Abstract
This study reports on trends in canine and feline urolithiasis in Canada during the past 10 years. Age, sex, breed of animals and mineral composition from 40 637 canine and 11 353 feline bladder uroliths submitted to the Canadian Veterinary Urolith Centre between 1998 and 2008 were recorded. Struvite and calcium oxalate uroliths comprised > 85% of all uroliths submitted. In dogs, the number of struvite submissions has declined and the number of calcium oxalate submissions has increased. Struvite uroliths were most common in female dogs and calcium oxalate uroliths in male dogs. The shih tzu, miniature schnauzer, bichon frisé, lhasa apso, and Yorkshire terrier were the breeds most commonly affected for both struvite and calcium oxalate uroliths. Urate uroliths were most common in male dalmatians. In cats, struvite submissions declined and calcium oxalate submissions remained constant. Struvite and calcium oxalate uroliths were common in domestic, Himalayan, Persian, and Siamese cats. Urate uroliths were over-represented in Egyptian maus.
Résumé
Urolithiase canine et féline : Examen de plus de 50 000 soumissions d’urolithes au Centre canadien vétérinaire d’urolithiase de 1998 à 2008. Cette étude présente un rapport sur les tendances de l’urolithiase canine et féline au Canada au cours des dix dernières années. L’âge, le sexe, la race des animaux et la composition minérale de 40 637 urolithes de vessie canins et de 11 353 urolithes de vessie félins soumis au Centre canadien vétérinaire d’urolithiase entre 1998 et 2008 ont été consignés. Les lithiases phospho-ammoniaco-magnésiennes et les urolithes d’oxalate calcique représentaient > 85 % de tous les urolithes soumis. Chez les chiens, le nombre de soumissions de lithiases phospho-ammoniaco-magnésiennes a chuté et le nombre de soumissions d’oxalate calcique a augmenté. Les lithiases phospho-ammoniaco-magnésiennes étaient les plus communes chez les chiens femelles et les urolithes d’oxalate calcique étaient les plus communs chez les chiens mâles. Le Shih Tzu, le Schnauzer miniature, le Bichon frisé, le Lhasa Apso et le Terrier du Yorkshire étaient les races les plus communément affectées par les lithiases phospho-ammoniaco-magnésiennes et les urolithes d’oxalate calcique. Les urolithes uratiques étaient les plus courants chez les Dalmatiens mâles. Chez les chats, les soumissions de lithiases phospho-ammoniaco-magnésiennes ont baissé tandis que les soumissions d’oxalate calcique sont demeurées stables. Les lithiases phospho-ammoniaco-magnésiennes et les urolithes d’oxalate calcique étaient courants chez les chats domestiques, les Himalayens, les Persans et les Siamois. Les urolithes uratiques étaient surreprésentés chez les Maus égyptiens.
(Traduit par Isabelle Vallières)
Introduction
The Canadian Veterinary Urolith Centre (CVUC) in Guelph, Ontario, Canada, opened in February 1998. The CVUC is a collaborative effort between Medi-Cal/Royal Canin Veterinary Diet and the University of Guelph. In the past 10 years, the CVUC has quantitatively analyzed over 50 000 submissions. Of these, 40 637 (78%) were from dogs and 11 353 (22%) from cats. Submissions to the CVUC were received from across Canada.
The purpose of this paper was to report on the number and mineral composition of bladder uroliths, passed, retrieved, or surgically removed, from Canadian dogs and cats over a 10-year period.
Materials and methods
A computer-assisted search of data from questionnaires submitted to the CVUC was used to compile information about all urinary bladder calculi analyzed between February, 1998 and August, 2008. The age, sex, and breed of dogs and cats were recorded.
Most of the uroliths analyzed had been surgically removed, voided, or retrieved with catheter assistance. In a small number of cases, electrohydraulic lithotripsy had been performed at the Faculté de médecine vétérinaire, Université de Montréal and the fragments were retrieved with a stone basket and urethroscopic guidance. Quantitative analysis was performed as has been previously described (1). Uroliths containing at least 70% of a single mineral were classified as that mineral type (2). Uroliths with nidus and shells of different mineral types were classified as compound and were included in the “other” category in this paper. Uroliths containing < 70% of a single mineral component and without an obvious nidus or shell were classified as mixed and were included in the “other” category (2). For purposes of this discussion, the term “calcium oxalate” includes calcium oxalate monohydrate, calcium oxalate dihydrate, or both; the term “urate” includes the salts of uric acid (ammonium, potassium, and sodium acid urate).
The change in prevalence of struvite, calcium oxalate, and other uroliths over time was evaluated using the chi-squared test for trend. A P-value of < 0.05 was considered significant for all comparisons.
Results
Canine submissions
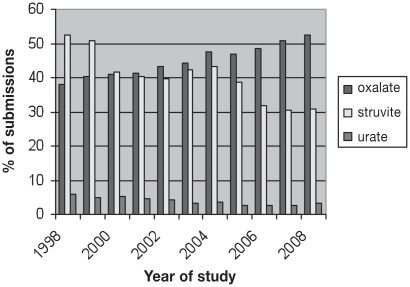
Approximately 85% of submissions (34 374/40 637) were classified as calcium oxalate or struvite in composition. There was a significant increase in the prevalence of calcium oxalate uroliths during the study period (P < 0.0001). There were significant decreases in the prevalence of struvite (P < 0.0001), urate (P < 0.0001), calcium phosphate (P < 0.001), silica (P < 0.001) and cystine (P = 0.005) uroliths during the study period, with no change in xanthine uroliths (P = 0.11) (Table 1; Figure 1).
Table 1.
Frequency of occurrence of canine uroliths of various composition over the 10-year period 1998 to 2008
| Mineral type | 1998a | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008b | 1998–2008 total numbers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxalate | 807 | 1192 | 1357 | 1453 | 1581 | 1719 | 1972 | 2082 | 2295 | 2451 | 1592 | 18 471 |
| Struvite | 1111 | 1499 | 1381 | 1416 | 1453 | 1639 | 1793 | 1709 | 1502 | 1466 | 934 | 15 903 |
| Urate | 124 | 143 | 178 | 157 | 161 | 121 | 145 | 119 | 130 | 129 | 95 | 1502 |
| Calcium phosphate | 43 | 138 | 81 | 62 | 84 | 91 | 54 | 130 | 102 | 40 | 26 | 851 |
| Silica | 24 | 34 | 31 | 34 | 26 | 31 | 20 | 24 | 34 | 17 | 14 | 289 |
| Cystine | 11 | 10 | 11 | 17 | 6 | 12 | 9 | 10 | 16 | 7 | 6 | 115 |
| Xanthine | 1 | 1 | 2 | 3 | 1 | 5 | 0 | 2 | 3 | 6 | 5 | 29 |
| DSBC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 |
| Sodium pyrophosphate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Totals | 2123 | 2956 | 3324 | 3508 | 3653 | 3873 | 4151 | 4433 | 4743 | 4833 | 3040 | 40 637 |
February to December.
Data for January to August.
Figure 1.
Changing trends in percentage of canine urolith submissions over the 10-year period 1998 to 2008.
Although mixed breed dogs accounted for most canine submissions (9832/40 637; 24%), small purebred dogs (shih tzu, miniature schnauzer, bichon frisé, lhasa apso, and Yorkshire terrier) were over-represented. These 5 breeds of dogs were associated with approximately 50% of all uroliths submitted (Table 2). There was a minimum of 1000 cases for each of these breeds. When additional breeds with at least 250 cases/breed were included (toy poodle, Jack Russell terrier, Maltese, Pekingese, pomeranian, pug) small breeds were associated with approximately 60% of all uroliths that were seen. There is a strong tendency for males in these breeds to form calcium oxalate, whereas the females are more prone to form struvite. The female schnauzer is an exception, as they develop calcium oxalate uroliths more frequently than struvite uroliths (1509/3157; 48% oxalate and 1228/3157; 39% struvite). The male dalmatian was at highest risk for urate urolithiasis (794/829; 96%). The female dalmatian was also at risk, although the total number of submissions was significantly lower (14/18; 78%). Very few struvite (9/847; 1%) and calcium oxalate (7/847; 0.8%) uroliths were identified in dalmatians. Other urolith types, including cystine, xanthine, silica, calcium phosphate, sodium pyrophosphate, and dried solidified blood calculi (DSBC) were less commonly reported (Table 1).
Table 2.
Stone composition and sex of mixed breed and top 6 canine breeds, 1998–2008
| Stone composition |
||||||
|---|---|---|---|---|---|---|
| Breed (total number of submissions) | Sex | Number of stones | Calcium oxalate | Struvite | Urates | Other |
| Mixed breeds | M | 3524 | 2933 | 286 | 60 | 254 |
| (9832) | F | 6308 | 893 | 4403 | 31 | 981 |
| Shih tzu | M | 1838 | 1403 | 176 | 89 | 170 |
| (5863) | F | 4025 | 618 | 2656 | 57 | 694 |
| Miniature schnauzer | M | 2491 | 2227 | 77 | 64 | 123 |
| (5648) | F | 3157 | 1509 | 1228 | 29 | 391 |
| Bichon frisé | M | 1561 | 1393 | 57 | 5 | 106 |
| (4627) | F | 3066 | 541 | 2025 | 8 | 492 |
| Lhasa apso | M | 1007 | 894 | 25 | 12 | 76 |
| (1962) | F | 955 | 326 | 462 | 9 | 158 |
| Yorkshire terrier | M | 899 | 748 | 43 | 51 | 57 |
| (1352) | F | 453 | 139 | 235 | 13 | 66 |
| Dalmatian | M | 829 | 6 | 7 | 794 | 22 |
| (847) | F | 18 | 1 | 2 | 14 | 1 |
| Totals | 30 131 | 13 631 | 11 682 | 1236 | 3582 | |
Feline submissions
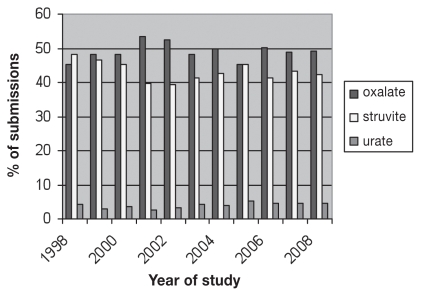
In cats, 92% of bladder uroliths were composed of magnesium ammonium phosphate (struvite) or calcium oxalate. In the 10-year study period, there was no significant change in the prevalence of calcium oxalate uroliths (P = 0.81) but there was a significant decrease in the prevalence of struvite uroliths (P = 0.031) and an increase in the prevalence of urate (P = 0.003) and DSBC uroliths (P = 0.004). No changes were found in the prevalence of calcium phosphate (P = 0.13), xanthine (P = 0.29), silica (P = 0.66), cystine (P = 0.46) and sodium pyrophosphate uroliths (P = 0.29) (Table 3; Figure 2). Most stones came from domestic short hair (DSH), medium hair (DMH), and longhair cats (DLH), followed by Himalayan, Persian, and Siamese cats (Table 4). Ragdoll cats had a higher than expected rate of calcium oxalate submissions (14/25; 56%) compared with other urolith types. Urate uroliths were over-represented in Egyptian maus (17/20; 85%) compared with domestic cats (392/9972; 4%) and other purebred cats (6/490; 1.2% Himalayan and 9/326; 3% Persian cats).
Table 3.
Frequency of occurrence of feline uroliths of various composition over the 10-year period 1998–2008)
| Mineral type | 1998a | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008b | 1998–2008 total numbers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxalate | 343 | 451 | 493 | 535 | 526 | 523 | 544 | 505 | 630 | 654 | 366 | 5570 |
| Struvite | 367 | 435 | 462 | 397 | 395 | 451 | 466 | 504 | 518 | 579 | 313 | 4887 |
| Urate | 33 | 28 | 36 | 27 | 33 | 46 | 43 | 59 | 59 | 63 | 35 | 462 |
| Calcium phosphate | 9 | 10 | 7 | 11 | 13 | 21 | 11 | 17 | 10 | 4 | 5 | 118 |
| DSBC | 1 | 0 | 0 | 0 | 2 | 5 | 2 | 2 | 4 | 7 | 2 | 25 |
| Xanthine | 0 | 0 | 0 | 4 | 0 | 2 | 3 | 0 | 2 | 1 | 2 | 14 |
| Silica | 2 | 2 | 0 | 0 | 1 | 1 | 3 | 0 | 3 | 0 | 1 | 13 |
| Cystine | 1 | 1 | 2 | 0 | 1 | 2 | 2 | 0 | 0 | 1 | 1 | 11 |
| Sodium pyrophosphate | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 4 | 0 | 0 | 1 | 11 |
| Totals | 760 | 933 | 1020 | 999 | 1005 | 1088 | 1095 | 1116 | 1258 | 1337 | 742 | 11 353 |
February to December.
Data for January to August.
Figure 2.
Changing trends in percentage of feline urolith submissions over the 10-year period 1998 to 2008.
Table 4.
Stone composition and sex of feline patients, 1998–2008
| Stone composition |
||||||
|---|---|---|---|---|---|---|
| Breed (total number submissions) | Sex | Number of stones | Calcium oxalate | Struvite | Urates | Other |
| DSH | M | 3910 | 2118 | 1454 | 178 | 160 |
| (7552) | F | 3642 | 1486 | 1926 | 125 | 105 |
| DLH and DMH | M | 1246 | 661 | 473 | 54 | 58 |
| (2420) | F | 1174 | 436 | 659 | 35 | 44 |
| Himalayan | M | 295 | 223 | 63 | 2 | 7 |
| (490) | F | 195 | 119 | 69 | 4 | 3 |
| Persian | M | 201 | 150 | 33 | 8 | 10 |
| (326) | F | 125 | 68 | 51 | 1 | 5 |
| Siamese | M | 130 | 88 | 22 | 16 | 4 |
| (211) | F | 81 | 41 | 22 | 14 | 4 |
| Ragdoll | M | 14 | 8 | 3 | 1 | 2 |
| (25) | F | 11 | 6 | 4 | 1 | 0 |
| Egyptian mau | M | 11 | 0 | 0 | 11 | 0 |
| (20) | F | 9 | 0 | 3 | 6 | 0 |
| Totals | 11 044 | 5404 | 4782 | 456 | 402 | |
DSH = domestic short hair.
DMH = domestic medium hair.
DLH = domestic long hair.
Male DSH, DMH, and DLH cats were 1.4 times more likely to develop a calcium oxalate (2779/5156; 54%) versus a struvite (1927/5156; 37%) urolith. Female DSH, DMH and DLH cats were 1.3 times more likely to develop a struvite (2585/4816; 54%) versus a calcium oxalate (1922/4816; 40%) urolith. Male and female purebred cats (Himalayan, Persian, Siamese, Ragdoll) appeared to be at risk for both struvite and calcium oxalate uroliths with higher numbers of calcium oxalate urolith submissions versus struvite submissions. Male Himalayan cats were 3.6 times more likely to develop a calcium oxalate (223/295; 76%) versus a struvite urolith (63/295; 21%). Female Himalayan cats were also more likely to develop a calcium oxalate (119/195; 61%) compared with a struvite (69/195; 35%) urolith. Male and female Persian cats were also more likely to develop a calcium oxalate urolith (150/201; 75% male and 68/125; 54% female) than a struvite urolith (33/201; 16% male and 51/125; 41% female). A similar trend was seen in the Siamese cat where 68% of males formed calcium oxalate and only 17% formed struvite uroliths. In female Siamese cats, 41/81 (51%) formed calcium oxalate uroliths; 22/81 (27%) formed struvite. Almost equal numbers of male and female Ragdoll cats formed calcium oxalate uroliths (8/14; 57% male and 6/11; 55% females). Although the numbers were small (11 submissions), 100% of male and 67% (6/9) of female Egyptian mau cats formed urate uroliths.
Other urolith types, including cystine, xanthine, silica, calcium phosphate, sodium pyrophosphate and dried solidified blood calculi (DSBC) were less commonly reported, although more DSBC and sodium pyrophosphate uroliths were seen in cats than in dogs (Tables 1 and 3).
Discussion
We combined our previously published data on CVUC submissions from February 1998 to April 2003 (3,4) with our most recent numbers to August 2008 in order to generate analyses with larger numbers and to identify trends in urolith submissions to the CVUC since its inception.
Although struvite uroliths predominated in the 1980s, there has been a progressive increase in the number of calcium oxalate urolith submissions in dogs and cats reported in North America. In the USA, the prevalence of canine calcium oxalate submissions increased dramatically from 5% in 1981 to 42% in 2005. During this period, canine struvite submissions declined from 78% to 38% (2,5–8). In cats, struvite uroliths predominated (> 80%) in the early 1980s and then slowly declined to ~34% of submissions by 2001 (9–11). Between 1984 and 2002, the proportion of calcium oxalate uroliths submitted to the University of Minnesota Urolith Center increased from ~3% to ~50% (10,11). However, since 2002, struvite uroliths have been on the rise and have surpassed calcium oxalate as the number one urolith submission in the USA. Based on ~9221 feline uroliths analyzed at the University of Minnesota Urolith Center in 2005, the most common mineral types were struvite (48%), calcium oxalate (41%), and purine (4.6%) (10).
Theories on the cause of the increasing incidence of calcium oxalate over the last couple of decades include increased feeding of acidified commercial diets; changes in dietary content of calcium, magnesium, phosphorus, or oxalate; decreased water consumption; an increase in sedentary lifestyles of many dogs and cats and, in dogs, trends favoring ownership of small breeds that are more prone to calcium oxalate uroliths (6,12–18).
In dogs, a predisposition for the increased incidence in small breeds has been reported (2,4,7,19–21). Lower urine volume and fewer numbers of micturitions resulting in increased mineral concentrations in smaller breed dogs compared with larger breed dogs may cause the predisposition (22). In a study comparing miniature schnauzers with Labrador retrievers, the miniature schnauzers urinated significantly less often than the Labrador retrievers and had a lower urine volume, a significantly higher urine pH, and significantly higher urinary calcium concentrations than the Labrador retrievers (22). Further work is necessary to determine the significance, genetics, and pathophysiology of calcium oxalate uroliths in Persian, Himalayan, Siamese and Ragdoll cats.
Females outnumbered males 16.4:1 in canine struvite submissions in this study. Struvite uroliths tend to occur primarily in female dogs due to the presence of a urinary tract infection as previously reported (4). This is not the case in cats in which urinary tract infections are uncommon. In purebred cats, females presented with higher numbers of calcium oxalate than struvite submissions.
Calcium oxalate uroliths tend to occur in an older population of pets compared with struvite uroliths. Smith et al (23) reported that senior cats (mean age 10.63 +/− 1.32 y) produced urine that had significantly lower struvite relative supersaturation (RSS) values (0.721 +/− 0.585 versus 4.984 +/− 4.028) and significantly higher calcium oxalate RSS values (3.449 +/− 1.619 versus 0.911 +/− 0.866) when compared with a group of younger (4.06 +/− 1.02 y) cats. The senior cats had a significantly lower urine pH compared with the younger cats (6.08 ± 0.22 versus 6.38 +/− 0.22, respectively). The decrease in urine pH in the senior cats may partially explain the increased risk for forming calcium oxalate uroliths with age (23).
The 3rd most common urolith in dogs and cats is ammonium urate. The most commonly affected breed of dog is the dalmatian in which the genetics has been confirmed (24–26). In non-dalmatian dogs, almost all urate formed from the degradation of purine nucleotides is metabolized by hepatic uricase to allantoin, which is very soluble and excreted by the kidneys. This is not the case in dalmatians where only 30% to 40% of uric acid is converted to allantoin, resulting in increased serum levels and urinary excretion of urate. The defective uric acid mechanism in dalmatians probably involves alterations in both the hepatic and renal pathways, but the exact mechanism is incompletely understood. A familial tendency has been suggested for English bulldogs, the 2nd most common breed associated with uroliths at the Minnesota Urolith Center (27). Younger animals with portosystemic vascular shunts are at risk (27). Regardless of cause, severe hepatic dysfunction may predispose dogs to urate lithogenesis (2). In cats, the pathophysiology of urate urolithiasis is not known and further work is needed in this area. This is the first report of Egyptian maus appearing to be predisposed to urate urolithiasis.
Less commonly submitted uroliths include xanthine, cystine, silica, calcium phosphate, sodium pyrophosphate, and dried solidified blood calculi (DSBC). There is one previous report of sodium pyrophosphate uroliths appearing in 4 cats and 1 dog; little is known about the cause of this type of stone (28). Previous reports indicate that DSBC have appeared in cats only (29). The report herein is the first, to the authors’ knowledge, that documents the detection of these uroliths in dogs. Further work is necessary to determine the predisposing factors for DSBC in dogs.
In conclusion, there has been a significant increase in canine calcium oxalate urolith submissions and a significant decrease in canine and feline struvite urolith submissions to the CVUC over the last 10 y. Small breed dogs and select purebred breeds of cats are over-represented in calcium oxalate and struvite urolith submissions. The Egyptian mau cat is over-represented in urate submissions. Sodium pyrophosphate and DSBC, although uncommon, have been identified in both dogs and cats. Further work is needed to elucidate the reasons behind the trends identified.
Acknowledgments
The authors thank Mike Favrin, Gabriel Ho, Jennifer Proulx, Carole White, and Dean Elliott for their assistance in quantitative analysis of the uroliths at the CVUC; Kelly Kirkpatrick, Jasmine Sun and Jennifer Weishar for data entry; and Dr. Scott Weese for assistance with statistical analysis. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Moore A. Quantitative analysis of urinary calculi in dogs and cats. Veterinary Focus. 2007;17:22–27. [Google Scholar]
- 2.Osborne CA, Lulich JP, Polzin DG, et al. Analysis of 77,000 canine uroliths: Perspectives from the Minnesota Urolith Center. Vet Clin North Am Small Anim Pract. 1999;29:17–38. doi: 10.1016/s0195-5616(99)50002-8. [DOI] [PubMed] [Google Scholar]
- 3.Houston DM, Moore AE, Favrin MG, Hoff B. Feline urethral plugs and bladder uroliths: A review of 5484 submissions 1998–2003. Can Vet J. 2003;44:974–977. [PMC free article] [PubMed] [Google Scholar]
- 4.Houston DM, Moore AEP, Favrin MG, Hoff B. Canine urolithiasis: A look at over 16000 urolith submissions to the Canadian Veterinary Urolith Centre from February 1998 to April 2003. Can Vet J. 2004;45:225–230. [PMC free article] [PubMed] [Google Scholar]
- 5.Lulich JP, Osborne CA, Bartges JW, Lekcharoensuk C. Canine lower urinary tract disorders. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 5th ed. Philadelphia: WB Saunders; 2000. pp. 1747–1781. [Google Scholar]
- 6.Ling GV, Thurmond MC, Choi YK, Franti CE, Ruby AL, Johnson DL. Changes in proportion of canine urinary calculi composed of calcium oxalate or struvite in specimens analyzed from 1981 through 2001. J Vet Intern Med. 2003;17:817–823. doi: 10.1111/j.1939-1676.2003.tb02520.x. [DOI] [PubMed] [Google Scholar]
- 7.Osborne CA. Improving management of urolithiasis: Canine struvite uroliths. DVM Magazine. Apr 1, 2004. [Last accessed October 7, 2009]. Available from http://veterinarynews.dvm360.com/dvm/article/articleDetail.jsp?id=90577.
- 8.Osborne CA, Lulich JP. Perspectives: Analysis of 275,000 uroliths. DVM 360. Jul, 2006. [Last accessed October 7, 2009]. Available from http://veterinarynews.dvm360.com/dvm/Medicine/Perspectives-Analysis-of-275000-uroliths/ArticleStandard/Article/detail/360567.
- 9.Cannon AB, Westropp JL, Ruby AL, Kass PH. Evaluation of trends in urolith composition in cats: 5,230 cases (1985–2004) J Am Vet Med Assoc. 2007;231:570–576. doi: 10.2460/javma.231.4.570. [DOI] [PubMed] [Google Scholar]
- 10.Forrester SD. Evidence-based nutritional management of feline lower urinary tract disease. Proc 24th ACVIM; May 31, 2006; Louisville, Kentucky ACVIM. pp. 510–512. [Google Scholar]
- 11.Osborne CA, Lulich JP, Thumachai R, et al. Feline Urolithiasis. In: Osborne CA, Kruger JM, Lulich JP, editors. Vet Clin North Am (Small Anim Pract) Philadelphia: WB Saunders; 1996. pp. 217–232. [PubMed] [Google Scholar]
- 12.Stevenson AE. The incidence of urolithiasis in cats and dogs and the influence of diet in the formation and prevention of recurrence. Institute of Urology and Nephrology, University College London; 2001. PhD thesis. [Google Scholar]
- 13.Jones BR, Sanson RL, Morris RS. Elucidating the risk factors of feline lower urinary tract disease. NZ Vet J. 1997;45:100–108. doi: 10.1080/00480169.1997.36003. [DOI] [PubMed] [Google Scholar]
- 14.Lekcharoensuk C, Osborne CA, Lulich JP. Epidemiologic study of risk factors for lower urinary tract diseases in cats. J Am Vet Med Assoc. 2001;218:1429–1435. doi: 10.2460/javma.2001.218.1429. [DOI] [PubMed] [Google Scholar]
- 15.Lekcharoensuk C. Association between patient-related factors and risk of calcium oxalate and magnesium ammonium phosphate urolithiasis in cats. J Am Vet Med Assoc. 2000;217:520–525. doi: 10.2460/javma.2000.217.520. [DOI] [PubMed] [Google Scholar]
- 16.Lekcharoensuk C, Osborne CA, Lulich JP, et al. Association between dietary factors and calcium oxalate and magnesium ammonium phosphate urolithiasis in cats. J Am Vet Med Assoc. 2001;219:1228–1237. doi: 10.2460/javma.2001.219.1228. [DOI] [PubMed] [Google Scholar]
- 17.Thumachai R, Lulich JP, Osborne CA, et al. Epizootiologic evaluation of urolithiasis in cats: 3948 cases (1982–1992) J Am Vet Med Assoc. 1996;208:547–551. [PubMed] [Google Scholar]
- 18.Kirk CA, Ling GV, Franti CE, Scarlett JM. Evaluation of factors associated with development of calcium oxalate urolithiasis in cats. J Am Vet Med Assoc. 1995;207:1429–1434. [PubMed] [Google Scholar]
- 19.Lulich JP, Osborne CA, Bartges JW, Lekcharoensuk C. Canine lower urinary tract disorders. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 5th ed. Philadelphia: WB Saunders; 2000. pp. 1747–1781. [Google Scholar]
- 20.Stevenson AE. The incidence of urolithiasis in cats and dogs and the influence of diet in the formation and prevention of recurrence. Institute of Urology and Nephrology, University College London; 2001. PhD thesis. [Google Scholar]
- 21.Lekcharoensuk C, Lulich JP, Osborne CA, et al. Patient and environmental factors associated with calcium oxalate urolithiasis in dogs. J Am Vet Med Assoc. 2000;217:515–519. doi: 10.2460/javma.2000.217.515. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson AE, Markwell PJ. Comparison of urine composition of healthy Labrador retrievers and miniature schnauzers. Am J Vet Res. 2001;62:1782–6. doi: 10.2460/ajvr.2001.62.1782. [DOI] [PubMed] [Google Scholar]
- 23.Smith BHE, Stevenson AE, Markwell PJ. Dietary sodium promotes increased water intake and urine volume in cats. J Nutr. 2004;134:2128S–2129S. doi: 10.1093/jn/134.8.2128S. [DOI] [PubMed] [Google Scholar]
- 24.Bannasch DL. The Genetic and Molecular Basis of Urate Calculi Formation in Dalmatians. Proc 26th ACVIM; San Antonio, Texas. 2008. pp. 475–477. [Google Scholar]
- 25.Sorenson JL, Ling GV. Diagnosis, prevention, and treatment of urate urolithiasis in Dalmatians. J Am Vet Med Assoc. 1993;203:863–869. [PubMed] [Google Scholar]
- 26.Bannasch DL, Ling GV, Bea J, Famula TR. Inheritance of urinary calculi in the Dalmatian. J Vet Intern Med. 2004;18:483–487. doi: 10.1892/0891-6640(2004)18<483:ioucit>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Albasan H, Lulich JP, Osborne CA, Lekcharoensuk C. Evaluation of the association between sex and risk of forming urate uroliths in Dalmatians. J Am Vet Med Assoc. 2005;227:565–569. doi: 10.2460/javma.2005.227.565. [DOI] [PubMed] [Google Scholar]
- 28.Frank A, Norrestam R, Sjodin A. A new urolith in four cats and a dog: Composition and crystal structure. J Biol Inorg Chem. 2002;7:437–444. doi: 10.1007/s00775-001-0315-1. [DOI] [PubMed] [Google Scholar]
- 29.Westropp JL, Ruby AL, Bailiff NL, Kyles AE, Ling GV. Dried solidified blood calculi in the urinary tract of cats. J Vet Intern Med. 2006;20:828–834. doi: 10.1892/0891-6640(2006)20[828:dsbcit]2.0.co;2. [DOI] [PubMed] [Google Scholar]