Abstract
In a previous study we demonstrated that the activity of pyramidal tract neurons (PTNs) of the motor cortex is modulated in relation to postural corrections evoked by periodical tilts of the animal. The modulation included an increase in activity in one phase of the tilt cycle and a decrease in the other phase. It is known that the motor cortex contains a large population of inhibitory GABAergic neurons. How do these neurons participate in periodic modulation of PTNs? The goal of this study was to investigate the role of GABAA inhibitory neurons of the motor cortex in the modulation of postural-related PTN activity. Using extracellular electrodes with attached micropipettes, we recorded the activity of PTNs in cats maintaining balance on a tilting platform both before and after iontophoretic application of the GABAA receptor antagonists gabazine or bicuculline. The tilt-related activity of 93% of PTNs was affected by GABAA receptor antagonists. In 88% of cells, peak activity increased by 75 ± 50% (mean ± SD). In contrast, the trough activity changed by a much smaller value and almost as many neurons showed a decrease as showed an increase. In 73% of the neurons, the phase position of the peak activity did not change or changed by no more than 0.1 of a cycle. We conclude that the GABAergic system of the motor cortex reduces the posture-related responses of PTNs but has little role in determining their response timing.
Keywords: balance, bicuculline, cat, gabazine, iontophoresis, motor cortex
Introduction
Pyramidal tract neurons (PTNs) have been the focus of many studies of motor cortex activity during motor tasks (e.g. Evarts, 1968; Fetz & Finocchio, 1972; Conrad et al., 1977; Martin & Ghez, 1985; Georgopoulos et al., 1988, 1992; Mountcastle, 1980; Scott & Kalaska, 1995, 1997; Kakei et al., 2003). These studies provide fundamental knowledge about the involvement of these cells and the motor cortex generally in the control of different motor behaviours. Considerable effort has been aimed at characterising the intracortical networks that underlie this involvement (e.g. Birt et al., 2003; Jacobs & Donoghue, 1991; Matsumura et al., 1991, 1992; Castro-Alamancos et al., 1995; Kubota, 1996; Connors & Telfeian, 2000; Schneider et al., 2002; Capaday, 2004). It has been shown that gamma-aminobutyric acid (GABA) plays an important role in regulating motor-related activity in the motor cortex. When the motor cortex was deprived of GABA by injection of bicuculline, a GABAA receptor antagonist, manual dexterity in monkeys was severely impaired (Matsumura et al., 1991). When bicuculline was applied iontophoretically onto individual neurons in the monkey motor cortex, their activity during lever pressing increased (Matsumura et al., 1992). Electrical stimulation studies in the rat motor cortex showed that bicuculline broadened output motor fields (Jacobs & Donoghue, 1991). In chloralose-anaesthetized cats, Capaday & Rasmusson (2003) found that bicuculline expands somatosensory receptive fields of cells in the motor cortex. Also in anaesthetized cat preparations, data were obtained suggesting that selective GABAA inhibition and disinhibition may functionally link different neuronal groups of the motor cortex in useful motor synergies (Schneider et al., 2002). To further understand of the role of GABAA-mediated influences in cortical motor-related activity, however, different motor behaviours in awake animals need to be studied.
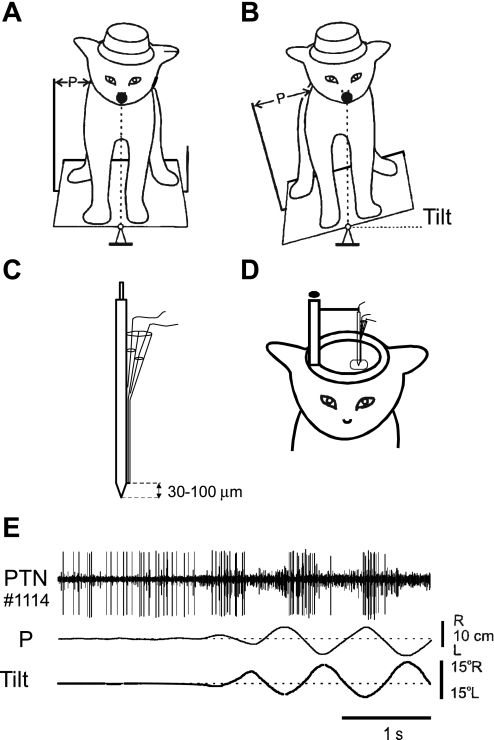
In quadrupeds, the dorsal-side-up body orientation is maintained by a closed-loop control system that performs a highly automatic, nonvoluntary motor function (Magnus, 1924; Bard & Macht, 1958). In this postural system the generation of a corrective motor response is based on various sensory feedback signals (reviewed in Horak & Macpherson, 1996; Macpherson et al., 1997; Massion, 1998; Deliagina & Orlovsky, 2002). Although the basic nervous mechanisms for feedback postural control reside in the brainstem, cerebellum and spinal cord, participation of the motor cortex in the control system has been demonstrated in lesion studies, by transcranial magnetic stimulation, and by intracortical electrical stimulation (e.g. Gahery & Nieoullon, 1978; Ioffe, 1973; Massion, 1979; Ioffe et al., 1988, 2002; Lavoie et al., 1995; Latash et al., 2003; Solopova et al., 2003). Using a single-cell recording technique in cats and rabbits, we have shown that the activity of descending efferent neurons of layer V of the motor cortex, including pyramidal tract neurons, is modulated during postural corrections (Beloozerova et al., 2003, 2005). When the supporting platform was tilted periodically, the activity of most PTNs changed with the rhythm of tilts and of the postural corrections evoked by the tilts (Fig. 1A, B and E).
Fig. 1.
Experimental design. (A) The cat stood on a platform. A mechanical sensor P measured displacements of the body in relation to the platform (postural corrections). (B) When the platform was tilted to the right, the cat kept the dorsal-side-up orientation by extending the limbs on the right side. A mechanical sensor measured the angular displacement of the platform (Tilt). (C) A system for recording the activity of PTNs and for iontophoretic application of gabazine or bicuculline. A two- or three-channel glass pipette was attached to an extracellular microelectrode. Not shown to scale. (D) The electrode and pipette were inserted into the motor cortex by a miniature microdrive attached to the head. (E) PTN activity was recorded along with platform tilts and postural corrections; R, right; L, left.
The goal of the present study was to investigate the role of the GABAA inhibitory neurons of the motor cortex in the modulation of PTN posture correction-related activity. A brief account of part of this study has been published in abstract form (Tamarova et al., 2004).
Materials and methods
Recordings were obtained from three adult cats, two males and a female. All experiments were conducted in accordance with NIH guidelines and were approved by the Barrow Neurological Institute Animal Care and Use Committee. Methods for behavioural training and general surgical procedures have been previously described (Beloozerova & Sirota, 1993; Beloozerova et al., 2005; Prilutsky et al., 2005). Surgery was performed under aseptic conditions using Isoflurane anesthesia (2–5%) administered by inhalation for the length of the surgery. Following the surgery, but prior to extubation, 0.005 to 0.01 mg/kg of analgesic Buprenorphine was administered intramuscularly and another dose was administered 12 h thereafter. After the extubation the cat was placed in a warm padded cage and respiration and reflexes were monitored until the cat regained conciousness.
The cat stood on a platform while continuously licking food ejected from a feeder in front of it (Fig. 1A). The platform periodically tilted in the frontal (roll) plane of the animal (Fig. 1B). A sine-like tilt trajectory with a period of 1 s and amplitude of ±15° was used. After some training, the cats effectively compensated for the platform tilts by extending limbs on the side moving down and flexing limbs on the opposite side (Beloozerova et al., 2005). Postural corrections helped the animal to maintain the dorsal-side-up orientation of the head and trunk and to keep the mouth against the feeder.
The neuronal activity in the forelimb representation of area 4γ of the motor cortex was recorded extracellularly using either platinum–tungsten quartz-insulated microelectrodes (40 µm outer diameter) pulled to a fine tip and mechanically sharpened (Reitboeck, 1983), or tungsten varnish-insulated electrodes (Frederick Haer & Co.). In order to identify the motor forelimb representations in each subject, three approaches have been used: (i) somatic receptive field mapping; (ii) observation of neuronal activity during voluntary movements; and (iii) intracortical microstimulation: trains of 10 50 mA cathodal pulses at 200 Hz, with each pulse of 0.2 ms duration (see Beloozerova et al., 2005 for details of identification procedures). A freshly pulled two- or three-barrelled pipette (glass BF-100-50-10; Sutter Instruments Co.) was glued to the recording electrode with a horizontal pipette–electrode tip separation of 15–70 µm and a vertical separation of 30–100 µm (Fig. 1C). Before gluing, the tip of the pipette was broken back to an inner diameter (ID) of each barrel of 2–5 µm. The horizontal distance between openings of neighbouring pipettes was 4–10 µm. The electrode–pipette assembly was inserted into the motor cortex through a hole in a plastic plate implanted above it, and advanced into the cortical tissue by a miniature manual microdrive (Fig. 1D). The criterion for identification of PTNs was the test for collision of spontaneous and antidromic spikes evoked by electrical stimulation of the pyramidal tract at the medullar level (Bishop et al., 1962; Fuller & Schlag, 1976).
The GABAA receptor antagonists gabazine and bicuculline methiodide (both obtained from Sigma) were separately and freshly dissolved in sterile 0.9% sodium chloride with pH 3–4 at a concentration of 20 mm. We were not aiming to compare the effects of gabazine and bicuculline; rather, we used the two substances as tools to achieve GABAA receptors blockade. Gabazine or bicuculline was delivered in the vicinity of the target neuron by positive currents of 50–100 nA (pipettes with ID 5 µm) or 100–200 nA (pipettes with ID 2–4 µm) applied for 50–100 s. Between the applications, a retaining negative current of 20 nA was used. In all experiments, positive current equal to the drug application current was applied for 100 s through a pipette filled with physiological solution, and changes in the activity of the neuron were monitored for 1–10 min after the application. If at any point after gabazine or bicuculline application any paroxysmal activity occurred, data were discarded, the electrode was withdrawn and the cat was returned to the home cage.
Postural tests included 50–100 tilt cycles before a GABAA receptor antagonist application (control) and 300–600 tilt cycles during and immediately after the application. If needed, an additional test for recovery consisting of 100–200 tilt cycles was performed 30–40 min after the GABAA antagonist application. All tilts were performed in a series of 15 cycles, which were separated by 15-s periods of stable platform. In selected experiments, applications were repeated after the activity of the neuron had returned to the control level, but no sooner than 1 h after the initial application.
For neuronal data analysis, each of the tilt cycles was divided into 10 equal bins, the peak of the right tilt being taken as the cycle onset. For each neuron, a histogram of spike activity in the tilt cycle was generated, and then the activity was averaged over all successive cycles of a given test. The following parameters were determined for each neuron: (i) the mean frequency of discharge on a stable platform (Frest); (ii) the mean frequency of discharge during tilts (Ftilt); (iii) the maximum and minimum frequency in the histogram, Fmax and Fmin; (iv) the differences between Frest and Fmax or Fmin: ΔFmax = Fmax − Frest and ΔFmin = Frest − Fmin (as shown in Fig. 2); (iv) the coefficient of modulation M = (1 − Fmin/Fmax) × 100% (the Rayleigh test for directionality was used to determine whether the activity of a neuron was modulated; Batshelet, 1981; Fisher, 1993); and (vi) the phase position of Fmax. All quantitative data are presented as the mean ± SD. The t-test was used to characterize the statistical significance when comparing different means; the significance level was set at P = 0.05.
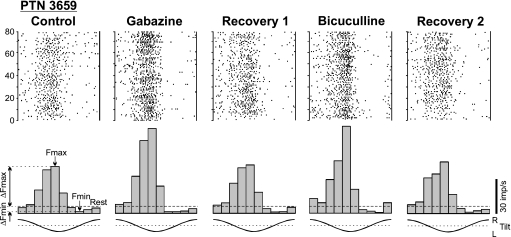
Fig. 2.
A representative example of the effect of gabazine and bicuculline on postural responses of a PTN. A raster and histogram of the PTN activity during 80 tilt cycles are shown for each of the following conditions: Control, before application; Gabazine, 2 min after gabazine application (20 mm, 100 nA, 100 s); Recovery 1 is 15 min after gabazine application; Bicuculline, 3 min after bicuculline application (20 mm, 100 nA, 100 s). Recovery 2 is 15 min after bicuculline application. Note the significant increase in peak activity in both Gabazine and Bicuculline tests while the rest, trough activity and timing of activity did not change. Fmax and Fmin, the maximum and minimum frequencies in the histogram; ΔFmax = Fmax − Frest; ΔFmin = Frest − Fmin.
Toward the end of experiments, several reference lesions were made, in the region of the motor cortex where neurons were sampled, by application of 30 µA DC cathodal current for 10 s through a recording electrode. At the termination of the experiment, cats were deeply anaesthetized with pentobarbital sodium. Cats were then perfused with isotonic saline followed by a 10% formalin solution. Frozen brain sections of 50 µm thickness were cut in the regions of recording and stimulating electrodes. The tissue was stained for Nissl substance with Cresyl Violet. The position of the stimulation electrode in the medullar pyramids was verified by observation of electrode track gliosis. Positions of recording tracks in the motor cortex were estimated in relation to the reference lesions.
Results
The posture-related activity of 28 PTNs from 11 tracks through the forelimb representation of the motor cortex was tested before and after iontophoretic application of gabazine (n = 17) or bicuculline (n = 19) in the immediate vicinity of the neurons. In no instance was there any systemic behavioural effect of a GABAA receptor antagonist application. The tested neurons were representative of the larger population of forelimb-related PTNs, whose posture-related activity on the tilting platform was described in a previous paper (Beloozerova et al., 2005). The Frest of these cells was 10.1 ± 5.3 imp/s, and the Ftilt was 12.4 ± 7.8 imp/s (not significantly different). In the control, the activity of all neurons except for one was modulated in relation to the tilt cycle, and the coefficient of frequency modulation (M) was 73 ± 18%.
Figure 2 provides a typical example of the effect of iontophoretic application of gabazine and bicuculline on the activity of one PTN. Before application (Control), neuronal activity was modulated by tilts, with the maximum in the first half of the cycle. Two minutes after gabazine application (Gabazine), the peak activity (Fmax) increased significantly while Frest, Fmin and the Fmax phase position were unchanged. Fmax returned to the control level 15 min later (Recovery 1). Three minutes after bicuculline application (Bicuculline), the Fmax of the neuron increased again to a value similar to that with gabazine; Frest, Fmin and the Fmax phase were again unchanged. Fmax returned to the control level 15 min later (Recovery 2).
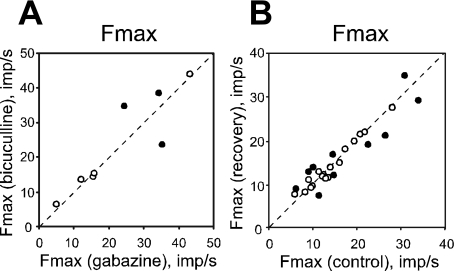
All neurons tested with both gabazine and bicuculline (n = 8) responded very similarly to the two substances. Figure 3A shows Fmax in the presence of gabazine or bicuculline for each of the tested cells. The activities were very close in six out of eight cells. Similar results were obtained with each of the other parameters calculated. Therefore, for further analysis we have pooled data obtained with gabazine and bicuculline, taking the gabazine data for the cells tested with both substances. We have analysed the responses to gabazine and/or bicuculline of only those cells whose complete (n = 18) or clear partial (n = 10) recovery was documented (Fig. 3B).
Fig. 3.
Maximum activity (Fmax) of individual PTNs under (A) Gabazine vs. Bicuculline, and (B) in Control vs. Recovery. In A and B, • denotes a PTN whose activity was statistically significantly different in the two conditions; ○, those whose activity was not significantly different.
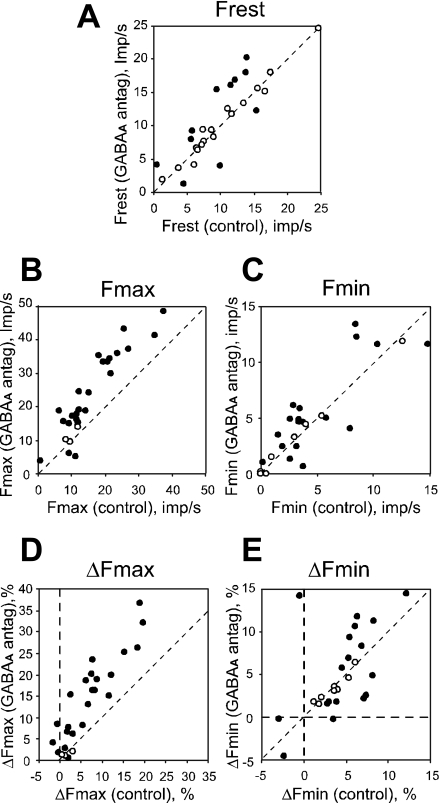
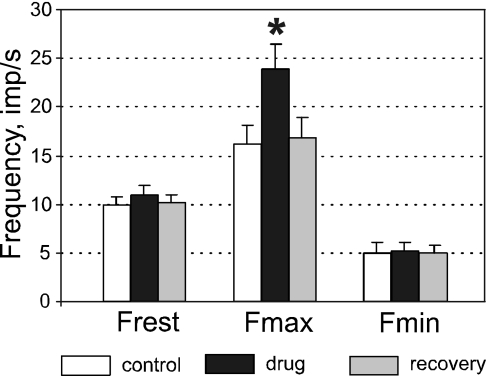
After treatment with a GABAA receptor antagonist, Frest did not change in 61% of cells; the activity of the remaining 39% increased (by 52 ± 22%, in 29% of cells) or decreased (by 25 ± 20%, in 10% of cells; Fig. 4A). Bicuculline caused an increase of Frest in 21% of the cells tested with it and a decrease in another 21%. The PTN population Frest stayed unchanged after treatment with a GABAA receptor antagonist (Fig. 5).
Fig. 4.
Effects of GABAA receptor antagonists on the activity of individual PTNs during postural corrections. (A) Effect on the rest activity of the neurons (Frest). (B) Effect on the maximum activity of the neurons (Fmax). (C) Effect on the minimum activity of the neurons (Fmin). (D) Effect on the difference between Frest and Fmax (ΔFmax). (E) Effect on the difference between Frest and Fmin (ΔFmin). In A–E, • denotes a PTN whose activity was statistically significantly different in the two conditions; ○, those whose activity was not significantly different.
Fig. 5.
Effects of GABAA receptor antagonists on PTN population activity: Frest, Fmax and Fmin. *P < 0.01 for the increase in Fmax after treatment with a GABAA receptor antagonist.
In contrast to resting activity, which in many individual neurons and in the total PTN population did not change after blockade of GABAA inhibition, activity during postural corrections was affected by GABAA receptor antagonism in 93% (all but two) of the PTNs. The maximum effect developed in 3–8 min and lasted for ∼5 min. A significant recovery could be achieved as soon as 15 min after application and typically was complete 30–40 min thereafter. Passing a current through a pipette filled with physiological solution had no effect on any of the cells in the time window tested (1–10 min after application). A repeated application of either gabazine or bicuculline ≥1 h after the previous one gave virtually identical results.
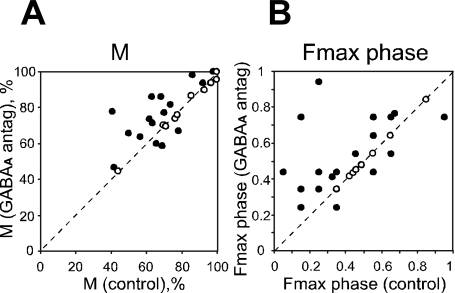
Out of 26 cells that responded to a GABAA receptor antagonist during postural corrections, 21 (81%) increased their mean activity (Ftilt) by 50 ± 26%, while three cells decreased and two did not change. An even larger proportion of cells (23/26, 88%) increased their Fmax (by 9 ± 4.5 imp/s or 75 ± 50%; Fig. 4B). The same time, Fmin rose in a smaller number of cells (12/26, 46%; P < 0.01, χ2 test) and by a significantly smaller value (2.1 ± 1.3 imp/s; P < 0.05, t-test; Fig. 4C). Moreover, in 6 (23%) cells Fmin decreased by 4.1 ± 5.4 imp/s. The peak activity in relation to the resting level (ΔFmax) was significantly greater with a GABAA receptor antagonist than in control in 22 (85%) cells (by 170 ± 130%; Fig. 4D, upper left part). In contrast, although the difference between the rest and the minimum activity (ΔFmin) was significantly altered by a GABAA receptor antagonist in 18 (69%) neurons, it was smaller than in control only in half of these cells (by 73 ± 52%; lower right of Fig. 4E) and greater in the other half (by 45 ± 26%; upper left of Fig. 4E). The magnitudes of ΔFmin changes, either increases or decreases, were significantly smaller than those of ΔFmax (P < 0.05, t-test). For the whole PTN population, Fmax in the presence of a GABAA receptor antagonist was significantly greater than in control, while Fmin was not different (Fig. 5). As a result of the Fmax increase (in the majority of the cells) and also the Fmin decrease (in some other cells) after GABAA receptor antagonist application, the coefficient of modulation, M, rose in 13 (50%) of the PTNs (Fig. 6A).
Fig. 6.
Effects of GABAA receptor antagonists on (A) the coefficient of modulation and (B) the phase of maximum activity of individual PTNs. In A and B, • denotes a PTN whose activity was statistically significantly different in the two conditions; ○, those whose activity was not significantly different.
Out of the 26 cells that responded to a GABAA receptor antagonist during postural corrections, the phase of the peak activity (Fmax phase) did not change as compared to control in 8 (31%). It changed by 0.1 of a cycle in 11 (42%) cells, by 0.2 of a cycle in four (15%) cells and by >0.2 of a cycle in three (12%) cells (Fig. 6B). When Fmax changed by >0.1 of a cycle, the phase shift typically occurred because the cell became more active in a different portion of its original period of elevated activity. In only one cell did treatment with a GABAA receptor antagonist lead to the appearance of a completely new activity peak; and in a different single cell, a change in the Fmax phase was the only effect of GABAA receptor antagonist application.
Discussion
We have found that the GABAA receptor antagonists gabazine and bicuculline caused phase-specific changes in the posture-related activity of PTNs. Most commonly, firing frequency increased within the peak; the effects on the frequency between peaks as well as peak timing were relatively small.
Several facts suggest that in our experimental conditions the predominant action of bicuculline was the blockade of GABAA receptors, rather than the blockade of Ca2+-dependent K+ current or other non-GABAergic effects. First, the resting activity of less than a quarter (21%) of PTNs increased after treatment with bicuculline. Second, across the cycle of posture-related corrections, bicuculline affected PTN responses not uniformly but in a strong phase-dependent manner, being maximal in the period of higher PTN activity. Third, in seven neurons we tested the effect of bicuculline during two different behaviours (posture maintenance and scratching or posture maintenance and locomotion). In five of these cells we observed a clear-cut effect of bicuculline on activity during one behaviour but not the other (I. Beloozerova, unpublished observation). Fourth, in eight neurons we compared the posture-related effects of bicuculline with those of another GABAA receptor antagonist, gabazine, and found them to be very similar (Figs 2 and 3B). Finally, with our application regimen only rarely (in <10% of all applications) did we observe paroxysmal activity in the neurons; these data have been excluded from the analysis.
In many previous studies similar to our experiments, GABAA-mediated influences have been found to decrease the amplitude of neuronal responses to stimulation or elevate the threshold for the responses (e.g. Dykes et al., 1984; Alloway & Burton, 1991; Jacobs & Donoghue, 1991; Matsumura et al., 1992; Kyriazi et al., 1996; Sato et al., 1995; Sawaguchi, 2001; Li et al., 2002). Studies have also shown in different compartments of the sensory cortex (somatosensory, visual and auditory) and in different animal models that GABAA-mediated influences inhibit responses to sensory stimulation, with strong effects within many neurons' primary receptive fields (e.g. Alloway et al., 1989; Ferster, 1986; Borg-Graham et al., 1998; Moore & Nelson, 1998; Martinez et al., 2002; Wang et al., 2002; Qin & Sato, 2004). A variety of other studies have also documented a positive correlation between levels of inhibition and excitation in the cortex (e.g. Haider et al., 2006; Weliky et al., 1995; Sato et al., 1996; Varela et al., 1999; Shu et al., 2003; Marino et al., 2005; Higley & Contreras, 2006). The possible role of reducing the neuronal responses at their focus in respect of different physiological functions has been discussed in the above and other reports. It is suggested that the dynamic balance of excitatory and inhibitory interactions may confer significant computational and plasticity advantages. Regarding the role of GABAA-mediated inhibition in the posture-related activity of PTNs, we suggest the following. We have previously proposed that the posture-related activity of PTNs provides a control signal for postural adjustments (Beloozerova et al., 2005). Here we report that cortical GABAA-mediated influences reduce this signal. This reduction might be related to a cortical mechanism for adaptive modulation of the control signal if the need arises. During complex motor behaviours, such as walking on an unstable surface, the phase-dependent corrective control signal to the limbs can be increased by tonic inhibition of GABAAergic influences on PTNs.
In our experiments, postural responses in the motor cortex after treatment with a GABAA receptor antagonist increased to a lesser degree than those reported by Matsumura et al. (1992) for a press–release task in monkeys (75 ± 50% vs. 300 ± 150%). This is unlikely to be because we did not apply a sufficient amount of GABAA receptor antagonist or because there was still significant room to increase the response by delivering more substance. We used a much stronger current and longer application time than did Matsumura et al. (1992). The effect of application in our experiment also lasted much longer, suggesting that a larger amount of GABAA receptor antagonist was applied. It seems likely that GABAA-mediated influences play different roles in different behaviours and in different neuronal networks. Other authors have also reported considerable differences in the effects of a GABAA receptor blockade between individual neurons and between neuronal networks (e.g. Sillito, 1975; Alloway & Burton, 1991; Matsumura et al., 1992; Sato et al., 1996; Murthy & Humphrey, 1999; Schiller & Tehovnik, 2003). For example, Alloway & Burton (1991) found fewer inhibitory effects in the slowly adapting neurons in the somatosensory cortex than in the rapidly adapting ones. Matsumura et al. (1992) found that the effects in the premotor cortex were weaker than those in the primary motor cortex.
During postural corrections, the interburst activity of PTNs was rather mildly and inconsistently affected by GABAA receptor antagonism. Thus, the troughs in the posture-related responses of PTNs for the most part were not caused by the cortical inhibitory GABAergic interneurons that are active during these troughs. GABAA-mediated input to PTNs seems to play a rather small role in causing their periods of lowered activity. This finding suggests that the main cause of the periods of reduced PTN activity is a decrease of the excitatory (most probably thalamic) drive.
In addition to the main effect (an increase in the peak discharge), treatment with a GABAA receptor antagonist caused a decrease in the trough activity in 23% of PTNs (Fig. 4C). Also, the peak activity decreased in two cells (Fig. 4B). These decreases in activity can be explained by the involvement of non-GABAAergic inhibitory neurons that synapse on PTNs and whose activity increased (disinhibited) after treatment with a GABAA receptor antagonist. With our application regimen, the GABAA antagonists could affect tissue several hundreds of microns in diameter (Jacobs & Donoghue, 1991). Indeed, in few cases when we recorded two cells simultaneously with the same electrode, the GABAA receptor antagonists typically affected the activity of both of them. In future studies, it would be interesting to continue examining the effects of a GABAA transmission blockade on the activity of different identified classes of cortical neurons.
In this study, the phase position of the peak activity changed slightly (by 0.1 of a cycle) or not at all in the majority of PTNs (73%) after treatment with a GABAA receptor antagonist. In only two neurons did the peak phase change substantially (Fig. 6B). The relatively small changes in the neuronal response timing and trough activity suggest that some source other than the cortical GABAAergic mechanisms, such as thalamic or cortico-cortical inputs, largely determine the basic pattern of PTN activity during postural corrections. In contrast to sensory cortices where the responses to sensory stimuli are ‘shaped’ in different domains by intracortical GABAAergic inhibition (e.g. Sillito, 1975; Dykes et al., 1984; Eysel, 1992; Kyriazi et al., 1996; Sato et al., 1996; Pernberg et al., 1998; Foeller et al., 2001; Tremere et al., 2001; Wang et al., 2002; Dehner et al., 2004; Chang et al., 2005), responses in the motor cortex are primarily ‘scaled’ while their timing is determined by input signals.
Acknowledgments
We thank Peter Wettenstein for invaluable engineering assistance and Dr Vladimir Maiorov for a useful discussion of the results. This work was supported by grants from NIH R01 NS-39340 and Barrow Neurological Foundation to I.N.B., and from NIH R01 NS-049884, the Swedish Research Council (no. 11554), and Gösta Fraenckels Foundation to T.G.D.
Glossary
Abbreviations
- Fmax
maximum frequency in the histogram
- Fmin
minimum frequency in the histogram
- Frest
mean frequency of discharge on a stable platform
- Ftilt
mean frequency of discharge during tilts
- GABA
gamma-aminobutyric acid
- M
coefficient of modulation
- PTN
pyramidal tract neuron.
References
- Alloway KD, Burton H. Differential effects of GABA and bicuculline on rapidly- and slowly-adapting neurons in primary somatosensory cortex of primates. Exp.Brain Res. 1991;85:598–610. doi: 10.1007/BF00231744. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Rosenthal P, Burton H. Quantitative measurements of receptive field changes during antagonism of GABAergic transmission in primary somatosensory cortex of cats. Exp.Brain Res. 1989;78:514–532. doi: 10.1007/BF00230239. [DOI] [PubMed] [Google Scholar]
- Bard P, Macht MB. The behavior of chronically decerebrated cat. In: Wolstenholme GEW, O'Connor CM, editors. Neurological Basis of Behaviour. London: Churchill; 1958. pp. 55–71. [Google Scholar]
- Batshelet E. Circular Statistics in Biology. New York: Academic Press; 1981. [Google Scholar]
- Beloozerova IN, Sirota MG. The role of the motor cortex in the control of accuracy of locomotor movements in the cat. J.Physiol.(Lond.) 1993;461:1–25. doi: 10.1113/jphysiol.1993.sp019498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Orlovsky GN, Deliagina TG. Activity of pyramidal tract neurons in the cat during postural corrections. J.Neurophysiol. 2005;93:1831–1844. doi: 10.1152/jn.00577.2004. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Swadlow HA, Orlovsky GN, Popova LB, Deliagina TG. Activity of different classes of neurons of the motor cortex during postural corrections. J. Neurosci. 2003;23:7844–7853. doi: 10.1523/JNEUROSCI.23-21-07844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birt D, Aou S, Woody CD. Intracellularly recorded responses of neurons of the motor cortex of awake cats to presentations of Pavlovian conditioned and unconditioned stimuli. Brain Res. 2003;969:205–216. doi: 10.1016/s0006-8993(03)02331-x. [DOI] [PubMed] [Google Scholar]
- Bishop PO, Burke W, Davis R. The identification of single units in central visual pathways. J.Physiol.(Lond.) 1962;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg-Graham LJ, Monier C, Fregnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature. 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- Capaday C. The integrated nature of motor cortical function. Neuroscientist. 2004;10:207–220. doi: 10.1177/107385403262109. [DOI] [PubMed] [Google Scholar]
- Capaday C, Rasmusson DD. Expansion of receptive fields in motor cortex by local blockade of GABAA receptors. Exp.Brain Res. 2003;153:118–122. doi: 10.1007/s00221-003-1634-y. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J.Neurosci. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc.Natl Acad.Sci.USA. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Telfeian AE. Dynamic properties of cells, synapses, circuits, and seizures in neocortex. Adv.Neurol. 2000;84:141–152. [PubMed] [Google Scholar]
- Conrad B, Wiesendanger M, Matsurami K, Brooks VB. Precentral unit activity related to control of arm movements. Exp.Brain Res. 1977;29:85–95. doi: 10.1007/BF00236877. [DOI] [PubMed] [Google Scholar]
- Dehner LR, Keniston LP, Clemo HR, Meredith MA. Cross-modal circuitry between auditory and somatosensory areas of the cat anterior ectosylvian sulcal cortex: a ‘new’ inhibitory form of multisensory convergence. Cereb.Cortex. 2004;14:387–403. doi: 10.1093/cercor/bhg135. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN. Comparative neurobiology of postural control. Curr.Opin.Neurobiol. 2002;12:652–657. doi: 10.1016/s0959-4388(02)00376-8. [DOI] [PubMed] [Google Scholar]
- Dykes RW, Landry P, Metherate R, Hicks TP. Functional role of GABA in cat primary somatosensory cortex: Shaping receptive fields of cortical neurons. J.Neurophysiol. 1984;52:1066–1093. doi: 10.1152/jn.1984.52.6.1066. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J.Neurophysiol. 1968;31:14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Eysel UT. Lateral inhibitory interactions in areas 17 and 18 of the cat visual cortex. Prog.Brain Res. 1992;90:407–422. doi: 10.1016/s0079-6123(08)63624-9. [DOI] [PubMed] [Google Scholar]
- Ferster D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J.Neurosci. 1986;6:1284–1301. doi: 10.1523/JNEUROSCI.06-05-01284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Finocchio DV. Operant conditioning of isolated activity in specific muscles and precentral cells. Brain Res. 1972;40:19–23. doi: 10.1016/0006-8993(72)90100-x. [DOI] [PubMed] [Google Scholar]
- Fisher NI. Statistical Analysis of Circular Data. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Foeller E, Vater M, Kossl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. J.Assoc.Res.Otolaryngol. 2001;2:279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller JH, Schlag J. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res. 1976;122:283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- Gahery Y, Nieoullon A. Postural and kinetic coordination following cortical stimuli which induce flexion movements in the cat's limbs. Brain Res. 1978;149:25–37. doi: 10.1016/0006-8993(78)90585-1. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Ashe J, Smyrnis N, Taira M. The motor cortex and the coding of force. Science. 1992;256:1692–1695. doi: 10.1126/science.256.5064.1692. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Kettner RE, Schwartz AB. Primate motor cortex and free arm movement to visual target in three-dimensional space. II. Coding of the direction of movement by neural population. J.Neurosci. 1988;8:2928–2937. doi: 10.1523/JNEUROSCI.08-08-02928.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J.Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J.Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak F, Macpherson J. Postural orientation and equilibrium. In: Shepard J, Rowell L, editors. Handbook of Physiology.Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 255–292. [Google Scholar]
- Ioffe ME. Pyramidal influences in establishment of new motor coordinations in dogs. Physiol.Behav. 1973;11:145–153. doi: 10.1016/0031-9384(73)90343-0. [DOI] [PubMed] [Google Scholar]
- Ioffe ME, Ivanova NG, Frolov AA, Birjukova EV, Kiseljova NV. On the role of motor cortex in the learned rearrangement of postural coordination. In: Gurfincel VS, Ioffe ME, Massion J, Roll J-P, editors. Stance and Motion, Fact and Concepts. New York, London: Plenum Press; 1988. pp. 212–226. [Google Scholar]
- Ioffe ME, Massion J, Schmitz C, Viallet F, Gantcheva R. Reorganization of motor patterns during motor learning. A specific role of the motor cortex. In: Latash ML, editor. Progress in Motor Control-III. Champaign, IL: Human Kinetics; 2002. pp. 123–146. [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Sensorimotor transformations in cortical motor areas. Neurosci.Res. 2003;46:1–10. doi: 10.1016/s0168-0102(03)00031-2. [DOI] [PubMed] [Google Scholar]
- Kubota K. Motor cortical muscimol injection disrupts forelimb movement in freely moving monkeys. Neuroreport. 1996;7:2379–2384. doi: 10.1097/00001756-199610020-00020. [DOI] [PubMed] [Google Scholar]
- Kyriazi HT, Carvell GE, Brumberg JC, Simons DJ. Quantitative effects of GABA and bicuculline methiodide on receptive field properties of neurons in real and simulated whisker barrels. J.Neurophysiol. 1996;75:547–560. doi: 10.1152/jn.1996.75.2.547. [DOI] [PubMed] [Google Scholar]
- Latash ML, Danion F, Bonnard M. Effects of transcranial magnetic stimulation on muscle activation patterns and joint kinematics within a two-joint motor synergy. Brain Res. 2003;961:229–242. doi: 10.1016/s0006-8993(02)03958-6. [DOI] [PubMed] [Google Scholar]
- Lavoie BA, Cody FW, Capaday C. Cortical control of human soleus muscle during volitional and postural activities studied using focal magnetic stimulation. Exp.Brain Res. 1995;103:97–107. doi: 10.1007/BF00241968. [DOI] [PubMed] [Google Scholar]
- Li CX, Callaway JC, Waters RS. Removal of GABAergic inhibition alters subthreshold input in neurons in forepaw barrel subfield (FBS) in rat first somatosensory cortex (SI) after digit stimulation. Exp.Brain Res. 2002;145:411–428. doi: 10.1007/s00221-002-1124-7. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Deliagina TG, Orlovsky GN. Control of body orientation and equilibrium in vertebrates. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, Networks, and Motor Behavior. Cambridge: MIT Press; 1997. pp. 257–267. [Google Scholar]
- Magnus R. Körperstellung. Berlin: Springer; 1924. [Google Scholar]
- Marino J, Schummers J, Lyon DC, Schwabe L, Beck O, Wiesing P, Obermayer K, Sur M. Invariant computations in local cortical networks with balanced excitation and inhibition. Nat.Neurosci. 2005;8:194–201. doi: 10.1038/nn1391. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Task-related coding of stimulus and response in cat motor cortex. Exp.Brain Res. 1985;57:427–442. doi: 10.1007/BF00237829. [DOI] [PubMed] [Google Scholar]
- Martinez LM, Alonso JM, Reid RC, Hirsch JA. Laminar processing of stimulus orientation in cat visual cortex. J.Physiol.(Lond.) 2002;540:321–333. doi: 10.1113/jphysiol.2001.012776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. Role of the motor cortex in the postural adjustment associated with movements. In: Asanuma H, Wilson VJ, editors. Integration in the Nervous System. Tokyo: Igaku-Shoin; 1979. pp. 239–260. [Google Scholar]
- Massion J. Postural control systems in developmental perspective. Neurosci.Biobehav.Rev. 1998;22:465–472. doi: 10.1016/s0149-7634(97)00031-6. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Sawaguchi T, Kubota K. GABAergic inhibition of neuronal activity in the primate motor and premotor cortex during voluntary movement. J.Neurophysiol. 1992;68:692–702. doi: 10.1152/jn.1992.68.3.692. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Sawaguchi T, Oishi T, Ueki K, Kubota K. Behavioral deficits induced by local injection of bicuculline and muscimol into the primate motor and premotor cortex. J.Neurophysiol. 1991;65:1542–1553. doi: 10.1152/jn.1991.65.6.1542. [DOI] [PubMed] [Google Scholar]
- Moore CI, Nelson SB. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J.Neurophysiol. 1998;80:2882–2892. doi: 10.1152/jn.1998.80.6.2882. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Sensory receptors and neural encoding: introduction to sensory processes. In: Mountcastle VB, editor. Medical Physiology. 14th edn. St. Louis: Mosby; 1980. pp. 327–347. [Google Scholar]
- Murthy A, Humphrey AL. Inhibitory contributions to spatiotemporal receptive-field structure and direction selectivity in simple cells of cat area 17. J.Neurophysiol. 1999;81:1212–1224. doi: 10.1152/jn.1999.81.3.1212. [DOI] [PubMed] [Google Scholar]
- Pernberg J, Jirmann KU, Eysel UT. Structure and dynamics of receptive fields in the visual cortex of the cat (area 18) and the influence of GABAergic inhibition. Eur.J.Neurosci. 1998;10:3596–3606. doi: 10.1046/j.1460-9568.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J.Neurophysiol. 2005;94:2959–2969. doi: 10.1152/jn.00704.2004. [DOI] [PubMed] [Google Scholar]
- Qin L, Sato Y. Suppression of auditory cortical activities in awake cats by pure tone stimuli. Neurosci.Lett. 2004;365:190–194. doi: 10.1016/j.neulet.2004.04.092. [DOI] [PubMed] [Google Scholar]
- Reitboeck HJ. Fiber microelectrodes for electrophysiological recordings. J.Neurosci.Meth. 1983;8:249–262. doi: 10.1016/0165-0270(83)90038-9. [DOI] [PubMed] [Google Scholar]
- Sato H, Katsuyama N, Tamura H, Hata Y, Tsumoto T. Mechanisms underlying orientation selectivity of neurons in the primary visual cortex of the macaque. J.Physiol.(Lond.) 1995;494:757–771. doi: 10.1113/jphysiol.1996.sp021530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T. Unmasking of silent ‘task-related’ neuronal activity in the monkey prefrontal cortex by a GABA (A) antagonist. Neurosci.Res. 2001;39:123–131. doi: 10.1016/s0168-0102(00)00204-2. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Cortical inhibitory circuits in eye-movement generation. Eur.J.Neurosci. 2003;18:3127–3133. doi: 10.1111/j.1460-9568.2003.03036.x. [DOI] [PubMed] [Google Scholar]
- Schneider C, Devanne H, Lavoie BA, Capaday C. Neural mechanisms involved in the functional linking of motor cortical points. Exp.Brain Res. 2002;146:86–94. doi: 10.1007/s00221-002-1137-2. [DOI] [PubMed] [Google Scholar]
- Scott SH, Kalaska JF. Changes in motor cortex activity during reaching movements with similar hand paths but different arm postures. J.Neurophysiol. 1995;73:2563–2567. doi: 10.1152/jn.1995.73.6.2563. [DOI] [PubMed] [Google Scholar]
- Scott SH, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. I. Activity of individual cells in motor cortex. J.Neurophysiol. 1997;77:826–852. doi: 10.1152/jn.1997.77.2.826. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Sillito AM. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J.Physiol.(Lond.) 1975;250:305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solopova IA, Kazennikov OV, Deniskina NB, Levik YS, Ivanenko YP. Postural instability enhances motor responses to transcranial magnetic stimulation in humans. Neurosci.Lett. 2003;337:25–28. doi: 10.1016/s0304-3940(02)01297-1. [DOI] [PubMed] [Google Scholar]
- Tamarova ZA, Sirota MG, Orlovsky GN, Deliagina TG, Beloozerova IN. Role of GABAA inhibition in modulation the activity of pyramidal tract neurons during postural corrections. Soc.Neurosci.Abstr. 2004:187.2. [Google Scholar]
- Tremere L, Hicks TP, Rasmusson DD. Role of inhibition in cortical reorganization of the adult raccoon revealed by microiontophoretic blockade of GABA (A) receptors. J.Neurophysiol. 2001;86:94–103. doi: 10.1152/jn.2001.86.1.94. [DOI] [PubMed] [Google Scholar]
- Varela JA, Song S, Turrigiano GG, Nelson SB. Differential depression at excitatory and inhibitory synapses in visual cortex. J.Neurosci. 1999;19:4293–4304. doi: 10.1523/JNEUROSCI.19-11-04293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;944:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- Weliky M, Kandler K, Fitzpatrick D, Katz LC. Patterns of excitation and inhibition evoked by horizontal connections in visual cortex share a common relationship to orientation columns. Neuron. 1995;15:541–552. doi: 10.1016/0896-6273(95)90143-4. [DOI] [PubMed] [Google Scholar]