Abstract
Formation of lasting memories is believed to rely on structural alterations at the synaptic level. We had found that increased neuronal activity down-regulates Nogo receptor-1 (NgR1) in brain regions linked to memory formation and storage, and postulated this to be required for formation of lasting memories. We now show that mice with inducible overexpression of NgR1 in forebrain neurons have normal long-term potentiation and normal 24-h memory, but severely impaired month-long memory in both passive avoidance and swim maze tests. Blocking transgene expression normalizes these memory impairments. Nogo, Lingo-1, Troy, endogenous NgR1, and BDNF mRNA expression levels were not altered by transgene expression, suggesting that the impaired ability to form lasting memories is directly coupled to inability to down-regulate NgR1. Regulation of NgR1 may therefore serve as a key regulator of memory consolidation. Understanding the molecular underpinnings of synaptic rearrangements that carry lasting memories may facilitate development of treatments for memory dysfunction.
Keywords: behavior, hippocampus, long-term potentiation, myelin inhibitors, synaptic plasticity
Events underlying formation of memories that last hours to days are partially understood. Less is known about mechanisms that allow such memories to become transformed into very long-term (months) memories. First demonstrated as reactive sprouting in response to injury (1), structural synaptic plasticity in the adult brain is known to be a normal feature of gray matter (2, 3) and may be how lasting memories are formed and maintained. Thus perturbations of sensory input, such as monocular deprivation, leave lasting traces in the cerebral cortex in the form of changes of synaptic contacts (4) and involves dynamic changes of the actin cytoskeleton (5).
The lack of regenerative capacity in the mammalian CNS is partly due to the growth-inhibitory proteins Nogo (6–8), MAG (9, 10), and OMgp (11). These ligands can all bind to Nogo receptor 1 (NgR1) (12, 13). Since NgR1 lacks a cytoplasmic domain, additional transmembrane molecules (14–17) are needed to mediate intracellular signaling, leading to growth cone collapse (18).
We have previously demonstrated robust transcription of NgR1 in brain neurons, rather than glial cells, particularly in cortex cerebri and hippocampus (19), regions endowed with marked synaptic plasticity (20). Because Nogo is not only expressed in myelin, but also by many neurons (21), we hypothesized that NgR signaling might regulate activity-dependent synaptic reorganization underlying long-term memory (22). We found that neuronal NgR1 mRNA levels were efficiently and transiently down-regulated in the hippocampal formation and cerebral cortex of rats by kainic acid (22). Such temporary down-regulation of NgR1 transcription also occurred during the learning phase of a running behavior (22). Using fMRI, we recently showed in rats subjected to thoracic spinal cord injury, that when forelimb sensory representation in cortex expands into neighboring areas, NgR1 becomes specifically down-regulated in those sensorimotor cortical areas undergoing the plastic changes (23). Moreover, mice lacking NgR1 maintain the ocular dominance shift response to monocular deprivation into adulthood (24), suggesting supranormal CNS plasticity in the absence of NgR1. A similar improvement of ocular dominance shift plasticity has also been found in mice lacking functional PirB (25), a recently identified additional receptor for Nogo, MAG, and OMgp (26). Here we test the hypothesis that NgR1 regulation plays an important role in long-term memory formation.
Results
Generation of Mice Overexpressing NgR1 in Forebrain Neurons.
We first tested whether the prompt down-regulation of NgR mRNA expression in response to kainic acid seen in rats (22) also occurs in mice and found a similar temporally and spatially coupled reciprocal regulation of the NgR1 and BDNF genes in response to kainic acid (Fig. S1). We thus generated mice in which the normal, neural activity-driven down-regulation of endogenous NgR1 would be counteracted by constitutive expression of a NgR1 transgene. CamKII becomes increasingly active after birth in rat (27) and mouse (Fig. S2) forebrain neurons and exerts a key role in LTP and synaptic plasticity (28–30). We therefore used the CamKII promoter to limit transgene expression to forebrain neurons. We reasoned that the rapid temporary down-regulation of NgR1 normally seen during plastic events might be without effect if a NgR1 transgene was expressed in those same forebrain neurons, and hypothesized that this should render mice less able to undergo activity-dependent synaptic remodeling. Transgene induction was obtained using the tet-off system (Fig. S3).
Four independent tet-off inducible NgR1 overexpressing mouse lines (L1–L4) were established to minimize risk of transgene genome integration errors. Transgenic mice were healthy with no obvious phenotype, although adult L1 and L2 mice, selected for further testing, weighed approximately 10% less than controls (P = 0.047 and 0.057, respectively, two-tailed t-test). We found no, or only modest, changes in levels of noradrenaline, dopamine, DOPAC, HVA, serotonin, 5HIAA, or the striatal HVA/DA ratio, suggesting that monoaminergic neurotransmission was essentially intact in transgenic mice (Fig. S4). Downstream events of NgR activation includes RhoA activation. However, there was no difference in degree of hippocampal RhoA activation between L1 and control mice (Fig. S4).
Robust Tet-Off Inducible Expression of Transgenic NgR1 mRNA and Protein.
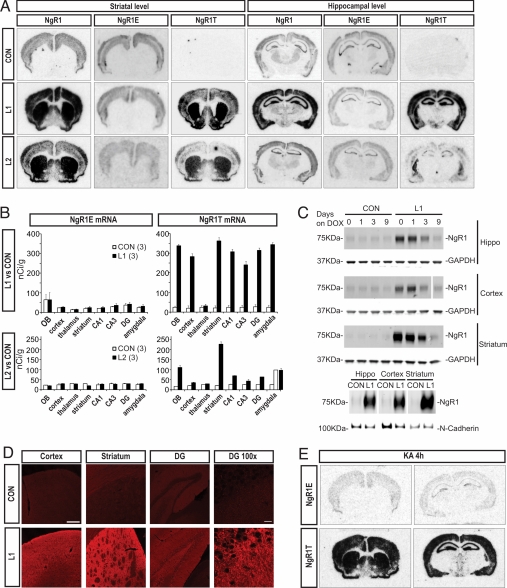
There were certain differences in the precise patterns and intensities of overexpression between lines (Fig. 1). NgR1 overexpressing (double transgenic: CamKIIa and pTRE/NgR1) mice and heterozygous (single transgenic: CamKIIa or pTRE/NgR1) littermates from the L1 and L2 lines were chosen and produced similar results. Consistent with choice of promoter, transgenic NgR (NgR1T) transcription was robust in striatum, hippocampus and cortex cerebri (Fig. 1 A and B), as well as in the olfactory bulb and amygdala (Fig. 1B). While transgenic mRNA levels were higher than endogenous levels in all areas of expression in both L1 and L2 mice, L2 NgR1 levels were generally considerably lower than L1 levels (Fig. 1B). Doxycycline counteracted the increased NgR1 mRNA levels in L1 mice (hippocampal CA3 levels in nCi/g: control 154, control + dox 1 month 145, L1 302, L1 + dox 1 month 149). We found strong increases of NgR1 protein levels in hippocampus, cortex, striatum, and olfactory bulb of L1 mice (Fig. S5). After delivery of doxycycline for 1 month, NgR protein levels were no longer increased in any of these areas (Fig. S5). NgR1 protein levels were below the detection level in cerebellum and spinal cord (Fig. S5). In hippocampus, cortex, and striatum, protein levels were markedly decreased 3 days after initiating doxycycline treatment, and protein overexpression could no longer be seen after 9 days (Fig. 1C). As expected, subcellular fractionation showed high amounts of NgR1 protein in the plasma membrane fraction of hippocampal, cortical and striatal tissue (Fig. 1C). We observed that neither endogenous NgR1 mRNA levels (Fig. 1 A and B), nor levels of Nogo, Lingo-1, Troy, or BDNF mRNA were significantly affected by transgene expression (Fig. S6). Using immunohistochemistry, we found supranormal levels of NgR-like immunoreactivity in cortical, hippocampal and striatal areas of L1 mice (Fig. 1D). Of note, NgR-like immunoreactivity was found not only in the striatal neuropil, presumably partly emanating from the cortico-striatal pathways, but also in substantia nigra pars reticulata, presumably reflecting NgR protein in projections from NgR expressing striatal output neurons, as indicated by the marked CamKII-driven NgR mRNA expression observed in striatal neurons (Fig. 1A). Importantly, when transgenic mice were challenged with kainic acid, NgR1 transgene transcription was maintained (Fig. 1E).
Fig. 1.
Characterization of inducible NgR1 overexpression. (A) In situ hybridization showing endogenous (NgRE) and transgenic (NgRT) mRNA in two transgenic mouse lines (L1 and L2) and controls (CON), quantified in (B) (OB: olfactory bulb, DG: dentate gyrus). The expression of transgene is specific in forebrain neurons and stronger in L1 than L2 (n = 3). Means ± SEM. (C) Upper three lanes: NgR1 protein in three brain areas of control and L1 mice before and after 1, 3, and 9 days of treatment with doxycycline (dox). Loading controls: GAPDH. For cortex, additional lanes between day 3 and 9 were digitally removed from the membrane picture. Lower lane: NgR1 protein in plasma membrane fractions from three brain areas of control and L1 mice. Loading control: N-Cadherin. (D) Strong NgR-like immunoreactivity in three brain areas of L1 compared to control mice. (Scale bar, 200 μm and 20 μm for DG enlargement.) (E) Robust transgene overexpression in L1 mice is maintained after kainic acid (KA).
No Impairment of LTP, L-LTP, De-Potentiation, or Cortical Spine Numbers.
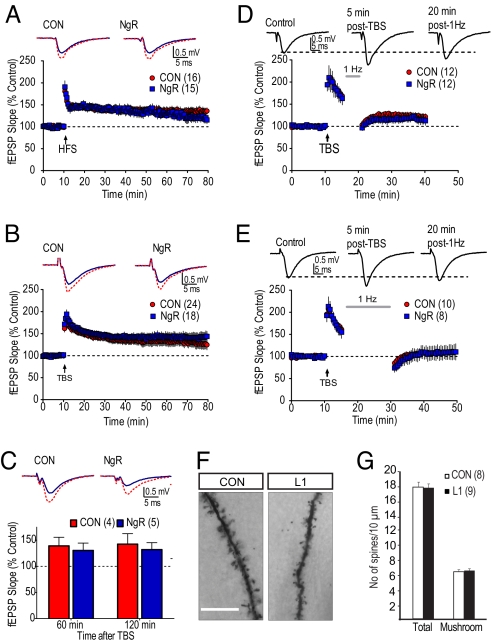
We did not expect NgR1 to affect the relatively rapid events that initiate and maintain LTP. To investigate this, we recorded extracellular field EPSPs from CA1 in hippocampal slices from control and L1 and L2 mice in response to 0.033-Hz electrical stimulation. After 10 min of stable baseline recordings, either high frequency stimulation or theta-burst stimulation induced robust LTP (Fig. 2 A and B), lasting at least 70 min, with no marked differences between overexpressing and control mice suggesting that NgR down-regulation is not necessary for LTP to become established. Furthermore, electrical stimulation of brain slices from control and NgR overexpressing mice produced fEPSPs with similar time courses and shapes (Fig. 2 A and B), and similar responses across a range of stimulus intensities (Fig. S7). This indicates that baseline synaptic processes were also not altered by the transgene. We also considered the possibility that the ability to maintain LTP for longer periods (L-LTP) might be compromised. However, as shown in Fig. 2C, stable LTP was maintained for at least 2 h following a single theta burst stimulation, and this was not significantly different between control and L2 mice (Fig. 2C) (P = 0.54, two-way RM-ANOVA, genotype × time). While loss of NgR prevents the expression of hippocampal LTD (31), LTD is typically only observed in slices obtained from juvenile animals (31, 32), and so would be unlikely to underlie the long-term behavioral changes observed in our adult mice. Since the reversibility (‘de-potentiation’) of LTP by low frequency stimulation (LFS) is well established in adult animals (33), and also reverses changes in spine morphology that may be regulated by NgR (31, 34), we next examined this phenomenon. As shown in Fig. 2 D and E, LFS (1 Hz) started 5 min after theta burst stimulation, resulted in de-potentiation of the previously potentiated response. The extent of de-potentiation was dependent on the number of stimuli (300 vs. 900 pulses); however, no significant differences were observed between L2 and control mice using either paradigm (300 pulse, P = 0.55; 900 pulse, P = 0.82; two-way RM-ANOVA, genotype × time). Together, these data suggest that the long-term memory deficits observed in L1 and L2 mice do not reflect intrinsic deficits in electrophysiological hippocampal plasticity or metaplasticity. We also determined spine density on apical dendrites of cortical pyramids. There was no significant difference in the total amount of spines (Fig. 2 F and G, P = 0.82, two-tailed t-test) or the amount of mushroom shaped spines (Fig. 2G, P = 0.81, two-tailed t-test).
Fig. 2.
LTP, L-LTP, de-potentiation, and spine counts. (A and B) No difference in LTP induced by either high frequency stimulation (HFS) or theta-burst stimulation (TBS). Representative fEPSPs are shown before (blue) or 60 min following (red) LTP-induction. (C) Similarly, there was no difference in L-LTP 60 or 120 min after TBS. fEPSPs are shown before (blue) and 2 h post induction (red). (D and E) No difference in de-potentiation is seen using 5 min (D) or 15 min (E) 1-Hz stimulations, delivered 5 min following TBS. Representative fEPSPs are shown from two different control mice. (F) Representative images of Golgi-stained apical dendrites from cortex cerebri (Scale bar, 50 μm.) (G) No difference was seen in the density of mushroom shaped spines or in total spine density.
Disturbed Running Behavior in NgR1 Overexpressing Mice.
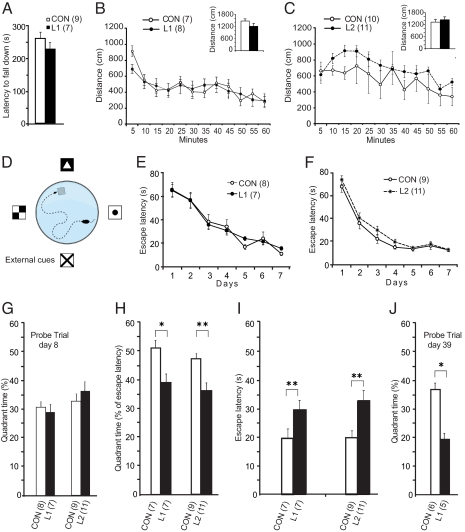
To test whether NgR1 overexpression would result in motor deficits we used the rotarod test (Fig. 3A) and found no significant difference, indicating that overexpressing mice have normal motor control. Similarly, there were no marked differences in spontaneous locomotion between L1 or L2 mice and controls (Fig. 3 B and C). This suggests that NgR1 overexpression does not disturb innate locomotor functions. Since we previously reported that NgR1 mRNA levels are down-regulated in hippocampus and cerebral cortex of rats by wheel running (22), we next exposed mice to running wheels for 5 weeks (Fig. S8). Both control and L1 mice significantly increased their running from week 1 to week 2. However, controls increased running significantly more (P < 0.05) and also plateaued at a higher level. Thus, while NgR1 overexpression does not appear to disturb innate locomotor activities, it appears to impair locomotor learning and/or the plasticity needed to develop a preference for running.
Fig. 3.
Rotarod, locomotion and swim maze; NgR1 transgene impairs long-term spatial memory. (A) L1 mice do not differ significantly from controls in latency before falling off a rotarod. (B and C) Spontaneous locomotor behavior of L1 and L2 mice does not differ markedly from that of controls. Insets show first 10 min. (D) Schematic illustration of Morris swim maze. (E and F) L1 and L2 mice do not differ from controls in Morris water maze training. (G) When exposed to a probe trial (platform removed) 1 day after the learning period there was no significant difference between the groups with regard to time spent in the target quadrant. (H and I) L1 and L2 mice retested at day 60, with the platform in its original position, spent less time in the platform-containing quadrant (H; quadrant time in % of total escape latency) and had longer escape latencies than controls (I). (J) Probe trial (platform removed) performed on day 39 on an additional group of mice showed that L1 mice spent less time in the target quadrant than controls. Means ± SEM *, P < 0.05, **, P < 0.01.
NgR Overexpression Impairs Long-Term Spatial Memory.
To determine whether presence of a NgR transgene compromises long-term learning and spatial information, we used the Morris water maze, a hippocampus-dependent reference memory task (Fig. 3D). L1 and L2 mice and controls all improved their day-to-day ability to find a hidden platform in a fixed location (Fig. 3 E and F). There were no marked group differences. Similarly, a probe trial (without platform) 24 h after the last training session, showed that all groups spent an equal proportion of their time in the target quadrant searching for the platform (Fig. 3G), indicating that 24-h memory of the task was not impaired. However, when tested in the water maze again, at day 60, with the platform in its original position, both L1 and L2 mice spent a lower proportion of their swim time in the target quadrant before finding the platform (Fig. 3H). They also needed a significantly longer time to find the platform (escape latency) compared to controls (Fig. 3I). Swim speed did not differ (P = 0.59). Retested at day 61, both L1 and L2 mice had relearned the task and performed as well as controls. In a separate experiment, other L1 mice were trained to find the platform and thereafter subjected to reversal learning, where the platform location had been moved 180°. When retested 40 days later with the platform back in its original location, there was no longer a difference in swim time between NgR1 overexpressing mice and controls [days × group, F (1, 6) = 0.8, P = 0.56], suggesting that it was the advantage of remembering platform position by controls that caused the differences in Fig. 3I. Finally, we repeated the long-term memory test with L1 mice, only this time a probe trial (platform removed) was carried out at day 39. Again, NgR1 overexpressing mice were significantly impaired compared to littermate controls (Fig. 3J) (P = 0.037). These mice were 9 months old at testing, and showed a tendency of impaired learning already during the first 7 training days. We therefore also calculated the ratio of time in the correct quadrant at day 39 compared to their final performance at day 7 (at completion of training); this ratio was also significantly lower in the NgR overexpressing group.
We retested swim maze performance of L1 NgR1 overexpressing and control mice (those depicted in Fig. 3 E, H, and I) when these animals became 18 months old. During the learning phase, there was a significant interaction between time and group (P < 0.05), such that the old controls performed better than the old L1 mice. By day 5, the escape latency time was identical for old controls and old L1 mice (Fig. S9). Retested 1 month later, old controls performed at the same level as day 5, suggesting that they remembered platform location well. In contrast, old L1 mice needed a much longer time to find the platform, indicating that their ability to form lasting memories was impaired (Fig. S9). This demonstrates that 18-month-old mice are able to form spatial memories lasting a month to the same extent as younger adults, and suggests that NgR1 signaling remains important for memory formation also in old mice.
Immediate, But Not 1-Week-Delayed, Transgene Inactivation Rescues Long-Term Memory in a Passive Avoidance Setting.
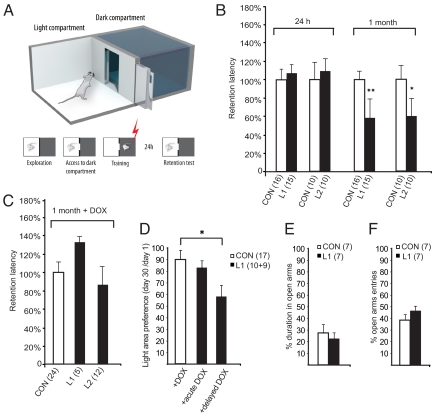
To examine the process of memory consolidation more closely, we used passive avoidance (35, 36), a behavioral paradigm in which a robust long-term memory for an unpleasant event is established and measured following a single training session (Fig. 4A). NgR1 overexpressing mice (L1 and L2) avoided re-entering the dark compartment 1 day after pairing it with a negative conditioning event (foot shock), similar to controls (Fig. 4B). Seven days later, there was a tendency for overexpressing mice to reenter the dark compartment faster than controls (L1: P = 0.13; L2 P = 0.24 versus controls). However, both L1 and L2 NgR1 overexpressing mice were clearly impaired 1 month later, entering the dark compartment significantly sooner than controls (Fig. 4B). Only 33% of the L1 NgR1 overexpressing mice, compared to 75% of the littermate controls, refrained from entering the dark compartment during the allotted trial time of 300 s. Similarly, only 25% of the L2 NgR1 overexpressing mice compared to 60% of the littermate controls remained in the light compartment. As mice did not get a new electric shock if they re-entered the dark compartment at interim tests carried out on day 1 or 7, it could be argued that they might have relearned that the dark compartment was no longer dangerous. To control for this, we performed an additional experiment where NgR1 overexpressing mice (L2 strain) and littermate controls received the initial conditioning shock but were not subjected to any interim passive avoidance tests before a memory test after 28 days. In this test, four of nine controls vs. seven of eight NgR1 overexpressing mice re-entered the dark compartment, suggesting that relearning was not an important factor in the previous tests. When transgenic NgR1 expression was turned off in adult mice by doxycycline, L1 and L2 mice no longer differed significantly from controls (Fig. 4C). These observations strongly suggest that presence of the NgR1 transgene per se, rather than for example transgene integration errors, impaired the establishment of very long-term memory. We next tested if doxycycline treatment starting directly after, or 7 days after, the conditioning event could rescue long-term memory formation and chose a passive avoidance protocol in which the door between the two compartments is kept open during test sessions. The total amount of time spent in the light compartment at day 30 was compared to that at day 1. Transgenic mice that received doxycycline immediately after the conditioning event performed similar to controls (P = 0.56; Fig. 4D) while transgenic mice that did not receive doxycycline until day 7 performed significantly worse (P = 0.049). One possible reason for these differences might be a difference in anxiety levels. We therefore examined mice in the elevated plus-maze (37), and found no difference between L1 and control mice (Fig. 4 E and F).
Fig. 4.
Long-term passive avoidance impairment is NgR1 transgene dependent, elevated plus maze behavior is not disturbed. (A) Passive avoidance set up. (B) There is no difference between L1 or L2 mice and controls 24 h after training but at 30 days transgenic mice enter the dark compartment significantly sooner than controls (data shown as % of control mean values ± SEM; *, P < 0.05 **, P < 0.01; Student's two-tailed t-test). (C) This difference was not seen in doxycycline treated animals. (D) L1 mice exposed to doxycycline beginning immediately (acute) after the shock performed as controls, while L1 mice that received doxycycline from day 7 (delayed) performed worse than controls. (E and F) Elevated plus maze behavior. Neither time spent in the open arms (E), nor entries into the open arms (F), differed between NgR overexpressing and control mice (ANOVA). Means ± SEM.
Discussion
Understanding the mechanisms that underlie formation and long-term maintenance of learned skills and other forms of memories may aid in understanding and in the development of treatments for memory impairments in aging and disease, including stroke (38). We hypothesized that down-regulation of NgR1, the key receptor component of the Nogo nerve growth inhibitory signaling system discovered by Schwab and colleagues (see ref. 39), may be a prerequisite for establishment of enduring memories (22). Here we provide genetic evidence for the hypothesis; presence of a NgR1 transgene that cannot be down-regulated by neuronal activity in forebrain neurons, neutralizes the effects of down-regulation of endogenous NgR1, and severely impairs the transition of newly obtained memories and skills into permanently stored engrams. NgR1-transgenic mice remember an aversive event or a spatial task for 24 h like normal mice, but fail to remember normally after a month. This suggests that activity-driven down-regulation of NgR1 constitutes a key permissive event allowing experience-dependent neuronal plasticity to lead to lasting memories.
Using the tet-off controlled fore-brain specific NgR overexpression, we were able to conclude that ongoing NgR1 overexpression in cortex and hippocampus does not impair the ability to form lasting memories, provided that transgene transcription is turned off by doxycycline directly after a conditioning event. However, if transgene transcription is not turned off in forebrain cortical areas until a week after the conditioning event, leading to loss of transgenic NgR protein a few days later, long-term memory formation is impaired, restricting the window of time during which NgR1 down-regulation is important for memory consolidation to days 3–10 after a memory-forming event.
Since LTP, a model for the acute plastic changes in synaptic strength that are thought to underlie memory formation in mammalian CNS (40, 41), occurs on a time scale of minutes, and our behavioral data suggested that 24 h memories were not affected by NgR1 overexpression, we hypothesized and demonstrated that LTP was unaffected by NgR1 overexpression. Likewise, we found L-LTP not to be affected. While LTD is difficult to observe in adult mice (32), a related phenomenon, de-potentiation, was also found not to be affected. These results support previous work showing that lack of NgR1 also does not impair acute electrophysiological characteristics in adult hippocampal slices (31). Together this suggests that NgR1 is not primarily involved in the initiation of memory.
An interesting mechanism for NgR1-mediated plasticity, whereby NgR1, but not NgR2, acts as a negative regulator of FGF2-induced neuritic growth was recently demonstrated (31). FGF2 and FGF1 were shown to have high affinity for NgR1 and to exert NgR1-regulated effects. Our findings are compatible with such a modus operandi for NgR1 in the formation of lasting memories but do not exclude a role for other NgR1 ligands, such as Nogo itself.
The establishment of very long-term memories involves several phases from immediate electrical and chemical bidirectional transsynaptic events, via intermediate phases, which may involve synaptic rearrangements, to a final stage in which memories presumably become represented by more or less permanent synaptic rearrangements. Our results using passive avoidance and the Morris swim maze suggest that these later stages, in which memories finally become stable constituents of CNS circuitry, are critically dependent on NgR1 signaling regulation. Because we find large amounts of transgenic NgR1 in the cell membrane, we hypothesize that loss of NgR1 in the pre- and/or postsynaptic membrane, in parallel with regulation of NCAM and other cell adhesion systems (42), are needed for boutons and dendritic spines to become temporarily insensitive to Nogo and/or other NgR1 ligands in neurite membranes, detach, and become capable of rearrangements in response to locally increased levels of BDNF (43), FGF (31), and other positive and negative neurotropic signals. Once new synaptic arrangements have been established, reversal of the activity-induced molecular changes would stabilize the connections.
The fact that there is a window in time during which retrograde amnesia can be induced (44), suggests that there are checkpoints along the road to lasting memories, such that newly developed structural changes need time to become stable. Studies of LTP/LTD show that chemical changes underlie the changes of synaptic strength recorded by electrophysiological techniques (34). Structural changes presumably constitute the intermediate step and become the long-term memory storage substratum. The time window for structural spine changes recently demonstrated in vivo for experience (monocular deprivation) to leave lasting changes in cortical neurons (4), approximately 4–16 days, is fully compatible with both the temporal characteristics of retrograde amnesia, the short memory span of our NgR1 overexpressing mice and the fact that immediate, but not 1-week-delayed silencing of the transgene normalizes long-term memory in these mice. The fact that targeting CamKII will cause mice to have impaired very long-term (10–50 days), but not 1- to 3-day memories (29) is evidence of a crucial involvement of CamKII in the process of forming very long-term memories. Our data show that NgR1 constitutes another key regulator of this process. PirB, a second receptor for Nogo, MAG and OMgp (25), is another putative long-term memory regulator in neurons that express this gene. Finally, our results show that NgR1 signaling continues to be important for the formation of lasting memories also in aged mice.
Better insight into mechanisms underlying structural plasticity and its consolidation at the synaptic level will help explain how lifelong memories are formed and maintained. Understanding the full repertoire of synaptic plasticity control exercised by NgR1 regulation may aid the development of methods to improve plasticity and long-term memory.
Methods
The two key long term memory tests used were passive avoidance and Morris water maze (see SI Text for details). For generation of transgenic mice, in situ hybridization, immunohistochemistry, immunoblotting, electrophysiology, RhoA assay, spine counts, HPLC, membrane fractionation, running wheel, rotarod, elevated plus-maze, locomotion tests, and statistical analysis, details, and descriptions are given in SI Text. Experiments were approved by the Stockholm Animal Ethics committee.
Supplementary Material
Acknowledgments.
We thank Eva Lindqvist and Karin Pernold for excellent technical assistance, and Mattias Karlén for the artwork. This work was supported by The Swedish Medical Research Council, Torsten and Ragnar Söderberg's Foundation, The Swedish Brain Foundation, the Håallsten Foundation, Swedish Brain Power, AFA (Arbetsmarknadens försäkringsaktiebolag) Sweden, U.S. Public Health Service grants, National Institute on Drug Abuse, National Institutes of Health, and the Karolinska Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905390106/DCSupplemental.
References
- 1.Raisman G. Neuronal plasticity in the septal nuclei of the adult rat. Brain Res. 1969;14:25–48. doi: 10.1016/0006-8993(69)90029-8. [DOI] [PubMed] [Google Scholar]
- 2.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 3.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 4.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matus A, Brinkhaus H, Wagner U. Actin dynamics in dendritic spines: A form of regulated plasticity at excitatory synapses. Hippocampus. 2000;10:555–560. doi: 10.1002/1098-1063(2000)10:5<555::AID-HIPO5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 6.Prinjha R, et al. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 7.Chen MS, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 8.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 9.McKerracher L, et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 11.Wang KC, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 12.Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 13.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 14.Mi S, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 15.Park JB, et al. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Shao Z, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 17.Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and Omgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- 18.Chivatakarn O, Kaneko S, He Z, Tessier-Lavigne M, Giger RJ. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci. 2007;27:7117–7124. doi: 10.1523/JNEUROSCI.1541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josephson A, et al. Nogo-receptor gene activity: Cellular localization and developmental regulation of mRNA in mice and humans. J Comp Neurol. 2002;453:292–304. doi: 10.1002/cne.10408. [DOI] [PubMed] [Google Scholar]
- 20.Martin KC, Kandel ER. Cell adhesion molecules, CREB, and the formation of new synaptic connections. Neuron. 1996;17:567–570. doi: 10.1016/s0896-6273(00)80188-9. [DOI] [PubMed] [Google Scholar]
- 21.Josephson A, Widenfalk J, Widmer HW, Olson L, Spenger C. NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury. Exp Neurol. 2001;169:319–328. doi: 10.1006/exnr.2001.7659. [DOI] [PubMed] [Google Scholar]
- 22.Josephson A, et al. Activity-induced and developmental down-regulation of the Nogo receptor. Cell Tissue Res. 2003;311:333–342. doi: 10.1007/s00441-002-0695-8. [DOI] [PubMed] [Google Scholar]
- 23.Endo T, Spenger C, Tominaga T, Brene S, Olson L. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signaling. Brain. 2007;130:2951–2961. doi: 10.1093/brain/awm237. [DOI] [PubMed] [Google Scholar]
- 24.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 26.Atwal JK, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 27.Burgin KE, et al. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990;10:1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 29.Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- 30.Miller S, et al. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- 31.Lee H, et al. Synaptic function for the Nogo-66 receptor NgR1: Regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner JJ, Alger BE. Homosynaptic LTD and depotentiation: Do they differ in name only? Hippocampus. 1996;6:24–29. doi: 10.1002/(SICI)1098-1063(1996)6:1<24::AID-HIPO5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Staubli U, Scafidi J. Time-dependent reversal of long-term potentiation in area CA1 of the freely moving rat induced by theta pulse stimulation. J Neurosci. 1999;19:8712–8719. doi: 10.1523/JNEUROSCI.19-19-08712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Wang XB, Frerking M, Zhou Q. Spine expansion and stabilization associated with long-term potentiation. J Neurosci. 2008;28:5740–5751. doi: 10.1523/JNEUROSCI.3998-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madjid N, et al. 5-Hydroxytryptamine 1A receptor blockade facilitates aversive learning in mice: Interactions with cholinergic and glutamatergic mechanisms. J Pharmacol Exp Ther. 2006;316:581–591. doi: 10.1124/jpet.105.092262. [DOI] [PubMed] [Google Scholar]
- 36.Stiedl O, Misane I, Spiess J, Ogren SO. Involvement of the 5-HT1A receptors in classical fear conditioning in C57BL/6J mice. J Neurosci. 2000;20:8515–8527. doi: 10.1523/JNEUROSCI.20-22-08515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 38.Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzenbach RR, Schwab ME. Disinhibition of neurite growth to repair the injured adult CNS: Focusing on Nogo. Cell Mol Life Sci. 2008;65:161–176. doi: 10.1007/s00018-007-7170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 41.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci. 2004;5:195–208. doi: 10.1038/nrn1349. [DOI] [PubMed] [Google Scholar]
- 43.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends Neurosci. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.