Abstract
In order to establish the possible gender influence on the activity of leflunomide (LEF) in rheumatoid arthritis (RA), we evaluated the proapoptotic activity of the active LEF metabolite A77 1726 (LEF-M), in combination with sex hormones, on cultures of human macrophages. In particular, we focussed our investigation on the triggering phase of the apoptosis. Cultures of macrophages from activated THP-1 cells and from RA synovial tissues were treated with LEF-M alone [30μM] or in presence of 17beta-estradiol (E2) (10-9M) or testosterone (T) (10-8M) for 24 hours. FAS, FAS-L, FADD (Fas-Associated via Death Domain) and FLICE (FADD-Like Interleukin-1 beta Converting Enzyme) were evaluated by immunocytochemistry (ICC), Western blot (WB) and reverse transcriptase-multiplex polymerase chain reaction (RT-MPCR). Regarding macrophages from THP-1 cells (M), the ICC showed that LEF-M exerted a significant up-regulation on all investigated apoptotic proteins, when compared to untreated cells (control) (p<0.001). On the contrary, E2 significantly increased FAS-L positivity (p<0.05) and down-regulated FADD (p<0.01), while others apoptotic proteins were not modulated when compared to control. Regarding RA synovial macrophages (SM), the ICC showed that LEF-M exerted a significant up-regulation on FAS and FAS-L when compared to control (p<0.001). On the contrary, E2 down-regulated significantly FAS-L (p<0.001), while FAS was not modulated respect to control. T significantly increased the apoptotic proteins in all conditions. The results of the present study suggests a less efficient therapeutic effect of LEF in female patients, due to the contrasting action of estrogens on LEF- induced apoptosis.
Keywords: Rheumatoid arthritis, leflunomide, apoptosis, macrophages, sex hormones
Introduction
Rheumatoid arthritis (RA) is a chronic, immune-mediated and inflammatory polyarthritis that induces articular damage and disability accompanied by destruction of joint integrity [1]. One of the major pathological changes in RA is the formation of synovial “pannus”, an exuberant hyperplastic immune/inflammatory tissue, which extends from the synovial/cartilage interface over the surface of the articular cartilage, leading to its consequent destruction and erosion. The inflammatory synovial tissue in particular is rich in lymphocytes, plasma cells, and macrophages. In RA there is also hyperplasia of the synovial intima, the synovial lining cells being almost entirely formed by macrophages [2]. Macrophages are known to produce and respond to different mediators that stimulate bone resorption and are a major source of proteinases [3].
Similarly, it is well known that synovial macrophages (SM) are capable of producing pro-inflammatory cytokines able of amplifying the inflammatory reaction by inducing the active-tion of other synovial cell populations [4].
The persistence of the inflammation during RA can be considered the expression of the balance between two major conditions: 1. The extravasation of circulating inflammatory cells into the tissue and in part the local prolife-ration of resident cells such as fibroblasts and endothelial cells; 2. The incapacity to activate apoptotic processes from recruited and resident synovial and inflammatory cells, resulting in prolonged and inappropriate cell survival into the tissue. Monocytes and macrophages, and also fibroblasts, seem to be crucial for these events [5,6]. Insufficient apoptosis seems to contribute to the pathogenesis of many disorders including auto-immune diseases [7]. RA synovial monocyte/macrophages are characterized by the persistent activation or, in other words, by the incapacity to switch off cellular activation, an event strictly linked to the inability of these cells to set up the normal program of apoptosis [8].
Although apoptosis is an intrinsic process present in all cells, it can be regulated by extrinsic factors, including growth factors, cytokines, cellular stress and hormones [7]. The neuroendocrine immune (NEI) system is regarded as a fundamental network for the maintenance of health status (homeostasis), and it plays an important role in several systemic diseases, including autoimmune disorders. Among the major players of NEI pathways are steroid hormones such as cortisol and sex hormones [9]. Clinical and experimental evidence indicates that estro-gens play an important role in the activation and maintenance of immune response and that lymphocytes and monocytes from female subjects (or tested in vitro with 17beta-estradiol) show higher immune/inflammatory reactivity [10,11].
Sex hormones, together with other factors, may play an important role in the regulation of the immune/inflammatory response in immune-mediated diseases such as RA [12,13]. Treatment of human monocyte/macrophages by sex hormones was found to influence apoptosis; in particular androgens have been found to increase apoptosis, and estrogens showed a protective trend on cell death [14]. Moreover, male RA patients have been shown to react better to specific treatments such as anti-cytokines (TNFalpha-blockers) and anti-proliferative drugs (i.e. methotrexate (MTX) or Leflunomide (LEF), increasing the interest for the complex roles played by gonadal hormones [15]. Up to date, the antiproliferative drug LEF is successfully employed in RA treatment [16]. Recent studies, suggest that the observed anti-inflammatory effects exerted by LEF in arthritis are strongly related to its ability to inhibit osteoclastogenesis, as well as metallo-proteinase and inflammatory cytokine production by cultured RA synovial cells [17,18].
In order to establish the possible gender influence on the activity of LEF, we evaluated the proapoptotic activity of the active meta-bolite of LEF A77 1726 (LEF-M) in combination with sex hormones on cultures of human macrophages. In particular, we focused our investigation on the triggering phase of apoptosis, involving the FAS/FAS-L apoptotic pathway.
Materials and Methods
Cultures of THP-1 cells (M)
THP-1 cells (human monocytes) were seeded partly into flexiperm chamber slides (International, PBI) (105 cells/well) and partly into multiwell plates (2×106 cells/well), treated with phorbol myristate acetate (PMA) [0,1 μg/ml] for 2 hours to differentiate in adherent macrophages (M) and subsequently cultured with LEF-M alone [30μM] and in presence of E2 (10-9M) or T (10-8M) for 24 hours in 5% CO2 air humidified atmosphere at 37°C. All concentrations have been previously tested as closer to the in vivo conditions in other experiments [14,18,19].
The flexiperm chamber slide cultures were used for immunocytochemistry (ICC) analysis and the multiwell plate cultures were employed for mRNA and protein extraction. Experiments were done in triplicate.
Primary cultures of synovial macrophages (SM)
SM were obtained from 5 RA patients (3 females and 2 males, mean age: 48±12 yrs, disease duration 4±6 yrs, DAS28>5.2) fulfilling the 1987 revised criteria of the American College of Rheumatology for adult RA (ACR; formerly, the American Rheumatism Association) and who underwent therapeutic arthroscopic synoviectomy or joint replacement surgery of the knee Arnett [20]. RA patients with similar disease duration, activity and therapy were enrolled in order to reduce the variability of the results.
At the time of surgery, all the patients were treated from 15 days with i.v. non steroidal anti-inflammatory drugs, and with low-dose glucocorticoids (prednisone 5–7.5 mg/day) and for short-time with antibiotics. No biological drugs, anti-proliferative drugs or other disease modifying anti-rheumatic drugs (DMARDs) were administered at the time of knee surgery. The Ethical Committee of the University of Genova approved the study and informed consent was obtained from patients.
After surgery, the synovial RA tissue samples were gently cut into small pieces (2–5 mm), washed in Dulbecco's phosphate buffered saline (DPBS; Sigma-Aldrich, Sigma Chemical Division, Milan, Italy) and incubated in collagenase [0.75 mg/ml] (type IV from Clostridium histolyticum; Sigma-Aldrich, Sigma Chemical Division, Milan, Italy) for 1 hour at 37°C. The digest was passed through a wire 150 mesh to separate the synovial cells from the debris tissue. The cells were then washed three times with DPBS, resuspended in RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% foetal bovine serum (containing <0.5 EU/ml endotoxin), 2 mmol/l L-glutamine, 100 μg/ml streptomycin, and 100 U/ml penicillin (Sigma-Aldrich). Viability of the cells (90–95%) was tested by trypan blue exclusion.
The SM were seeded partly into flexiperm chamber slides (International, PBI) (105 cells/well) and at the same time further aliquots were seeded into multiwell plates (2×106 cells/well) and were incubated in 5% CO2 air humidified atmosphere at 37°C. After 1 hour, non-adherent cells were washed out, while adherent cells (SM) were cultured with LEF-M alone [30μM] and in presence of E2 (10-9M) or T (10-8M) for 24 hours. The flexiperm chamber slide cultures were used for the ICC analysis and the multiwell plate cultures were performed for mRNA and protein extraction.
Immunocytochemistry assay (ICC)
Slides with cellular spots, obtained from differentiated human monocytes (THP-1 cells) (M) and RA SM in different culture conditions, were fixed in cold acetone for 30 seconds, air dried, and stored at -20°C until the immune-detection of some apoptosis markers.
After rehydratation in PBS the slides were incubated with a solution of 3% H2O2 for 15 minutes to prevent non-specific reaction due to endogenous peroxidases. Then the cellular spots were incubated with primary human antibodies (Santa Cruz Biotechnology, CA, USA) anti-FAS (dilution 1:100), anti-FAS-L (dilution 1:500), anti-FADD (Fas-Associated via Death Domain) (dilution 1:100), anti-FLICE (FADD-Like Interleukin-1 beta Converting Enzyme) (dilution 1:100), also known as caspase-8, at room temperature for 45 minutes. Only anti-bodies anti-FAS and anti-FAS-L were used for RA SM. Linked antibodies were detected by biotinylated universal (pan-specific) secondary antibody and subsequently horseradish peroxidase-streptavidin complex (Vector Laboratories, Burlingame, CA, USA). Each step was followed by two washes in PBS. The staining reaction was developed by the diaminobenzidine system (DakoCytomation, Dako North America, Inc., CA, USA). Finally, slides were counter-stained with haematoxylin, fixed with ethanol, and cover-slipped with Eukitt. Negative controls were treated identically; however the primary antibodies were omitted.
Image analysis was performed with the Leica Q-Win Image Analysis System (Leica, Cambridge, UK). Almost 100 cells were analyzed for each sample, and pixels/μm2 (positive area) were quantified by the Leica Q-Win software.
Western blot analysis (WB)
After the different treatments, macrophage pellets were lysed in a buffer containing 20 mmol/l Tris-HCl pH 8, 150 mmol/l NaCl, 1 mmol/l phenylmethylsulphonylfluoride, 5 mg/ml aprotinin, and 0.5% Nonidet P-40 (Promega, Milan, Italy) for 1 hour at 4°C. The lysates were then centrifuged for 10 minutes at 13,000 rpm. Protein samples (20 μg) were diluted with sample buffer and separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis. The proteins were transferred to a Hibond-C nitrocellulose membrane (GeHealthCare Europe). After transfer, the membrane was blocked for 1 hour at room temperature in PBS containing 5% non-fat powdered milk.
For immunoblot analysis, the membranes were incubated with primary antibodies anti-FAS (dilution 1:500) and anti-FAS-L (dilution 1:200) (Santa Cruz, Biotechnology) overnight at 4°C, washed in 0.05% PBS/Tween 20 pH 7.4 and incubated for 1 hour at room temperature with secondary antibody (1:65000) (Sigma, Milan, Italy). After three further washes with PBS/Tween, bound secondary antibody was detected by emitting chemiluminescent reaction (Immobilon, Millipore, CA, USA).
Reverse transcriptase-multiplex polymerase chain reaction (RT-MPCR)
mRNA extraction from the cultured cells in different experimental conditions was performed with the Rneasy Mini Kit Qiagen (Qiagen, USA).
The apoptotic proteins were evaluated by reverse transcriptase-multiplex polymerase chain reaction (RT-MPCR) (MPCR Kit for Human apoptotic Genes Set-4, Maxim, Biotech Inc., Rockville, MD), including primer for FAS, FAS-L, FLICE, FADD and TRADD (TNFR1-Associated Death Domain Protein). This method is an accurate and valid system for detecting multiple gene expression simultaneously by amplifying all genes under the same conditions. Primers specific for β-actin (Promega, Milan, Italy) was used as internal positive control.
In relation to the primary cultures we have studied RA SM samples by RT-MPCR limited to the effects of E2 alone and E2 combined with LEF-M.
Statistical analysis
The statistical analysis was carried out by non-parametric test. Wilcoxon test was performed to compare the paired treatments, and Mann-Whitney test to compare different treatments. A probability value p<0.05 was considered statistically significant.
Results
THP-1 cells (M)
The quantified (Q-Win image analysis) ICC analysis on M showed that LEF-M exerted a significant up-regulation on all investigated apoptotic proteins, when compared to untreated cells (control) (p<0.001) (Figures 1, 2, 3, 4).
Figure 1.
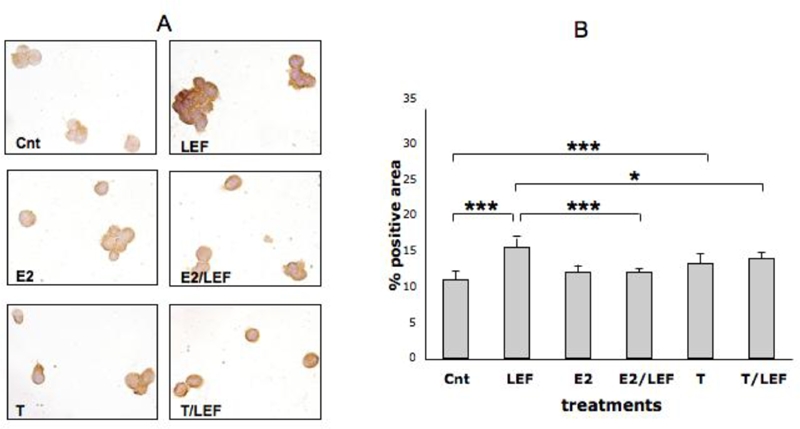
FAS expression by ICC in cultures of THP-1 cells (M). FAS expression by ICC and relative quantification by image analysis (Leica Q-Win system) in cultures of THP-1 cells (M) in different conditions: untreated cells (Cnt), Leflunomide metabolite (LEF) treatment, 17beta-estradiol (E2) treatment, 17beta-estradiol treatment in combination with Leflunomide metabolite (E2/LEF), testosterone (T) treatment, testosterone treatment in combination with Leflunomide metabolite (T/ LEF). Full report of the significance statistical analysis: Cnt vs LEF: ***. Cnt vs E2: ns. Cnt vs E2/LEF: ns. Cnt vs T: ***. Cnt vs T/LEF: ***. LEF vs E2: ***. LEF vs E2/LEF: ***. LEF vs T: ***. LEF vs T/LEF: ***. E vs E2/LEF: ns. E2 vs T: ns. E2 vs T/LEF: ******. E2/LEF vs T: ns. E2/LEF vs T/LEF: ****** T vs T/LEF: ns.*** = p<0.001; ****** = p<0.01; *** = p<0.05; ns= not significant.
Figure 2.
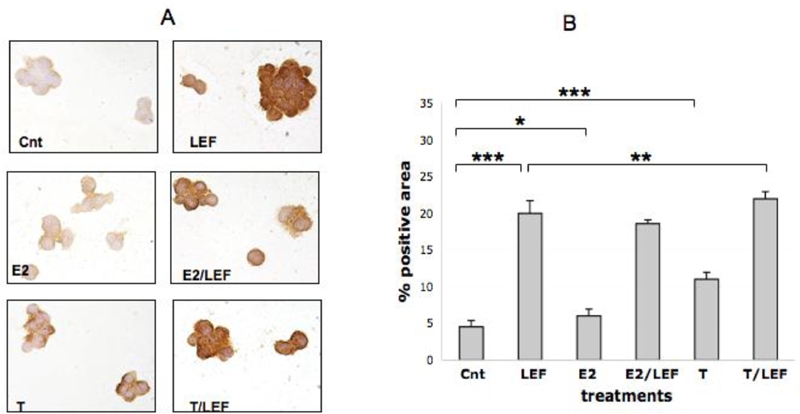
FAS-L expression by ICC in cultures of THP-1 cells (M). FAS-L expression by ICC and relative quantification by image analysis (Leica Q-Win system) in cultures of THP-1 cells (M) in different conditions: untreated cells (Cnt), Leflunomide metabolite (LEF) treatment, 17beta-estradiol (E2) treatment, 17beta-estradiol treatment in combination with Leflunomide metabolite (E2/LEF), testosterone (T) treatment, testosterone treatment in combination with Leflunomide metabolite (T/LEF). Full report of the significance statistical analysis: Cnt vs LEF: ***. Cnt vs E2: *. Cnt vs E2/LEF: ***. Cnt vs T: ***. Cnt vs T/LEF: ***. LEF vs E2: ***. LEF vs E2/LEF: ns. LEF vs T: ***. LEF vs T/LEF: **. E2 vs E2/LEF: ***. E2 vs T: ***. E2 vs T/LEF: ***. E2/LEF vs T: ***. E2/LEF vs T/LEF: ***. T vs T/LEF: ***. *** = p<0.001; ** = p<0.01; * = p<0.05; ns= not significant.
Figure 3.
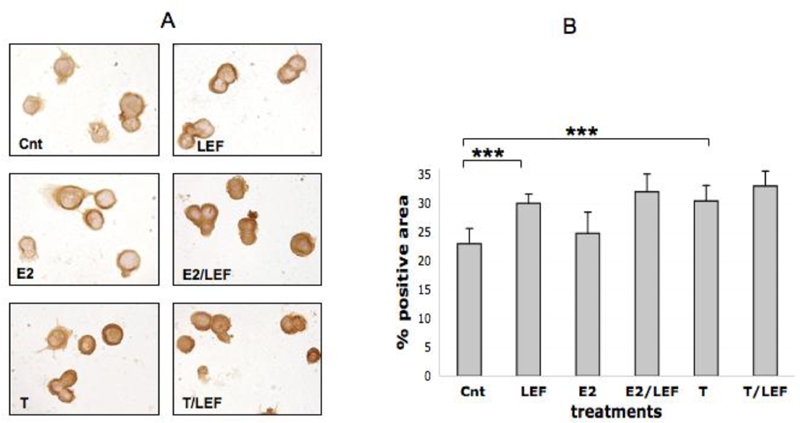
FLICE expression by ICC in cultures of THP-1 cells (M). FLICE (caspase 8) expression by ICC and relative quantification by image analysis (Leica Q-Win system) in cultures of THP-1 cells (M) in different conditions: untreated cells (Cnt), Leflunomide metabolite (LEF) treatment, 17beta-estradiol (E2) treatment, 17beta-estradiol treatment in combination with Leflunomide metabolite (E2/LEF), testosterone (T) treatment, testosterone treatment in combination with Leflunomide metabolite (T/LEF). Full report of the significance statistical analysis: Cnt vs LEF: ***. Cnt vs E2: ns. Cnt vs E2/LEF:***. Cnt vs T: ***. Cnt vs T/LEF: ***. LEF vs E2: **. LEF vs E2/LEF: ns. LEF vs T: ns. LEF vs T/LEF: ns. E vs E2/LEF: ***. E2 vs T:***. E2 vs T/LEF: ***. E2/LEF vs T: ns. E2/LEF vs T/LEF: ns. T vs T/LEF: ns. *** = p<0.001; ** = p<0.01; * = p<0.05; ns= n not significant.
Figure 4.
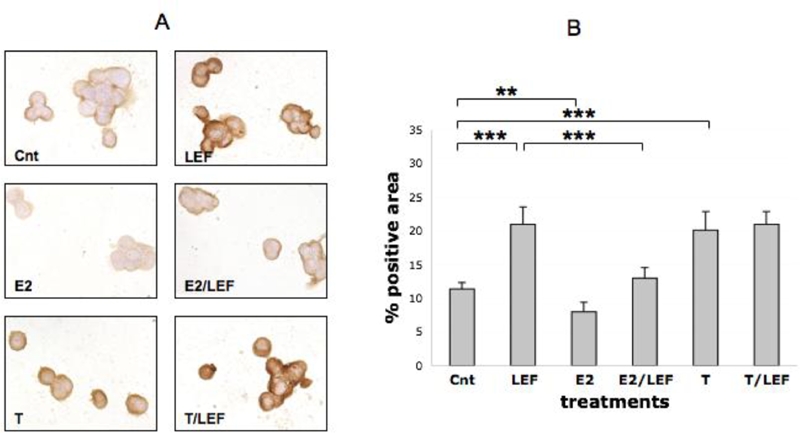
FADD expression by ICC in cultures of THP-1 cells (M). FADD expression by ICC and relative quantification by image analysis (Leica Q-Win system) in cultures of THP-1 cells (M) in different conditions: untreated cells (Cnt), Leflunomide metabolite (LEF) treatment, 17beta-estradiol (E2) treatment, 17beta-estradiol treatment in combination with Leflunomide metabolite (E2/LEF), testosterone (T) treatment, testosterone treatment in combination with Leflunomide metabolite (T/LEF). Full report of the significance statistical analysis: Cnt vs LEF: ***. Cnt vs E2: **. Cnt vs E2/LEF: ns. Cnt vs T: ***. Cnt vs T/LEF: ***. LEF vs E2: ***. LEF vs E2/LEF: ***. LEF vs T: ns. LEF vs T/LEF: ns. E vs E2/LEF: ***. E2 vs T: ***. E2 vs T/LEF: ***. E2/LEF vs T: ***. E2/LEF vs T/LEF: ***. T vs T/LEF: ns.*** = p<0.001; ** = p<0.01; * = p<0.05; ns= not significant.
The treatment of the cultured cells with E2 increased significantly FAS-L positivity (p<0.05) (Figure 2), down-regulated significantly FADD (p<0.01) (Figure 4), while others apoptotic proteins were not modulated when compared to control (Figures 1 and 3). Moreover, E2 and LEF-M combination decreased FAS-L and significantly FAS and FADD (p<0.001), when compared with LEF-M alone (Figures 1, 2, 4).
On the contrary, T treatment induced a significant increase of all investigated apoptotic proteins (p<0.001), when compared to untreated cells (Figures 1, 2, 3, 4). Interestingly, the addition of T to LEF-M treatment induced a further significant increase only of FAS-L protein when compared to the LEF-M treated cells (Figure 2). The results of the ICC analysis were confirmed by WB analysis for FAS and FAS-L (Figure 5).
Figure 5.
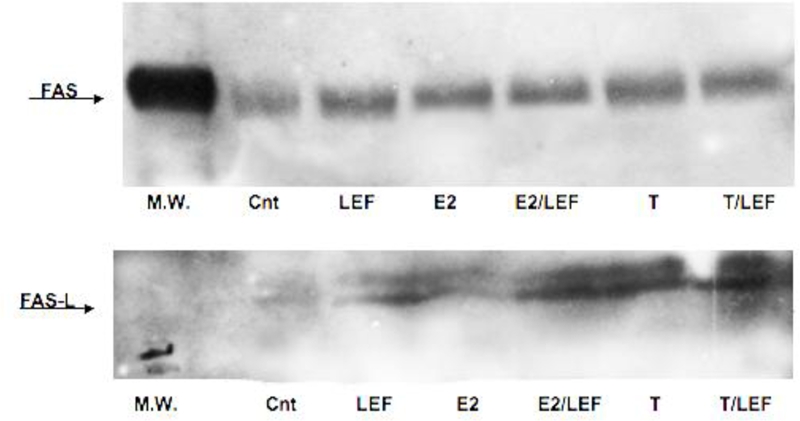
FAS and FAS-L expression by WB in cultures of THP-1 cells (M). FAS and FAS-L expression by WB in cultures of THP-1 cells (M) in different conditions. Untreated cells (Cnt), Leflunomide metabolite (LEF) treatment, 17beta-estradiol (E2) treatment, 17beta-estradiol treatment in combination with Leflunomide metabolite (E2/LEF), testosterone (T) treatment, testosterone treatment in combination with Leflunomide metabolite (T/LEF).
The RT-MPCR analysis did not show any statistical significant difference of mRNA expression between the different experimental conditions (Figure 6). However, further experiments at shorter incubation times might be necessary to better detect the mRNA expression of different molecules involved in the apoptotic signaling. Notwithstanding, FLICE PCR product was amplified, confirming a mild increase expression after LEF-M treatment (Figure 6).
Figure 6.
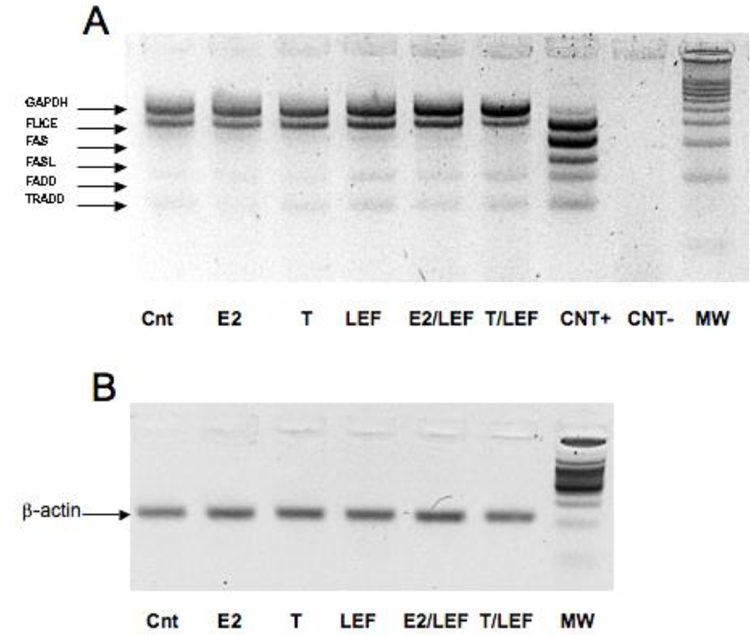
FAS, FAS-L, FLICE (caspase 8), FADD and TRADD mRNA expression by RT-MPCR in cultures of THP-1 cells (M). Reverse transcriptase-multiplex polymerase chain reaction (RT-MPCR) assay for FAS, FAS-L, FLICE (caspase 8), FADD and TRADD mRNA expression (A) in cultures of THP-1 cells (M) in different conditions: untreated cells (Cnt), 17beta-estradiol (E2) treatment, testosterone (T) treatment, Leflunomide metabolite (LEF) treatment, 17beta-estradiol treatment in combination with Leflunomide metabolite (E2/LEF), testosterone treatment in combination with Leflunomide metabolite (T/ LEF). beta-actin as control (B).
Synovial macrophages (SM)
The quantified (Q-Win image analysis) ICC analysis on RA SM showed that LEF-M exerted a significant up-regulation on FAS and FAS-L when compared to control (p<0.001) (Figures 7, 8). FAS-L was down-regulated significantly in the cultured cells treated with E2 (p<0.001), while FAS was not modulated respect to control (Figures 7, 8). Interestingly, when E2 was added to LEF-M, FAS was not modulated while FAS-L showed a significant decrease (p<0.001), when compared with LEF-M alone (Figures 7, 8).
Figure 7.
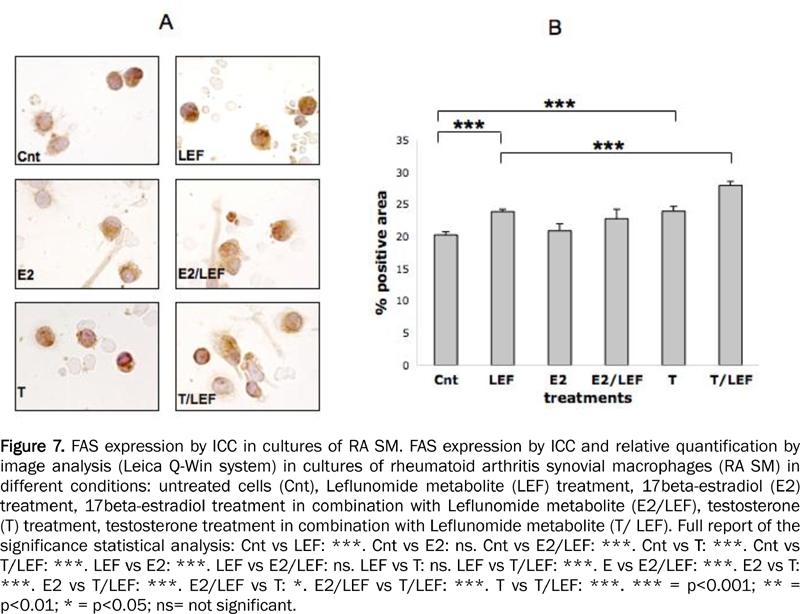
FAS expression by ICC in cultures of RA SM. FAS expression by ICC and relative quantification by image analysis (Leica Q-Win system) in cultures of rheumatoid arthritis synovial macrophages (RA SM) in different conditions: untreated cells (Cnt), Leflunomide metabolite (LEF) treatment, 17beta-estradiol (E2) treatment, 17beta-estradiol treatment in combination with Leflunomide metabolite (E2/LEF), testosterone (T) treatment, testosterone treatment in combination with Leflunomide metabolite (T/ LEF). Full report of the significance statistical analysis: Cnt vs LEF: ***. Cnt vs E2: ns. Cnt vs E2/LEF: ***. Cnt vs T: ***. Cnt vs T/LEF: ***. LEF vs E2: ***. LEF vs E2/LEF: ns. LEF vs T: ns. LEF vs T/LEF: ***. E vs E2/LEF: ***. E2 vs T: ***. E2 vs T/LEF: ***. E2/LEF vs T: *. E2/LEF vs T/LEF: ***. T vs T/LEF: ***. *** = p<0.001; ** = p<0.01; * = p<0.05; ns= not significant.
Figure 8.
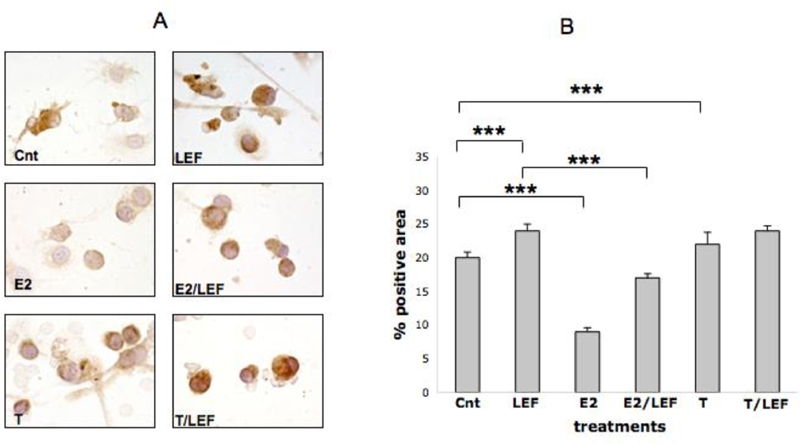
FAS-L expression by ICC in cultures of RA SM. FAS-L expression by ICC and relative quantification by image analysis (Leica Q-Win system) in cultures of rheumatoid arthritis synovial macrophages (RA SM) in different conditions: untreated cells (Cnt), Leflunomide metabolite (LEF) treatment, 17beta-estradiol (E2) treatment, 17beta-estradiol treatment in combination with Leflunomide metabolite (E2/LEF), testosterone (T) treatment, testosterone treatment in combination with Leflunomide metabolite (T/ LEF). Full report of the significance statistical analysis: Cnt vs LEF: ***. Cnt vs E2: ***. Cnt vs E2/LEF: ***. Cnt vs T: ***. Cnt vs T/LEF: ***. LEF vs E2: ***. LEF vs E2/LEF: ***. LEF vs T: ***. LEF vs T/LEF: ns. E vs E2/LEF: ***. E2 vs T: ***. E2 vs T/LEF: ***. E2/LEF vs T: ***. E2/LEF vs T/LEF: ***. T vs T/LEF: ***. *** = p<0.001; ** = p<0.01; * = p<0.05; ns= not significant.
The ICC analysis showed that T treatment induced a significant increment of FAS and FAS-L (p<0.001) when compared to untreated cells (Figures 7, 8). The addition of T to LEF-M treatment induced a significant increase only of FAS (p<0.001) when compared with the LEF-M treated cells (Figures 7, 8).
The RT-MPCR analysis confirmed ICC results for FAS and FAS-L expression and revealed modulation of the other mRNA expression of apoptotic proteins. In particular, LEF-M increased mRNA expression of all the proteins investigated, while the addition of E2 to LEF-M seemed to contrast the apoptotic effect of LEF-M (Figure 9).
Figure 9.
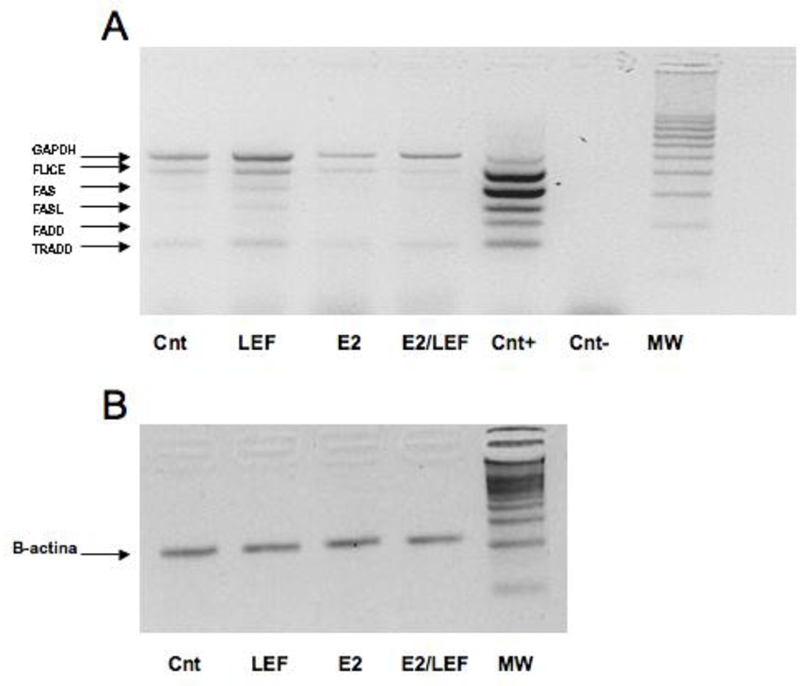
FAS, FAS-L, FLICE (caspase 8), FADD and TRADD mRNA expression by RT-MPCR in cultures of RA SM. Reverse transcriptase-multiplex polymerase chain reaction (RT-MPCR) assay for FAS, FAS-L, FLICE (caspase 8), FADD and TRADD mRNA expression (A) in cultures of rheumatoid arthritis synovial macrophages (RA SM) in different conditions. Untreated cells (Cnt), Leflunomide metabolite (LEF) treatment, 17beta-estradiol (E2) treatment, 17beta-estradiol treatment in combination with Leflunomide metabolite (E2/LEF). Beta-actin as control (B).
Discussion
The present study shows that LEF-M treatment induces a significant increase of all the investigated apoptotic proteins by cultured macrophages (including RA synovial tissue macrophages). Therefore, the anti-inflamma-tory mechanism exerted by LEF seems to be also related to its proapoptotic activity, at least related to recruitment of the early signal-transducing molecules [21]. Moreover, sex hormones seem to interfere with expression of proapoptotic factors and modulate the up-regulatory activity exerted by LEF-M on the apoptosis. In particular, the addition of E2 seems to contrast LEF-M effects at level of FAS-L/FAS/ FADD interaction.
Concerning the combination of LEF-M with T, the modulation was not always evident respect to treatment with LEF-M alone. These results were investigated and obtained by the combined use of ICC, WB and mRNA analysis, in order to better define the sex hormone and LEF-M effects on human cultured macrophages. Monocytes/macrophages contribute to the autoimmune process, mainly acting as antigen-processing/presenting cells and sources of inflammatory cytokines, particularly at the level of the synovial tissue in RA [5]. The persistence of the inflammation during rheu-matoid synovitis can be considered also as the incapacity to activate apoptotic processes from recruited and resident synovial and inflammatory cells, resulting in prolonged and inappropriate cell survival into the tissue.
Apoptosis plays a critical role in the maintenance of tissue homeostasis and is also a physiological mechanism that eliminates excess or damaged cells. Therefore, insufficient apoptosis can contribute to the pathogenesis of many disorders including autoimmune diseases. Macrophages are known to respond to different mediators such as sex hormones in the tissues in which they are formed, including the synovial tissue [19,22].
Recently, we tested the effects of E2 and T on activated macrophages from human monocytic cells (THP-1), in order to evaluate the influence of both hormones on cell proliferation and apoptosis [14]. Effects were evaluated using activity of NFk-B, a complex of molecules that affects cellular activation. T was found to exert proapoptotic effects, whereas E2 induced the opposite effects by interfering with NFk-B activities [14]. E2 increased the expression of markers of cell growth and proliferation, whereas T induced an increase of the PARP–cleaved expression indicating DNA damage and apoptosis [14].
Last studies showed that the estrogen metabolite 16alpha-hydroxyestrone, was far more potent than E2 in exerting an influence on cell proliferative activities [23]. Therefore, these results supported the hypothesis that sex hormones modulate cell growth and apoptosis.
Recent studies further reinforce these results, showing that E2 inhibits apoptosis in different cell types (cardiac myocytes and other cells) whereas androgens have been found to induce apoptosis [24,25]. On the other hand, prevalence of immune-mediated inflammatory diseases is lower in male RA patients, and the illness has been shown to react better to specific treatments, such as anticytokines and anti-proliferative drugs [15,26]. Up to date, the antiproliferative drug LEF is successfully employed in RA treatment. The efficacy of LEF in the treatment of RA patients might be due to the fact that it acts at several levels of the cell cycle.
Indeed, in addition to the well-described effects on many inflammatory mediators, and in order to establish the possible gender influence on the activity of LEF, we have evaluated the apoptotic activity of LEF-M alone and in combination with sex hormones by cultures of human macrophages. The apoptotic process requires the activation of a family of cysteine proteases, termed caspases, that can be induced by engagement of cell surface death receptors [27]. We have also investigated the triggering phase of apoptosis, involving FAS/FAS-L apoptotic pathway, leading to formation of FAS-FADD-FLICE/caspase-8 complex, known as Death-Inducing Signal Complex (DISC).
The results of the current study confirm all the previous data discussed here and support the complex role exerted by LEF in modulating the immune/inflammatory response, at least in RA. On the other hands, the our results also suggest a less efficient therapeutic effect of LEF in female patients, due to the contrasting action of estrogens on apoptosis, as also observed during the DMARD therapy in a recent large multicenter RA study [28]. Gender, of course, has been shown to have a variety of other related issues in autoimmune disease [29].
References
- 1.Klareskog L, Catrina AI, Paget S. Rheumatoid Arthritis. Lancet. 2009;373:659–72. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 2.Lundy SK, Sarkar S, Tesmer LA, Fox DA. Cells of the synovium in rheumatoid arthritis. T lymphocytes. Arthritis Res Ther. 2007;9:202. doi: 10.1186/ar2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang JS, Qin JM, Demazziere A, Bullstrode CJ, Francis MJ, Duthie RB, Athanasou NA. Bone resorption by cells isolated from rrheumatoid synovium. Ann Rheum Dis. 1992;51:1223–1229. doi: 10.1136/ard.51.11.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan FM, McInnes J IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008 Nov;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinne Rw, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in Rheumtoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 7.Katsumi Eguchi. Apoptosis in Autoimmune Disease. Internal Medicine. 2001;Vol. 40(Nº 4) doi: 10.2169/internalmedicine.40.275. [DOI] [PubMed] [Google Scholar]
- 8.Pope RM. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nature Rev. Immunol. 2002;2:527–535. doi: 10.1038/nri846. [DOI] [PubMed] [Google Scholar]
- 9.Cutolo M, Straub R H. Insight into endocrine-immunological disturbances in autoimmunity and their impact on treatment. Arthritis Research & Terapy. 2009;Vol 11(Nº 2) doi: 10.1186/ar2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanda N, Tsuchida T, Tamaki K. Estrogen enhancement of anti-double-stranded DNA antibody and immunoglobulinG production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42:328–337. doi: 10.1002/1529-0131(199902)42:2<328::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Kramer PR, Kramer SF, Guan G. 17 Beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum. 2004;50:1967–1975. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- 12.Cutolo M, Villaggio B, Craviotto C, Pizzorni C, Seriolo B, Sulli A. Sex hormones and rheumatoid arthritis. Autoimmunity Rev. 2002;1:284–289. doi: 10.1016/s1568-9972(02)00064-2. [DOI] [PubMed] [Google Scholar]
- 13.Laivoranta-Nyman S, Luukkainen R, Hakala M, Hannonen P, Möttönen T, Yli-Kerttula U, Ilonen J, Toivanen A. Differences between female and male patients with familial rheumatoidarthritis. Ann Rheum Dis. 2001;60:413–415. doi: 10.1136/ard.60.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutolo M, Capellino S, Montagna P, Ghiorzo P, Sulli A, Villaggio B. Sex hormone modulation of cell growth and apoptosis of the human monocytic/macrophage cell line. Arthritis Res Ther. 2005;7:R 1124–1132. doi: 10.1186/ar1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, Gogus F, Skakic V, Badsha H, Peets T, Baranauskaite A, Géher P, Ujfalussy I, Skopouli FN, Mavrommati M, Alten R, Pohl C, Sibilia J, Stancati A, Salaffi F, Romanowski W, Zarowny-Wierzbinska D, Henrohn D, Bresnihan B, Minnock P, Knudsen LS, Jacobs JW, Calvo-Alen J, Lazovskis J, Pinheiro Gda R, Karateev D, Andersone D, Rexhepi S, Yazici Y, Pincus T, QUEST-RA Group Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA Study. Arthritis Res Ther. 2009;11(1):R7. doi: 10.1186/ar2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherwinski HM, Byars N, Ballaron SJ, Nakano GM, Young JM, Ransom JT. Leflunomide interferes with pyrimidine nucleotide biosyntesis. Inflamm Res. 1995;44:317–322. doi: 10.1007/BF01796261. [DOI] [PubMed] [Google Scholar]
- 17.Burger D, Begue-Pastor N, Benavent S, Gruaz L, Dayer JM. The active metabolite of leflunomide, A77 1726, inhibits the production of prostaglandin E2, matrix metalloproteinase 1 and interleukin 6 in human fibroblast-like synoviocytes. Rheumatology. 2003;42:89–96. doi: 10.1093/rheumatology/keg038. [DOI] [PubMed] [Google Scholar]
- 18.Cutolo M, Sulli A, Ghiorzo P, Pizzorni C, Craviotto C, Villaggio B. Antiinflammatory effects of leflunomide on cultured synovial macrophages from patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:297–302. doi: 10.1136/ard.62.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castagnetta L, Carruba G, Granata OM, Stefano M, Miele M, Schmidt M, Cutolo M, Straub RH. Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. J Rheumatol. 2003;30:2597–2605. [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Cutolo M, Montagna P, Brizzolara R, Sulli A, Seriolo B, Villaggio B, et al. Sex hormones modulate the effects of Leflunomide on cytokine production by cultures of differentiated monocyte/macrophages and synovial macrophages from rheumatoid arthritis patients”. Journal of Autoimmunity. 2009;32:254–260. doi: 10.1016/j.jaut.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Castagnetta L, Cutolo M, Granata O, Di Falco M, Bellavia V, Carruba G. Endocrine end-points in rheumatoid arthritis. Ann NY Acad Sci. 1999;876:180–192. doi: 10.1111/j.1749-6632.1999.tb07637.x. [DOI] [PubMed] [Google Scholar]
- 23.Capellino S, Montagna P, Villaggio B, Soldano S, Straub RH, Cutolo M. Hydroxylated estrogen metabolites influence the proliferation of cultured human monocytes: possible role in synovial tissue hyperplasia. Clin Exp Rheumatol. 2008;26:903–909. [PubMed] [Google Scholar]
- 24.Pelzer T, Schumann M, Neumann M, De Jager T, Stimpel M, Serfling E, Neyses L. 17 beta-estradiol prevents programmed cell death in cardiac myocytes. Biochem Biophys Res Commun. 2000;268:192–200. doi: 10.1006/bbrc.2000.2073. [DOI] [PubMed] [Google Scholar]
- 25.Ling S, Dai A, Williams MR, Myles K, Dilley RJ, Komesaroff PA, Sudhir K. Testosterone (T) enhances apoptosis-related damage in human vascular endothelial cells. Endocrinology. 2002;143:1119–1125. doi: 10.1210/endo.143.3.8679. [DOI] [PubMed] [Google Scholar]
- 26.Kvien TK, Uhling T, Odegard S. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann NY Acad Sci. 2006;1069:212–222. doi: 10.1196/annals.1351.019. [DOI] [PubMed] [Google Scholar]
- 27.Mor G, Straszewski S, Kamsteeg M. Role of the Fas/Fas ligand system in female reproductive organs: survival and apoptosis. Biochem Pharmacol. 2002;64:1305–1315. doi: 10.1016/s0006-2952(02)01267-4. [DOI] [PubMed] [Google Scholar]
- 28.Mancarella L, Bobbio-Pallavicini F, Ceccarelli F, Falappone PC, Ferrante A, Malesci D, Massara A, Nacci F, Secchi ME, Manganelli S, Salaffi F, Bambara ML, Bombardieri S, Cutolo M, Ferri C, Galeazzi M, Gerli R, Giacomelli R, Grassi W, Lapadula G, Cerinic MM, Montecucco C, Trotta F, Triolo G, Valentini G, Valesini G, Ferraccioli GF, GISEA group Good clinical response, remission, and predictors of remission in rheumatoid arthritis patients treated with tumor necrosis factor-alpha blockers: the GISEA study. J Rheumatol. 2007;34:1670–1673. [PubMed] [Google Scholar]
- 29.Ortona E, Margutti P, Matarrese P, Franconi F, Malorni W. Redox state, cell death and autoimmune diseases: a gender perspective. Autoimmun Rev. 2008;7:579–584. doi: 10.1016/j.autrev.2008.06.001. [DOI] [PubMed] [Google Scholar]


