Abstract
Embryonic development of the pancreas is marked by an early phase of dramatic morphogenesis, in which pluripotent progenitor cells of the developing pancreatic epithelium give rise to the full array of mature exocrine and endocrine cell types. The genetic determinants of acinar and islet cell lineages are somewhat well defined; however, the molecular mechanisms directing ductal formation and differentiation remain to be elucidated. The complex ductal architecture of the pancreas is established by a reiterative program of progenitor cell expansion and migration known as branching morphogenesis, or tubulogenesis, which proceeds in mouse development concomitantly with peak Pdx1 transcription factor expression. We therefore evaluated Pdx1 expression with respect to lineage-specific markers in embryonic sections of the pancreas spanning this critical period of duct formation and discovered an unexpected population of nonislet Pdx1-positive cells displaying physical traits of branching. We then established a 3D cell culture model of branching morphogenesis using primary pancreatic duct cells and identified a transient surge of Pdx1 expression exclusive to branching cells. From these observations we propose that Pdx1 might be involved temporally in a program of gene expression sufficient to facilitate the biochemical and morphological changes necessary for branching morphogenesis.
INTRODUCTION
The primary function of the exocrine pancreas is to produce and secrete digestive enzymes for export to the small intestine. The collection and transport of these enzymes are facilitated by an intricate ductal network of branched epithelial tubules, such that enzymes secreted into smaller peripheral ducts ultimately feed into the larger main pancreatic duct, which in turn flows into the duodenum. This complex structure is established during embryonic development by a coordinated mechanism of progenitor cell proliferation and migration known as branching morphogenesis (Jorgensen et al., 2007). Some of the genetic and biochemical events directing this process are shared among the many organs served by ductal networks—such as the lung, breast, and kidney—and are also somewhat conserved across species (Lu and Werb, 2008). In general, branching morphogenesis is initiated by mesenchymal signaling to epithelial cell growth factor receptors, inducing multiple responses within those epithelial cells to 1) undertake temporary cytoskeletal reorganization that will facilitate cell motility, 2) inhibit proliferation, and 3) suppress the original mesenchymal growth factor signal (Metzger and Krasnow, 1999; Affolter et al., 2003). The specific mesenchymal growth factors and epithelial growth factor receptors, their cognate signaling pathways, and transcription factors involved in such processes contributing to branching morphogenesis in the pancreas are not well understood and remain to be elucidated.
Pancreatic organogenesis is dependent on the homeodomain transcription factor Pdx1, as demonstrated by pancreatic agenesis observed in Pdx1-null mice (Ahlgren et al., 1996; Offield et al., 1996). In the developing mouse embryo, Pdx1-expressing cells are first observed at embryonic day 8.5 (E8.5), prior even to the earliest indication of morphogenesis, in endodermal cells designated to give rise to the pancreas (Ahlgren et al., 1996; Li et al., 1999). Although Pdx1 is detected in cells of E8.5, the emerging pancreatic dorsal and ventral buds (E9.5) and even the early stages of invaginating pancreatic epithelium (E10.5), Pdx1 is not required for these developmental stages, as demonstrated by early pancreatic bud formation and invagination observed in Pdx1−/− mice (Ahlgren et al., 1996; Offield et al., 1996; Kim and MacDonald, 2002). However, the subsequent program of branching morphogenesis that establishes the more complex ductal network does not occur in the absence of Pdx1, as demonstrated by complete absence of these structures in Pdx1-null mice. Importantly, recombinant cultures of Pdx1−/− pancreatic epithelium (E10.5) and wild-type pancreatic mesenchyme (E10.5) fail to proliferate in vitro, indicating that the Pdx1 transcription factor is an essential mediator of mesenchymal signaling at this critical stage of epithelial proliferation and ductal network formation (Ahlgren et al., 1996). Additionally, it is at just this time of progression from bud formation to ductal network that branching cells are thought to transiently lose their epithelial nature and acquire a more motile and invasive phenotype, similar to a full or partial epithelial–mesenchymal transition (EMT; Pollack et al., 1998; Affolter et al., 2003; O'Brien et al., 2004). Thus, the spatiotemporal requirement for Pdx1 function at the commencement of branch initiation, and the general nature of cells at this developmental transition to shed their epithelial characteristics, suggest that Pdx1 may govern the transcriptional program necessary to induce these morphogenetic changes. It has been suggested that the molecular mechanism of branching morphogenesis represents a process of controlled invasion during embryonic development and that these same mechanisms might be exploited in a neoplastic setting to produce the uncontrolled invasion observed in tumorigenic cells (Fata et al., 2004). Therefore, it is of interest to both developmental biology and pancreatic cancer biology that these mechanisms are more clearly defined.
We propose that expression of Pdx1 initiates branching morphogenesis in the developing pancreas by activating this program of cytoskeletal reorganization and cell migration. We have previously developed and characterized primary mouse pancreatic duct cells that have the capacity to form spheroid cysts when cultured in three-dimensional (3D) matrices (Schreiber et al., 2004; Deramaudt et al., 2006). Such cysts are distinguished by a well-organized scheme of contiguous polarized epithelial cells surrounding a hollow lumen. Now we have established a model system that recapitulates branching morphogenesis in primary pancreatic ductal epithelial cells, and using this approach, we now describe that these spheroid cysts can form tubules or branches, both primary and secondary, that display striking expression of Pdx1 at the initiation of branching morphogenesis, which is followed by loss of Pdx1 during mature branching. We also report remarkable similarity during branching morphogenesis in the developing mouse embryonic pancreas.
MATERIALS AND METHODS
3D Cell Culture
Primary pancreatic ductal epithelial cells (PDCs) were isolated from wild-type animals and then cultured and passaged on collagen-coated plates in fully supplemented medium, as described previously (Schreiber et al., 2004). For 3D culture of PDC cysts, cells grown in 2D on collagen-coated plates were treated sequentially with 1 mg/ml collagenase and 0.05% trypsin to generate single-cell suspensions. 3D PDC cultures were established from single-cell suspensions in one of three ways as specified in the text: embedding in a 2 mg/ml bovine collagen solution (Nutragen from INAMED, Palo Alto, CA) as described by O'Brien et al., (2006); embedding in a 50% Matrigel Basement Membrane Matrix solution (BD Biosciences, San Jose, CA); or as “on-top” Matrigel cultures in which PDCs resuspended in a 5% Matrigel solution are seeded in culture dishes precoated with a 100% Matrigel layer (Lee et al., 2007). All 3D cultures were maintained in full PDC medium that was changed every other day and were incubated at 37°C and 5% CO2.
Immunofluorescence and Confocal Microscopy
PDC cysts embedded in collagen and cultured in Lab-Tek chamber slides (Nunc, Rochester, NY) were fixed and stained according to the methods previously published by O'Brien and Mostov (O'Brien et al., 2006). In brief, collagen cultures were treated with collagenase, fixed in 4% paraformaldehyde (PFA), then incubated in a 0.025% saponin, fish skin gelatin permeabilization solution, and finally treated with RNase A before incubation with primary and secondary antibodies diluted in permeabilization solution: goat anti-Pdx1 1:250 (Santa Cruz Biotechnology, Santa Cruz, CA), Cy2-donkey anti-goat 1:600 (Molecular Probes, Eugene, OR), FITC-phalloidin and AlexaFluor-phalloidin 1:600 (Invitrogen, Carlsbad, CA). After antibody incubations, cultures were postfixed in 4% PFA, counterstained with DAPI, and mounted directly onto slides with ProLong mounting media (Invitrogen, Carlsbad, CA). PDC cysts embedded in Matrigel and cultured in Lab-Tek chamber slides were fixed in 4% PFA for 10 min, permeabilized in 0.1% Triton X-100 in PBS for 5 min, and blocked in 1% BSA in PBS for 1 h, all steps at room temperature (RT). Cultures were then incubated for 1 h each with primary and secondary antibodies diluted in 1% BSA in PBS, washed, DAPI-stained, and mounted directly onto chamber slides with ProLong mounting media. Fixed and stained cysts were photographed on the Zeiss LSM-510 Meta confocal microscope (Thornwood, NY).
Immunohistochemistry
Pancreatic tissue was harvested from Pdx1:lacZ reporter mice (Offield et al., 1996) at different embryonic stages and processed for histochemical detection of β-galactosidase activity in conjunction with other lineage markers as previously described (Song et al., 1999). Dissected embryonic pancreas from wild-type E10.5–E16.5 embryos were fixed in 4% PFA overnight at 4°C, cryoprotected in 30% sucrose-PBS for 4–6 h at 4°C, OCT-embedded, and cut into 3–4-μm sections. Sections were permeabilized for 15–30 min in 0.2% Triton X-100 in PBS and blocking of unspecific reactivity was performed for 1 h in 10% FBS-0.2% Triton X-100 in PBS at RT. Primary antibodies were incubated at the appropriate dilutions in 5% FBS-0.2% Triton X-100 in PBS overnight: rabbit anti-amylase 1:400 (Sigma, St. Louis, MO), rabbit anti-Pan-Keratin 1:300 (Dako, Carpinteria, CA), rat anti-K19 1:200 (monoclonal anti-Troma-III, University of Iowa), goat anti-Pdx1 1:10000 (gift from C. V. Wright, Vanderbilt University), guinea pig anti-insulin 1:400 (Biomeda, Foster City, CA), rat anti-E-cadherin 1:400 (Zymed, South San Francisco, CA). The next morning slides were washed three times in 0.2% Triton X-100 in PBS, and sections were incubated with the appropriate secondary cy2- and/or cy3- and/or cy5-conjugated secondary IgG antibodies at 1:200 dilution for 1 h at RT in the dark. After three more washes in PBS the nuclei were labeled with DAPI (1:1000) and mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Images were acquired using a Zeiss Axiovert 200 M imaging microscope.
RESULTS
Pdx1 Is Differentially Expressed in the Ductal Epithelium throughout Pancreatic Organogenesis
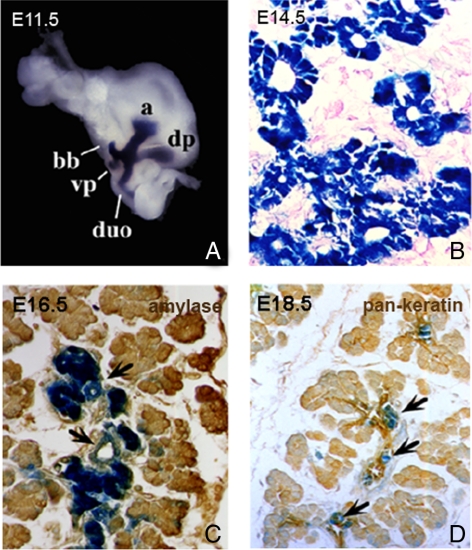
We first sought to determine if Pdx1 expression correlates with ductal epithelial formation and maturation during pancreatic development. For this purpose we utilized Pdx1:lacZ transgenic mice, in which a β-galactosidase reporter gene is expressed under regulation of a 4.5-kb Pdx1 promoter element (Offield et al., 1996), to assess dynamic Pdx1 expression in the developing mouse pancreas in conjunction with additional markers of acinar (amylase) and ductal (keratin) differentiation (Figure 1). To that end, we determined that Pdx1 (as assessed by lacZ with ∼24 h of prolonged expression) was expressed throughout the epithelium at E11.5 and E14.5, before widespread detection of amylase or keratin (Figure 1, A and B). By E16.5, Pdx1 expression was down-regulated in amylase-positive acinar cells as they achieved a fully differentiated phenotype, but still was expressed in ductal epithelium and in islet tissue (Figure 1C). In this series of lacZ staining, we could not detect the pan-keratin marker of duct cells until E18.5, which coincided with Pdx1 down-regulation in pancreatic ducts (Figure 1D). Therefore, Pdx1 is expressed during the formation of the ductal epithelium and is down-regulated upon maturity of pancreatic ducts, similar to that observed for precursor versus mature acinar cells, as expected because adult pancreatic ducts and acini typically do not express Pdx1.
Figure 1.
Onset of differentiated gene expression in exocrine pancreas. (A) Pdx1 expression domain indicated by Xgal staining in Pdx1:lacZ/+ transgenic mice. E11.5, isolated foregut from E11.5 mouse embryo demonstrating Pdx1 expression in posterior foregut. a, gastric antrum; dp, dorsal pancreatic bud; vp, ventral pancreatic bud; bb, biliary bud; duo, duodenum. (B) E14.5, Xgal/eosin-stained section demonstrates Pdx1 expression in entire pancreatic epithelium. (C) E16.5, Xgal/anti-amylase–stained section shows down-regulation of Pdx1 expression in developing acinar cells, ongoing expression in immature ductal epithelium (arrows), and budding islet cells. (D) E18.5, Xgal/anti-keratin–stained section shows down-regulation of Pdx1 expression in keratin-positive mature ductal epithelium. Note ongoing expression in budding islets (arrows). Original magnification 40×.
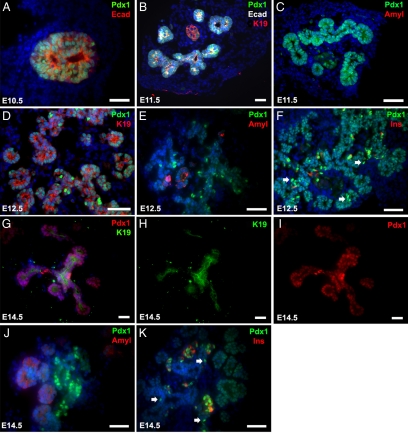
We next sought to closely evaluate Pdx1 expression in the pancreatic epithelium throughout the more precise period of branching morphogenesis. Early morphogenesis of the pancreatic epithelium commences at approximately E10.5, after formation of the dorsal and ventral buds (Pictet et al., 1972; Gittes, 2008) and proceeds until at least E14.5 when the bulk of the ductal network has been established. Pdx1 is reported to be uniformly expressed in the developing pancreas beginning at E8.5 when expression is evident in pancreatic progenitor cells of the foregut endoderm, until E12.5 when islet and acinar cell differentiation programs commence (Ahlgren et al., 1996). At this time, Pdx1 expression begins to vary among the differentiating cell types of the emerging pancreas. With the aim of elucidating distinct Pdx1 expression patterns unique to emerging ductal structures, we evaluated Pdx1 expression within embryonic sections from the E10.5–E14.5 period of branching morphogenesis with respect to markers specific for epithelial (E-cadherin), ductal (keratin-19, K19), acinar (amylase), and islet (insulin) cells (Figure 2). All pancreatic epithelial cells at E10.5 and E11.5 are uniformly Pdx1-positive (Figure 2, A and B). As expected at this early stage of development, neither amylase (Figure 2C) nor insulin (data not shown) are yet detectable at E11.5, although the ductal marker K19 is observed in all Pdx1-positive epithelial cells (Figure 2B).
Figure 2.
Differential Pdx1 expression in the developing pancreas. (A and B) E10.5, Pdx1/E-cadherin–stained section and E11.5, Pdx1/E-cadherin/Keratin-19–stained section demonstrate uniform Pdx1 staining throughout pancreatic epithelium and positive K19 ductal marker staining throughout epithelium beginning at E11.5. Note Pdx1-negative DAPI-staining cells are surrounding pancreatic mesenchyme. (C) E11.5, Pdx1/Amylase-stained section shows complete absence of amylase-positive acinar cells at this developmental stage. (D and G) Pdx1/K19-stained sections at E12.5 and E14.5, respectively, illustrate emergence of Pdx1hi subpopulation of cells at E12.5, and persisting at E14.5, within otherwise uniformly Pdx1-positive and K19-positive epithelia. (E and J) Pdx1/Amylase-stained sections at E12.5 and E14.5, respectively, show that no Pdx1hi cells coexpress the acinar cell marker amylase. (F and K) Pdx1/Insulin-stained sections at E12.5 and E14.5, respectively, display a majority of cells that are both Pdx1hi- and insulin-positive, indicative of differentiating islet cells, but also demonstrate a subpopulation of cells that are Pdx1hi- and insulin-negative, denoted by arrows. (H) K19-positive cells (for comparison to G); (I) Pdx-positive cells (for comparison to G). Scale bar, 50 um.
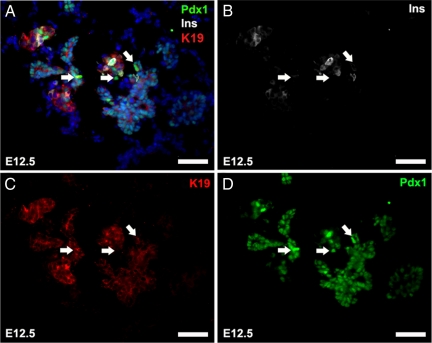
The first evidence of differential Pdx1 expression within embryonic pancreas sections is observed at E12.5 (Figure 2, D–F). Although Pdx1 expression persists in all epithelial cells at this developmental stage, there are cells evident within each section in which the signal is distinctly more intense, henceforth referred to as Pdx1hi cells. Already at E12.5 and continuing at E14.5, a striking pattern of normal versus Pdx1hi cells is established (Figure 2, D and G). With respect to ductal marker costaining, no obvious pattern of Pdx1hi cells is evident (Figure 2, G and I); however, when compared with acinar marker staining, amylase is never detectable in Pdx1hi cells (Figure 2, E and J). Intriguingly, we observe with insulin costaining that all insulin-positive cells are Pdx1hi, but not all Pdx1hi cells are insulin-positive (Figure 2, F and K). The subpopulations of Pdx1hi/insulin-negative epithelial cells that are observed at E12.5 and E14.5 coincide with a significant phase of branching morphogenesis, suggesting that at these time points a surge in Pdx1 expression serves a purpose other than islet differentiation. On closer inspection of such cells at E12.5, when both branching morphogenesis and islet differentiation are in play, we observed several examples of Pdx1hi/insulin-negative epithelial cells that appear to be at an initial stage of migrating away from neighboring epithelial cells (Figure 3, A–C; arrows identify specific examples). These Pdx1hi cells may represent branching epithelia.
Figure 3.
Pdx1 expression in nonislet pancreatic epithelial cells. E12.5 pancreas sections stained for Pdx1, insulin, K19 and DAPI. (A) Merged view. (B) insulin. (C) K19. (D) Pdx1. Arrows denote some Pdx1hi cells that are insulin-negative. Scale bar, 50 μm.
3D PDC Cultures Recapitulate Branching Morphogenesis
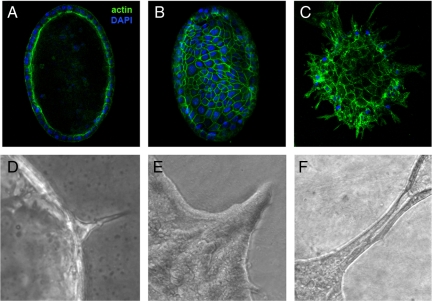
We next attempted to model his phenomenon in vitro. We established 3D cell culture conditions for early-passage primary PDCs isolated from wild-type mice and cultured them in standard medium for up to 3 wks (Figure 4). Within 3 days of culture in Matrigel matrix, PDCs formed hollow spheres of polarized cells, or cysts (Figure 4, A and B), which continued to grow after additional days of culture. PDCs grown in 2% bovine collagen, however, also formed hollow cysts within 3–4 days of culture but then proliferation slowed and cyst cells began to form spikes and extensions, which represent the initial stages of branching morphogenesis (Figure 4C). Branching morphogenesis proceeded in standard medium, without the supplementation of additional growth factors. This suggests that factors produced by the PDCs, components of the complex PDC media, and/or the collagen matrix provide sufficient signals to initiate branching within individual cells. Notably, PDCs embedded in Matrigel proliferate but do not form branches or ducts (data not shown), indicating that specific cell–matrix interactions are critical to activate signal transduction pathways that will ultimately induce branch initiation. Branch formation in our 3D collagen cultures begins as a process of cytoskeletal rearrangement within individual cells, resulting in the formation of long extensions or spikes reaching outward into the collagen matrix (Figure 4D). Branching cells continue to migrate outward into the matrix environment and proliferate to form cords of polarizing cells that begin to surround a luminal space (Figure 4E). Ultimately a fully formed duct or tubule of polarized cells encompassing a hollow lumen is evident (Figure 4F), demonstrating that our 3D collagen systems are sufficient to support the process of branching morphogenesis to completion.
Figure 4.
In vitro branching morphogenesis of PDCs when cultured in 3D. PDCs form hollow spheroid cysts of polarized cells when cultured in 3D matrices and undergo branching morphogenesis when cultured in collagen gels. (A) Central plane of cyst grown for 3 days in Matrigel depicting hollow lumen. (B) Top surface of same cyst. (C) The same cells form cysts when grown in collagen gels, and after 3–4 d in culture commence branching morphogenesis. Actin (green) and DNA (blue). Branching morphogenesis within collagen gels proceeds through all stages from early extensions (D) or spikes to cords of polarized cells (E) beginning to form a hollow lumen to mature tubules (F). Original magnification, ×20.
Pdx1 Is Expressed in Branching Pancreatic Duct Cells
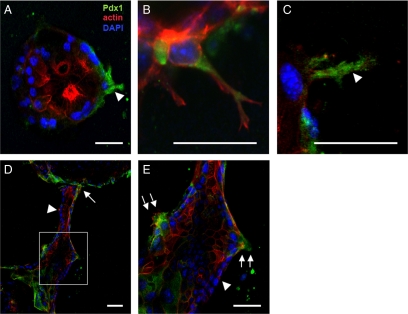
To evaluate Pdx1 expression in branching pancreatic epithelial cells, we prepared 3D collagen cultures of PDCs maintained for up to 2 weeks to allow for cyst formation and branching morphogenesis. These cultures were then fixed and stained for Pdx1, which is not typically expressed in mature adult duct cells. We found that Pdx1 is detected in fully formed cysts exclusively in individual cells commencing branch initiation (Figure 5). Examination of branch formation at multiple stages of morphogenesis indicates that Pdx1 is expressed at the earliest stages of branching (Figure 5, A–C) during early spike formation and extension into the extracellular matrix space. However, as branching duct cells transition to proliferation and repolarize to surround a luminal space, Pdx1 expression is no longer detectable (Figure 5, D and E). In extended 3D collagen cultures, secondary branches emerge from ducts established between two cysts (Figure 5, D and E). Again, Pdx1 expression is detected exclusively in duct cells forming these secondary branches, whereas the cells of the newly formed hollow duct are negative for Pdx1.
Figure 5.
Pdx1 expression in PDC cysts at initial points of branching morphogenesis. (A) Pdx1, not typically expressed in adult PDCs, is expressed exclusively in cells commencing tubulogenesis. (B and C) Pdx1 is expressed in initial extension and early cord. Note in B that Pdx1 expression in nucleus of cell adjacent to branching PDC. (D) A hollow tubule forms between two spheroid cysts. (E) Enlargement of inset in D. Pdx1 is expressed only at sites of branch initiation and not in epithelial cell wall of mature tubules. (D and E) Arrowheads, Pdx1-negative PDCs lining the hollow tubule. Single arrows, Pdx1-positive cells at sites of primary branching (D); double arrows, Pdx-1 positive cells at sites of secondary branching (E). Pdx1 (green), actin (red), and DAPI (blue). Arrowheads, Pdx1 cytoplasmic staining. Scale bar, 10 μm.
DISCUSSION
Pancreatic endocrine and early exocrine development is dependent on proper spatial and temporal expression of the Pdx1 transcription factor. Subsequent exocrine acinar lineage specification is dependent further on the Ptf1a transcription factor. Yet the regulation of ductal lineage specification is not known, either during development or during regeneration. To better understand the establishment of the pancreatic ductal network, we investigated the mechanism of branching morphogenesis, the process of progenitor cell proliferation, migration and differentiation that ultimately establishes the ductal architecture of the adult pancreas. To this end, we first examined a panel of lineage-specific markers throughout the peak stages of branching morphogenesis, E10.5–E14.5, and identified at E12.5 and E14.5 the emergence of a subpopulation of cells with apparent upregulation of Pdx1 expression, but without coexpressing insulin markers that would indicate a differentiating islet cell. We also observed in this subpopulation of cells, physiological features suggestive of migration or branching, such as extension outward into the mesenchyme and away from adjacent epithelial cells.
To support these observations, we established a 3D cell culture system to model the process of branching morphogenesis in vitro, utilizing primary pancreatic ductal epithelial cells isolated from wild-type mice. 3D PDC cultures were established from single-cell suspensions and embedded in type I collagen gels or in Matrigel. These cultures formed spheroid cysts demonstrating proper ductal epithelial cell polarity with a central lumen. We first observed that branching morphogenesis proceeded spontaneously and to completion of a fully formed tubule, only in collagen gel matrices as opposed to those cysts grown in Matrigel, which do not form branches. This suggests that specific type I collagen matrix–PDC interactions are necessary and sufficient to activate as yet undetermined signal transduction pathways that ultimately activate the genetic and biochemical program of branching morphogenesis. Similar specificities for matrix-dependent signal pathway activation cascades have been reported elsewhere, for example, the activation of specific matrix metalloproteinases in response to collagen but not Matrigel matrix in vascular endothelial cells (Haas et al., 1998).
We observed in our 3D model system that, although Pdx1 is not typically detected in mature differentiated PDCs, the initiation site of branching morphogenesis is distinctly highlighted by expression of Pdx1. Pdx1 expression is apparent at the junction of the spheroid cyst and the initial developing tubules, but wanes with a differentiated tubule. The sudden, dramatic appearance of Pdx1 in cells normally lacking it could indicate a potential dedifferentiation event, or reversion to a progenitor cell state, in order to genetically support the initiation of branching morphogenesis.
We note that, in our in vitro observations, Pdx1 is detected in the cytoplasm of branching epithelial cells, an unexpected localization. However, several observations of cytoplasmic localization have been reported for Pdx1 in islet cells, under conditions of oxidative stress or glucose stimulation (Kawamori et al., 2003; Guillemain et al., 2004), as a mechanism of Pdx1 regulation by nuclear export. In addition, we also observe cytoplasmic staining of Pdx1 in a subpopulation of cells in our series of embryonic sections, most consistently at E11.5 in individual cells that are not bound to adjacent epithelial cells, possibly representing the first observable branching epithelial cells (data not shown). Perhaps Pdx1 is initially up-regulated in response to mesenchymal signals that induce branching morphogenesis, followed by export to the cytoplasm as a means of rapid down-regulation. Given the previously reported mechanisms of nuclear export as a means of Pdx1 down-regulation, perhaps its presence in the cytoplasm is indicative of nuclear export after a brief surge transcriptional activity in the nucleus. Modulation of Pdx-1 expression via RNA interference in 3D cysts would help support further the role of Pdx1 in tubulogenesis.
It is tempting to speculate that a transient burst of Pdx1 expression in duct cells might transactivate a partial EMT program of gene expression that would induce the morphological and biochemical changes necessary to facilitate branching morphogenesis, similar to pEMT observations described in other branching cell lines (O'Brien et al., 2004; Leroy and Mostov, 2007). Such a phenomenon, if confirmed, could provide reasonable mechanistic support for previous reports of aberrant Pdx1 expression in nearly half of all human pancreatic cancer specimens tested, a finding which significantly correlated with lymph node metastasis and poor prognosis for patients (Koizumi et al., 2003; Wang et al., 2005). In the study by Koizumi et al., pancreatic cancer cell lines transfected with Pdx1 demonstrated a significantly increased ability to migrate, which complements our observations of Pdx1 up-regulation in cells acquiring a new capability for motility. Conversely, perhaps the pathway is reversed and it is Pdx1 that is induced by EMT-associated transcription factors. This type of scenario would emulate a phenomenon recently described by Mani et al. in which differentiated epithelial cells assume a progenitor stem cell-like state concomitant with a mesenchymal phenotype, after EMT induction (Mani et al., 2008). Regardless, it will be very interesting to identify novel gene transcription in duct cells induced to express Pdx1, to test whether classic regulators of pEMT or cell migration are activated.
ACKNOWLEDGMENTS
Pdx1:lacZ mice were generously provided by Chris Wright (Vanderbilt University). We thank the Center's Morphology, Molecular Biology, and Cell Culture Core Facilities, along with Dr. Xinyu Zhao at the Penn Biomedical Imaging Core Facility. We are grateful to Drs. Ben Stanger and Doris Stoffers for helpful discussions, along with members of the Rustgi lab. This work was supported by National Institutes of Health (NIH) Grant R01 DK060694 (A.K.R., M.P.W., M.R., J.v.B.), the National Pancreas Foundation (M.P.W., M.R.), NIH Grant R01 DK56211 (S.D.L.), the Chicago Diabetes Project (MRC), and NIH Grant P30 DK050306 Center for Molecular Studies in Digestive and Liver Diseases.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-03-0203) on September 30, 2009.
REFERENCES
- Affolter M., Bellusci S., Itoh N., Shilo B., Thiery J. P., Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell. 2003;4:11–18. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Ahlgren U., Jonsson J., Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- Deramaudt T. B., et al. N-cadherin and keratinocyte growth factor receptor mediate the functional interplay between Ki-RASG12V and p53V143A in promoting pancreatic cell migration, invasion, and tissue architecture disruption. Mol. Cell Biol. 2006;26:4185–4200. doi: 10.1128/MCB.01055-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata J. E., Werb Z., Bissell M. J. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes G. K. Developmental biology of the pancreas: a comprehensive review. Dev. Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Guillemain G., Da Silva Xavier G., Rafiq I., Leturque A., Rutter G. A. Importin beta1 mediates the glucose-stimulated nuclear import of pancreatic and duodenal homeobox-1 in pancreatic islet beta-cells (MIN6) Biochem. J. 2004;378:219–227. doi: 10.1042/BJ20031549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas T. L., Davis S. J., Madri J. A. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J. Biol. Chem. 1998;273:3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- Jorgensen M. C., Ahnfelt-Ronne J., Hald J., Madsen O. D., Serup P., Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocr. Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- Kawamori D., Kajimoto Y., Kaneto H., Umayahara Y., Fujitani Y., Miyatsuka T., Watada H., Leibiger I. B., Yamasaki Y., Hori M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes. 2003;52:2896–2904. doi: 10.2337/diabetes.52.12.2896. [DOI] [PubMed] [Google Scholar]
- Kim S. K., MacDonald R. J. Signaling and transcriptional control of pancreatic organogenesis. Curr. Opin. Genet. Dev. 2002;12:540–547. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Doi R., Toyoda E., Masui T., Tulachan S. S., Kawaguchi Y., Fujimoto K., Gittes G. K., Imamura M. Increased PDX-1 expression is associated with outcome in patients with pancreatic cancer. Surgery. 2003;134:260–266. doi: 10.1067/msy.2003.231. [DOI] [PubMed] [Google Scholar]
- Lee G. Y., Kenny P. A., Lee E. H., Bissell M. J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy P., Mostov K. E. Slug is required for cell survival during partial epithelial-mesenchymal transition of HGF-induced tubulogenesis. Mol. Biol. Cell. 2007;18:1943–1952. doi: 10.1091/mbc.E06-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Arber S., Jessell T. M., Edlund H. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat. Genet. 1999;23:67–70. doi: 10.1038/12669. [DOI] [PubMed] [Google Scholar]
- Lu P., Werb Z. Patterning mechanisms of branched organs. Science. 2008;322:1506–1509. doi: 10.1126/science.1162783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S. A., et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger R. J., Krasnow M. A. Genetic control of branching morphogenesis. Science. 1999;284:1635–1639. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Tang K., Kats E. S., Schutz-Geschwender A., Lipschutz J. H., Mostov K. E. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev. Cell. 2004;7:21–32. doi: 10.1016/j.devcel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Yu W., Tang K., Jou T. S., Zegers M. M., Mostov K. E. Morphological and biochemical analysis of Rac1 in three-dimensional epithelial cell cultures. Methods Enzymol. 2006;406:676–691. doi: 10.1016/S0076-6879(06)06053-8. [DOI] [PubMed] [Google Scholar]
- Offield M. F., Jetton T. L., Labosky P. A., Ray M., Stein R. W., Magnuson M. A., Hogan B. L., Wright C. V. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Pictet R. L., Clark W. R., Williams R. H., Rutter W. J. An ultrastructural analysis of the developing embryonic pancreas. Dev. Biol. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Pollack A. L., Runyan R. B., Mostov K. E. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev. Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- Schreiber F. S., Deramaudt T. B., Brunner T. B., Boretti M. I., Gooch K. J., Stoffers D. A., Bernhard E. J., Rustgi A. K. Successful growth and characterization of mouse pancreatic ductal cells: functional properties of the Ki-RAS(G12V) oncogene. Gastroenterology. 2004;127:250–260. doi: 10.1053/j.gastro.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Song S. Y., et al. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1999;117:1416–1426. doi: 10.1016/s0016-5085(99)70292-1. [DOI] [PubMed] [Google Scholar]
- Wang X. P., Li Z. J., Magnusson J., Brunicardi F. C. Tissue MicroArray analyses of pancreatic duodenal homeobox-1 in human cancers. World J. Surg. 2005;29:334–338. doi: 10.1007/s00268-004-7823-4. [DOI] [PubMed] [Google Scholar]