Epithelial Cells
To understand the mechanism by which Dkk-1 attenuates epithelial restitution, we next investigated signaling molecules known to regulate cell migration. Rho family small GTPases, especially Cdc42, Rac-1, and RhoA, are critically involved in directional cell migration (Nobes and Hall, 1999; Evers et al., 2000; Fukata et al., 2003). Specifically, Cdc42 and Rac-1 induce the formation of filopodia and lamellipodia via activation of Arp2/3, whereas RhoA directs the assembly of contractile actin-myosin filaments (Jaffe and Hall, 2005). Additionally, Cdc42 has been identified as a key regulator of directional polarization in migrating cells (Stowers et al., 1995; Etienne-Manneville and Hall, 2001, 2003). Thus, to determine whether Dkk-1 inhibits cell migration by altering Rho GTPase activation, we performed pull-down assays for activated Rac-1 and Cdc42 by using PAK-GBD beads (Figure 4A). We observed that 1 h after wounding, the activity of Cdc42 and Rac-1 in migrating Caco-2 cells was strongly enhanced in the presence of rDkk-1 (Cdc42, 3.3 ± 0.9-fold; Rac-1, 3.6 ± 1.4-fold of control). In contrast, no persistent difference in RhoA activity was seen (data not shown). We next localized active Cdc42-GTP in migrating cells by using a GFP-WASP-GBD construct (Kim et al., 2000), because the correct distribution of active Cdc42 at the leading edge is crucial for the initiation of directional cell migration (Figure 4, B and C). In contrast to control monolayers, where active Cdc42 mainly localized to the leading edge of cells, Cdc42-GTP was redistributed to the cytosol and along the entire plasma membrane, including the rear of cells, in the presence of rDkk-1. No specific staining was seen when cells were transfected with an empty eGFP plasmid (data not shown). Overexpression of GFP-WASP-GBD did not alter the distribution of endogenous Cdc42, and GFP-WASP-GBD colocalized with total Cdc42 at intercellular junctions in confluent monolayers, as well as at the leading edge of migrating cells (Supplemental Figure 2). The observation that Cdc42 activity and localization is affected by Dkk-1 suggests that Dkk-1 may inhibit the directional polarization of migrating epithelial cells. To address this possibility, we investigated the distribution of the polarity protein Par6, which is recruited to the leading edge of migrating cells by Cdc42 (Etienne-Manneville and Hall, 2001) (Figure 4D). Whereas Par6 localized mainly to the front of control monolayers, the protein was displaced from the leading edge in the presence of rDkk-1. In contrast, lateral membrane association of Par6 in confluent monolayers was not affected by Dkk-1. Western blot analysis revealed that protein levels of Par6 were not affected in the time frame of these experiments (Supplemental Figure 3). Finally, we analyzed the phosphorylation state of the atypical protein kinase PKCζ, which is activated and recruited into the Cdc42/Par6 polarity complex in migrating cells (Figure 4E). Consistent with an increase in Cdc42 activity, we observed an increased phosphorylation of PKCζ after 1 h in the presence of rDkk-1 (2.2 ± 0.4-fold of control; p < 0.05). The activation of Cdc42 and PKCζ by Dkk-1 was specific for migrating cells, because no effect was observed in stationary (postconfluent) and spreading cells (Supplemental Figure 4). Together, these results suggest that Dkk-1 interferes with the correct assembly of the Cdc42/Par6/PKCζ polarity complex at the leading edge, thus attenuating directional migration.
Figure 4.
Dkk-1 affects the activity and localization of the Cdc42/Par6/aPKC polarity complex. (A) Migrating Caco-2 cells were treated with buffer or rDkk-1 (100 ng/ml) for 1 h, and activity of Cdc42 and Rac-1 was determined by pull-down of the GTP-bound protein. (B) Active Cdc42-GTP was localized after 1 h by transfection with a GFP-WASP-GBD plasmid. Active Cdc42 (green) accumulated at the front of the cell in control monolayers (arrowheads) but was distributed in the cytoplasm and along the entire cell membrane in the presence of rDkk-1. Phase contrast images were overlaid, and dashed lines were added to indicate cell borders. Bars, 20 μm. (C) Quantification of active Cdc42 distribution revealed a significant displacement from the leading edge in the presence of rDkk-1 (*p < 0.01; **p < 0.05 vs. control). A schematic representation of the analyzed areas is shown on the right. (D) Par6 (green) was localized at the leading edge and in the confluent monolayer of migrating cells. Nuclei are shown in blue. Leading edge (arrowheads), but not lateral membrane association (arrows) was lost in the presence of rDkk-1. Bars, 20 μm. (E) Activity of PKCζ was assessed by Western blot using a phospho-specific antibody. **p < 0.05 versus control.
Dkk-1 Induces Random Directional Polarity in Migrating Epithelial Cells
Formation of the Cdc42/Par6/PKCζ polarity complex at the leading edge drives the reorientation of the MTOC and Golgi apparatus in the direction of migration (Cau and Hall, 2005). We therefore localized the MTOC and Golgi in migrating Caco-2 cells treated with increasing concentrations of rDkk-1 (Figure 5A). We observed that rDkk-1 dose-dependently inhibited the orientation of cells toward the wound, as determined by the location of the MTOC after 24 h of migration (p < 0.01 vs. control). An almost complete loss of directional polarization was seen at high concentrations of rDkk-1. This phenotype was restored by addition of an inhibitory Dkk-1 antibody. In agreement with these results, we also observed some inhibition of microtubule reorientation in the presence of Dkk-1 (Supplemental Figure 5). Failure to establish directional polarity was observed as early as 6 h after wounding, at which point the majority of untreated Caco-2 cells have relocated their MTOC in the direction of migration (data not shown). To assess if Dkk-1 can inhibit directional polarization of nontransformed epithelial cells, the Golgi apparatus was localized in migrating primary IEC-6 cells after 24 h (Figure 5B). In good agreement with the results from Caco-2 cells, directional orientation was attenuated in the presence of rDkk-1 (control, 78.0 ± 3.3%; rDkk-1, 47.7 ± 3.5%; p < 0.01). We further tested whether directional orientation of cells after wounding was regulated by secreted Dkk-1, by depleting the intracellular protein pool in Caco-2 cells using siRNA (Figure 5C). Dkk-1 knockdown had no effect on directional polarization in response to incubation of cells with rDkk-1 protein. Although the role of Dkk-1 on epithelial cell polarization has not been investigated thus far, a previous report has shown that the protein does not affect the directional migration of fibroblasts (Schlessinger et al., 2007). Thus, to determine whether the observed effects are cell type specific, we repeated the above-mentioned migration and polarization experiments with a mouse fibroblast cell line. In agreement with the study by Schlessinger et al. (2007), the directional orientation of 3T6 cells was not altered in the presence of rDkk-1 (Supplemental Figure 6A). Furthermore, the rate of cell migration was equally unaffected by Dkk-1 (Supplemental Figure 6B), suggesting that the underlying mechanisms may have cell type-specific differences. The above-mentioned observations indicate that Dkk-1 inhibits epithelial cell migration by affecting early events during directional cell polarization. To investigate this idea, we added rDkk-1 at different time points after wounding, and we determined the rate of migration after 48 h (Figure 5D). Confirming our hypothesis, we observed that rDkk-1 only inhibited cell migration if applied within 1 h.
Figure 5.
Exogenous Dkk-1 inhibits the directional polarization of migrating IEC. (A) Caco-2 cells were allowed to migrate for 24 h in the absence or presence of different concentrations of rDkk-1. Images are taken from the leading edge of cells stained for MTOC (green) and Golgi (red). Nuclei are shown in blue, and the position of the leading edge is indicated with a dashed line. Arrowheads indicate orientation of cells. Bars, 20 μm. rDkk-1 dose-dependently inhibited directional orientation of migrating cells. *p < 0.01 versus control; †p < 0.01 versus rDkk-1 + Anti-Dkk-1 antibody. (B) The directional orientation of migrating primary IEC-6 cells was determined by localization of the Golgi apparatus after 24 h. rDkk-1 (100 ng/ml) significantly inhibited directional polarization (*p < 0.01 vs. control). (C) Caco-2 cells transfected with scramble or Dkk-1 siRNA were allowed to migrate for 24 h in the presence of buffer or rDkk-1 (100 ng/ml). Dkk-1 knockdown had no apparent effect on directional polarization. *p < 0.01 versus control. (D) Rate of migration of Caco-2 cells after 48 h was analyzed when rDkk-1 (100 ng/ml) was added at different time points after wounding. *p < 0.01; **p = 0.05 versus control.
Loss of Directional Polarization Is Not Caused by Induction of Apoptosis
It is conceivable that loss of directional orientation in the presence of Dkk-1 is secondary to induction of cell death. Indeed, Dkk-1 has been found to induce apoptosis in a variety of cell types (Peng et al., 2006; Mikheev et al., 2007; Kwack et al., 2008; Weng et al., 2009). We therefore examined whether Dkk-1 can promote cell death in intestinal epithelial cells and whether loss of directional orientation is a result of apoptosis induction. We observed a dose-dependent activation of caspase-3 in migrating Caco-2 cells treated with rDkk-1 for 1 h (Figure 6A). We also found significantly increased numbers of apoptotic cells at the leading edge of migrating Caco-2 monolayers after 24 h of treatment with high concentrations of rDkk-1 (control, 9.3 ± 1.0; 160 ng/ml, 20.6 ± 7.7; 1 μg/ml, 25.6 ± 6.5 apoptotic cells/mm2; p < 0.01) (Figure 6B). To determine whether caspase activation and apoptosis induction are the underlying cause of loss of directional polarization, migrating Caco-2 monolayers were concomitantly treated with rDkk-1 and the pan-Caspase inhibitor zVAD-fmk for 24 h (Figure 6C). Despite effective inhibition of apoptosis at the leading edge, cells treated with rDkk-1 still failed to establish directional polarity. These findings suggest that apoptosis induction is not responsible for lack of polarization of migrating IECs.
Figure 6.
Induction of apoptosis by Dkk-1 does not cause loss of directional polarity. (A) rDkk-1 dose-dependently induced caspase-3 activation in migrating Caco-2 cells after 1 h. (B) The number of apoptotic cells at the leading edge after 24 h was determined by counting of pyknotic nuclei. *p < 0.01; **p < 0.05 versus control. (C) Directional orientation of migrating IEC treated with buffer of rDkk-1 (100 ng/ml) was assessed by MTOC localization (white pseudocolor). Images were taken after 24 h of migration. Inhibition of caspase activation by zVAD-fmk did not attenuate loss of directional polarization. A single M30-positive apoptotic cell (green) can be seen on the right. Nuclei are shown in blue. Arrow heads indicate orientation of cells. Bars, 20 μm.
DISCUSSION
Epithelial restitution requires the controlled, directed migration of coherent cell sheets into the denuded area. In this report, we identify the secreted Wnt inhibitor Dkk-1 as a novel regulator of epithelial cell migration. Dkk-1 attenuates cell migration in both transformed and primary intestinal epithelial cells, which is caused by a failure to establish directional polarity after wounding of the monolayer. In previous studies, Wnt signaling has been identified as a positive regulator of cell migration (Ouko et al., 2004; Endo et al., 2005; Cheng et al., 2008; He et al., 2008). However, the role of secreted Wnt inhibitors in the modulation of cell motility is less well understood. Dkk-1 expression is controlled by the canonical Wnt pathway through β-catenin/TCF4 and is down-regulated in confluent, postmitotic Caco-2 cells (Gonzalez-Sancho et al., 2005; Saaf et al., 2007). Consistent with this model, wounding of the epithelial monolayer returned the leading edge cells to a nonquiescent state with active β-catenin signaling, and consequently, led to the induction and secretion of Dkk-1. Interestingly, we saw a similar increase in Dkk-1 in mucosal sections from chronic inflammatory bowel disease patients (Supplemental Figure 7). This agrees with a previous report showing that TNF-α rapidly induces Dkk-1 in arthritis (Diarra et al., 2007). In the large intestine, the Dkk-1 receptors LRP6 and Kremen-1 are primarily expressed in crypt enterocytes, suggesting that these cells are primary targets of Dkk-1 during inflammation.
Because Dkk-1 is a potent Wnt inhibitor, we hypothesized that the protein can inhibit epithelial cell migration. We therefore investigated this hypothesis by using recombinant Dkk-1 protein at physiologically low concentrations. These concentrations were based on preliminary studies investigating Dkk-1 protein levels in serum samples from mice with dextran sulfate sodium-induced colitis (control, 4 ng/ml; colitis, 16 ng/ml), and human patients suffering from Crohn's colitis (control, 8 ng/ml; colitis, 30 ng/ml; unpublished data). In agreement with previously published data (Qin et al., 2007), we observed that exposure of IECs to Dkk-1 inhibited cell migration, and conversely, depletion of the protein using antibodies or siRNA promoted wound closure. Several distinct and interconnected mechanisms drive cell migration after wounding. These include the reorientation of leading edge cells toward the wound, forward propulsion by the organized detachment from and reattachment to the extracellular matrix, and changes in proliferation and apoptosis. Previous studies have mainly focused on the latter aspect, because β-catenin/TCF4 transcription targets include a variety of proteins that control proliferation, such as c-myc and cyclin D1 (for a comprehensive list of targets, the reader is referred to http://www.stanford.edu/∼rnusse/wntwindow.html). In good agreement with this model, Dkk-1 has been found to inhibit Wnt-activated cell replication in vitro and in vivo (Pinto et al., 2003; Kuhnert et al., 2004; Qiao et al., 2008; Wang et al., 2008). However, restitution of small wounds is a rapid migratory process that is independent of proliferation (Silen and Ito, 1985). Consequently, although Dkk-1 effectively inhibited IEC migration, we observed no changes in proliferation. This agrees with a recent report showing that physiologically low concentrations of Dkk-1, as used in this study, do not affect the replication of colorectal carcinoma cell lines (Zhang et al., 2009). We therefore hypothesized that the Wnt inhibitor may regulate other signaling pathways which are involved in cell migration. Small GTPases of the Rho family are key modulators of cell polarity, adhesion, and motility, among others. Any signaling molecule that regulates the activity of these proteins may thus have profound effects on epithelial restitution. We found that exogenous Dkk-1 rapidly increased the activity of Cdc42 and Rac-1. This finding was surprising, because we and others have observed that activation of these GTPases is associated with increased migration of epithelial cells (Babbin et al., 2007; Itoh et al., 2008). It is interesting to note that increased IEC restitution through Rac-1 requires phospholipase Cγ1-induced Ca2+ release (Rao et al., 2008); however, we did not find any changes in intracellular Ca2+ release after wounding of confluent monolayers when cells were treated with Dkk-1 (control, Supplemental Video 3; rDkk-1, Supplemental Video 4). It is thus possible that Rac-1 may not regulate cell migration in this model system, or may have other functions not investigated here.
Given the above-mentioned observations, we performed experiments to localize active Cdc42 in migrating cells, because this GTPase has been identified as the initiator of directional cell polarity (Etienne-Manneville and Hall, 2001, 2003). We determined that Cdc42-GTP, which accumulated at the leading edge of untreated migrating IECs, redistributed randomly along the plasma membrane in the presence of Dkk-1. Consequently, in agreement with current models of epithelial cell polarization, Par6 was displaced from the leading edge in monolayers treated with Dkk-1. Furthermore, although we were not able to localize phosphorylated PKCζ in our cells, we observed an increased activation of PKCζ consistent with higher levels of Cdc42-GTP. We reasoned that interference with the correct assembly of this polarization complex should dramatically affect directional orientation of migrating cells. Indeed, the relocation of the MTOC and the Golgi apparatus in the direction of migration was significantly and dose-dependently attenuated in IECs treated with Dkk-1. Based on the results from our live cell microscopy studies, it is thus possible that the reduced net forward movement of the epithelial monolayer is caused by randomized migration of cells at the leading edge, rather than a reduced speed of migration of individual cells. Interestingly, Dkk-1 had no effect on the polarization of fibroblasts, in agreement with a previous report by Schlessinger et al. (2007). The notion that mechanisms governing cellular orientation are cell type specific is supported by a recent study showing that in epithelial cells, both Cdc42 and E-cadherin are essential in establishing directional polarity (Desai et al., 2009). In contrast, fibroblasts, which do not form cell-cell adhesions, may require different cues such as gradients of soluble proteins. Considering that noncanonical Wnt signaling pathways regulate directional polarity in a variety of cell types (Montcouquiol et al., 2006; Schlessinger et al., 2007; Yu et al., 2009), it is reasonable to assume that individual secreted Wnt agonists and antagonists have discrete effects on different target cell populations within the same tissue. Alternatively, Dkk-1 may modulate noncanonical Wnt signaling, which has been previously suggested (Caneparo et al., 2007; Korol et al., 2008).
Another mechanism by which Dkk-1 might inhibit cell migration is through induction of apoptosis. Indeed, we observed a rapid activation of Caspase-3 in migrating IECs treated with Dkk-1 and an increased number of apoptotic cells at the front of the cell sheet. However, the relatively small increase in apoptotic cells observed in this study suggests that although such mechanisms may contribute to attenuated wound closure, they do not explain the magnitude of the effect. In summary, we propose that Dkk-1 plays a key role in governing epithelial cell migration by controlling directional polarization. Our data suggest that mobilization of the quiescent epithelial monolayer by wounding causes an activation of β-catenin signaling at the leading edge, and the rapid movement of the cell sheet into the denuded area. We hypothesize that in the later stages of epithelial restitution, the secretion of Dkk-1 induced by β-catenin/TCF4 facilitates the return to a resting state by inhibiting directional orientation. This mechanism is not protein synthesis or cell division dependent, and it may thus be critical for the closure of small wounds of the intestinal mucosa, such as those caused by mechanical injury or mild inflammatory lesions. Inhibition of cell migration in later stages of wound closure could ensure establishment of the epithelial barrier and prevent continued movement of cells. In this context, it is interesting to note that epigenetic silencing of Wnt inhibitors is commonly observed in gastrointestinal cancer (Aguilera et al., 2006; Maehata et al., 2008). We therefore hypothesize that altered Dkk-1 expression influences not only closure of small mucosal wounds but also cancer growth and metastasis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kirsten Gerner-Smidt for expert technical assistance. This work was supported by National Institutes of Health grants DK-61379, DK-79392, and DK-72564 (to C.T.C., and C.A.P.) and DK-055679 (to A. N.) and by the Crohn's & Colitis Foundation of America (to S. K., S. S., P. N.).
Abbreviations used:
- Dkk-1
Dickkopf-1
- IEC
intestinal epithelial cell
- MTOC
microtubule organizing center
- rDkk-1
recombinant Dkk-1.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-05-0415) on September 23, 2009.
REFERENCES
- Aguilera O., Fraga M. F., Ballestar E., Paz M. F., Herranz M., Espada J., Garcia J. M., Munoz A., Esteller M., Gonzalez-Sancho J. M. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25:4116–4121. doi: 10.1038/sj.onc.1209439. [DOI] [PubMed] [Google Scholar]
- Babbin B. A., Jesaitis A. J., Ivanov A. I., Kelly D., Laukoetter M., Nava P., Parkos C. A., Nusrat A. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J. Immunol. 2007;179:8112–8121. doi: 10.4049/jimmunol.179.12.8112. [DOI] [PubMed] [Google Scholar]
- Bafico A., Liu G., Yaniv A., Gazit A., Aaronson S. A. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Caneparo L., Huang Y. L., Staudt N., Tada M., Ahrendt R., Kazanskaya O., Niehrs C., Houart C. Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/beta catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes Dev. 2007;21:465–480. doi: 10.1101/gad.406007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau J., Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- Cheng C. W., Yeh J. C., Fan T. P., Smith S. K., Charnock-Jones D. S. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem. Biophys. Res. Commun. 2008;365:285–290. doi: 10.1016/j.bbrc.2007.10.166. [DOI] [PubMed] [Google Scholar]
- de Santa Barbara P., van den Brink G. R., Roberts D. J. Development and differentiation of the intestinal epithelium. Cell Mol. Life Sci. 2003;60:1322–1332. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R. A., Gao L., Raghavan S., Liu W. F., Chen C. S. Cell polarity triggered by cell-cell adhesion via E-cadherin. J. Cell Sci. 2009;122:905–911. doi: 10.1242/jcs.028183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir M., Montgomery E. A., Glockner S. C., Schuebel K. E., Hooker C. M., Herman J. G., Baylin S. B., Gearhart S. L., Ahuja N. Epigenetic regulation of WNT signaling pathway genes in inflammatory bowel disease (IBD) associated neoplasia. J. Gastrointest. Surg. 2008;12:1745–1753. doi: 10.1007/s11605-008-0633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra D., et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- Dignass A. U. Mechanisms and modulation of intestinal epithelial repair. Inflamm. Bowel Dis. 2001;7:68–77. doi: 10.1097/00054725-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Endo Y., Wolf V., Muraiso K., Kamijo K., Soon L., Uren A., Barshishat-Kupper M., Rubin J. S. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J. Biol. Chem. 2005;280:777–786. doi: 10.1074/jbc.M406391200. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- Evers E. E., Zondag G. C., Malliri A., Price L. S., ten Klooster J. P., van der Kammen R. A., Collard J. G. Rho family proteins in cell adhesion and cell migration. Eur. J. Cancer. 2000;36:1269–1274. doi: 10.1016/s0959-8049(00)00091-5. [DOI] [PubMed] [Google Scholar]
- Fedi P., Bafico A., Nieto Soria A., Burgess W. H., Miki T., Bottaro D. P., Kraus M. H., Aaronson S. A. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J. Biol. Chem. 1999;274:19465–19472. doi: 10.1074/jbc.274.27.19465. [DOI] [PubMed] [Google Scholar]
- Fukata M., Nakagawa M., Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr. Opin. Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Glinka A., Wu W., Delius H., Monaghan A. P., Blumenstock C., Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gollob J. A., Sciambi C. J., Huang Z., Dressman H. K. Gene expression changes and signaling events associated with the direct antimelanoma effect of IFN-gamma. Cancer Res. 2005;65:8869–8877. doi: 10.1158/0008-5472.CAN-05-1387. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho J. M., Aguilera O., Garcia J. M., Pendas-Franco N., Pena C., Cal S., Garcia de Herreros A., Bonilla F., Munoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24:1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- He F., Xiong W., Yu X., Espinoza-Lewis R., Liu C., Gu S., Nishita M., Suzuki K., Yamada G., Minami Y., Chen Y. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development. 2008;135:3871–3879. doi: 10.1242/dev.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh R. E., Kiyokawa E., Aoki K., Nishioka T., Akiyama T., Matsuda M. Phosphorylation and activation of the Rac1 and Cdc42 GEF Asef in A431 cells stimulated by EGF. J. Cell Sci. 2008;121:2635–2642. doi: 10.1242/jcs.028647. [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Li Z., Sacks D. B. E-cadherin-mediated cell-cell attachment activates Cdc42. J. Biol. Chem. 2000;275:36999–37005. doi: 10.1074/jbc.M003430200. [DOI] [PubMed] [Google Scholar]
- Korol O., Gupta R. W., Mercola M. A novel activity of the Dickkopf-1 amino terminal domain promotes axial and heart development independently of canonical Wnt inhibition. Dev. Biol. 2008;324:131–138. doi: 10.1016/j.ydbio.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F., Davis C. R., Wang H. T., Chu P., Lee M., Yuan J., Nusse R., Kuo C. J. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwack M. H., Sung Y. K., Chung E. J., Im S. U., Ahn J. S., Kim M. K., Kim J. C. Dihydrotestosterone-inducible Dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Invest. Dermatol. 2008;128:262–269. doi: 10.1038/sj.jid.5700999. [DOI] [PubMed] [Google Scholar]
- Maehata T., Taniguchi H., Yamamoto H., Nosho K., Adachi Y., Miyamoto N., Miyamoto C., Akutsu N., Yamaoka S., Itoh F. Transcriptional silencing of Dickkopf gene family by CpG island hypermethylation in human gastrointestinal cancer. World J. Gastroenterol. 2008;14:2702–2714. doi: 10.3748/wjg.14.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev A. M., Mikheeva S. A., Rostomily R., Zarbl H. Dickkopf-1 activates cell death in MDA-MB435 melanoma cells. Biochem. Biophys. Res. Commun. 2007;352:675–680. doi: 10.1016/j.bbrc.2006.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M., Crenshaw E. B., 3rd, Kelley M. W. Noncanonical Wnt signaling and neural polarity. Annu. Rev. Neurosci. 2006;29:363–386. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Niida A., Hiroko T., Kasai M., Furukawa Y., Nakamura Y., Suzuki Y., Sugano S., Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouko L., Ziegler T. R., Gu L. H., Eisenberg L. M., Yang V. W. Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J. Biol. Chem. 2004;279:26707–26715. doi: 10.1074/jbc.M402877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Miao C., Li J., Fan X., Cao Y., Duan E. Dickkopf-1 induced apoptosis in human placental choriocarcinoma is independent of canonical Wnt signaling. Biochem. Biophys. Res. Commun. 2006;350:641–647. doi: 10.1016/j.bbrc.2006.09.087. [DOI] [PubMed] [Google Scholar]
- Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L., Xu Z. L., Zhao T. J., Ye L. H., Zhang X. D. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;269:67–77. doi: 10.1016/j.canlet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Qin X., Zhang H., Zhou X., Wang C., Zhang H., Zhang X., Ye L. Proliferation and migration mediated by Dkk-1/Wnt/beta-catenin cascade in a model of hepatocellular carcinoma cells. Transl. Res. 2007;150:281–294. doi: 10.1016/j.trsl.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Rao J. N., Liu S. V., Zou T., Liu L., Xiao L., Zhang X., Bellavance E., Yuan J. X., Wang J. Y. Rac1 promotes intestinal epithelial restitution by increasing Ca2+ influx through interaction with phospholipase C-(gamma)1 after wounding. Am. J. Physiol. Cell Physiol. 2008;295:C1499–C1509. doi: 10.1152/ajpcell.00232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaf A. M., Halbleib J. M., Chen X., Yuen S. T., Leung S. Y., Nelson W. J., Brown P. O. Parallels between global transcriptional programs of polarizing Caco-2 intestinal epithelial cells in vitro and gene expression programs in normal colon and colon cancer. Mol. Biol. Cell. 2007;18:4245–4260. doi: 10.1091/mbc.E07-04-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K., McManus E. J., Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J. Cell Biol. 2007;178:355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T. G., Skerra A. The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nat. Protoc. 2007;2:1528–1535. doi: 10.1038/nprot.2007.209. [DOI] [PubMed] [Google Scholar]
- Semenov M. V., Tamai K., Brott B. K., Kuhl M., Sokol S., He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Silen W., Ito S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu. Rev. Physiol. 1985;47:217–229. doi: 10.1146/annurev.ph.47.030185.001245. [DOI] [PubMed] [Google Scholar]
- Stowers L., Yelon D., Berg L. J., Chant J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc. Natl. Acad. Sci. USA. 1995;92:5027–5031. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. E., Wang X. D., Ernst J. A., Polakis P., Gao W. Q. Regulation of epithelial branching morphogenesis and cancer cell growth of the prostate by Wnt signaling. PLoS ONE. 2008;3:e2186. doi: 10.1371/journal.pone.0002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng L. H., Wang C. J., Ko J. Y., Sun Y. C., Su Y. S., Wang F. S. Inflammation induction of Dickkopf-1 mediates chondrocyte apoptosis in osteoarthritic joint. Osteoarthritis Cartilage. 2009;17:919–929. doi: 10.1016/j.joca.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Yu J., Carroll T. J., Rajagopal J., Kobayashi A., Ren Q., McMahon A. P. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. H., Walker F., Kiflemariam S., Whitehead R. H., Williams D., Phillips W. A., Mikeska T., Dobrovic A., Burgess A. W. Selective inhibition of proliferation in colorectal carcinoma cell lines expressing mutant APC or activated B-Raf. Int. J. Cancer. 2009;125:297–307. doi: 10.1002/ijc.24289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.