Abstract
Klein, Dolph (Rutgers, The State University, New Brunswick, N. J.) and David Pramer. Some products of the bacterial dissimilation of streptomycin. J. Bacteriol. 83:309–313. 1962.—Bacterial dissimilation of streptomycin resulted in products detectable with Folin-Ciocalteu's phenol reagent, ninhydrin, and Ehrlich's reagent; these products absorbed ultraviolet light, with a maximum at 265 mμ. Evidence was also obtained for the production of a volatile base, possibly methylamine.
Urea and streptamine were formed by growing cultures and washed-cell suspensions supplied with streptomycin. More than 90% of the amidine-nitrogen of the antibiotic was recovered as urea-nitrogen in culture filtrates. It was evident that the bacterial dissimilation of streptomycin involved hydrolysis of the guanido groups of the streptidine moiety of the antibiotic molecule. The site of action was the bond between the amidine group and the secondary amino-nitrogen of each guanido group, and the products of the reaction were urea and streptamine.
Full text
PDF

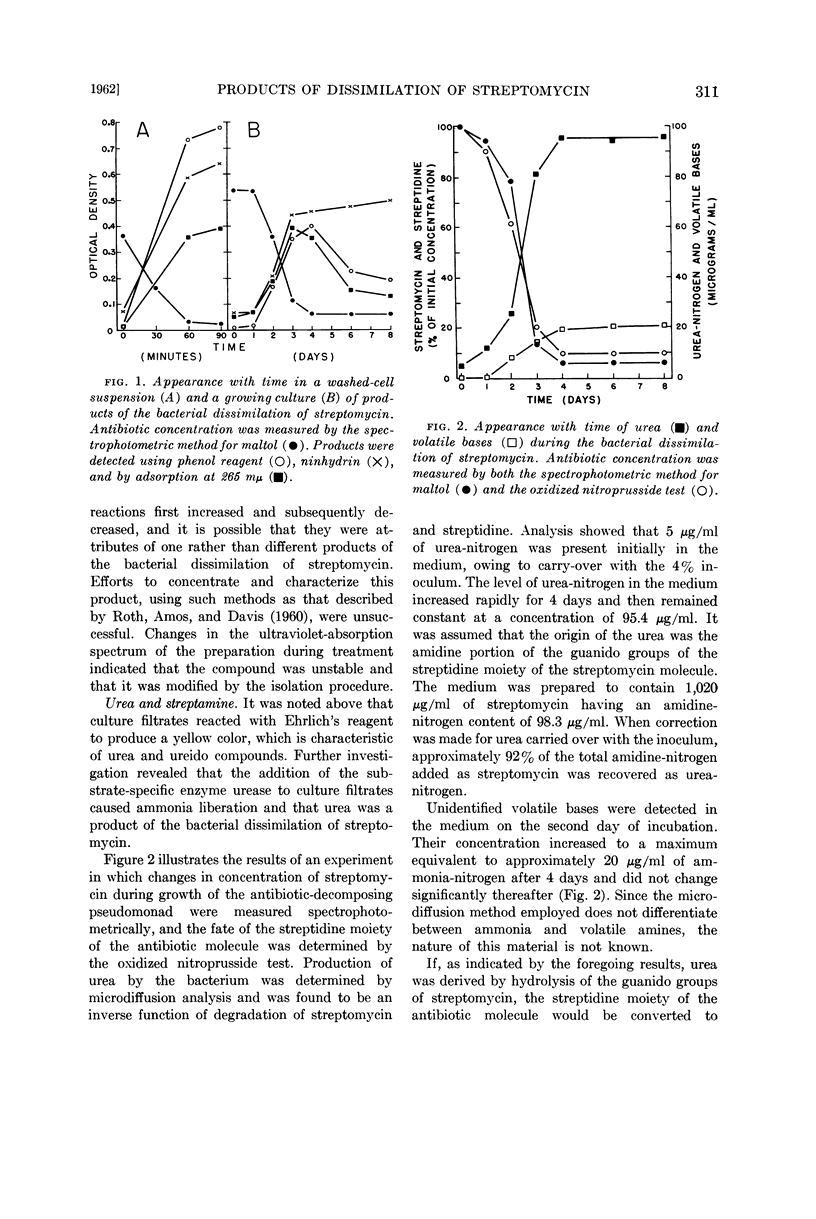
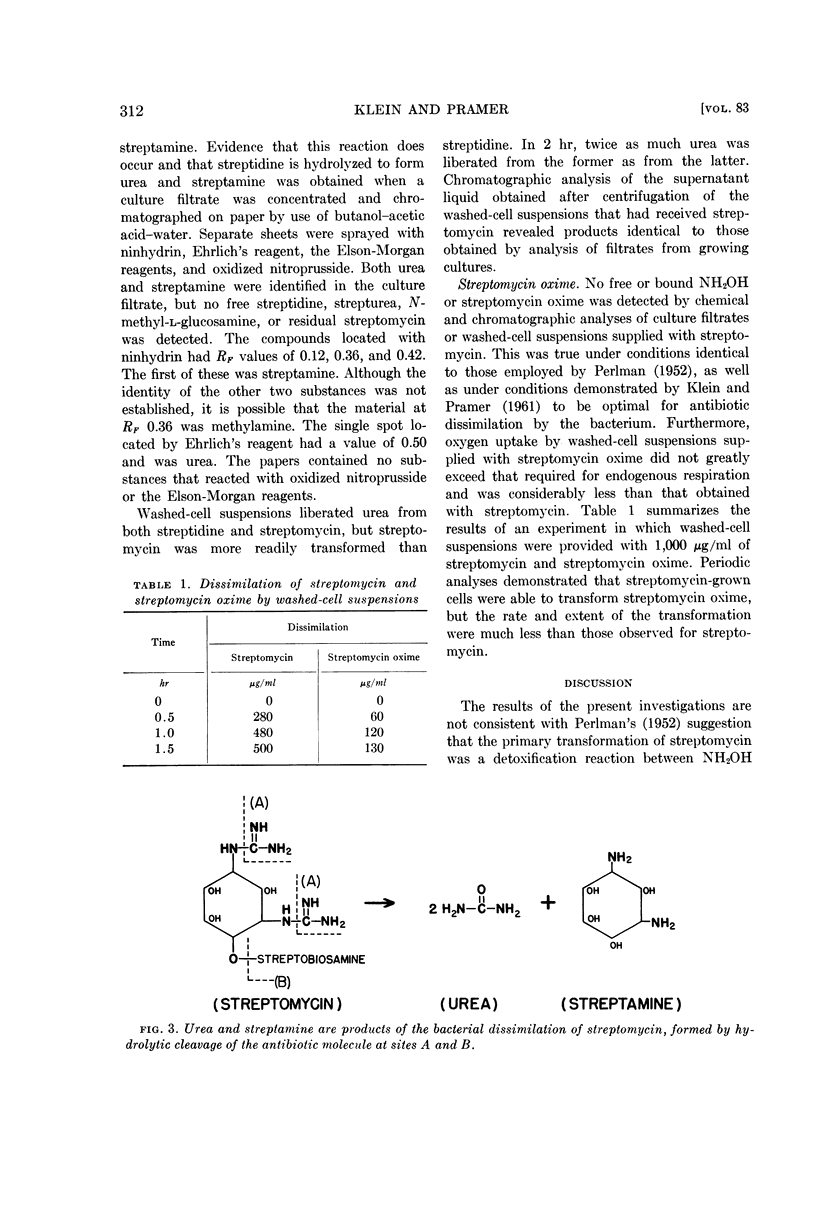

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- KLEIN D., PRAMER D. Bacterial dissimilation of streptomycin. J Bacteriol. 1961 Oct;82:505–510. doi: 10.1128/jb.82.4.505-510.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramer D., Starkey R. L. Decomposition of Streptomycin. Science. 1951 Feb 2;113(2927):127–127. doi: 10.1126/science.113.2927.127. [DOI] [PubMed] [Google Scholar]
- ROTH H., AMOS H., DAVIS B. D. Purine nucleotide excretion by Escherichia coli in the presence of streptomycin. Biochim Biophys Acta. 1960 Jan 29;37:398–405. doi: 10.1016/0006-3002(60)90495-9. [DOI] [PubMed] [Google Scholar]


