Abstract
Background
The risk-benefit ratio of aspirin therapy in prevention of cardiovascular disease (CVD) remains contentious, especially in type 2 diabetes. This study examined the benefit and harm of low-dose aspirin (daily dose < 300 mg) in patients with type 2 diabetes.
Methods
This is a longitudinal observational study with primary and secondary prevention cohorts based on history of CVD at enrolment. We compared the occurrence of primary composite (non-fatal myocardial infarction or stroke and vascular death) and secondary endpoints (upper GI bleeding and haemorrhagic stroke) between aspirin users and non-users between January 1995 and July 2005.
Results
Of the 6,454 patients (mean follow-up: median [IQR]: 4.7 [4.4] years), usage of aspirin was 18% (n = 1,034) in the primary prevention cohort (n = 5731) and 81% (n = 585) in the secondary prevention cohort (n = 723). After adjustment for covariates, in the primary prevention cohort, aspirin use was associated with a hazard-ratio of 2.07 (95% CI: 1.66, 2.59, p < 0.001) for primary endpoint. There was no difference in CVD event rate in the secondary prevention cohort. Overall, aspirin use was associated with a hazard-ratio of 2.2 (1.53, 3.15, p < 0.001) of GI bleeding and 1.71 (1.00, 2.95, p = 0.051) of haemorrhagic stroke. The absolute risk of aspirin-related GI bleeding was 10.7 events per 1,000 person-years of treatment.
Conclusion
In Chinese type 2 diabetic patients, low dose aspirin was associated with a paradoxical increase in CVD risk in primary prevention and did not confer benefits in secondary prevention. In addition, the risk of GI bleeding in aspirin users was rather high.
Background
Type 2 diabetes is associated with 2-3 fold increased risk of cardiovascular disease (CVD) and is a leading cause of mortality and morbidity [1,2]. In a meta-analysis consisting of Caucasian populations, aspirin therapy reduced the combined risk of myocardial infarction by 28% but had no effect on total mortality and stroke [3]. Despite the high risks for CVD in type 2 diabetes, most of the data relating to the use of aspirin and anti-platelet agents in diabetes come from post-hoc analysis of large clinical trials with small numbers and insufficient statistical power [4]. In a recent multicentre, placebo-controlled, randomized trial, aspirin treatment with or without anti-oxidant did not confer beneficial effects in diabetic patients who had no clinical evidence of CVD [5].
In both primary and secondary prevention trials, aspirin use is associated with a 2-fold increased risk for gastrointestinal (GI) bleeding and 80% increased risk of haemorrhagic stroke [4,6]. Besides, studies from Asian populations suggest high rates of GI bleeding in patients receiving aspirin (38.5% patients on aspirin had gastroduodenal mucosal injury) [7]. This may in part explain the relatively low rate of aspirin usage in Asian patients with CVD or associated risk factors [8]. Other researchers have reported higher rates of haemorrhagic stroke in Chinese than their Caucasian counterparts (23-52% vs. 9-18% of all strokes) [9,10]. In the CHARISMA Study which compared dual therapy of clopidogrel plus aspirin versus placebo plus aspirin, a higher rate of primary end point (fatal or non-fatal CVD) was observed in patients with multiple risk factors including diabetes treated with dual therapy (6.6%) than those treated with aspirin alone (5.5%) [11].
In light of the uncertain clinical effects of aspirin therapy and relative paucity of data in diabetic patients, we undertook a prospective analysis to examine the benefit (avoidance of vascular events) and harm (occurrence of major bleeding) of low-dose aspirin therapy (75 to 325 mg per day) for primary and secondary prevention of CVD in Chinese Type 2 diabetic patients.
Methods
Subjects
Type 2 diabetic patients aged 30 years or above were selected from the Hong Kong Diabetes Registry established since 1995. Using an electronic prescription database, we identified patients who had received low-dose aspirin (75-325 mg per day) [5] during the observational period ("aspirin users") and those who had not ("non-users"). For each subject, the index (baseline) date referred to the date of enrolment to the Registry; or the date of aspirin initiation if patients were started on aspirin after the diabetes assessment. This avoided the error of giving the aspirin users "immortal" person-time (i.e. the error in overestimating the time to development of endpoint if the index date taken was earlier than the time when aspirin was initiated) [12]. History of GI bleeding was confirmed using databases of hospital admission and endoscopy reports, dated back to 1995. Exclusion criteria included known history of malignancy, haemorrhagic stroke, upper GI bleeding, or any medical condition predisposing to GI bleeding, including alcoholism, oesophageal varices, Mallory-Weiss syndrome, liver cirrhosis or coagulopathies [13], as recorded in the endoscopy reports. The exclusion criteria were identified from discharge diagnosis based on International Classification of Diseases, Ninth Revision (ICD-9) codes, except for upper GI bleeding for which endoscopy reports were used to improve the sensitivity of event detection. The analysis of the Registry was approved by the Clinical Research Ethics Committee, the Chinese University of Hong Kong and complied with the Declaration of Helsinki. All patients, upon enrolment to the Registry, provided written informed consent for data analysis and research purpose.
Hong Kong Diabetes Registry
The Hong Kong Diabetes Registry was established in 1995 for quality assurance and improvement purposes at the Prince of Wales Hospital [14], which is a regional hospital serving a 1.2 million population. All patients undergo a 4-hour assessment modified from the European DIABCARE protocol [15]. Once a diabetic subject had undergone the comprehensive assessment, he/she was considered to have entered this study cohort and would be followed up till death.
Due to the heavily subsidized hospital care and less well developed primary care system in Hong Kong, the majority of patients with chronic diseases such as diabetes are managed in public hospital clinics where medications are dispensed on site. Since December 1996, all medication history, laboratory results and discharge summaries had been computerized and are available at the Hong Kong Hospital Authority (HA) Headquarter which is the governing body of 41 public hospitals which account for 95% hospital beds.
Primary and secondary prevention
The study population was stratified into the primary and secondary prevention cohorts. The former was defined as patients with no known history of occlusive vascular disease at enrolment. These included coronary heart disease (CHD) defined as angina with positive stress test or imaging, hospitalization with myocardial infarct (MI), stroke, transient ischaemic attack (TIA), or peripheral vascular disease (PVD) defined as history of lower extremity amputation, absent foot pulses confirmed by ankle:brachial ratio less than 0.9 on Doppler ultrasound scan. The secondary prevention cohort was defined as patients with known history of one or more of the above predefined vascular diseases at baseline. Patients treated with nitrate at baseline were also included in the secondary prevention cohort [16].
Clinical endpoints
Follow-up time was calculated as the period from enrolment to the first CHD event, death or 30th July 2005, whichever came first. Clinical endpoints were ascertained using the computerised records of endoscopy, hospital discharges and death as retrieved from the HA Central Computer System. Primary and secondary endpoints were defined to examine the potential benefit (in reducing major vascular events) and harm (in causing upper GI bleeding and haemorrhagic stroke) associated with aspirin use. The "primary composite endpoint" was defined as a combination of death from a vascular cause and major vascular events, including hospitalizations due to non-fatal MI and/or non-fatal stroke, whichever occurred earlier. Vascular causes included cardiac, cerebrovascular, venous thromboembolic, haemorrhagic, and other vascular causes, in accordance with the meta-analysis by the Antithrombotic Trialists' Collaboration [17].
"Secondary endpoints" included upper GI bleeding and haemorrhagic stroke. Since upper GI bleeding is a non-specific diagnosis which can be due to oesophagitis, varices, and malignancy in addition to gastroduodenal ulcer, we defined ulcer bleeding based on the endoscopy reports which are computerized in all HA hospitals. Hence, ulcer bleeding was defined as gastric or duodenal ulcer presented as haematemesis, melena, or coffee ground vomiting as documented in the upper GI endoscopy reports by gastroenterologist [18]. All patients admitted with suspected stroke underwent computer tomography (CT) scan imaging to distinguish haemorrhagic from ischaemic stroke according to HA clinical guidelines [19].
The occurrence of vascular endpoints was identified from the principal discharge diagnoses using the ICD-9 codes including fatal or non-fatal CHD (codes 410-414); congestive heart failure (code 428), all stroke [fatal or non-fatal] (codes 430-438), haemorrhagic stroke (codes 430-432), ischaemic stroke (codes 433-435) or coronary revascularization (codes 36.0-36.1). CHD was defined as MI (code 410) or ischemic heart disease (code 411-414).
Sample size estimation
In a pilot analysis of 5,000 patients enrolled in the Hong Kong Diabetes Registry [20], amongst aspirin non-users, the primary annual CVD event rate was 2.5% and 10% amongst those with prior history of CVD. For a 10-year observational study and assuming a 25% risk reduction with aspirin [5], 5,000 patients would be required to achieve 80% power at a 5% two-sided, alpha level based on the proportional hazards model for the primary prevention analysis and 900 patients for secondary prevention analysis.
Statistical analysis
Analyses were performed using the Statistical Package for Social Sciences (SPSS, version 14.0) software. All data are expressed as mean ± standard deviation (SD), median (interquartile range IQR) or geometric mean (95% confidence interval [CI]) as appropriate. Serum triglyceride (TG) and creatinine, and spot urine albumin-creatinine ratio (ACR) were logarithmically transformed due to skewed distributions. Chi-square (χ2) test and Student's t-test were used for between-group comparisons, as appropriate. Cox proportional hazards models were used to calculate the hazard ratios (HR) with 95% CI for the primary and secondary endpoints associated with aspirin use. For each of the outcome measures, Kaplan-Meier procedure was used to estimate the survival curves for aspirin users and non-users. The proportional hazards assumption was checked by examining the plots of the hazard functions for each group. The primary composite endpoint and its components were analysed separately for the primary and secondary prevention cohorts.
Multivariable techniques were used to control for potential confounders including age, gender, smoking habit, alcohol intake, duration of diabetes, retinopathy, sensory neuropathy, PVD, history of CVD, body mass index, blood pressure (BP), serum lipids, glycated haemoglobin (HbA1c), albuminuria, serum creatinine, and usage of antihypertensive, antidiabetic, anticoagulant, and lipid-lowering drugs at baseline. For the analysis of GI bleeding, the usage of nonsteroidal anti-inflammatory drugs, corticosteroids, and acid-suppressing agents were included as covariates to adjust for potential confounding effects [21]. A p value < 0.05 (2-tailed) was considered significant.
Results
Demographics
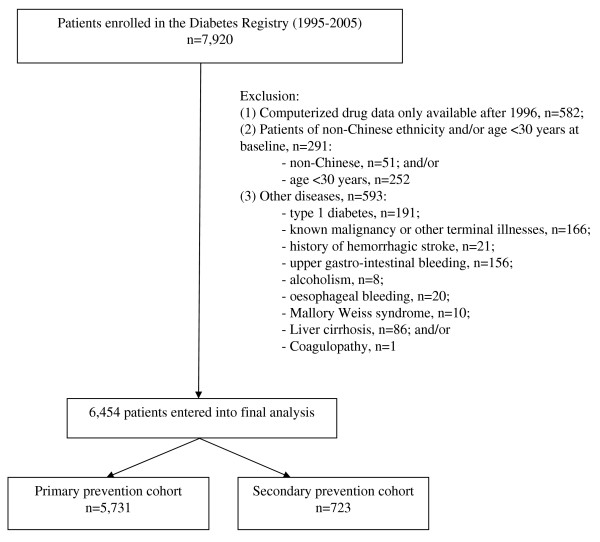
Figure 1 summarises the patients included in the final analysis. From the original database of 6454 patients, 5731 patients (age: 58.0 ± 12.8 years; disease duration: 8.1 ± 6.9 years) entered the primary prevention cohort and 723 patients, secondary prevention cohort. The percentage of aspirin usage was 18% (n = 1034) and 81% (n = 585) in the secondary prevention cohort. The mean follow-up time from baseline to death or study end (July 2005) was 4.6 ± 2.5 years (median [IQR]: 4.7 [4.4] years), giving a total of 29,618 person-years of follow-up. In both the primary and secondary cohorts, aspirin users had more adverse cardio-metabolic risk profiles, increased usage of cardiovascular drugs, anti-diabetic drugs and histamine H2 receptor blockers than aspirin non-users (Tables 1, 2).
Figure 1.
Patients included into the final analysis according to the inclusion and exclusion criteria.
Table 1.
Baseline clinical and biochemical characteristics between aspirin users and non-users in the complete cohort (n = 6,454)
|
Overall (n = 6,454) |
Aspirin users (n = 1,619) |
Non-aspirin users (n = 4,835) |
p-value‡ | |
| Age, years | 58.0 ± 12.8 | 65.7 ± 10.2 | 55.4 ± 12.6 | < 0.001 |
| Male, n (%) | 2,928 (45.4) | 819 (50.6) | 2,109 (43.6) | < 0.001 |
| Duration of diabetes, years | 8.1 ± 6.9 | 11.4 ± 7.5 | 7.0 ± 6.3 | < 0.001 |
| Smoking (current), n (%) | 1,033 (16.1) | 253 (15.7) | 780 (16.2) | < 0.001 |
| Alcohol (current), n (%) | 517 (8.1) | 100 (6.2) | 417 (8.7) | < 0.001 |
| Systolic blood pressure, mmHg | 136.2 ± 20.3 | 142.6 ± 21.7 | 134.1 ± 19.4 | < 0.001 |
| Diastolic blood pressure, mmHg | 75.1 ± 10.6 | 75.1 ± 11.2 | 75.1 ± 10.3 | 0.848 |
| Body weight, kg | 63.4 ± 12.3 | 63.3 ± 11.8 | 63.5 ± 12.5 | 0.583 |
| Body mass index, kg/m2 | 25.2 ± 3.9 | 25.3 ± 3.7 | 25.2 ± 4.0 | 0.135 |
| Waist circumference (cm): Males | 88.7 ± 9.6 | 90.1 ± 8.7 | 88.4 ± 9.8 | < 0.001 |
| Females | 83.8 ± 10.0 | 85.5 ± 9.9 | 83.6 ± 9.9 | < 0.001 |
| Glycosylated haemoglobin, % | 7.59 ± 1.69 | 7.72 ± 1.68 | 7.57 ± 1.69 | < 0.001 |
| Fasting plasma glucose, mmol/L | 8.59 ± 3.10 | 8.57 ± 3.18 | 8.59 ± 3.09 | 0.046 |
| Total cholesterol, mmol/L | 5.21 ± 1.05 | 5.09 ± 1.06 | 5.23 ± 1.05 | 0.167 |
| LDL cholesterol, mmol/L | 3.19 ± 0.95 | 3.06 ± 0.97 | 3.21 ± 0.94 | 0.286 |
| HDL cholesterol, mmol/L | 1.30 ± 0.38 | 1.26 ± 0.33 | 1.31 ± 0.39 | < 0.001 |
| Serum triglycerides, mmol/L † | 1.45 (1.43, 1.47) | 1.58 (1.53, 1.64) | 1.43 (1.41, 1.45) | < 0.001 |
| Serum creatinine, μmol/L † | 83.2 (82.4, 83.9) | 97.1 (95.0, 99.2) | 79.1 (78.3, 79.8) | < 0.001 |
| Estimated GFR# | 74.6 (73.8, 75.3) | 58.6 (56.8, 60.5) | 77.6 (76.8, 78.5) | < 0.001 |
| Spot urine ACR, mg/mmol † | 3.7 (3.6, 3.9) | 8.3 (7.4, 9.2) | 2.9 (2.7, 3.0) | < 0.001 |
| Blood haemoglobin, g/dL | 13.7 ± 1.6 | 13.4 ± 1.7 | 13.8 ± 1.6 | < 0.001 |
| Peripheral vascular disease, n (%) | 400 (6.2) | 223 (13.8) | 177 (3.7) | < 0.001 |
| Lower extremity amputation, n (%) | 26 (0.4) | 13 (0.8) | 13 (0.3) | 0.003 |
| Ischaemic heart disease, n (%) | 281 (4.4) | 240 (14.8) | 41 (0.8) | < 0.001 |
| Myocardial infarction, n (%) | 67 (1) | 65 (4) | 2 (0) | < 0.001 |
| Coronary revascularisation, n (%) | 77 (1.2) | 75 (4.6) | 2 (0) | < 0.001 |
| Congestive heart failure, n (%) | 119 (1.8) | 85 (5.3) | 34 (0.7) | < 0.001 |
| Stroke, n (%) | 256 (4) | 221 (13.7) | 35 (0.7) | < 0.001 |
| Atrial fibrillation, n (%) | 70 (1.1) | 35 (2.2) | 35 (0.7) | < 0.001 |
Mean ± SD or number of patients (%) or † geometric mean (95% confidence interval).
‡ By Chi-square test for categorical variables and t-test for continuous variables
#Based on the abbreviated MDRD (Modification of Diet in Renal Disease) equation for Chinese (ref. 42)
LDL and HDL = low- and high-density lipoprotein, ACR = albumin-to-creatinine ratio, GFR = glomerular filtration rate.
Table 2.
Baseline patterns of drug usage between users and non-users of aspirin in the complete cohort (n = 6,454).
|
Overall (n = 6,454) |
Aspirin users (n = 1,619) |
Non-aspirin users (n = 4,835) |
p-value† | |
| Anti-diabetic drugs | ||||
| Insulin | 1,203 (18.6%) | 458 (28.3%) | 745 (15.4%) | < 0.001 |
| Any oral agent | 4,301 (66.6%) | 1,128 (69.7%) | 3,173 (65.6%) | 0.003 |
| Sulphonylurea | 3,461 (53.6%) | 928 (57.3%) | 2,533 (52.4%) | 0.001 |
| Metformin | 3,189 (49.4%) | 839 (51.8%) | 2,350 (48.6%) | 0.025 |
| Thiazolidinedione | 27 (0.4%) | 9 (0.6%) | 18 (0.4%) | 0.322 |
| Anti-hypertensive drugs | ||||
| Any anti-hypertensive drugs | 3,103 (48.1%) | 1,135 (70.1%) | 1,968 (40.7%) | < 0.001 |
| Number of anti-hypertensive drugs* | 0.8 ± 1.0 | 1.3 ± 1.1 | 0.6 ± 0.9 | < 0.001 |
| Any RAS inhibitor | 1,632 (25.3%) | 615 (38%) | 1,017 (21%) | < 0.001 |
| ACE inhibitor | 1,519 (23.5%) | 564 (34.8%) | 955 (19.8%) | < 0.001 |
| AII antagonist | 123 (1.9%) | 57 (3.5%) | 66 (1.4%) | < 0.001 |
| Thiazide or related diuretic | 162 (2.5%) | 61 (3.8%) | 101 (2.1%) | < 0.001 |
| Loop diuretic | 217 (3.4%) | 129 (8%) | 88 (1.8%) | < 0.001 |
| Beta-blocker | 756 (11.7%) | 386 (23.8%) | 370 (7.7%) | < 0.001 |
| Alpha-blocker | 189 (2.9%) | 83 (5.1%) | 106 (2.2%) | < 0.001 |
| Calcium channel blocker | 1,838 (28.5%) | 697 (43.1%) | 1,141 (23.6%) | < 0.001 |
| Centrally-acting agent | 309 (4.8%) | 117 (7.2%) | 192 (4%) | < 0.001 |
| Vasodilator | 19 (0.3%) | 12 (0.7%) | 7 (0.1%) | < 0.001 |
| Other cardiovascular drugs | ||||
| Statin | 902 (14%) | 434 (26.8%) | 468 (9.7%) | < 0.001 |
| Fibrate | 232 (3.6%) | 76 (4.7%) | 156 (3.2%) | 0.006 |
| Digoxin | 65 (1%) | 33 (2%) | 32 (0.7%) | < 0.001 |
| Anti-arrhythmic drug | 20 (0.3%) | 9 (0.6%) | 11 (0.2%) | 0.040 |
| Oral anticoagulant | 59 (0.9%) | 9 (0.6%) | 50 (1%) | 0.080 |
| Antiplatelet drug other than aspirin | 24 (0.4%) | 14 (0.9%) | 10 (0.2%) | < 0.001 |
| Other drugs | ||||
| NSAID | 157 (2.4%) | 46 (2.8%) | 111 (2.3%) | 0.218 |
| Oral corticosteroid | 83 (1.3%) | 19 (1.2%) | 64 (1.3%) | 0.643 |
| Proton pump inhibitor | 25 (0.4%) | 10 (0.6%) | 15 (0.3%) | 0.085 |
| Histamine H2-receptor antagonist | 191 (3%) | 87 (5.4%) | 104 (2.2%) | < 0.001 |
| Misoprostol | 1 (0%) | 0 (0%) | 1 (0%) | 0.563 |
Data are number of patients (%) or mean ± SD
† By Chi-square test for categorical variables and t-test for continuous variables
RAS = renin-angiotensin system, ACE = angiotensin-converting enzyme, AII = angiotensin II, NSAID = non-steroidal anti-inflammatory drug, RAS inhibitor = either an ACE inhibitor or AII antagonist
Clinical endpoint of vascular outcomes and major gastrointestinal bleeding
In the primary prevention cohort, the primary composite endpoint occurred more frequently in aspirin users (13.3%, n = 138) than in non-users (5.5%, n = 260) (Table 3). Vascular death also occurred more frequently in aspirin users (5.2%, n = 54) than in non-users (1.3%, n = 61). A similar trend was observed for non-fatal MI (aspirin users, 2.6%, n = 27; non-users, 0.9%, n = 40) and non-fatal stroke (users, 6.9%, n = 71; non-users, 3.8%, n = 180). In the secondary prevention cohort, there were 145 primary composite endpoint with 116 (19.8%) events in users and 29 (21%) in non-users of aspirin. Vascular death occurred in 55 (9.4%) aspirin users and 14 (10.1%) non-users. Non-fatal MI occurred in 20 (3.4%) aspirin users and 3 (2.2%) non-users. Non-fatal stroke occurred in 55 (9.4%) aspirin users and 18 (13%) non-users. The event rates were higher in non-users except for non-fatal MI, albeit not significant.
Table 3.
Event rates and adjusted hazard ratio (95% confidence interval, CI) for clinical endpoints using multivariable Cox regression analysis in 6454 type 2 diabetic patients stratified by use of aspirin for primary or secondary prevention of cardiovascular disease.
| Overall | Aspirin users | Non-aspirin users |
Hazard ratio* (95% CI) |
p-value† | |
| Primary prevention cohort: | n = 5,731 | n = 1,034 | n = 4,697 | - | - |
| Primary composite endpoint | 398 (6.9%) | 138 (13.3%) | 260 (5.5%) | 2.07 (1.66 to 2.59) | < 0.001 |
| Vascular death | 115 (2%) | 54 (5.2%) | 61 (1.3%) | 2.61 (1.70 to 4.01) | < 0.001 |
| Non-fatal myocardial infarct | 67 (1.2%) | 27 (2.6%) | 40 (0.9%) | 2.05 (1.11 to 3.79) | 0.023 |
| Non-fatal stroke | 251 (4.4%) | 71 (6.9%) | 180 (3.8%) | 1.52 (1.14 to 2.04)# | 0.005# |
| Secondary prevention cohort: | n = 723 | n = 585 | n = 138 | - | - |
| Primary composite endpoint | 145 (20.1%) | 116 (19.8%) | 29 (21%) | 0.91 (0.60 to 1.37)# | NS |
| Vascular death | 69 (9.5%) | 55 (9.4%) | 14 (10.1%) | 0.92 (0.51 to 1.69)# | NS |
| Non-fatal myocardial infarct | 23 (3.2%) | 20 (3.4%) | 3 (2.2%) | 1.42 (0.42 to 4.85)# | NS |
| Non-fatal stroke | 73 (10.1%) | 55 (9.4%) | 18 (13%) | 0.71 (0.42 to 1.23)# | NS |
| Complete cohort: | n = 6,454 | n = 1,619 | n = 4,835 | - | - |
| Upper gastrointestinal bleeding | 138 (2.1%) | 70 (4.3%) | 68 (1.4%) | 2.19 (1.53 to 3.15) | < 0.001 |
| Endoscopically-confirmed ulcer bleeding | 68 (1.1%) | 33 (2%) | 35 (0.7%) | 1.72 (1.02 to 2.91) | 0.043 |
| Haemorrhagic stroke | 61 (0.9%) | 25 (1.5%) | 36 (0.7%) | 1.71 (1.00 to 2.95)# | 0.051# |
Data are number (%) of patients
Primary composite endpoint was defined as the composite of vascular death, non-fatal myocardial infarct or non-fatal stroke in accordance with the definition adopted by the Antiplatelet Trialists' Collaboration. Endpoints were identified from the principal diagnosis and principal procedure using the ICD-9 codes.
*Multivariable Cox proportional hazards model with age, gender, smoking, alcohol, duration of diabetes, retinopathy, sensory neuropathy, peripheral vascular disease, cardiovascular history, body mass index, blood pressure, serum lipids, HbA1c, albuminuria, serum creatinine, and baseline usage of antihypertensive, antidiabetic, anticoagulant, and lipid-lowering drugs adjusted as covariates.
#Hazard ratios and p-values were from univariate analysis.
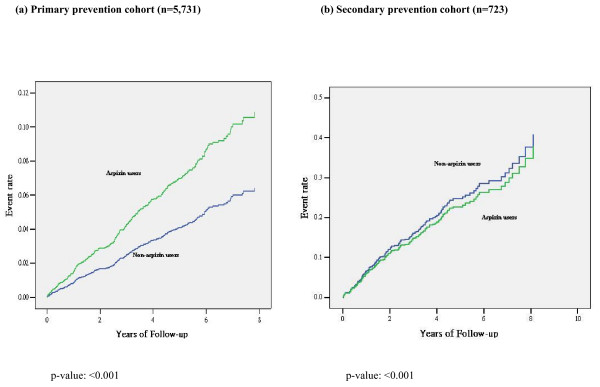
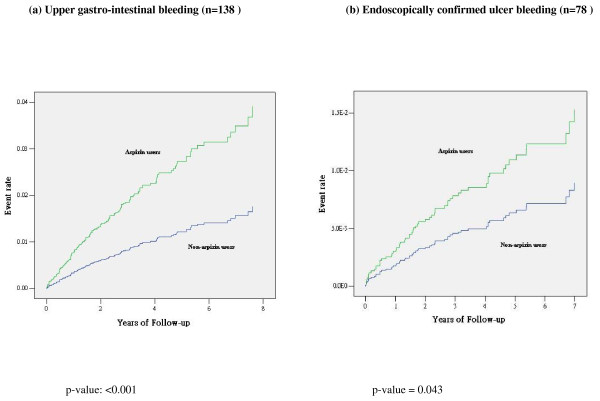
On multivariable analysis, aspirin use was associated with a 2-fold increased risk for the primary endpoint in the primary cohort (Table 3). The significance persisted with subgroup analysis by gender: HR (95% CI) = 1.86 (1.36, 2.54), men (n = 2560); 2.32 (1.70, 3.17), women (n = 3171) and 2.07 (1.66, 2.59), all (n = 5731). There were significant risk associations of aspirin with vascular death and non-fatal MI but not non-fatal stroke (Table 3). In the secondary prevention cohort, these risk associations were not observed (Figure 2 and Table 3). The primary composite endpoint in secondary prevention cohort remained insignificant after adjustment of gender. In the entire cohort of 6,454 patients, the rates of upper GI bleeding and endoscopically-confirmed ulcer bleeding were higher in aspirin users than non-users (Figure 3). There was also a trend for increased risk of haemorrhagic stroke in aspirin users than non-users, albeit not significant.
Figure 2.
Adjusted Kaplan-Meier curves for the primary composite endpoint (vascular death, non-fatal myocardial infarction and non-fatal stroke) in the primary and secondary prevention cohorts, stratified by aspirin usage during the observational period.
Figure 3.
Adjusted Kaplan-Meier curves for upper gastro-intestinal bleeding and endoscopically confirmed ulcer bleeding in the whole cohort of type 2 diabetic patients (n = 6,454), stratified by aspirin usage at baseline or during observational period.
Discussion
Cardiovascular events are major causes of mortality and morbidity in type 2 diabetes. Using this comprehensive Diabetes Registry, we have reported an annual CVD event rate averaging 2-3% increasing to 10% in those with cardio-renal complications at enrolment [14]. In the present analysis, the overall aspirin usage was 18% in the primary, and 80% in the secondary prevention cohort. During a 5-year observational period, aspirin use was associated with increased risk of CVD events and related death in the primary prevention cohort. In the secondary prevention cohort, similar event rates were observed between aspirin users and non-users. In the entire cohort, there was increased risk of hemorrhagic stroke and GI bleeding in the aspirin users.
Clinical guidelines in the United State recommend aspirin use for secondary prevention in type 2 diabetic patients with history of CVD and for primary prevention in those with a 10-year CHD risk of 15% or more [22]. However, these recommendations were not supported by evidence since most of the data in diabetic patients came from post-hoc analysis of large trials with marked heterogeneity [5,17,23,24]. In the meta-analysis by the Antiplatelet Trialists' Collaboration, only 6.5% of 68,814 study subjects with history of CVD had diabetes [5]. In primary prevention trials, the proportion of diabetic subjects was only 2-17% [23,24]. In a recent multicentre, randomised, placebo-controlled study with 1276 type 1 or type 2 diabetic patients who had no clinical evidence of CHD or PVD, aspirin treatment with or without anti-oxidants did not confer benefits on death or CVD event rates [5].
In our study, apart from lack of benefits, we observed a paradoxical increase in vascular risk amongst aspirin users in the primary prevention cohort. These findings are in agreement with current literature. In a subgroup analysis of the Antiplatelet Trialists' Collaboration which included data from diabetic patients (n = 1,365), the CVD event rates was 4.9% in aspirin users and 4.4% in aspirin non-users [5]. In our analysis, the non-fatal MI and stroke rates were 2.6% and 6.9% in aspirin users and the respective rates were 0.9% and 3.8% in aspirin non-users. In the Collaborative Group of the Primary Prevention Project which has the highest proportions of aspirin-treated diabetic patients [23,24], aspirin was associated with a non-significant increase in CVD deaths (RR [95% CI]: 1.23 [0.69 to 2.19]) in diabetic patients, as compared to a significant 68% reduction in aspirin-treated, non-diabetic subjects [25].
In our secondary prevention cohort, aspirin use was associated with a non-significant 9% reduction in vascular events. This lack of significance may be due to insufficient sample size (n = 723), when 900 patients are required to give the analysis an 80% statistical power. However, the very similar event rates between aspirin users (22.2%) and non-users (25.4%) strongly suggest a lack of benefit of aspirin. In the first meta-analysis performed in 1994 by the Antiplatelet Trialists' Collaboration involving 4502 diabetic patients and 42,323 non-diabetic subjects in the secondary prevention cohort, anti-platelet therapy modestly reduced the risk of vascular events by 3.8% in both groups (from 22.3% to 18.5% in diabetic group and from 16.4% to 12.6% in non-diabetic group, p < 0.001 in both groups) (5). In 2002, the same group performed a second meta-analysis of a larger cohort of 135,000 subjects. In a sub-group analysis of 9 trials which included 4,961 diabetic patients, antiplatelet therapy was associated with a non-significant 7% reduction in serious vascular events [17].
Aspirin and bleeding complications
Aspirin therapy is associated with elevated risks of major bleeding. Aspirin may also increase risk of haemorrhagic stroke [26], which carries poor prognosis [27]. Several lines of evidence suggest that Asian including Chinese patients have higher prevalence of GI bleeding and haemorrhagic stroke than their Caucasian counterparts. In a hospital-based survey involving Hong Kong Chinese, GI bleeding accounted for 37% of all adverse drug reactions leading to admissions [28] compared to 6.0-14.5% reported elsewhere [29]. In Mainland China, a stroke surveillance program also reported a higher age-adjusted stroke incidence (Chinese vs. Caucasians: 480-800 vs. 440-650 per 100,000 person-years) and intracerebral haemorrhage (160-450 vs. 40-120 per 100,000 person-years) in Chinese aged ≥ 55 years as compared to Caucasians [30].
In our cohort, usage of low-dose aspirin was associated with a 2.2-fold increased risk of upper GI bleeding. When we restricted the analyses to endoscopically-confirmed ulcer bleeding, we still observed an adjusted 72% increased risk amongst aspirin users. Other researchers have reported 1.5-2.6 increased risk of upper GI bleeding or perforation with aspirin use at daily dosage less than 300 mg [4,13]. It remains to be proven whether this high rate of GI bleeding might have confounded the increased risk of CVD in the primary prevention cohort and that treatment non-compliance may have contributed to the lack of benefits of aspirin in secondary prevention. In our limited analysis, use of other drugs such as NSAID was not found to be associated with clinical events though the observational nature of this study precludes any conclusion.
Apart from GI bleed, there was a non-significant 70% increased risk of haemorrhagic stroke amongst aspirin users (p = 0.051) probably due to insufficient sample size. This is similar to a reported risk of 84% in a meta-analysis of low dose aspirin (average daily dosage of 273 mg) in Caucasian patients [6]. The absolute risk of haemorrhagic stroke associated with aspirin appeared to be higher in Chinese than Caucasians (3.2 vs. 1.2 additional events per 1,000 person-years treated) [6].
Limitations
Our results need to be interpreted with caution. Firstly, our study did not distinguish between patients who received plain or enteric-coated aspirin although most studies suggested similar risks of GI bleed with different aspirin formulations [13,31,32]. Secondly, in agreement with most studies [13], we combined both cohorts in our analysis due to their similar risks of GI bleeding. Thirdly, there are considerable variations in the definitions of upper GI bleeding which may confound the risk estimation although we have used endoscopy reports to identify cases of ulcer bleeding.
While our overall results are in line with the literature, there is inevitable selection bias inherent with observational studies. Nevertheless, our comprehensive database has probably provided the largest unselected cohort of diabetic patients with relatively long duration of follow-up to address this unresolved therapeutic question. The detailed clinical and drug information including hospitalization and endoscopy reports have enabled us to adjust for confounding factors to draw important conclusions which have implications on clinical management. Our findings, which are particularly relevant to Chinese, may also explain the relatively low usage of aspirin in Asians than Caucasians [8]. Although we have not systemically examined doctors' rationale of prescribing, clinical experiences with adverse effects of aspirin may have led to cautious use of this class of drug.
Conclusion and Implications
The baseline data of our cohort showed the aspirin users had more risk factors for CVD than non-users. After statistical analysis with adjustment of these variables, aspirin use, among Chinese type 2 diabetic patients, for primary prevention was associated with 74% increased risk of incident CVD events while no benefit was observed in the secondary prevention cohort. This conclusion needs prospective study to be confirmed. In addition, overall speaking, aspirin use was associated with a 2.2-fold increased risk of upper GI bleeding and a non-significant trend for increased risk of haemorrhagic stroke. These results are not incompatible with the current body of knowledge based on either meta-analyses or randomized clinical trials and call for re-evaluation of the current recommendation regarding the use of aspirin in diabetic patients. Given their multiple comorbidities and high risk for CVD, there is an urgent need to design studies to evaluate the risk-benefit equation regarding the use of aspirin and other anti-platelet agents in diabetic patients with detailed documentation of potential confounders. In this respect, silent GI bleed with aspirin therapy may lead to anaemia which is a major predictor for CVD events in Chinese type 2 diabetic patients [33]. Meanwhile, there is a need to draw up protocols to identify high risk subjects for GI bleeding (e.g. smokers and carriers of Helicobacter Pyloris) for co-administration of ulcer-healing drugs, to maximize benefits and minimize harm associated with aspirin therapy.
Abbreviations
CVD: cardiovascular disease; GI: gastrointestinal; HA: Hospital Authority; CHD: coronary heart disease: MI: myocardial infarct; TIA: transient ischaemic attack; PVD: peripheral vascular disease; TG: triglyceride; ACR: albumin-creatinine ratio; HR: hazard ratios; BP: blood pressure
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
WL and JC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. WL, WS and JC designed the study. WS, AL and SC collected the data. WL, XY and GK did the data analysis. WL and GK drafted the manuscript. DS, FC and JC critically reviewed the manuscript. AK, RM and PT gave the administrative, technical, or material support. All authors provided suggestions during the preparation of the manuscript and approved the final version submitted for publication.
Acknowledgments
Acknowledgements
We thank Ms. Jessica Ching who facilitated retrieval of data on upper GI bleeding and endoscopically confirmed ulcer. Special thanks are extended to Mr. Kelvin Poon and Ms. Karen Szeto for their invaluable advice on data retrieval from the Hospital Authority. We thank all medical and nursing staff at the Diabetes Centre of the Prince of Wales Hospital for patient recruitment and management.
Contributor Information
Wilson Y Leung, Email: wilson_lg@hotmail.com.
Wing-yee So, Email: wingyeeso@cuhk.edu.hk.
Derek Stewart, Email: d.stewart@rgu.ac.uk.
Augustine Lui, Email: luicy@ha.org.hk.
Peter C Tong, Email: ptong@cuhk.edu.hk.
Gary T Ko, Email: gtc_ko@yahoo.com.hk.
Alice P Kong, Email: alicekong@cuhk.edu.hk.
Ronald C Ma, Email: rcwma@cuhk.edu.hk.
Francis K Chan, Email: fklchan@cuhk.edu.hk.
Xilin Yang, Email: yang.xilin@cuhk.edu.hk.
Sau-chu Chiang, Email: chiangsc@ha.org.hk.
Juliana C Chan, Email: jchan@cuhk.edu.hk.
References
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- Brand FN, Abbott RD, Kannel WB. Diabetes, intermittent claudication, and risk of cardiovascular events. The Framingham Study. Diabetes. 1989;38:504–509. doi: 10.2337/diabetes.38.4.504. [DOI] [PubMed] [Google Scholar]
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:161–172. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- Antiplatelet Trialists' Collaboration Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Br Med J. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O'Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R, Prevention of Progression of Arterial Disease and Diabetes Study Group. Diabetes Registry Group. Royal College of Physicians Edinburgh The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. Br Med J. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA. 1998;280:1930–1935. doi: 10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sanaka M, Nagasawa K, Abe K, Fukami M, Nakayama S, Tsuchiya A, Ishii T, Kuyama Y. Gastroduodenal mucosal injury in patients on antiplatelet therapy. Thromb Res. 2007;120:465–469. doi: 10.1016/j.thromres.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, Wilson PW, REACH Registry Investigators International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA. Incidence of the major stroke subtypes: initial findings from the North East Melbourne stroke incidence study (NEMESIS) Stroke. 2001;32:1732–1738. doi: 10.1161/01.str.32.8.1732. [DOI] [PubMed] [Google Scholar]
- Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ, CHARISMA Investigators Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- Ray WA. Observational studies of drugs and mortality. N Engl J Med. 2005;353:2319–2321. doi: 10.1056/NEJMp058267. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Hernandez-Diaz S, de Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br J Clin Pharmacol. 2001;52:563–571. doi: 10.1046/j.0306-5251.2001.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, So WY, Kong AP, Ma RC, Ko GT, Ho CS, Lam CW, Cockram CS, Chan JC, Tong PC. Development and validation of a total coronary heart disease risk score in type 2 diabetes mellitus. Am J Cardiol. 2008;101:596–601. doi: 10.1016/j.amjcard.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Piwernetz K, Home PD, Snorgaard O, Antsiferov M, Staehr-Johansen K, Krans M, for the DiabCare Monitoring Group of the St. Vincent Declaration Steering Committee Monitoring the targets of the St. Vincent declaration and the implementation of quality management in diabetes care: the DiabCare initiative. Diabet Med. 1993;10:371–377. doi: 10.1111/j.1464-5491.1993.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–1411. doi: 10.1001/jama.295.12.1404. [DOI] [PubMed] [Google Scholar]
- Antiplatelet Trialists' Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Br Med J. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallal HJ, Palmer KR. ABC of the upper gastrointestinal tract: Upper gastrointestinal haemorrhage. Br Med J. 2001;323:1115–1117. doi: 10.1136/bmj.323.7321.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KS, Mok V, Lam WW, Kay R, Tang A, Chan YL, Woo J. Aspirin-associated intracerebral hemorrhage: clinical and radiologic features. Neurology. 2000;54:2298–2301. doi: 10.1212/wnl.54.12.2298. [DOI] [PubMed] [Google Scholar]
- So WY, Ozaki R, Chan NN, Tong PC, Ho CS, Lam CW, Ko GT, Chow CC, Chan WB, Ma RC, Chan JC. Effect of angiotensin-converting enzyme inhibition on survival in 3773 Chinese type 2 diabetic patients. Hypertension. 2004;44:294–299. doi: 10.1161/01.HYP.0000137192.19577.c3. [DOI] [PubMed] [Google Scholar]
- Weil J, Langman MJ, Wainwright P, Lawson DH, Rawlins M, Logan RF, Brown TP, Vessey MP, Murphy M, Colin-Jones DG. Peptic ulcer bleeding: accessory risk factors and interactions with non-steroidal anti-inflammatory drugs. Gut. 2000;46:27–31. doi: 10.1136/gut.46.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson A, McIntosh A, Griffiths CJ. Clinical guidelines and evidence review for Type 2 diabetes. Blood pressure measurement. Sheffield: ScHARR, University of Sheffield; 2002. [Google Scholar]
- de Gaetano G, Collaborative Group of the Primary Prevention Project Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001;357:89–95. doi: 10.1016/S0140-6736(00)03539-X. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- Sacco M, Pellegrini F, Roncaglioni MC, Avanzini F, Tognoni G, Nicolucci A. Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diabetes Care. 2003;26:3264–3272. doi: 10.2337/diacare.26.12.3264. [DOI] [PubMed] [Google Scholar]
- The Swedish Aspirin Low-Dose Trial Collaborative Group Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet. 1991;338:1345–1349. doi: 10.1016/0140-6736(91)92233-R. [DOI] [PubMed] [Google Scholar]
- Anderson CS, Jamrozik KD, Burvill PW, Chakera TM, Johnson GA, Stewart-Wynne EG. Determining the incidence of different subtypes of stroke: results from the Perth Community Stroke Study, 1989-1990. Med J Aust. 1993;158:85–89. doi: 10.5694/j.1326-5377.1993.tb137529.x. [DOI] [PubMed] [Google Scholar]
- Chan TY, Critchley JA. Drug-related problems as a cause of hospital admissions in Hong Kong. Pharmacoepidemiol Drug Saf. 1995;4:165–170. doi: 10.1002/pds.2630040309. [DOI] [Google Scholar]
- Levy M, Kewitz H, Altwein W, Hillebrand J, Eliakim M. Hospital admissions due to adverse drug reactions: a comparative study from Jerusalem and Berlin. Eur J Clin Pharmacol. 1980;17:25–31. doi: 10.1007/BF00561673. [DOI] [PubMed] [Google Scholar]
- Jiang B, Wang WZ, Chen H, Hong Z, Yang QD, Wu SP, Du XL, Bao QJ. Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke. 2006;37:63–68. doi: 10.1161/01.STR.0000194955.34820.78. [DOI] [PubMed] [Google Scholar]
- Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. Br Med J. 2000;321:1183–1187. doi: 10.1136/bmj.321.7270.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper-gastrointestinal bleeding with enteric-coated or buffered product. Lancet. 1996;348:1413–1416. doi: 10.1016/S0140-6736(96)01254-8. [DOI] [PubMed] [Google Scholar]
- Tong PC, Kong AP, So WY, Ng MH, Yang X, Ng MC, Ma RC, Ho CS, Lam CW, Chow CC, Cockram CS, Chan JC. Hematocrit, independent of chronic kidney disease, predicts adverse cardiovascular outcomes in Chinese patients with type 2 diabetes. Diabetes Care. 2006;29:2439–2444. doi: 10.2337/dc06-0887. [DOI] [PubMed] [Google Scholar]