Abstract
The majority of the leukocytes cross the endothelial lining of the vessels through cell-cell junctions. The junctional protein Vascular Endothelial (VE)-cadherin is transiently re-distributed from sites of cell-cell contacts during passage of leukocytes. VE-cadherin is part of a protein complex comprising p120-catenin and beta-catenin as intracellular partners. Beta-catenin connects VE-cadherin to alpha-catenin. This VE-cadherin-catenin complex is believed to dynamically control endothelial cell-cell junctions and to regulate the passage of leukocytes, although not much is known about the role of alpha- and beta-catenin during the process of transendothelial migration (TEM). In order to study the importance of the interaction between alpha- and beta-catenin in TEM, we used a cell-permeable version of the peptide encoding the binding site of alpha-catenin for beta-catenin (S27D). The data show that S27D interferes with the interaction between alpha- and beta-catenin and induces a reversible decrease in electrical resistance of the endothelial monolayer. In addition, S27D co-localized with beta-catenin at cell-cell junctions. Surprisingly, transmigration of neutrophils across endothelial monolayers was blocked in the presence of S27D. In conclusion, our results show for the first time that the association of alpha-catenin with the cadherin-catenin complex is required for efficient leukocyte TEM.
Keywords: Transendothelial migration, VE-cadherin, catenin, cell-cell junction, permeability.
Introduction
Vascular Endothelial Cadherin (VE-cadherin, Cadherin-5) is specifically expressed in endothelial cells and localizes at adherens junctions. VE-cadherin is a calcium-dependent and homophilic transmembrane adhesion molecule that bridges adjacent endothelial cells. It associates to the armadillo-family proteins beta- and gamma-catenin that bind the actin-binding protein alpha-catenin 1,2. Together, these proteins form the so-called cadherin-catenin complex. Because VE-cadherin-mediated cell-cell junctions hold the endothelial cells tightly together, the endothelial layer functions as a barrier for macromolecules and leukocytes and thereby protects the underlying tissue from damage. However, under certain (pathologic) conditions, such as inflammation, traffic of leukocytes across the endothelial layer through the cell-cell junctions is required. To avoid leakage of the endothelial layer, the opening and closure of VE-cadherin-mediated junctions is tightly regulated through controlled intracellular signaling 3-5. We and others have previously reported that inhibition of VE-cadherin function by the use of blocking antibodies promotes transendothelial migration of neutrophils and CD34+ cells in vitro and in vivo 6-8, suggesting that VE-cadherin mediates transendothelial migration of leukocytes.
The catenins are thought to play an important role in the regulation of VE-cadherin function. Lampugnani and co-workers showed that tyrosine phosphorylation of VE-cadherin and associated catenins is increased in loosely confluent endothelial monolayers, whereas the phosphorylation is diminished in tightly confluent monolayers 9. Ozawa and Kemler showed that increased levels of tyrosine phosphorylation, induced by pervanadate promote the dissociation of alpha-catenin from the cadherin-catenin complex 10. Moreover, their results indicated that the binding of alpha-catenin to the cadherin-catenin complex is a prerequisite for proper functioning cadherin-catenin complex, since an E-cadherin-alpha-catenin chimera was insensitive to pervanadate treatment leaving E-cadherin trans-interactions intact, whereas cells expressing the normal cadherin-catenin complex, including beta-catenin, showed loss of cell-cell contact after pervanadate treatment 10.
Several papers have shown that beta- and alpha-catenin become transiently and locally dispersed from cell-cell contacts upon passage of leukocytes 11,12. However, it is unclear if the interaction between the catenins is necessary for optimal transendothelial migration.
In this study, we used a peptide encoding the beta-catenin binding site of alpha-catenin (S27D) 13, fused to a cell-permeable protein transduction domain and tagged with biotin to study the role of the alpha- and beta-catenin interaction in transendothelial migration of leukocytes. The results show that S27D binds beta-catenin and dissociates it from alpha-catenin, which is accompanied by a reversible drop of the electrical resistance of the endothelial cell monolayer. In addition, we show that the loss of beta-catenin from alpha-catenin, induced by the peptide, decreases leukocyte transmigration across the endothelium, despite the fact that the electrical resistance is reduced.
These data indicate that the passage of leukocytes through the cell-cell junctions requires the presence of alpha-catenin in the cadherin-catenin complex in order to modulate endothelial cell-cell junctions. This report is the first to show that alpha-catenin plays an important role in leukocyte transendothelial migration.
Methods
Reagents and Abs - mAb to beta-catenin, PY20, p120-catenin, VE-cadherin, plakoglobin and Annexin-V-FITC were purchased from Transduction Laboratories (Becton Dickinson Company, Amsterdam, The Netherlands). mAb to alpha-catenin was purchased from Zymed (South California, CA). Rabbit polyclonal Abs to alpha- and beta-catenin were purchased from Santa Cruz Biotechnology (CA, USA). Actin mAb was purchased from Sigma-Aldrich (Zwijndrecht, the Netherlands). ICAM-1 and VCAM-1 Abs were purchased from R&D systems (Abingdon, UK). Recombinant Tumor-Necrosis-Factor (TNF)-α was from PeproTech (Rocky Hill, NJ); Texas-Red Phalloidin, ALEXA 488-labelled GαM-Ig, ALEXA 568-labelled GαM-Ig, ALEXA 568-labelled GαR-Ig, ALEXA 488-labelled GαR-Ig and ALEXA 488-labelled streptavidin secondary Abs were from Molecular Probes (Leiden, The Netherlands) and all used in a 1:500 dilution. PECAM-1 Ab (Hec170) and Fibronectin (FN) were obtained from Sanquin (Amsterdam, The Netherlands). Propidium iodide was purchased from Calbiochem (San Diego, CA).
Cell cultures - Primary pooled HUVEC were purchased from Lonza (Allendale, USA) and cultured in FN-coated culture flasks (NUNC, Life Technologies) in EGM2 containing singlequots (Lonza) and used until passage 9. Freshly isolated endothelial cells from umbilical cord, according to Brinkman et al. 14, were cultured similarly and used until passage 5. The difference in passage time may be due to donor variation and that Lonza HUVECs were pooled. In TEM assays, endothelial cells were pre-treated with 10ng/ml TNF-α overnight as indicated. All cell lines were cultured at 37ºC and at 5% CO2. Primary neutrophils were isolated as described previously 15.
Measurement of apoptosis - Apoptosis of HUVECs was measured by flow cytometry with the annexin-V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis assay kit (Bender Medsystems, Vienna, Austria) as described 15. Confluent HUVECs were treated as indicated and detached with 10mM EDTA. Next, cells were centrifuged mildly and washed once in ice-cold HEPES medium with 2.5 mM Ca2+. All further steps were performed in this medium on ice. The cells were then incubated for 45 minutes with annexin-V-FITC, 1:250, which specifically binds phosphatidylserine residues on the cell membrane. During the last 10 minutes PI was added to a final concentration of 1 µg/mL. PI is a fluorescent dye that intercalates into DNA once the cell membrane has become permeable. After incubation, the cells were washed once and analyzed by FACScan (Becton Dickinson, San Jose, CA). Viable cells were defined as negative for Annexin-V-FITC and PI staining. Cell survival was expressed as a percentage of viable cells in relation to the total number of counted cells.
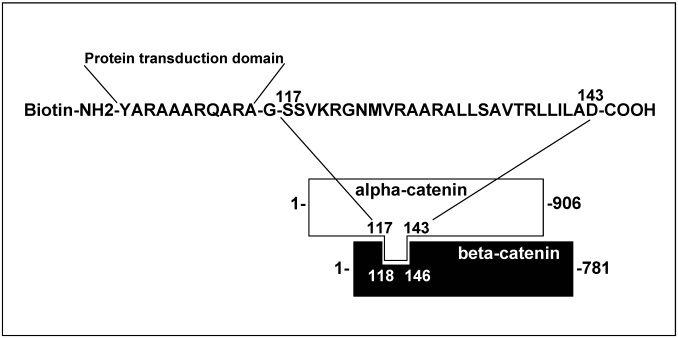
S27D peptide - The peptide encodes 27 amino-acids, which reflect the binding site of alpha-catenin to beta-catenin, according to Huber et al 13 (Figure 1). The peptide was synthesized at the Netherlands Cancer Institute (Amsterdam, The Netherlands) as a fusion with a protein transduction domain to make it cell-permeable 16 and tagged with biotin. Peptide stocks were in DMSO at 100mg/mL. As control peptide, the transduction domain, tagged with biotin and dissolved in DMSO was used. (See also previous report: 16). The peptides, either S27D or control, was used at a final concentration of 5μg/mL and added to the culture medium for indicated time periods.
Figure 1.
Schematic overview of the cell-permeable S27D peptide. The amino acid sequence of the beta-catenin-binding domain in alpha-catenin is shown. The peptide is N-terminally fused to a protein transduction domain and tagged with a biotin as indicated. Glycine (-G-) is used as a linker.
Immunofluorescence - HUVEC were cultured on FN-coated glass cover slips, fixed and immuno-stained as described 8 with Abs as indicated. Staining was followed by secondary staining with fluorescently labeled Abs. Images were recorded with a ZEISS LSM510 confocal microscope with appropriate filter settings. Cross-talk between the green and red channel was avoided by use of sequential scanning.
Flow Cytometry - HUVEC were cultured on FN-coated glass cover slips, treated with TNF-α overnight and incubated for 30 minutes with S27D, control peptide or DMSO. Next, cells were trypsinized, fixed, stained as indicated, and analyzed by FACS CANTO II (Becton Dickinson).
Electric Cell-substrate Impedance Sensing (ECIS) - Endothelial cells were added at 100.000 cells per well (0.8cm2) to a FN-coated electrode-array, containing L-cysteine-pretreated 10 gold-electrodes per array, and grown to confluency. After the electrode-check of the array and the basal trans-electrical resistance (TER) of the endothelial monolayer, the peptide or DMSO were added and TER was measured on line at 37ºC at 5% CO2 with the ECIS-Model-100 Controller from BioPhysics, Inc. (Troy, NY). Detailed
Immunoprecipitation and Western blot analysis - Cells were grown to confluency on FN-coated dishes (50cm2), washed twice gently with ice-cold Ca2+-and Mg2+-containing PBS and lysed in 300μL of lysis buffer (25mM Tris, 150mM NaCl, 10mM MgCl2, 2mM EDTA, 0.02% SDS, 0.2% deoxycholate, 1% NP-40, 0.5mM orthovanadate with the addition of fresh protease-inhibitor-cocktail tablets (Boehringer) pH 7.4). After 10 minutes on ice, cell lysates were collected and pre-cleared for 30 minutes at 4ºC with Streptavidin beads (Sigma-Aldrich, 15μl for each sample). The supernatant, cleared by centrifugation (14.000*g, 5 minutes at 4ºC) was incubated with 15μl protein Streptavidin beads that were coated with the biotin-tagged S27D or the control peptide, for 1h at 4ºC under continuous mixing. The beads were washed 3 times in lysis buffer and proteins were eluted by boiling in SDS-sample buffer containing 4% 2-mercaptoethanol (Bio-Rad). The samples were analyzed by 10% SDS-PAGE. Proteins were transferred to 0.45 μm nitro-cellulose (Schleicher and Schnell Inc., NH) and the blots were first blocked with 5% (w/v) low-fat milk in Tris-Buffered Saline Tween-20 (TBST) for 1h, subsequently incubated at room temperature with the appropriate Abs for 1h, followed by 30 minutes incubation with HRP-conjugated secondary Abs at RT. Between the various incubation steps, the blots were washed 5 times with TBST and finally developed with an enhanced chemiluminescence (ECL) detection system (Amersham). For the Triton-X100 insoluble fractions, HUVECs were lysed for 10 minutes on ice using 25mM Tris, 150mM NaCl, 10mM MgCl2, 2mM EDTA, 1% Triton-X100, 0.5mM orthovanadate with the addition of fresh protease-inhibitor-cocktail tablets at pH 7.4. Next, cells were scraped from the culture plate and centrifuged at 14.000*g, 10 minutes at 4ºC to separate the soluble from the insoluble fraction. Fractions were diluted in SDS-sample buffer containing 4% 2-mercaptoethanol and analyzed by Western blotting. As a control, RhoGDI, as a cytosol marker, was used in the Triton-X100 insoluble fraction to determine the isolation procedure.
Transendothelial migration assay - Assays were performed in 6.5-mm Transwell plates with 5-µm pore filters. HUVEC were plated at 20,000-30,000 cells/Transwell on FN-coated filters. The adherent cells were cultured for 2-3 days to obtain confluent endothelial monolayers and pre-treated overnight with TNF-α. 100.000 Neutrophils, isolated as described previously 7, or HL60 cells, differentiated by 1.3% DMSO for 3-5 days as described previously 8, were added to the upper compartment in 0.1 ml of assay medium, and 0.6 ml of assay medium with or without 50ng/mL human CXCL12 (SDF-1α) (Strathmann Biotech, Hannover, Germany) or 10nM fMLP (Sigma) was added to the lower compartment. Transwells were incubated at 37°C, 5% CO2, for 1h for neutrophils and 4h for HL60 cells. Cells that migrated to the lower compartment were collected and FACS analysis was used to determine how many cells migrated, as described before 8. After the assay, the filters were fixed and stained with Texas-Red phalloidin to confirm HUVEC monolayer confluency by confocal laser scanning microscopy.
Statistics - Student's t-test for paired samples (two-tailed) was used independent samples was used where indicated.
Results
The formation of adherens junctions and the integrity of endothelial monolayers are controlled by trans-interactions between VE-cadherin molecules on adjacent endothelial cells 2. Previous studies have shown that VE-cadherin-mediated cell-cell junctions are transiently disrupted to allow leukocyte TEM 4,8. Beta-catenin, which associates to the intracellular tail of VE-cadherin and alpha-catenin, which binds to beta-catenin, contribute to the connection of VE-cadherin with the cortical actin cytoskeleton 2. However, the role for the catenins in leukocyte TEM is unclear. Huber and co-workers defined the domain by which alpha-catenin binds beta-catenin 15. They used this domain to construct a peptide, S27D, that specifically binds beta-catenin. To study if this domain is important in TEM, we constructed a biotinylated fusion peptide of S27D with a protein-transduction domain (PTD), which renders the peptide cell-permeable 13 (Figure 1). PTD is originally cloned from the TAT-HIV sequence by Nagahara et al. 17. It exists of a hydrophobic amino acid stretch and is frequently used in studies to deliver peptides into mammalian cells 18. However, the delivery process is unknown 19. Nevertheless, small peptides such as S27D are delivered with high efficiency into mammalian cells 20.
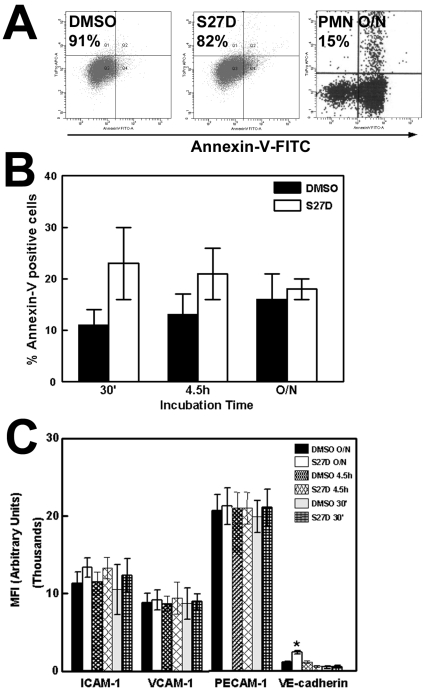
Firstly, we tested if S27D affected the viability of the endothelial cells. Using flow cytometry to measure Annexin-V binding, a marker for early apoptosis, we found that the majority of the endothelial cells (77%) were Annexin-V negative after 30 minutes S27D treatment compared to approximately 88% of the cells treated with DMSO or control peptide (Figure 2A). All cells remained propidium iodide-negative, showing that the treatment did not induce necrosis (Figure 2A). Additional analysis revealed no increase in apoptosis in S27D-treated endothelial cells, compared to DMSO-treated cells (Figure 2B). Moreover, the presence of S27D for up to 60 minutes did not change the morphology of the endothelial cell monolayer, as was judged by real-time phase-contrast recordings (data not shown). These data show that the peptide did not induce apoptosis or gross morphological changes in these cells. In addition, the surface expression of the adhesion molecules ICAM-1 and VCAM-1, as well as of PECAM-1, was unaltered after S27D treatment (Figure 2C). Interestingly, VE-cadherin expression was increased after S27D treatment. Whether this is the result of reduced internalization of cell-surface VE-cadherin is unclear. Xiao and colleagues showed that p120-catenin regulates the internalization of VE-cadherin 21. Therefore, we tested if the association of p120-catenin to VE-cadherin increased after S27D treatment. However, our results showed that S27D did not alter the interaction between these proteins (Supplemental figure 1).
Figure 2.
Effects of S27D on viability on endothelial cells. (A) TNF-α-stimulated HUVEC were treated for 30 minutes with S27D (5μg/mL), control peptide (CTRL) or DMSO and subsequently trypsinized, fixed and stained with Annexin-V-FITC and Propidium iodide (PI). Plots show that after S27D treatment, 77% of the cells were negative for Annexin-V. After DMSO and control peptide (CTRL) treatment, respectively 89% and 88% of the cells were negative. All cells were negative for PI, showing that the treatment did not induce necrosis. Neutrophils (PMN) were left overnight (O/N) and used as positive Annexin-V control. Only 15% of the PMNs were Annexin-V negative. (B) Effect of S27D on apoptosis of endothelial cells at different time points are analyzed using Annexin-V-FITC labeling. Experiment is carried out three times. Data are mean ± SEM. (C) HUVEC were treated as described under A. Expression of ICAM-1, VCAM-1, PECAM-1 and VE-cadherin was measured by flow cytometry analysis and expressed as Mean fluorescent Intensity (MFI) arbitrary units on the Y-axis. S27D was incubated for different time points as indicated. VE-cadherin expression is increased after overnight treatment with S27D. Experiment was carried out three times in triplicate. Data are mean ± SEM. *p<0.05.
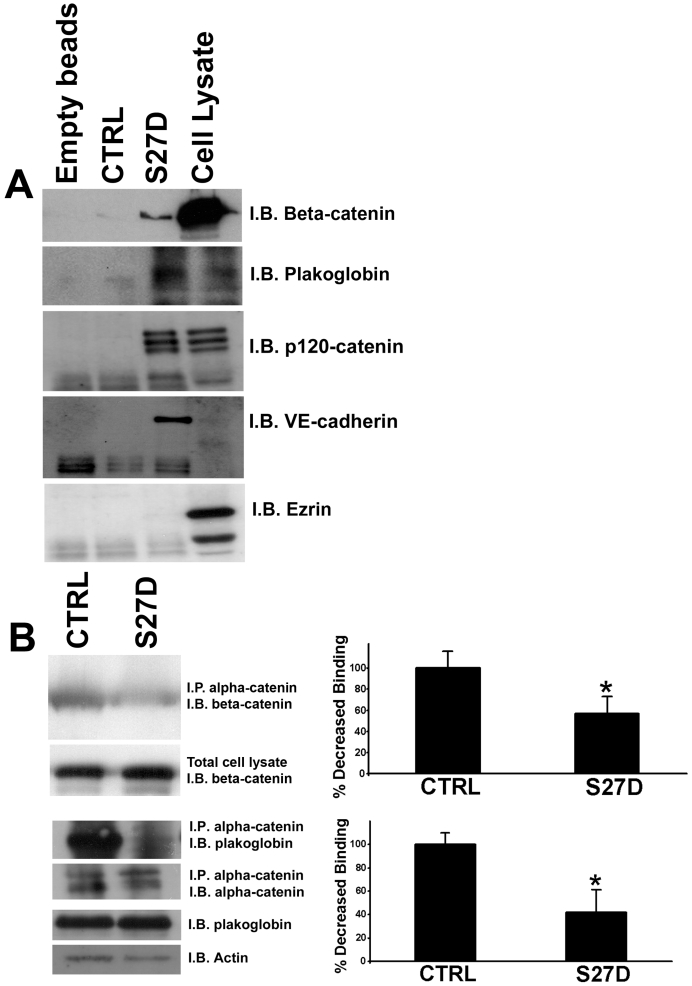
To confirm that S27D binds to beta-catenin, a pull-down experiment with endothelial cell lysates was performed. This experiment showed that S27D, but not the control peptide, binds to beta-catenin, but not the actin binding protein ezrin (Figure 3A). In addition, the peptide also binds to plakoglobin (Figure 3A). In the pull down experiment, also p120-catenin and VE-cadherin are found (Figure 3A). This showed that the peptide can precipitate the intact VE-cadherin complex. To elucidate if the peptide interferes with the interaction between beta-catenin and alpha-catenin, an immuno-precipitation (IP) assay for alpha-catenin was performed. Intact endothelial cells were pre-treated with the cell-permeable S27D or control peptide for 30 minutes after which the cells were lysed and alpha-catenin was immunoprecipitated. IPs were analyzed by Western blotting for binding of beta-catenin to alpha-catenin, which showed that S27D reduced the association between alpha- and beta-catenin in intact endothelial cells (Figure 3B). Also plakoglobin was precipitated by S27D. To study if S27D induced a shift of the cell-cell junction proteins form the cytoskeletal fraction (Triton-X-100 insoluble fraction) to the cytosolic fraction (Triton-X-100 soluble fraction), we analyzed the pellet fraction after S27D treatment by Western blotting. No changes in the levels of VE-cadherin, plakoglobin, alpha-catenin or actin in the cytoskeletal fraction after S27D treatment can be measured, as was judged by analyzing the Triton-X100-insoluble fraction by Western blotting (Supplemental figure 2).
Figure 3.
S27D binds to beta-catenin and dissociates alpha-catenin from beta-catenin. (A) HUVEC lysates were incubated with S27D and pull-down assays as described in Methods were performed. The samples were analyzed by Western blotting, which showed that S27D bound to beta-catenin, plakoglobin, p120-catenin and VE-cadherin. Control peptide (CTRL), encoding only the protein transduction domain, or empty beads did not bind to the proteins. Right lane shows protein expression in total cell lysate. Ezrin (bottom panel) did not bind to the peptide. (B) HUVEC were treated for 30 minutes with S27D or control peptide (CTRL) and then lysed. Subsequently, alpha-catenin was immuno-precipitated (I.P.) as described in Methods. Western blot was immunostained for beta-catenin (I.B.), which showed that S27D dissociates beta-catenin from alpha-catenin, but that the control peptide did not interfere with this interaction. Also plakoglobin binding to alpha-catenin was reduced after S27D administration. Lower panels showed controls for IP of alpha-catenin and expression of beta-catenin, plakoglobin and actin in total cell lysates. Data are representative for three independent experiments. Blots are scanned and the intensity were quantified using ImageJ and plotted in the graphs on the right. Results show that S27D significantly reduces the binding of beta-catenin or plakoglobin to alpha-catenin. Data are mean ± SEM. *p<0.05.
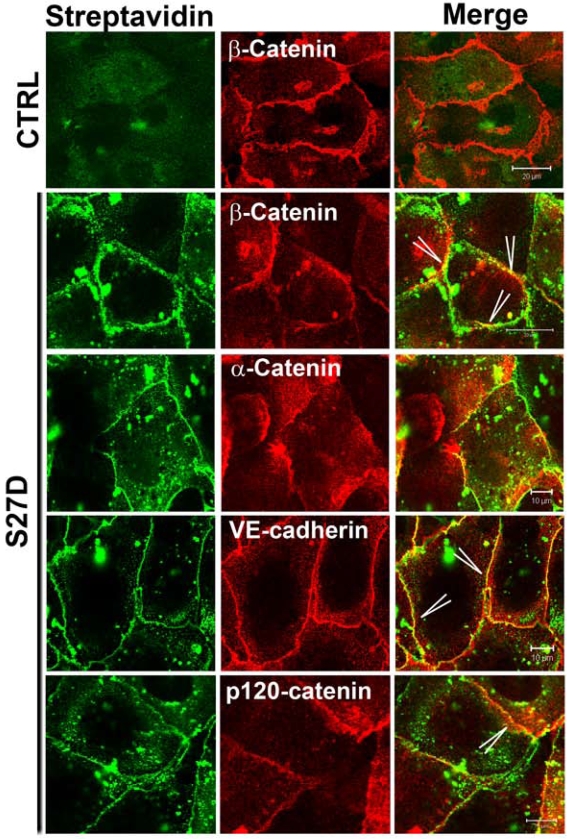
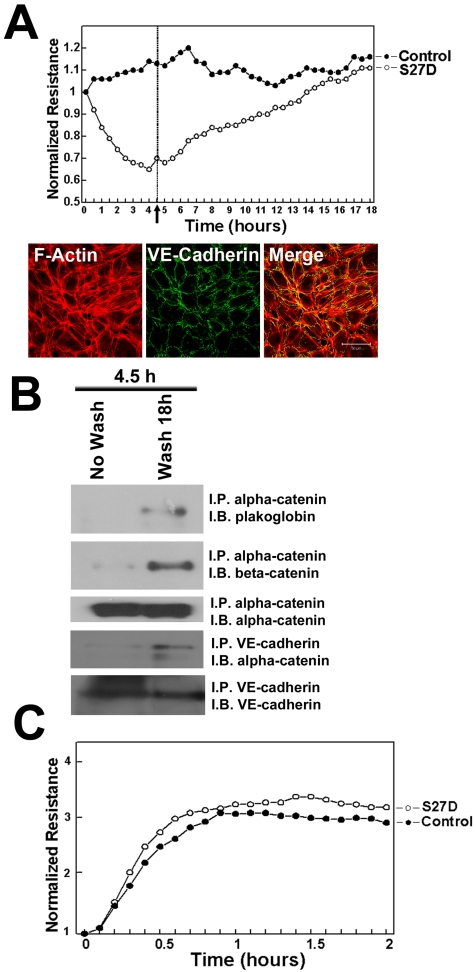
To study the localization of S27D, endothelial cells were incubated for 30 minutes with S27D and then fixed and permeabilized for fluorescent streptavidin-based image analysis. Using this approach, we confirmed that the biotin- and PTD-tagged S27D entered live cells and, in contrast to the control peptide accumulated at cell-cell junctions where it co-localized with beta-catenin (Figure 4). Alpha-catenin showed a more diffuse pattern away from the cell-cell junctions, indicating that S27D removed alpha-catenin from the cell-cell junctions (Figure 4). VE-cadherin and p120-catenin also showed a diffuse staining, although the majority of the proteins remained localized at cell-cell junctions. Finally, S27D did not induce major changes in F-actin levels or its distribution (data not shown). To study if the trans-electrical resistance (TER) of the endothelial monolayer is affected by S27D, TER was measured in time using ECIS (electrical cell-substrate impedance sensing) 20. When the endothelial monolayer reached confluency, the basal resistance was approximately 20 Ohms/cm2. At that time, S27D was added to the cell monolayers. After a few minutes, TER started to decline and reached lowest levels at approximately 4.5h after addition of S27D, which corresponded with approximately 16.2 Ohms/cm2 (Figure 5A). Interestingly, VE-cadherin was still localized at cell-cell junctions at this time point, as was analyzed in parallel by confocal microscopy (Figure 5A). To test if S27D affected the tyrosine phosphorylation level of VE-cadherin, VE-cadherin was immunoprecipitated and the samples were analyzed by Western blot using phosphotyrosine antibodies. No change in phospho-VE-cadherin levels could be detected in cells that have been treated with S27D for 30 minutes (Supplemental figure 3A). Next, the medium was refreshed and S27D was washed out. TER recovered and reached a plateau level, which was equal to the control peptide-treated cells at approximately 12 hours after wash-out (Figure 5A). Biochemical analysis of the VE-cadherin complex showed that the interaction between beta-catenin and alpha-catenin is restored after wash-out (Figure 5B). Alpha-catenin was also found in the VE-cadherin immunoprecipitate, indicating that the VE-cadherin-catenin complex was restored (Figure 5B). However, no change in VE-cadherin tyrosine phosphorylation is detected upon wash-out (Supplemental figure 3B). These results showed that the S27D peptide induced a reversible drop in TER and in the dissociation of the VE-cadherin complex, which is independent of VE-cadherin tyrosine phosphorylation. S27D did not affect the cell-matrix adhesion or viability, since all cells still adhered to the electrode and no floating cells were detected. Finally, addition of S27D upon plating of the cells to the fibronectin-coated electrodes did not affect the initial adhesion and cell-spreading 23 or the adhesion to the matrix (Figure 5C). Thus, S27D does not interfere with endothelial cell adhesion and spreading, but selectively targets the composition of the VE-cadherin-catenin complex and thereby junctional integrity.
Figure 4.
S27D induces diffused localization of alpha-catenin. Endothelial cells were cultured and grown to confluency on FN-coated glass cover slips and subsequently incubated with the control peptide (CTRL; upper panel) or S27D (lower panels) for 30 minutes. Next, cells were fixed, permeabilized and stained with streptavidin to detect S27D (green) and Abs to beta-catenin, VE-cadherin, alpha-catenin and p120-catenin (red). Merge shows the co-localization of S27D and the indicated proteins in yellow (Arrowheads). Bar, 20μm.
Figure 5.
S27D reduces TER of endothelial monolayers. (A) HUVECs were cultured to confluency on 10E golden ECIS electrodes and TER was recorded in real-time as described in Methods. Cells were treated as described in Figure 3. The electrical resistance is normalized and set as 1. Peptides remained present throughout the experiment. Results show that S27D, added at T=0 induces a drop in electrical resistance, which recovers after the peptide was washed-out (arrow and dashed line at 4.5 hours). Data are the average of triplicates of one experiment and are representative of four experiments, performed in triplicate. Lower panels show localization of VE-cadherin in green at cell-cell junctions after 4 hours of S27D treatment. F-actin is shown in red and co-localization appears in yellow in the merged image. Bar, 50μm. (B) HUVEC were treated for 4.5 hours with S27D (No Wash) or S27D was washed away after 4.5 hours and left untreated overnight (Wash 18h). Next, alpha-catenin or VE-cadherin was immuno-precipitated (I.P.) and Western blot membrane was stained as indicated (I.B.). Results showed that washing away S27D restored the VE-cadherin complex. (C) Upon plating, HUVECs were treated with S27D (open circles) or control peptide (closed circles), seeded on 10E-gold electrodes and cell-spreading was recorded in real-time using ECIS as described in Methods. Peptides remained present throughout the experiment. Results show that S27D did not affect HUVEC spreading on FN-coated surfaces.
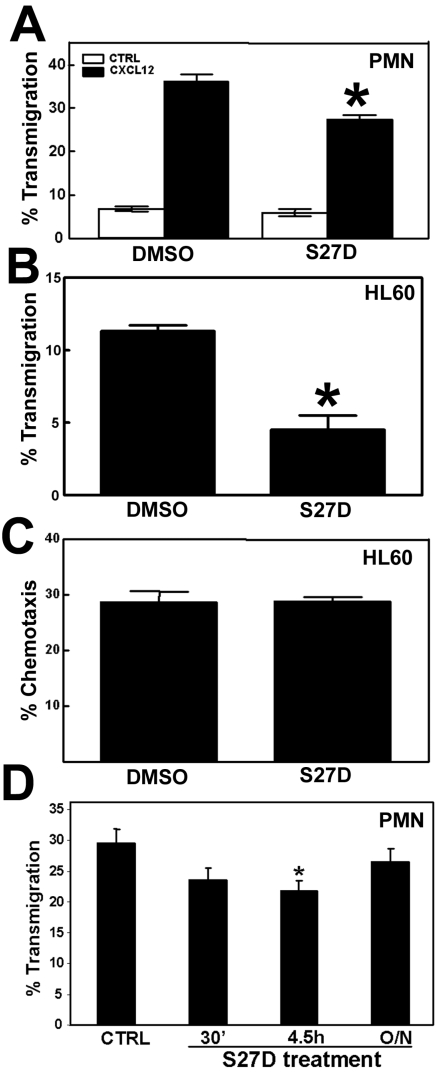
The majority of leukocytes that cross endothelial monolayers use the paracellular route, i.e. through the cell-cell junctions 23,24. Several groups, including ours, have shown that VE-cadherin is locally dispersed at sites where leukocytes transmigrate 4,8. Also beta- and alpha-catenin are locally dispersed from sites of cell-cell contact upon passage of leukocytes 11,12. However, it is not clear to what extent alpha-catenin contributes to the process of transendothelial migration. To study this, transendothelial migration assays using Transwells were performed and the effect of S27D was analyzed. Primary neutrophils were isolated and allowed to migrate across TNFα-stimulated HUVEC to the chemokine CXCL12. The results show that S27D significantly reduced the extravasation of neutrophils across endothelial cells (Figure 6A). Spontaneous migration of neutrophils was also reduced when endothelial cells were pretreated with S27D, albeit to a limited extent (Figure 6A). Migration of differentiated HL60 cells across endothelial monolayers was also significantly blocked in the presence of S27D (Figure 6B). To exclude an effect of S27D on the migration efficiency of the leukocytes, S27D was tested in a chemotaxis assay. Transwell-filters were coated with fibronectin and HL60 cells were allowed to migrate to CXCL12 in the presence or absence of S27D. Figure 6C shows that S27D did not affect the chemotactic capacity of the leukocytes, indicating that the effect of S27D observed in Figure 6A and 6B was due to the dissociation of alpha- and beta-catenin in the endothelial cells. The adhesion of neutrophils to pretreated endothelial cells was not affected by the presence of S27D (data not shown). Washing away S27D resulted in restoration of the cell-cell junctions and of the S27D-induced inhibition of neutrophil TEM (Figure 6D). Together, these data indicate that the presence of alpha-catenin in the VE-cadherin-catenin complex is required for efficient passage of leukocytes across endothelial monolayers.
Figure 6.
S27D reduces TEM of leukocytes. (A) Primary human neutrophils were allowed to migrate toward 50ng/mL CXCL12 for 1h across TNF-α-pre-treated HUVEC that are cultured on a FN-coated 5-μm pore Transwell filters. Chemotaxis was quantified as described in Methods. Endothelial cells were pre-treated for 30 minutes with DMSO or with S27D, which remained present throughout the experiment. Neutrophil migration was blocked significantly in the presence of the peptide. Data represent mean ± SEM from triplicates. Experiment was repeated three times. *, p<0.05. (B) Differentiated HL60 cells were allowed to migrate across TNFα-treated HUVEC as described under (A). HL60 migration was blocked significantly in the presence of S27D. Data represent mean ± SEM from triplicates. Experiment was repeated three times. *, p<0.01. (C) Differentiated HL60 cells were allowed to migrate across fibronectin-coated Transwell filters toward 50ng/mL CXCL12 for 2 hours in the absence or presence of S27D. Chemotaxis was quantified as described in Methods. Data represent mean ± SEM from triplicates. Experiment was repeated three times. (D) Primary human neutrophils were allowed to migrate as described under A, but as chemokine 10 nM fMLP was used. HUVECs were treated with S27D for 30 minutes, 4.5 hours present during the assay, or washed away after 4.5 hours and left untreated for O/N. S27D inhibited TEM significantly with 4.5 hours but not when washed away. Data represent mean ± SEM. Experiment was repeated three times. *, p<0.05.
Discussion
Endothelial cell-cell junctions are instrumental in regulating the passage of leukocytes across the vascular wall. Leukocytes cross the endothelium either through the cell-cell junctions, the paracellular route, or through the cell body of individual endothelial cells, the transcellular route 24. Recently, Carman and colleagues showed that the majority of lymphocytes transmigrate by the paracellular route, but that there is a difference in the relative contribution of this route depending on the type of endothelium 25. Lymphocytes that cross micro-vascular endothelium use the paracellular pathway for 70%, whereas more than 90% of the same lymphocytes choose this route when migrating across HUVEC. Irrespective of the type of endothelium, the majority of leukocytes prefer to migrate across the endothelial cell-cell junctions. Thus, the regulation of the cell-cell junctions during transmigration is important for efficient leukocyte extravasation. In this paper, we focused on the role of the adherens junctions and on the association between alpha- and beta-catenin in particular, in leukocyte transendothelial migration.
Our results indicate that the connection between alpha- and beta-catenin is required for proper migration of leukocytes across endothelial cell monolayers. These results were surprising, because it has been reported that breaking the link between alpha- and beta-catenin weakens the cell-cell junctions 10,13, which conceivably would result in more efficient TEM. In line with this, our results show that S27D reduces TER of endothelial monolayers. However, S27D inhibits TEM. The resistance of the monolayer is measured by ECIS, known to be a highly sensitive technique. S27D induces a drop in the resistance, even though immuno-fluorescence images show that VE-cadherin and beta-catenin are still present at cell-cell junctions. However, compared to thrombin-induced drop in resistance, S27D induces a small drop in resistance. Thus, we conclude from these results that the signals that lead to the local weakening of the cell-cell junctions, to allow leukocyte TEM, require the presence of alpha-catenin in the VE-cadherin-catenin complex.
VE-cadherin interacts with beta-catenin, which in turn is linked to the actin cytoskeleton through alpha-catenin. Loss of VE-cadherin function results in unstable endothelial junctions and an increase of leukocyte transmigration 6,8. Sandig and colleagues already reported that the cadherin-catenin complex remains intact during diapedesis. This was judged by co-localization studies, where alpha-catenin co-localizes with VE-cadherin during the passage of a leukocyte 12. It has been generally accepted that the strength of VE-cadherin-based cell-cell junctions depends on the interaction of the cadherin-catenin complex with the actin cytoskeleton 2. A simple explanation for the effect of S27D in the TEM assays is that under normal conditions, tension, which is generated through actomyosin-based contractility, weakens homotypic VE-cadherin interactions by pulling on alpha-catenin. When alpha-catenin is competed out of the cadherin-catenin complex by S27D, contractility towards cell-cell junctions is lost and the junctions remain relatively impermeable for leukocytes. The dogma that alpha-catenin links the cadherin-catenin complex to the actin cytoskeleton has been challenged recently by Drees and colleagues, showing that alpha-catenin cannot bind actin and beta-catenin at the same time 26. Nevertheless, Abe and Takeichi recently showed that EPLIN (Epithelial Protein Lost in Neoplasm) may be the linker between alpha-catenin and the actin cytoskeleton 27. If this is also the case in endothelial cells remains to be investigated.
Our data show that VE-cadherin still localizes at cell-cell junctions after S27D treatment. Moreover, S27D may increase VE-cadherin levels at junctions, although no change in total VE-cadherin protein is measured by Western blotting. This may point to a role for the alpha-beta-catenin interaction in VE-cadherin internalization. The expression of VE-cadherin on the surface is regulated by p120-catenin 21. Alcaide and colleagues recently reported that over-expression of p120-catenin results in increased VE-cadherin expression at the cell-cell junctions and subsequent inhibition of leukocyte TEM 28. Interestingly, the expression of the intracellular catenins was unaltered. Thus, these data suggest that p120-catenin-induced VE-cadherin is not bound to the catenins, which results in reduced TEM. This finding is in line with our data, in which VE-cadherin is uncoupled from alpha-catenin by S27D, and may remain present at the plasma membrane, resulting in reduced TEM. However, our biochemical data indicate that S27D induces no difference in p120-catein and VE-cadherin binding. Gavard and Gutkind showed that VE-cadherin internalization can be regulated through clathrin-coated pits 29. This pathway involves beta-arrestin and Rac1 signalling. Whether S27D is involved in this pathway needs further research.
Together, our results show that the process of leukocyte transendothelial migration requires an intact VE-cadherin-catenin complex, including alpha-catenin, to allow efficient passage of leukocytes.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Acknowledgments
We thank Erik Mul for excellent technical support. JDvB and FPvA are supported by the Dutch Heart Foundation (grant no. 2005T039) and NWO-ZonMW Veni grant 916.76.053.
Abbreviations
- RT
Room Temperature
- TBST
Tris-buffered Saline Tween-20 solution
- HRP
Horse-Radish-Peroxidase
- PBS
Phosphate-Buffered Saline solution
- FN
Fibronectin
- ICAM-1
Inter-Cellular Adhesion Molecule-1
- VCAM-1
Vascular Cell Adhesion Molecule-1
- PECAM-1
Platelet Endothelial Cell Adhesion Molecule-1
- HUVEC
Human Umbilical Cord Endothelial Cells
- Ab
Antibodies
- TER
Trans-endothelial Electrical Resistance
- TEM
Transendothelial Migration.
References
- 1.Dejana E, Bazzoni G, Lampugnani M.G. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp. Cell Res. 1999;252:13–19. doi: 10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- 2.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Arterioscler. Thromb. Vasc. Biol. 2007;28:223–232. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson-Leger C, Aurrand-Lions M, Imhof BA. The parting of the endothelium: miracle, or simply a junctional affair? J. Cell Sci. 2000;113:921–933. doi: 10.1242/jcs.113.6.921. [DOI] [PubMed] [Google Scholar]
- 4.Shaw SK, Bamba PS, Perkins BN. et al. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J. Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 5.Luscinskas FW, Ma S, Nusrat A. The role of endothelial cell lateral junctions during leukocyte trafficking. Immunol. Rev. 2002;186:57–67. doi: 10.1034/j.1600-065x.2002.18606.x. [DOI] [PubMed] [Google Scholar]
- 6.Gotsch U, Borges E, Bosse R. et al. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J. Cell Sci. 1997;110:583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 7.Hordijk PL, Anthony E, Mul FP. et al. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J. Cell Sci. 1999;112:1915–1923. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- 8.Van Buul JD, Voermans C, Van den Berg V. et al. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J. Immunol. 2002;168:588–596. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- 9.Lampugnani MG, Corada M, Andriopoulou P. et al. Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J. Cell Sci. 1997;110:2065–2077. doi: 10.1242/jcs.110.17.2065. [DOI] [PubMed] [Google Scholar]
- 10.Ozawa M, Kemler R. Altered cell adhesion activity by pervanadate due to the dissociation of alpha-catenin from the E-cadherin-catenin complex. J. Biol. Chem. 1998;273:6166–6170. doi: 10.1074/jbc.273.11.6166. [DOI] [PubMed] [Google Scholar]
- 11.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J. Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandig M, Negrou E, Rogers KA. Changes in the distribution of LFA-1, catenins, and F-actin during transendothelial migration of monocytes in culture. J. Cell Sci. 1997;110:2807–2818. doi: 10.1242/jcs.110.22.2807. [DOI] [PubMed] [Google Scholar]
- 13.Huber O, Krohn M, Kemler R. A specific domain in alpha-catenin mediates binding to beta-catenin or plakoglobin. J. Cell Sci. 1997;110:1759–1765. doi: 10.1242/jcs.110.15.1759. [DOI] [PubMed] [Google Scholar]
- 14.Brinkman HJ, Mertens K, Holthuis J, Zwart-Huinink LA, Grijm K, van Mourik JA. The activation of human blood coagulation factor X on the surface of endothelial cells: a comparison with various vascular cells, platelets and monocytes. Br J Haematol. 1994;87(2):332–342. doi: 10.1111/j.1365-2141.1994.tb04918.x. [DOI] [PubMed] [Google Scholar]
- 15.Maianski NA, Mul FP, van Buul JD. et al. Granulocyte colony-stimulating factor inhibits the mitochondria-dependent activation of caspase-3 in neutrophils. Blood. 2002;99(2):672–679. doi: 10.1182/blood.v99.2.672. [DOI] [PubMed] [Google Scholar]
- 16.van Hennik PB, ten Klooster JP, Halstead JR. et al. The C-terminal domain of Rac1 contains two motifs that control targeting and signaling specificity. J Biol. Chem. 2003;278:39166–39175. doi: 10.1074/jbc.M307001200. [DOI] [PubMed] [Google Scholar]
- 17.Nagahara H, Vocero-Akbani AM, Snyder EL. et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;12:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 18.El-Andaloussi S, Järver P, Johansson HJ, Langel U. Cargo-dependent cytotoxicity and delivery efficacy of cell-penetrating peptides: a comparative study. Biochem J. 2007;407(2):285–292. doi: 10.1042/BJ20070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62(16):1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta B, Levchenko TS, Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliv Rev. 2005;57(4):637–651. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Xiao K, Allison DF, Buckley KM. et al. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenowicz MJ, Fernandez-Borja M, Kooistra MR. et al. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur. J. Cell Biol. 2008;10:779–792. doi: 10.1016/j.ejcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 23.ten Klooster JP, Jaffer ZM, Chernoff J. et al. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J. Cell Biol. 2006;5:759–769. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelhardt B, Wolburg H. Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur. J. Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- 25.Carman CV, Sage PT, Sciuto TE. et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drees F, Pokutta S, Yamada S. et al. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U.S.A. 2008;105:13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alcaide P, Newton G, Auerbach S. et al. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3