Abstract
Summary
This study was undertaken to investigate the radiologic and clinical outcomes of vertebroplasty with calcium phosphate (CaP) cement in patients with osteoporotic vertebral compression fractures. The morphological changes of injected CaP cement in osteoporotic compressed vertebral bodies were variable and unpredictable. We suggest that the practice of vertebroplasty using CaP should be reconsidered.
Introduction
Recently, CaP, an osteoconductive filler material, has been used in the treatment of osteoporotic compression fractures. However, the clinical results of CaP-cement-augmented vertebrae are still not well established. The purpose of this study is to assess the clinical results of vertebroplasty with CaP by evaluating the morphological changes of CaP cement in compressed vertebral bodies.
Methods
Fourteen patients have been followed for more than 2 years after vertebroplasty. The following parameters were reviewed: age, sex, T score, compliance with osteoporosis medications, visual analog scale score, compression ratio, subsequent compression fractures, and any morphological changes in the filler material.
Results
The morphological changes of injected CaP included reabsorption, condensation, bone formation (osteogenesis), fracture of the CaP solid hump, and heterotopic ossification. Out of 14 patients, 11 (78.6%) developed progression of the compression of the CaP-augmented vertebral bodies after vertebroplasty.
Conclusions
The morphological changes of the injected CaP cement in the vertebral bodies were variable and unpredictable. The compression of the CaP-augmented vertebrae progressed continuously for 2 years or more. The findings of this study suggest that vertebroplasty using CaP cement should be reconsidered.
Keywords: Calcium phosphate, Osteoporosis, Spinal fracture, Vertebroplasty
Introduction
Percutaneous vertebroplasty (PVP) is a common and popular procedure in osteoporotic vertebral compression fractures [1–4]. Traditionally, polymethylmethacrylate (PMMA) cement has been used in vertebroplasty as a filler material. However, PMMA cement has several disadvantages, such as the possibility of exothermal injury, lack of osteoconductivity, and the alteration of normal biomechanics [5–8]. Therefore, calcium phosphate (CaP), an osteoconductive filler material, has been used in the treatment of osteoporotic compression fractures instead of PMMA [9–11]. It has been reported that there are advantages to the use of calcium phosphate cement [12–15]. CaP cement has osteoconductivity and might not alter the normal spinal biomechanics. However, the clinical results of CaP-cement-augmented vertebrae are still not well established. The fact that CaP has a weaker strength than PMMA may also be a disadvantage [16]. The clinical and radiological results of vertebroplasty using CaP cement have rarely been reported, and there are some controversies about the therapeutic validity of CaP in vertebroplasty [16]. The authors analyzed the radiological and clinical results of vertebroplasty using CaP cement. The purpose of this study is to assess the clinical validity of vertebroplasty with CaP by evaluating the morphological changes of the CaP cement in compressed vertebral bodies.
Clinical materials and methods
The authors performed 96 vertebroplasty or kyphoplasty procedures in osteoporotic vertebral compression fracture patients from December 2005 to November 2006. Among them, 45 levels of 44 patients were treated by vertebroplasty with CaP cement. We included only the patients who were followed for more than 2 years. A total of 14 levels in 14 patients were enrolled in our study. All of the patients had a single-level osteoporotic vertebral compression fracture. The patients with multilevel vertebral compression fractures were excluded from this study. The patients who were treated by kyphoplasty or who had pathologic vertebral compression fractures from spinal metastatic cancer, osteolytic bone tumors, and hemangioma were excluded from this study. Also, patients who had a secondary osteoporosis were excluded. All of the patients participated in follow-up care via an outpatient clinic once a month for 2 months after the PVP for the regular administration of osteoporosis medications and postoperative radiological evaluations.
Before vertebroplasty, all of the patients were evaluated with plain X-ray films, bone mineral density scans, magnetic resonance imaging (MRI), and radionuclide bone scanning to define the acute osteoporotic compression fracture. The authors performed a PVP in patients who complained of disabling back pain refractory to conservative management with analgesics and bed rest. We used a unilateral percutaneous vertebral body access technique through the posterolateral extrapedicular approach in all patients. The filler material used in the vertebroplasty was CaP cement (55% dicalcium phosphate dehydrate and 45% tricalcium phosphate, JectOS®, Kasios, France).
Clinical and radiological analysis
We reviewed the preoperative clinical parameters such as age, sex, bone mineral density, compliance of osteoporosis medications, visual analog scale (VAS) score, neurologic symptoms, and filler material (CaP cement) volume. The VAS score was checked preoperatively, immediately postoperatively, and postoperatively at 6, 12, and 24 months or more (the final follow-up period). We compared the preoperative VAS scores with the postoperative scores.
In addition, we also reviewed many radiological parameters such as the compression ratio, kyphotic angle, morphological changes of the injected CaP cement in the vertebral bodies, and the incidence of any subsequent adjacent or remote vertebral compression fractures.
All of the patients underwent serial follow-up plain radiographs immediately after the vertebroplasty, and postoperatively at 6, 12, and 24 months or more (the final follow-up period). We analyzed the morphological changes of the injected CaP cement in the vertebral bodies in the serial follow-up plain X-ray films.
The anterior and posterior heights of the fractured vertebral body were assessed in order to calculate the compression ratio (anterior/posterior (AP) height) before and after the vertebroplasty. All of the heights were measured using the Picture Archiving and Communication System and its computer software (PiviewSTAR™ 5.0, INFINITT, Seoul, Korea). The degree of compression progression of the cemented vertebral bodies, which is the compression ratio difference between the immediate postvertebroplasty measurement and the follow-up period measurements (12 months and the final follow-up period after the vertebroplasty), was calculated for all of the patients. The compression ratio difference between 12 months after the vertebroplasty and the final follow-up period was calculated as well. We compared each of the compression ratio differences.
Statistical analysis was performed using the Friedman test, the Mann Whitney U test, and the Wilcoxon rank sum test. P < 0.05 was considered statistically significant. SPSS 13.0 for Windows (SPSS, Chicago, IL, USA) was used for the statistical analysis.
Results
The mean age of the patients was 69.42 ± 10.26 years, and there were ten females and four males. The treated levels were distributed from T8 to L5: one in T8; one in T11; two in T12; four in L1; four in L2; one in L4; and one in L5. The mean follow-up period was 25.43 ± 1.91 months (24–30 months). The mean T score of the bone mineral density was −3.19 ± 0.66. The mean volume of the injected CaP cement was 3.98 ± 0.88 mL (Table 1).
Table 1.
Characteristics of patients
| Characteristics | Value |
|---|---|
| Age (year) | 69.42 ± 10.26 |
| Sex (M/F) | 4/10 |
| Bone mineral density (T score) | −3.19 ± 0.66. |
| Filler material volume (mL) | 3.98 ± 0.88 |
| Mean follow-up period (month) | 25.43 ± 1.91 (24–30 months) |
| Location of compression fracture | From T8 to L5 |
| 1 (T8); 2 (T11); 2 (T12); 4 (L1); 4 (L2); 1 (L1) | |
| Morphological changes of injected CaP (number of patients) | Seven of 14 patients (50%) |
| Reabsorption (6) | |
| Osteogenesis (2) | |
| Condensation (2) | |
| Bone cement fracture (1) | |
| Heterotopic ossification (3) | |
| Progression of compression of treated vertebrae | 11 of 14 patients (78.6%) |
Morphological changes of the injected CaP
Seven patients (50.0%) showed morphological changes of the injected CaP cement for the follow-up period, and seven patients (50.0%) did not. The morphological changes of the injected CaP cement in the vertebral bodies were variable and unpredictable. The morphological changes of the injected CaP included reabsorption, condensation, bone formation (osteogenesis), fracture of the CaP solid hump, and heterotopic ossification (Table 1, Figs. 1, 2, 3, and 4). These phenomena occurred in complex and serial fashions (Figs. 1, 2, and 3). Six patients presented with reabsorption of the CaP cement (Figs. 1, 2, 3, and 4). Osteogenesis in the augmented vertebral body developed after reabsorption of the CaP and could be detected by serial follow-up plain X-ray films showing an increasing density of the vertebral body when compared with the initial X-ray films (Figs. 1 and 2). Two patients presented with osteogenesis. Condensation of the CaP cement was seen in two cases; the diffusely injected CaP was condensed and reduced in size in the vertebral body. Heterotopic ossification occurred in three patients (Figs. 1, 2, and 3). The heterotopic ossification developed around the CaP-cement-augmented vertebral body. In one case (Fig. 3), as a result of the heterotopic ossification, bone fusion occurred below and above the CaP-augmented vertebral body. This patient developed new compression fractures at those two levels (Fig. 3). Two out of three of the patients who developed heterotopic ossifications had osteonecrosis in the compressed vertebrae (Figs. 2 and 3). In one case, an acute fracture of the CaP-cemented vertebral body occurred, and a fracture of the solid hump of the CaP cement was detected at the refractured vertebral body (Fig. 4).
Fig. 1.

Lateral plain films of a 57-year-old man with an L1 compression fracture. a Initially, the L1 vertebral body was compressed. b Immediate postoperative lateral plain X-ray showed well-deposited CaP cement. c Twelve months after the vertebroplasty, recollapse and heterotopic ossification occurred (arrow), and the injected CaP was reabsorbed. d Twenty-four months after the vertebroplasty, the heterotopic ossification was condensed and osteogenesis had developed in the vertebral body
Fig. 2.
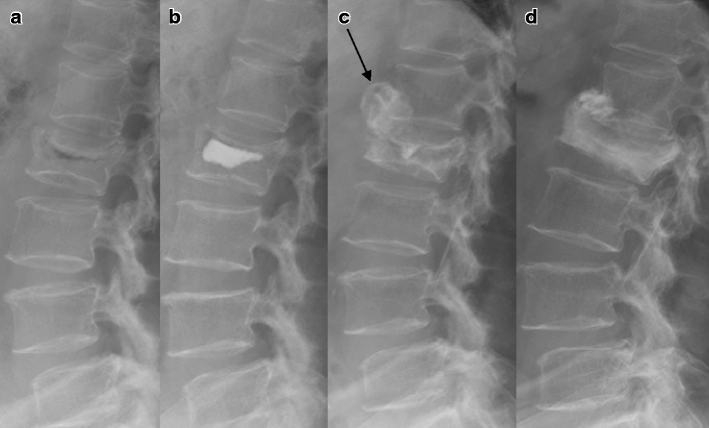
Lateral plain films of a 69-year-old woman with an L2 compression fracture. a The initial lateral plain X-ray showed an acute compression fracture and air cleft sign in the L2 vertebral body. b Immediate postoperative lateral plain X-ray showed well-deposited CaP cement. c Three months after the vertebroplasty, recollapse and heterotopic ossification occurred (arrow) and the injected CaP was reabsorbed. d Thirty months after the vertebroplasty, the heterotopic ossification was condensed and osteogenesis had developed in the vertebral body
Fig. 3.
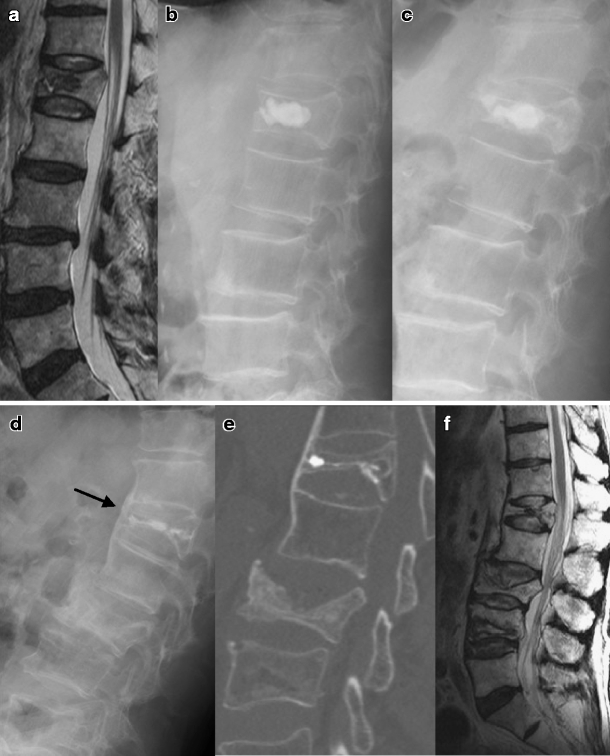
Radiologic studies of an 80-year-old man with an L1 compression fracture. a The initial MRI showed an acute compression fracture with osteonecrosis in the L1 vertebral body. b Immediate postoperative lateral plain X-ray showed well-deposited CaP cement. c Six months after the vertebroplasty, recollapse and heterotopic ossification occurred. The lateral plain X-ray (d), computed tomography (e) and MRI (f) were taken after 26 months after the vertebroplasty. The injected CaP was reabsorbed. Heterotopic ossification progressed and bone fusion developed (arrow). A subsequent vertebral compression fracture occurred at the L3 and L4 vertebrae
Fig. 4.
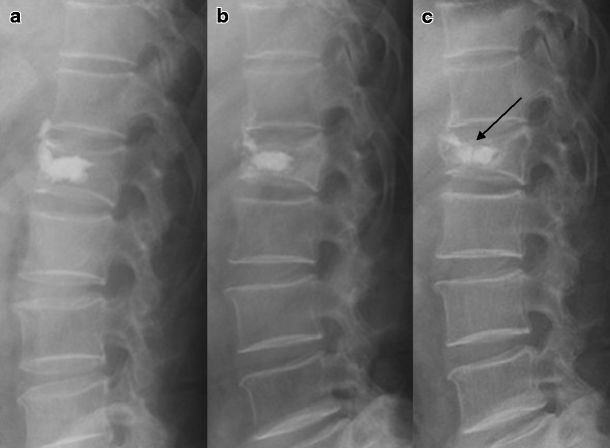
Lateral plain films of a 77-year-old man with an L1 compression fracture. a Immediate postoperative lateral plain X-ray. b Twelve months after the vertebroplasty, recollapse occurred and the injected CaP was partially reabsorbed. c Twenty-seven months after the vertebroplasty, he presented with back pain after a fall. Lateral plain X-ray showed that the CaP-augmented L1 vertebral body was more compressed than the immediately postoperative and follow-up X-rays, and the solid hump of the CaP cement was fractured as well (arrow)
Progression of the compression of the augmented vertebral body
Out of 14 patients, eleven (78.6%) developed progression of the compression of the CaP-augmented vertebral bodies after vertebroplasty. Progression of the compression of the cemented vertebral bodies was confirmed by serial follow-up plain X-ray films. The mean AP ratio of the CaP-augmented vertebrae decreased until 2 years or more postoperatively. The immediate postoperative AP ratio was 68.65 ± 6.71 and decreased to 60.98 ± 9.52 at 1 year after the vertebroplasty. Also, the postoperative AP ratio continued to decrease to 59.03 ± 11.19 at 2 years after the vertebroplasty (P < 0.05, Table 2). The mean ratio difference between the immediate postoperative status and at 1 year postoperatively was 7.6 ± 6.8, and difference between the postoperative 1- and 2-year measurements was 1.9 ± 2.9 (Table 2). The mean difference in the AP ratio of the compression of the vertebrae from the immediate postoperative to the 1-year postoperative period was significantly higher than from the postoperative 1 to 2 years or more (P < 0.05, Table 2). The mean difference in the AP ratio of the six vertebrae which developed reabsorption of the CaP cement was 16.84 ± 2.57, and, in the eight vertebrae which did not develop reabsorption, it was 4.95 ± 1.75 (P < 0.05, Table 3). The treated vertebrae which developed reabsorption of the CaP had a greater progression of the compression after the vertebroplasty than the vertebrae which did not develop reabsorption. The predisposing factor for the progression of the compression of the vertebrae was the reabsorption of the CaP cement.
Table 2.
Progression of compression of treated vertebrae
| Immediate postvertebroplasty | One year after vertebroplasty | Two years or more after vertebroplasty | |
|---|---|---|---|
| Compression ratio* | 68.65 ± 6.71 | 60.98 ± 9.52 | 59.03 ± 11.19 |
| Difference of compression ratio* | 7.6 ± 6.8 | 1.9 ± 2.9 |
*P < 0.05
Table 3.
Relationship between reabsorption of CaP and recollapse of treated vertebrae
| Patients with reabsorption of CaP | Patients without reabsorption of CaP | |
|---|---|---|
| Number of patient | Six of 14 patients | Eight of 14 patients |
| The mean difference of AP ratio of compressed vertebrae (P < 0.05) | 16.84 ± 2.57 | 4.95 ± 1.75 |
Although we encouraged the patients to maintain their regular osteoporosis medications, six patients were intermittently administrated medications. Eight patients maintained good compliance with their osteoporosis medications after the vertebroplasty. Six (75.0%) out of the eight patients with good compliance with their osteoporosis medications had progression of the compression of the augmented vertebrae. There was no statistical significance.
Clinical outcomes
The mean preoperative VAS score was 8.4 ± 0.6, and on postoperative day 1 it was 2.9 ± 1.1. The mean VAS score was significantly decreased postoperatively (P < 0.05, Table 4). The mean VAS scores were 2.9 ± 1.2 at 6 months postoperative, 3.1 ± 1.3 at 12 months postoperative, and 3.0 ± 2.4 at the final follow-up (more than 24 months; Table 4). The mean of the VAS scores at 6 and 12 months postoperative was slightly higher than at day 1 after the vertebroplasty. However, there was no statistical significance (P > 0.05). Fortunately, although serial recollapses occurred after the vertebroplasty with CaP, the mean score of the VAS of the back remained low, and there were no neurologic symptoms. However, in the cases of heterotopic ossifications with new vertebral compression fractures and fracture of injected CaP solid hump, the patients presented with high VAS scores (9 and 8 points).
Table 4.
The changes of VAS score of back during followed period
| Period | Preoperative | Immediate postoperative | Postoperative 6 months | Postoperative 12 months | Final followed period |
|---|---|---|---|---|---|
| VAS score | 8.4 ± 0.6 | 2.9 ± 1.1* | 2.9 ± 1.2 | 3.1 ± 1.3 | 3.0 ± 2.4 |
*P < 0.05
Discussion
PMMA was commonly used as a filler material for vertebroplasty. However, there are complications related with PMMA [1–4,17]. Recently, several studies have reported concerns about subsequent vertebral compression fractures after vertebroplasty [18–20]. Augmentation using PMMA can alter the normal spinal biomechanics and may result in subsequent vertebral compression fractures [7,8,12,14,21]. In contrast, osteoconductive filler materials such as CaP may prevent subsequent vertebral compression fractures. CaP cement has additional advantages including the absence of exothermic effects and osteoconductive activity [11–13,15].
One advantage of the CaP cement is that it is less stiff than PMMA, but this can also be seen as a disadvantage [16]. A case of recollapse of the vertebral body after kyphoplasty using CaP was reported [16]. In that case, an additional extensive surgical treatment was needed for the CaP-augmented vertebrae, which was severely collapsed and had a compressed thecal sac. CaP may not provide enough initial stiffness, and therefore recollapse may occur in the CaP-augmented vertebrae. In some patients, recollapse occurred 1 year after the vertebroplasty. The degree of the progression of the compression was more severe 1 year after the vertebroplasty than after more than a year postoperatively. Although the degree of progression of the compression was small after 1 year postoperatively, we think patients need regular follow-ups for serial reviews of plain X-rays. Furthermore, we suggest if reabsorption of the CaP cement occurs, the CaP cement may not provide enough stiffness to support the compressed vertebrae. Even though reabsorption of the CaP in the vertebral body is not a pathologic condition, it may result in the recollapse of the cemented vertebrae. It seems likely that reabsorption of the CaP may have adverse effects and may be a high-risk factor for the development of recollapse after vertebroplasty.
The bioactivity of the injected CaP cannot be controlled factitiously; therefore, the morphological changes of the CaP in the augmented vertebrae may be unpredictable and variable. The morphological changes of the injected CaP included reabsorption, condensation, bone formation (osteogenesis), fracture of the CaP solid hump, and heterotopic ossification. Reabsorption, osteogenesis, and heterotopic ossification were related with the bioactive properties of the CaP. In contrast, condensation and fracture of the CaP cement were related with the physical properties of the CaP. In two cases, condensation of the CaP occurred with concomitant recollapse of the vertebrae, possibly related to the fact that the strength of the CaP is not sufficient to support the compressed vertebral body. Also, the fracture of the solid hump of the CaP cement occurred after trauma.
It is well known that the bioactivity of CaP cement is one of its beneficial properties. However, we think that the bioactivity of CaP may not always be beneficial. CaP may not only have osteoconductive properties but osteoinductive properties as well [22,23]. In animal studies, it has been reported that CaP can result in ectopic bone formation in the muscular layers due to its osteoinductive properties [22,23]. Similarly, we suggest that the osteoinductivity of CaP can induce unwanted heterotopic ossifications in humans. In our study, heterotopic ossifications developed in three patients after vertebroplasty, and, in one case, heterotopic ossification resulted in bony fusions (Fig. 3). We suggest that bony fusion after heterotopic ossification may alter the normal biomechanics. In our study, subsequent vertebral compression fractures occurred in the patient with bony fusions after heterotopic ossification developed (Fig. 3). Furthermore, the mass of the heterotopic ossification may compress any adjacent structures. Fortunately, our cases did not present symptoms related with the compression of any adjacent structures. Although we cannot reveal the exact pathogenesis of the heterotopic ossification in vertebroplasty with CaP, we suggest that any CaP cement leakage into the adjacent tissue area is one possible cause of the heterotopic ossification. Although leakage of the CaP did not occur grossly during the vertebroplasty, we suggest that micro-leakage of the CaP might have occurred after the vertebroplasty via puncture, fracture, or osteonecrosis sites of the vertebral body and may have induced the heterotopic ossification. In our opinion, the leakage of CaP cement should be prevented during vertebroplasty, and CaP should not be used in patients with vertebral osteonecrosis.
We do not know the strength of the vertebrae that underwent osteogenesis after the injection of CaP. The osteoconductive effect of the CaP cement augmentation on the biomechanics is uncertain. The strength of the CaP-augmented vertebrae which developed osteogenesis after the vertebroplasty might be stronger than the normal vertebrae and therefore may alter the normal biomechanics. Thus, we think that the bioactivity of CaP may result in no better of an end point than PMMA biomechanically.
The morphological changes of the augmented CaP have progressed not simply but in complex and serial fashions. The authors suggest that the injected CaP will be able to change for a long time due to its bioactivity, and patients who were treated with CaP need a long-term follow-up and regular serial X-ray film screening. We do not yet know the final changes of the injected cement.
In this study, we were only able to follow up and assess 14 patients. Therefore, the results of our study cannot be generalized to all the CaP cements. For the clinical and radiologic outcomes to be better established, more patients should be studied and the follow-up period should be required.
Conclusions
The morphological changes of the injected CaP cement in the vertebral bodies were variable and unpredictable and included reabsorption, condensation, bone formation (osteogenesis), fracture of the CaP solid hump, and heterotopic ossification. These phenomena occurred in complex and serial fashions. The compression of the CaP-augmented vertebrae progressed continuously for 2 years or longer. The findings of this study suggest that the practice of performing vertebroplasty using CaP cement should be reconsidered. Additionally, we believe that the strength of the CaP cement may be too weak for vertebroplasty; bioactivity may not be necessary for filler materials, and new ideal filler materials should be developed.
Acknowledgments
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00198-009-1148-y
References
- 1.Amar AP, Larsen DW, Esnaashari N, et al. Percutaneous transpedicular polymethylmethacrylate vertebroplasty for the treatment of spinal compression fractures. Neurosurgery. 2001;49:1105–1114. doi: 10.1097/00006123-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Deramond H, Depriester C, Galibert P, et al. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am. 1998;36:533–546. doi: 10.1016/S0033-8389(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 3.Chin DK, Kim YS, Cho YE, et al. Efficacy of postural reduction in osteoporotic vertebral compression fractures followed by percutaneous vertebroplasty. Neurosurgery. 2006;58:695–700. doi: 10.1227/01.NEU.0000204313.36531.79. [DOI] [PubMed] [Google Scholar]
- 4.Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18:1897–1904. [PMC free article] [PubMed] [Google Scholar]
- 5.Polikeit A, Nolte LP, Ferguson SJ. The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit: finite-element analysis. Spine. 2003;28:991–996. doi: 10.1097/00007632-200305150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Hulme PA, Krebs J, Ferguson SJ, et al. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006;31:1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b. [DOI] [PubMed] [Google Scholar]
- 7.Tanigawa N, Komemushi A, Kariya S, et al. Radiological follow-up of new compression fractures following percutaneous vertebroplasty. Cardiovasc Intervent Radiol. 2006;29:92–96. doi: 10.1007/s00270-005-0097-x. [DOI] [PubMed] [Google Scholar]
- 8.Berlemann U, Ferguson SJ, Nolte LP, et al. Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J Bone Joint Surg Br. 2002;84:748–752. doi: 10.1302/0301-620X.84B5.11841. [DOI] [PubMed] [Google Scholar]
- 9.Nakano M, Hirano N, Ishihara H, et al. Calcium phosphate cement-based vertebroplasty compared with conservative treatment for osteoporotic compression fractures: a matched case-control study. J Neurosurg Spine. 2006;4:110–117. doi: 10.3171/spi.2006.4.2.110. [DOI] [PubMed] [Google Scholar]
- 10.Heini PF, Berlemann U, Kaufmann M, et al. Augmentation of mechanical properties in osteoporotic vertebral bones—a biomechanical investigation of vertebroplasty efficacy with different bone cements. Eur Spine J. 2001;10:164–171. doi: 10.1007/s005860000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieberman IH, Togawa D, Kayanja MM. Vertebroplasty and kyphoplasty: filler materials. Spine J. 2005;5:305S–316S. doi: 10.1016/j.spinee.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Hong SJ, Park YK, Kim JH, et al. The biomechanical evaluation of calcium phosphate cements for use in vertebroplasty. J Neurosurg. 2006;4:154–159. doi: 10.3171/spi.2006.4.2.154. [DOI] [PubMed] [Google Scholar]
- 13.Libicher M, Hillmeier J, Liegibel U, et al. Osseous integration of calcium phosphate in osteoporotic vertebral fractures after kyphoplasty: initial results from a clinical and experimental pilot study. Osteoporos Int. 2006;17:1208–1215. doi: 10.1007/s00198-006-0128-8. [DOI] [PubMed] [Google Scholar]
- 14.Lim TH, Brebach GT, Renner SM, et al. Biomechanical evaluation of an injectable calcium phosphate cement for vertebroplasty. Spine. 2002;27:1297–1302. doi: 10.1097/00007632-200206150-00010. [DOI] [PubMed] [Google Scholar]
- 15.Tomita S, Kin A, Yazu M, et al. Biomechanical evaluation of kyphoplasty and vertebroplasty with calcium phosphate cement in a simulated osteoporotic compression fracture. J Orthop Sci. 2003;8:192–197. doi: 10.1007/s007760300032. [DOI] [PubMed] [Google Scholar]
- 16.Heo DH, Kuh SU. Progressive, repeated lumbar compression fracture at the same level after vertebral kyphoplasty with calcium phosphate cement. Case report. J Neurosurg. 2007;6:559–562. doi: 10.3171/spi.2007.6.6.7. [DOI] [PubMed] [Google Scholar]
- 17.Heo DH, Chin DK, Yoon YS, et al. Recollapse of previous vertebral compression fracture after percutaneous vertebroplasty. Osteoporos Int. 2008;20:473–480. doi: 10.1007/s00198-008-0682-3. [DOI] [PubMed] [Google Scholar]
- 18.Fribourg D, Tang C, Sra P, et al. Incidence of subsequent vertebral fracture after kyphoplasty. Spine. 2004;29:2270–2276. doi: 10.1097/01.brs.0000142469.41565.2a. [DOI] [PubMed] [Google Scholar]
- 19.Lee WS, Sung KH, Jeong HT, et al. Risk factors of developing new symptomatic vertebral compression fractures after percutaneous vertebroplasty in osteoporotic patients. Eur Spine J. 2006;15:1777–1783. doi: 10.1007/s00586-006-0151-7. [DOI] [PubMed] [Google Scholar]
- 20.Uppin AA, Hirsch JA, Centenera LV, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 21.Lavelle WF, Cheney R. Recurrent fracture after vertebral kyphoplasty. Spine J. 2006;6:488–493. doi: 10.1016/j.spinee.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Le Nihouannen D, Daculsi G, Saffarzadeh A, et al. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone. 2005;36:1086–1093. doi: 10.1016/j.bone.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Yuan H, van Blitterswijk CA, de Groot K, et al. Cross-species comparison of ectopic bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) scaffolds. Tissue Eng. 2006;12:1607–1615. doi: 10.1089/ten.2006.12.1607. [DOI] [PubMed] [Google Scholar]


