Summary
Generation of NO by nitric oxide synthase (NOS) is implicated in gamete interaction and fertilisation. Exposure of human spermatozoa to NO donors caused mobilisation of stored Ca2+ by a mechanism that did not require activation of guanylate cyclase but was mimicked by S-nitroso-glutathione (GSNO; an S-nitrosylating agent). Application of dithiothreitol, to reduce protein –SNO groups, rapidly reversed the actions of NO and GSNO on [Ca2+]i. The effects of NO, GSNO and dithiothreitol on sperm protein S-nitrosylation, assessed using the biotin switch method, closely paralleled their actions on [Ca2+]i. Immunofluorescent staining revealed constitutive and inducible NOS in human oviduct and cumulus (the cellular layer investing the oocyte). 4,5-diaminofluorescein (DAF) staining demonstrated production of NO by these tissues. Incubation of human sperm with oviduct explants induced sperm protein S-nitrosylation resembling that induced by NO donors and GSNO. Progesterone (a product of cumulus cells) also mobilises stored Ca2+ in human sperm. Pre-treatment of sperm with NO greatly enhanced the effect of progesterone on [Ca2+]i, resulting in a prolonged increase in flagellar excursion. We conclude that NO regulates mobilisation of stored Ca2+ in human sperm by protein S-nitrosylation, that this action is synergistic with progesterone and that this synergism is potentially highly significant in gamete interactions leading to fertilisation.
Introduction
Nitric oxide (NO), synthesised by nitric oxide synthases (endothelial, neuronal and inducible types; eNOS, nNOS and iNOS) acts as a rapid paracrine (and probably autocrine) cellular messenger, typically by activation of soluble guanylate cyclase (sGC; Arnold et al., 1977; Miki et al., 1977; Braughler et al., 1979; Ahern et al., 2002). It is a small, uncharged molecule and therefore highly diffusible. However, NO is a free radical and its reactivity is such that lifetime of the molecule is brief, biological effects probably being confined to a volume with a radius of 100-200 μm from the point of synthesis (Lancaster, 1997). Though the action of NO is of particular importance in the cardiovascular tissue (where it was first called endothelium derived relaxing factor [EDRF]) and nervous system (Calebrese et al., 2007; Li and Moore, 2007; Rastaldo et al., 2007), it is now clear that NO plays a role in most tissue and cell types. The expression of NOS has been demonstrated both in cells of the male and female mammalian reproductive tracts and also in gametes of vertebrates and invertebrates, leading to the suggestion that NO may play important physiological roles in fertilisation (Creech et al., 1998; Rosselli et al., 1998; Thaler and Epel, 2003; Kim et al, 2004).
In mammalian sperm, production of NO endogenously and/or by cells of the female tract may contribute to capacitation, inducing tyrosine phosphorylation by mechanisms involving and/or independent of the cAMP-protein kinase A pathway (Funahashi, 2002; Thundathil et al, 2003; O'Flaherty et al., 2004, 2005, 2006, Roy and Atreja, 2008). NO also induces or contributes to induction of acrosome reaction (Revelli et al, 2001; Funahashi, 2002; Herrero et al., 2003; O'Flaherty et al., 2004; Yang et al, 2005). With regard to effects of NO on motility of mammalian sperm, a number of studies have shown that application of NO in vitro has functional effects, but the data here are complex. Treatment with NO-donors at high doses, or prolonged exposure to NO, suppresses motility, probably simulating cytotoxic effects that may occur in the testis or in sperm held in semen (Herrero et al., 1994; Weinberg et al., 1995; Zhang & Zheng, 1996; Joo et al., 1999; Calabrese, 2001; Wu et al., 2004). However, low concentrations of NO may stimulate motility (Herrero et al., 1994; Zhang & Zheng, 1996; Calabrese, 2001). In this context the study of Creech et al. (1998) on sperm of the Fathead Minnow (Pimephelus promelus) is particularly interesting. In ova of this fish, NOS is localised to the micropyle, the route of entry into the oocyte for the sperm. NO is produced during a critical 5 minute period after laying of the eggs and enhances sperm motility. The spatial and temporal ‘precision’ of the NO signal thus potentially plays a key role in fertilisation in this species (Creech et al., 1998). Reports that NOS is present in the mammalian oviduct, (Rosselli et al., 1996; Ekerhovd et al., 1999; Lapointe et al,, 2006) and also in the oocyte and the cumulus and corona cells that surround it (Hattori et al., 2001; Reyes et al., 2004; Tao et al., 2004) raise the intriguing possibility that NO plays a similar role in mammalian fertilisation, regulating sperm motility or even inducing chemotaxis (Miraglia et al., 2007).
Participation of Ca2+, a key regulator of sperm motility and hyperactivation (Darszon et al., 2007; Publicover et al., 2007), in modulation by NO has not yet been investigated. Here we report that NO is a potent regulator of Ca2+ signalling in human sperm, acting synergistically with progesterone, and we propose a role for NO in regulating the interaction of human gametes.
Materials and Methods
Female tract and cumulus cells
Oviduct and endometrial explants were obtained with informed consent from pre-menopausal patients undergoing hysterectomy or bilateral salpingectomy (for reasons unconnected with tubal pathology) at the Birmingham Women's Hospital, (Shropshire REC Reference: 06/Q2601/51).
Surplus cumulus cells were obtained during intracytoplasmic sperm injection (ICSI) cycles performed at The Assisted Conception Unit, Birmingham Women's Hospital [Human Fertilization and Embryology Authority (HFEA) Centre 0199].
COV434 cells (immortalised human granulosa cell line; a gift from the Clinical Oncology Unit, LUMC, the Netherlands; Zhang et al., 2000) were grown in DMEM-F12 (10% fetal bovine serum; 1% penicillin/streptomycin; 1% non essential amino acids) at 37°C, 5% CO2.
Detection of NOS in human female tract and cumulus
Loose human cumulus from oocyte retrieval were stored in phosphate buffered saline (PBS) at 4°C. Cells were then smeared onto standard microscope slides, air-dried and fixed with 100% methanol (−20°C, 6 minutes). The slides were treated with 50% (v/v) methanol in PBS (20°C, 5 minutes) and washed 3 times in 0.1% (v/v) Triton X-100 in PBS and subsequently re-hydrated with PBS for 15 minutes (20°C).
COV434 cells were released by scraping and centrifuged at 300 g for 5 minutes at room temperature. Cells were then resuspended in PBS, smeared onto standard microscope slides, air-dried and fixed with 4% formaldehyde for 6 minutes at room temperature then permeabilised using 0.2% Triton X-100 for 15 minutes and washed with 0.1% (v/v) triton X-100 in PBS.
Ampullary explants were washed in Hanks balanced salt solution (HBSS, Gibco) before being incubated with 0.25% collagenase type I (Gibco: 17100-017) in Dulbecco's phosphate buffered saline (DPBS) w/o CaCl2 or MgCl2 (Gibco 14190) for 1h at 37oC with gentle agitation. The supernatant was collected and pelleted by centrifugation at 500g for 5min. This was then plated in DMEM F12 supplemented with 150 pg/ml 17β-oestradiol and left to adhere and grow at 37°C 6% CO2 for two days. These cells formed a monolayer and retained functioning cilia. Fixation/permeabilisation was as for COV343 cells
Slides were blocked in 1% (w/v) bovine serum albumin (BSA), 5% (v/v) goat serum in PBS (30 minutes, 37°C in 5% CO2 in air) then incubated with rabbit polyclonal anti-eNOS, -nNOS or -iNOS (1:50 dilution in 1% (w/v) BSA in PBS, 37°C in 5% CO2 in air, 60 minutes). Slides were washed with PBS then secondary-antibody (donkey anti-rabbit Texas red or FITC – 1:200 dilution in 1% (w/v) BSA in PBS) was applied (37°C in 5% CO2 in air, 60 minutes). Finally slides were washed and coverslips mounted using DakoCytomation fluorescence mounting medium.
Detection of NO production in cumulus and oviductal epithelium
Human cumulus masses and ampullary explants were washed in supplemented Earle's Balanced Salt Solution (sEBSS) and incubated in the dark at 37°C and 6% CO2 with 5 μM 4,5-diaminofluorescein (DAF)-FM diacetate for 30 minutes. Excess DAF-FM was removed by 3 washes in sEBSS and the cumulus was transferred to microscope slides under a cover slip supported on spots of vacuum grease so as gently to compress it. The slides were examined under a Nikon inverted fluorescence microscope (488 nm excitation / 540 nm emission).
Sperm preparation and capacitation
Donors were recruited at the Birmingham Women's Hospital (HFEA Centre 0119), in accordance with the Human and Embryology Authority Code of Practice. All donors gave informed consent (LREC 2003/239) and sperm were obtained by direct swim-up into sEBSS (pH 7.3-7.4) with 0.3% BSA and adjusted to 6 million cells/ml (Kirkman-Brown et al., 2000). Sperm were allowed to capacitate at 37°C and 5% CO2 for 5-6 hours. For the biotin switch assay semen was layered over 1 ml fractions of 45 and 90% Percoll (made isotonic with M medium 1X: 137 mM NaCl, 2.5 mM KCl, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10 mM glucose). Samples were centrifuged (2000 g for 20 minutes), further washed with PBS then diluted and incubated in PBS.
Single cell imaging
Cell density was reduced to 4 million cells/ml and 200 μl aliquots were then loaded with 12 μM Oregon Green BAPTA (OGB) 1-AM (0.6% dimethyl sulfoxide (DMSO), 0.12% Pluronic F-127) for 40 minutes and transferred to an imaging chamber (180 μl), incorporating a coverslip coated with 1% poly-D-lysine (PDL), for further 20 minutes (all at 37°C and 5% CO2). The imaging chamber was then perfused with fresh medium (25°C) to remove unattached cells and excess dye. All experiments were performed at 25±1°C, with a perfusion rate of approximately 0.4 ml/minute. Cells were imaged with a Nikon TE200 inverted fluorescence microscope. Images were obtained every 10 seconds using a X40 objective and a Hamamatsu Orca 1 cooled CCD camera controlled by iQ software (Andor Technology, Belfast, UK).
Data were processed offline using iQ as described previously (Kirkman-Brown et al., 2000). For each cell, Microsoft Excel was used to calculate the mean and 95% confidence interval of fluorescence intensity for (i) 14 images during the control period (Con ± con), (ii) 14 images from minute 3 after treatment (A ± a) and, (iii) 14 images from minute 6 after treatment (B ± b). At each sampling point the response was considered significant if
where ‘X’ and ‘x’ are the mean and 95% confidence interval for that sampling point, and ‘Con’ and ‘con’ are the mean and 95% confidence interval for the control period. In experiments with two or more treatments the same procedure was followed, by defining additional ‘control’ periods. Cells were sorted into those showing increase, decrease or no change in fluorescence after treatment. Visual examination of fluorescence-time plots confirmed that this procedure provided an accurate sorting of responses. More complex [Ca2+]i responses (such as oscillations) were quantified by direct observation of time-fluorescence intensity plots. Values in the text giving frequency of each response type are mean ± s.e.m.. Microsoft Excel was used to perform paired or unpaired t tests as appropriate.
Assay of sperm protein S-nitrosylation and visualisation of S-nitrosoproteins
S-nitrosylation of proteins in human spermatozoa was assessed using the biotin switch assay as described previously (Lefièvre et al., 2007).
To visualise S-nitrosoproteins in sperm exposed to female tract-synthesised NO, sperm (50 million cells/ml) were incubated with fresh human tubal and endometrial explants (fragments approx 3 mm3) in 50 μl DMEM F12 medium (Gibco # 11320). supplemented with 150 pg/ml 17β-oestradiol (Sigma, E8875) at 37°C 5% O2 6% CO2 balance N2 for 2 hours. Sperm were then retrieved and fixed on slides using 4% formaldehyde and S-nitrosoproteins were detected using a method adapted from Yang and Loscalzo (2005), as decribed previously (Lefièvre et al., 2007). This method depends on blocking thiols with a thiol-reactive agent (MMTS) followed by reduction of S-nitrosothiols with ascorbate, and labelling with fluorescently tagged methanethiosulfonate (MTSEA).
Flagellar activity assessment
Samples were prepared and capacitated as described above and spermatozoa were introduced into the chamber and observed under phase-contrast microscopy. Loosely-attached cells with a freely motile flagellum were then selected to assess flagellar activity (images acquired at 1 Hz). Using the mid-point of the midpiece as a reference point, frame-to-frame displacement was measured throughout the experiments using the ImageJ MTrackJ plugin and plotted against time.
Chemotaxis
Sperm chemotaxis was assayed as described previously (Teves et al, 2006)
Materials
sEBSS contained (mM) 1 NaH2PO4, 5.4 KCl, 0.81 MgSO4.7H2O, 5.5 C6H12O6, 2.5 C3H3NaO3, 19 CH3CH(OH)COONa, 1.8 CaCl2.2H2O, 25. NaHCO3 and 116.4 NaCl (pH 7.3-7.4, 285-295 mOsm). In Ca2+-free sEBSS, CaCl2 was replaced with NaCl (118.4 mM). Fatty acid-free BSA was from SAFC Biosciences (Lenexa, KS, USA) Catalog N° 85041C-50G.
Rabbit polyclonal anti-eNOS, nNOS and iNOS and donkey anti-rabbit Texas red were from Santa Cruz Biotechnologies Inc, California, USA. SYTOX Green was from Molecular Probes, Oregon, USA.
OGB 1-AM was from Invitrogen Molecular Probes (Paisley, UK). PDL was from BD Biosciences (Oxford, UK). Protease inhibitor cocktail tablets were from Roche Diagnostics Ltd. (Lewes, East Sussex, UK) and EZ-Link Biotin-N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide (EZ-Link Biotin-HPDP) from Perbio Science UK Ltd. (Cramlington, Northumberland, UK). Nitrocellulose membrane was supplied by GE Healthcare UK Ltd. (St.Giles, Bucks, UK), IgG Fraction Monoclonal Mouse Anti-Biotin by Jackson Immunoresearch Laboratories (Stratech Scientific, Soham, Cambrigshire, UK) and Lumi-GLO, an enhanced chemiluminescence kit, from Insight Biotechnology Ltd. (Wembley, Middlesex, UK).
All other chemicals referred in the text were from Merck Biosciences Ltd. (Beeston, Nottingham, UK), except progesterone, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), DMSO, Pluronic F-127 and 8-bromoguanosine-3′,5′-cyclophosphate sodium salt (8-bromo cGMP) which were from Sigma-Aldrich (Poole, Dorset, UK).
Results
Human oviduct and cumulus express NOS
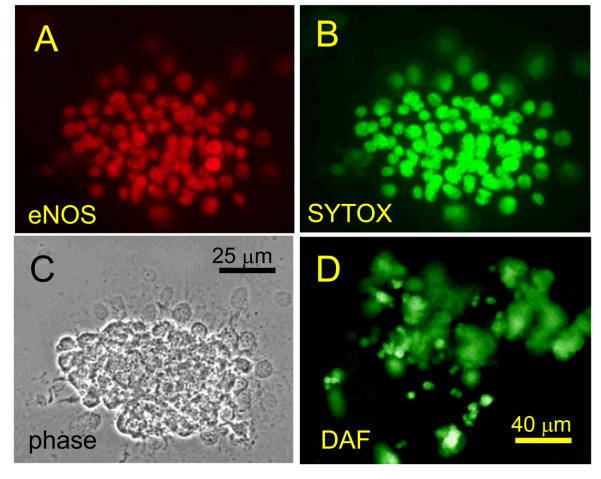
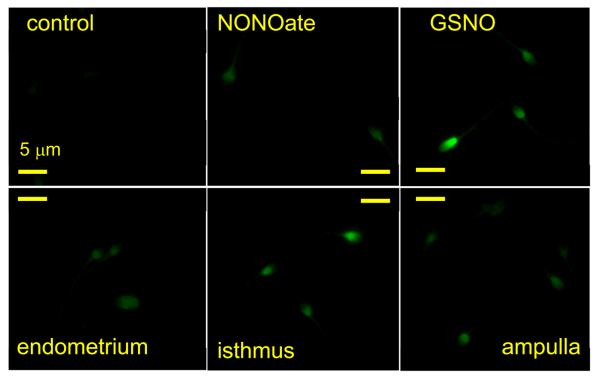
To confirm that human sperm encounter increased NO concentrations upon approaching the oocyte, we first assessed the expression of NOS in oviduct explants and in isolated fragments of human cumulus. Antibodies for eNOS showed the presence of this isoform in virtually every cell of human oviductal (ampullary) primary cultures, human cumulus fragments and COV 434 (human granulosa) cell line (Fig. 1). Similar results were obtained with antibodies specific for nNOS and iNOS was clearly present in oviduct (Figs. S1, S2). We used DAF to visualise NO synthesis in human cumulus fragments and oviduct explants. After DAF loading we observed a rapid increase in fluorescence in all cells, indicative of NO synthesis (Fig. S3). In cumulus fragments this was blocked by 1 mM L_NAME but generation of NO by oviduct explants appeared to be insensitive to this inhibitor (not shown). Incubation of human sperm under the same conditions did not result in a detectable fluorescent signal (not shown) indicating that NOS activity in sperm is much lower than in the cumulus or oviduct. Mouse cumulus also active synthesised NO but only a subset of cells (≈10%) became stained by DAF (Fig. S3).
Fig. 1.
Expression of eNOS in human oviductal and cumulus cells. A, C and E show staining of human oviductal (ampullary) primary culture (A), human cumulus (C) and human granulosa cell line (COV 434; E) for eNOS. B, D and F show corresponding phase images of these samples.
NO donors cause elevation of human sperm [Ca2+]i
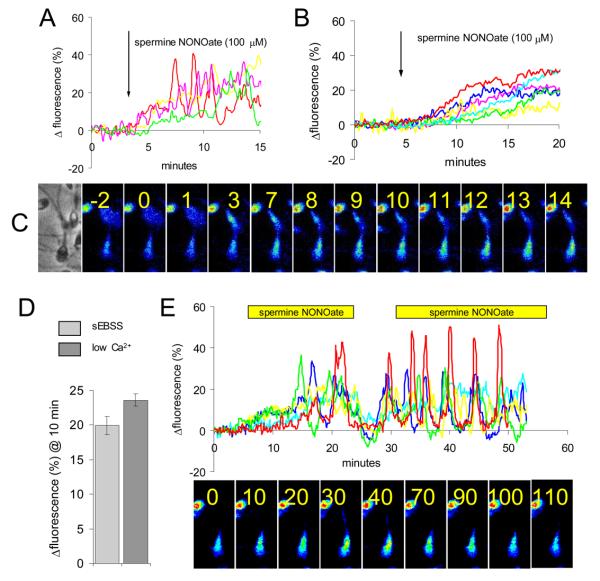
Expression of active NOS in oviduct and cumulus indicates that sperm will encounter NO as they approach the oocyte. To investigate the possible effect of this stimulus on sperm [Ca2+]i (which regulates motility) we applied NO donors to sperm loaded with OGB. 100 μM spermine NONOate caused a gradual but significant rise in [Ca2+]i (73±5% of cells; n=8). Latency of the effect was 0-2 minutes and fluorescence typically stabilised at ≈20% above control levels after 10 minutes (Fig. 2A). In 19% of cells oscillations were superimposed on the NO-induced elevation of [Ca2+]i (Fig. 2A; red trace). This action of NONOate was dose-dependent, application of 1 uM inducing a discernible increase in fluorescence in only 25% of cells. When NONOate concentration was subsequently raised to 10 μM the majority of cells (>70%) showed a gradual increase in fluorescence of 1-30% over 5-10 minutes. Subsequent application of 100 μM NONOate caused little further increase in fluorescence but clearly enhanced the occurrence of [Ca2+]i oscillations (Fig. S4). Similar results were obtained in three experiments. To determine whether this rise in [Ca2+]i was dependent primarily upon influx of Ca2+ at the plasmalemma, we incubated cells in saline with no added Ca2+ (Fig. 2B), where [Ca2+]o is ≤5 μM (Harper et al, 2004). Under these conditions, a slow elevation in [Ca2+]i occurred in 64±12% of cells (n=4; NS compared to sEBSS) but oscillations were rarely seen. The localisation (primarily neck/midpiece, spreading into the posterior head; Fig. 2C) and mean amplitude (Fig. 2D) of the response to NO were similar under the two conditions. Upon washout of spermine NONOate, [Ca2+]i fell rapidly but then showed a partial recovery in many cells. When the NONOate was reintroduced most cells again responded, the increase in [Ca2+]i being more pronounced and usually occurring as a series of oscillations in the neck/midpiece (Fig. 2E).
Fig. 2.
NO mobilises stored Ca2+ in sperm. (A) Spermine NONOate causes a slowly-developing rise in [Ca2+]i in human sperm. Responses of 4 separate cells are shown. Red trace shows example of cell generating [Ca2+]i oscillations. (B) In low-Ca2+ medium ([Ca2+]o≤5 μM) the response to NONOate was similar, but oscillations were rarely seen. Responses of 7 cells shown. (C) Pseudocolour image series showing NONOate-induced rise in [Ca2+]i in the sperm neck/midpiece. Numbers show minutes since application of 100 μM spermine NONOate. (D) Mean normalised increase in fluorescence 10 minutes after application of 100 μM spermine NONOate to cells bathed in sEBSS (271 cells; 3 experiments) and low-Ca2+ sEBSS (214 cells; 3 experiments). (E) A rapid decrease in [Ca2+]i followed washout of NONOate, followed by slow recovery. Upon re-introduction of NONOate many cells generated oscillations in the neck/midpiece region. Responses of 5 individual cells shown. Lower panel shows pseudocolour images series of a single [Ca2+]i oscillation (numbers show time in seconds).
Mobilisation of Ca2+ by NO is not dependent on stimulation of guanylate cyclase
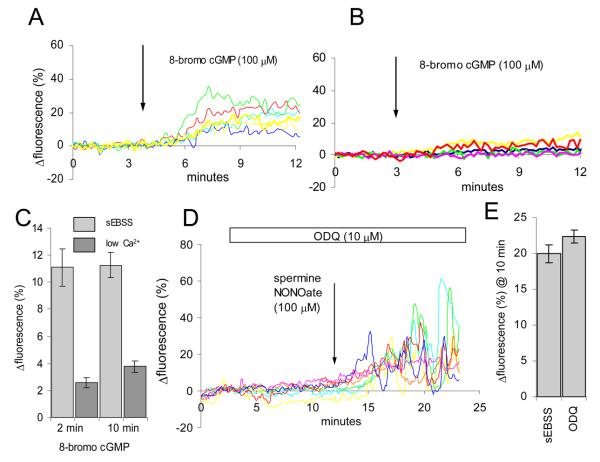
The ‘classic’ target for NO in its role as a messenger is soluble guanylate cyclase (sGC). Though cGMP (and therefore sGC activity) is low in mammalian sperm, there is evidence that effects of NO on acrosome reaction and possibly other sperm functions are exerted through this pathway (Herrero et al., 1998; Revelli et al., 2001). To investigate whether sGC might mediate NO-induced elevation of [Ca2+]i, we first examined the effects of the membrane-permeant analogue 8-bromo cGMP (100 μM). Upon application of 8-bromo cGMP, OGB fluorescence increased (77±9% of cells; n=4) to a plateau, stabilising after 2-3 minutes (Fig. 3A). However, unlike the action of NONOate, when the experiments were repeated in low-Ca2+ saline the effect of cGMP, though detectable (63±9% of cells; n=3), was reduced in amplitude by >70% (Fig. 3B,C). Thus, cGMP does elevate [Ca2+]i in human sperm but appears to do so by increased Ca2+ influx rather than by mobilisation of intracellular stores. To confirm that the response to NONOate was not due to activation of sGC, we used the sGC inhibitor ODQ. Approximately 10 minutes pre-treatment with 10 μM ODQ, a dose in excess of that required to inactivate the enzyme (Schrammel et al., 1996; Garthwaite et al., 1995), exerted no inhibitory effect on the response to NO (Fig. 3D,E). We repeated these experiments using 100 μM ODQ and again a clear response to NO was apparent (data not shown).
Fig. 3.
Mobilisation of stored Ca2+ by NO does not involve cGMP. (A) 100 μM 8-bromo cGMP causes rapid elevation of [Ca2+]i in human sperm. Responses of 6 cells shown. (B) Response to 8-bromo cGMP is greatly reduced and slowed in cells exposed to cGMP in low-Ca2+ saline. Responses of 5 cells shown. (C) Ca2+-dependence of the response to 100 μM 8-bromo cGMP. Light grey bars show responses of cells bathed in sEBSS (72 cells; 2 experiments), dark grey bars show cells bathed in low-Ca2+ sEBSS (122 cells; 3 experiments). (D) Pre-treatment with the sGC inhibitor ODQ (10 μM; white bar) does not inhibit the increase in [Ca2+]i induced by exposure to 100 μM spermine NONOate (arrow). Responses of 7 cells are shown. (E) Mean normalised increase in fluorescence 10 minutes after application of 100 μM spermine NONOate under control conditions (208 cells; 3 experiments) and after pre-treatment with 10 μM ODQ (267 cells; 3 experiments). Pre-treatment did not modify the amplitude of the response.
S-nitrosylglutathione (GSNO), an S-nitrosylating agent, mobilises Ca2+
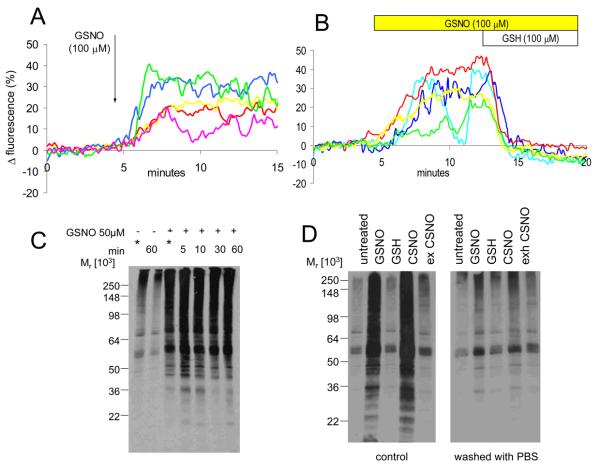
An alternative action of NO is directly to modify protein function by S-nitrosylation of specific target motifs, an action of NO that we recently detected in human sperm (Lefièvre et al. 2007). S-nitrosylglutathione (GSNO) a membrane-impermeant protein S-nitrosylating agent (Ji et al., 1999; Zaman et al., 2006) can act at intracellular targets, probably by generation of the membrane permeant product cys-NO (Zhang and Hogg, 2004). Upon application of 100 μM GSNO a significant elevation of [Ca2+]i occurred in 70±5% of sperm (n=7). This effect was rapid in comparison to the action of NO, reaching a plateau in approximately 3 minutes in most cells (Fig. 4A).
Fig. 4.
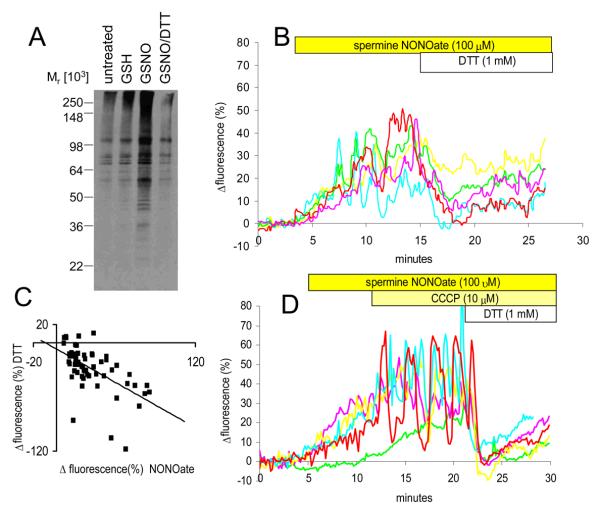
NO and protein S-nitrosylation in sperm. (A) 100 μM GSNO, a nitrosylating agent, causes a rise in [Ca2+]i similar to that seen with NONOate but onset of the effect is more rapid. Responses of 6 cells shown. (B) 100 μM GSH rapidly reverses the action of 100 μM GSNO on sperm [Ca2+]i. Responses of 5 cells shown. (C) GSNO causes rapid S-nitrosylation of sperm proteins: Lane 1 shows background levels in cells processed immediately for assay (indicated by *). Lane 2 shows that, after 60 minutes of incubation of the cells in sEBSS, this level does not change. Lanes 3, 4, 5, 6 and 7 show increased S-nitrosylation in cells processed for assay immediately upon exposure to 50 μM GSNO (*) and those incubated with GSNO for 5, 10, 30 and 60 minutes respectively. S-nitrosylation reaches near steady-state levels in the sample processed immediately (approximately 5 minutes for preliminary centrifugation; see methods). (D) S-nitrosylation of sperm proteins is rapidly reversible. Left panel shows S-nitrosylated proteins in untreated cells incubated for 10 minutes (lane 1), cells exposed to GSNO and cys-SNO (lanes 2 and 4) and cells exposed to GSH and exhausted cys-NO (lanes 3 and 5; controls). Right panel shows same treatments but cells were washed in PBS immediately before processing for the assay. S-nitrosylation caused by GSNO and CSNO is rapidly reversed upon removal of the agent.
Decomposition of GSNO can lead to release of NO. To test the possibility that GSNO was acting as an NO donor (rather than as an S-nitrosylating agent), we co-applied 100 μM glutathione (GSH). Under these conditions decomposition of GSNO (and generation of NO) is accelerated (Singh et al., 1996), but formation of membrane permeant product cys-NO, leading to direct S-nitrosylation of intracellular proteins is suppressed (Zhang and Hogg, 2004). When GSH was applied in the presence of GSNO there was a rapid fall in [Ca2+]i (Fig. 4B). We conclude that mobilisation of Ca2+ by GSNO is by a ‘direct’ action to nitrosylate target proteins in the sperm.
Kinetics of sperm protein S-nitrosylation parallel those of Ca2+ mobilisation
[Ca2+]i responses to GSNO were rapid (approximately 3 minutes to peak; Fig. 4A). Reversal of [Ca2+]i elevation in the presence of GSH was similarly rapid (Fig. 4B), as was reduction in [Ca2+]i upon washout of spermine NONOate (Fig. 2E). We therefore investigated the kinetics and reversibility of protein S-nitrosylation in sperm exposed to GSNO. When sperm were processed for the biotin switch assay immediately after exposure to 50 μM GSNO (≈5 minutes for preliminary centrifugation; see methods), S-nitrosylation was already at steady-state, further incubation (up to 60 minutes) having very little effect (Fig. 4C). Conversely, when cells incubated under S-nitrosylating conditions were washed in PBS, S-nitrosylation was immediately reversed (Fig. 4D).
Mobilisation of Ca2+ by NO and GSNO are reversed by DTT
Dithiothreitol (DTT) is a cell-permeant thiol-reducing agent that, even at low doses (1 mM) effectively reverses biological effects induced by protein S-nitrosylation (Stoyanovsky et al., 1997). After a 1 hour exposure of intact sperm to 100 μM GSNO, application of 1 mM DTT caused complete reversal of S-nitrosylation within 5 minutes (Fig. 5A). Similarly, when 1 mM DTT was applied to sperm 10-15 minutes after exposure to spermine NONOate (when mobilisation of Ca2+ by NO was well established) we observed a rapid fall in [Ca2+]i. Mean fluorescence (Rtot) fell to approximately 5% above control levels and some cells returned to levels recorded before application of NO (Fig. 5B). Similar effects were seen in 4 other experiments. The amplitude of the fall in fluorescence induced by DTT was correlated with that of the preceding NONOate-induced rise (Fig. 5C), consistent with an action of DTT to reverse the effect of exposure to NO. In most cells there was then a small increase in fluorescence of 5-10% over the following 10 minutes. A rapid reversal of the action of GSNO on [Ca2+]i also occurred upon application of 1 mM DTT (not shown).
Fig. 5.
Thiol reducing agents reverse NO effects. (A) DTT rapidly reverses nitrosylation of sperm proteins. Lane 1 shows endogenous S-nitrosylation in cells incubated in sEBSS for 60 minutes. Lanes 2 and 3 show cells incubated in the presence of 1 mM GSH (control) and 100 μM GSNO. Lane 4 shows cells incubated as for lane 3 but 1 mM DTT was added to the incubation 5 minutes before processing for the assay. (B) DTT reverses the action of 100 μM spermine NONOate. Upon application of 1 mM DTT, the increase in fluorescence induced by spermine NONOate is rapidly reduced or completely reversed. Responses of 5 separate cells shown. (C) DTT induced-decrease in fluorescence is correlated with the preceding NONOate-stimulated increase in fluorescence. Scattergram shows data from a single experiment, representative of 5 repeats. R2=0.33. (D) Action of DTT is not due to e−-dependent mitochondrial Ca2+ accumulation. After application of 100 μM spermine NONOate to mobilise Ca2+, the cells were exposed to 10 μM CCCP to collapse the mitochondrial inner membrane potential. The effect of subsequent application of 1 mM DTT resembled that seen in cells with functioning mitochondria. Responses of 5 cells shown.
Oxidation of thiols can cause Ca2+ mobilisation from mitochondria. This effect is reversed by DTT (Halestrap et al., 1997; Pariente et al., 2001; McStay et al., 2002) and might thus contribute to the observed [Ca2+]i responses. We therefore exposed NO-treated cells to 10 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) prior to DTT exposure to collapse the mitochondrial inner membrane potential and mobilise mitochondrial Ca2+ (Konji et al., 1985). In most cells there was a visible increase in [Ca2+]i upon CCCP application, consistent with participation of mitochondria in [Ca2+]i buffering (Wennemuth et al., 2003) and many of the cells then showed [Ca2+]i oscillations (Fig. 5D; red and blue traces). When 1 mM DTT was then applied to these cells, recovery of [Ca2+]i occurred as before, despite the inability of uncoupled mitochondria to accumulate Ca2+ (Fig. 5D).
The efficacy of DTT to reduce [Ca2+]i was such that we investigated whether an effect could be seen in cells not previously exposed to NO. In most cells (63±7%, n=3) this was the case, but the amplitude of this effect was small (≈ 5%). In most of these cells there was then a slight recovery of (≈2%) over the following 5 minutes (not shown).
Incubation of sperm with human oviduct explants causes protein S-nitrosylation.
To determine whether NO production by tissues encountered by the sperm is sufficient to induce protein S-nitrosylation, we incubated sperm with human oviduct explants. Sperm retrieved from these incubations and processed for labelling of S-nitrosothiols (Lefievre et al, 2007) showed levels of labelling equivalent in intensity and distribution to that induced by parallel incubation with 100 μM GSNO and slightly greater than that seen with 100 μM NONOate (Fig. 6). Oviduct showed higher levels of sperm S-nitrosylation (labelling with MTSEA) than that seen with endometrium.
Fig. 6.
NO production by female tract cells induces S-nitrosylation in human sperm. S-nitrosylated proteins were identified using fluorescently-tagged methanethiosulfonate, as described in the text. Negligible levels of labelling were present in controls but treatment with 100 μM spermine NONOate or GSNO caused clear labelling, particularly at the back of the sperm head. Incubation of sperm with primary cultures derived from endometrial of tubal explants (ampulla and isthmus) induced levels of S-nitrosyaltion at least as great as those seen with NONOate.
Interaction of the Ca2+-mobilising effects of NO and progesterone
We have shown previously that progesterone cyclically mobilises Ca2+ stored in a membranous compartment in the sperm neck/midpiece region (Harper et al., 2004; Harper & Publicover, 2005; Bedu-Addo et al., 2007), an effect that involves activation of ryanodine receptors (RyRs: Harper et al., 2004; reviewed in Harper & Publicover, 2005). RyRs are known to be positively regulated by S-nitrosylation (Stoyanovsky et al., 1997; Meissner, 2004) and RyR2 was identified in the nitrosoproteome of human sperm (Lefièvre et al., 2007). Since the action of NO on sperm [Ca2+]i is by S-nitrosylation (leading to mobilisation of stored Ca2+), interaction or synergism between the effects of these two agents, both of which will be encountered by sperm approaching the oocyte, might be anticipated.
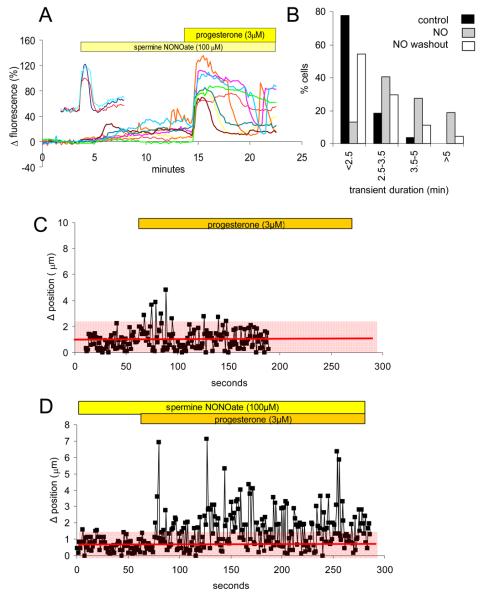
In control experiments the [Ca2+]i transient induced by 3 μM progesterone was typically of 2-2.5 minutes duration (Kirkman-Brown et al., 2000; Fig. 7A – insert). When cells were pre-treated with 100 μM spermine NONOate (10 minutes) then exposed to progesterone in the continued presence of the NO donor, this response were clearly altered. Though 2-3 minute transients were still observed (Fig. 7A; brown and yellow traces), the majority of cells showed a [Ca2+]i plateau (Fig. 7A; red and green traces) or a normal peak with a pronounced ‘shoulder’ (Fig. 7A; orange, pink and blue traces). Analysis of transient duration (from start of rise to inflexion at end of falling phase) showed that after exposure to NONOate prolonged responses became much more common (Fig. 7B). In 3 pairs of experiments (control and NO treated cells from the same sample; see Fig. 7B), pre-treatment with NONOate increased the proportion of responses of ≥2.5 minutes from 42±8% to 92±3% (P<0.025, paired-t). To investigate whether the effect of pre-treatment with NONOate would persist in the absence of the NO donor, we carried out parallel experiments in which NONOate was washed off simultaneously with the introduction of 3 μM progesterone. In these experiments responses to progesterone resembled those of non-pretreated cells (Fig. 7B), the proportion of transients ≥2.5 min in two experiments being 40% and 45% (NS compared to parallel experiments without pretreatment; paired t).
Fig. 7.
Pre-treatment with 100 μM spermine NONOate potentiates responses of sperm to 3 μM progesterone. (A) When sperm were exposed to 3 μM progesterone after pre-treatment with spermine NONOate (100 μM for 10 minutes), the initial [Ca2+]i transient was enlarged (in some cells) and significantly prolonged compared to that seen in control cells (insert: 3 single cell responses, scales as for main plot). Responses of 8 cells shown. (B) Co-stimulation with spermine NONOate increases the proportion of cells in which a prolonged [Ca2+]i transient occurs in response to stimulation with 3 μM progesterone. Data are plotted as percentage of cells in each class (defined by [Ca2+]i transient duration). Control cells (black bars; n=27) were from the same sample as cells exposed to NO before and during progesterone stimulation (grey bars; n=69) and cells in which NO was washed off as progesterone was applied (white bars; 44 cells). (C) Progesterone (3 μM) causes a brief increase in flagellar displacement. Red line and shading show the mean ± 2 s.d. of frame-to-frame midpiece displacement during the control period. Graph shows response of 1 cell (representative of > 100 cells in 2 experiments). (D) Pre-treatment with spermine NONOate (100 μM) prolonged and intensified the effect of progesterone on flagellar activity. Red line and shading show the mean ± 2 s.d. of frame-to-frame midpiece displacement during the control period. The graph shows response of 1 sperm cell (representative of > 100 cells in 2 experiments).
Synergism of NO and progesterone in regulating flagellar beat mode
Mobilisation of Ca2+ stored in the neck/midpiece region of human sperm by progesterone or by 4-aminopyridine causes an increase in midpiece bending and flagellar displacement, which is clearly visible in loosely tethered cells (Harper et al., 2004; Bedu-Addo et al., 2007; Bedu-Addo et al., 2008). We therefore imaged cells under phase contrast (1 Hz acquisition rate) to assess the midpiece (and thus flagellar) displacement. In ≈70% of cells exposed to 3 μM progesterone there was a brief (30-50 seconds) increase in frame-to-frame flagellar displacement (Fig. 7C; representative cell from >150 cells in 2 experiments), consistent with increased flagellar activity during the [Ca2+]i transient (Fig. 7A insert). When cells were treated with 100 μM spermine NONOate for 10 minutes there was no significant effect on flagellar beat mode. Subsequent application of 3 μM progesterone (in the continued presence of the NO donor) did not alter the proportion of cells showing a response (≈80%), but the enhancement of flagellar activity (measured as an increase in frame-to-frame midpiece displacement) was maintained for the duration of recording (≈4 minutes), including a series of peaks (Fig. 7D; representative cell from >100 cells in 2 experiments). The kinetics of this increase in midpiece displacement were consistent with those of the enhanced [Ca2+]i response to progesterone seen in sperm pre-treated with NONOate (Fig.7A,B). Supplementary movies show examples of cells responding to progesterone in the presence and absence of 100 μM spermine NONOate.
Discussion
Application of spermine NONOate to capacitated human sperm induced a clear rise in [Ca2+]i in the neck/midpiece of most cells (Fig. 2), leading to modulation of flagellar activity (Fig. 7; see below). Elevation of [Ca2+]i was not sensitive to omission of Ca2+ from the medium (≈1000-fold reduction in [Ca2+]o; Fig. 2B,D) so this effect reflects mobilisation of stored Ca2+ by NO.
The primary actions of NO in target tissues are (i) activation of soluble guanylate cyclase (sGC), leading to a rise in [cGMP] and actions mediated through PKG or through direct action on cyclic nucleotide-gated channels and (ii) direct modulation of protein function by S-nitrosylation of exposed cysteine residues (Davis et al., 2001; Ahern et al., 2002). It has been suggested that NO acts as a chemoattractant for human sperm, acting through stimulation of sGC (Miraglia et al., 2007) and when we exposed human sperm to cGMP we observed a sustained rise in [Ca2+]i not dissimilar to that seen with NO. However, this effect was clearly dependent on [Ca2+]o, consistent with generation by Ca2+ influx, not store mobilisation (Fig. 3A-C). Furthermore, saturating doses of ODQ, an effective inhibitor of sGC, did not modify the response to NO (Fig. 3D-E). In contrast, induction of AR by NO was blocked by the same drug (W. C. Ford, unpublished data). We have shown recently that NO, at the concentrations used here, causes S-nitrosylation of a number of sperm proteins (Lefièvre et al., 2007), suggesting that this alternative effect of NO could underlie our observations. Consistent with this interpretation we found that:
i) GSNO, an S-nitrosylating agent but very poor generator of NO (Jarboe et al., 2008) acted similarly to NO but more rapidly (Fig. 4A).
ii) Assessment of sperm protein S-nitrosylation by the biotin switch method (Foster and Stamler; 2004; Lefievre et al., 2007) showed that, like the effect of GSNO on [Ca2+]i, GSNO-induced S-nitrosylation was rapid, occurring in ≤5 minutes (the minimum period that could be assessed using the assay; Fig. 4C) and was immediately reversed by washout of GSNO or co-application of 1 mM DTT (Fig. 5A).
iii) The effect of GSNO on [Ca2+]i was largely reversed by co-application of GSH (Fig. 4B). GSH enhances breakdown of GSNO to liberate NO (Singh et al., 1996) but will inhibit generation of membrane permeant cys-NO, which is required for protein S-nitrosylation by GSNO in intact cells (Zhang and Hogg, 2004)
iv) The action of NO was rapidly reversed by 1 mM DTT (Fig, 5B). Of particular significance here is the relationship between the increase in OGB fluorescence caused by NO and the subsequent fall upon application of DTT. The characteristics of non-ratiometric fluorescence [Ca2+] measurements are such that, as [Ca2+]i increases, the change in fluorescence for a given increment in [Ca2+] is reduced (Fig. S5A). The observed negative correlation (Fig. 5C) is consistent with reversal of the action of NO by DTT (Fig. S5D), whereas action of DTT to reduce [Ca2+]i by a mechanism unrelated to the preceding effect of NO would lead to a positive correlation (Fig. S5E).
We conclude that DTT reverses the action of NO and that NO-induced mobilisation of stored Ca2+ reflects direct modulation of protein function by S-nitrosylation. It is of interest that, when NONOate was washed off and reintroduced after 5-10 min the effect of NO was apparently enhanced, particularly the generation of [Ca2+]i oscillations (Fig 2E). Protein S-nitrosylation reverses rapidly upon washout of NONOate (Fig. 4D) so this persistence of effect may reflect increased Ca2+ leak at the plasmalemma (and consequent filling of the store), perhaps due to increased [cGMP].
Progesterone mobilises Ca2+ stored in the neck/midpiece of human sperm, by a mechanism involving activation of RyRs, leading to [Ca2+]i oscillations strikingly similar to those described here (Harper et al., 2004; Fig. 2A). RyRs are localised to the neck/midpiece (Harper et al,, 2004; Lefiévre et al. in prep) and we have shown recently that RyR2 is a target for S-nitrosylation in human sperm (Lefiévre et al., 2007). Since S-nitrosylation (or S-oxidation by HNO) of RyRs increases open probability and mobilises microsomal Ca2+ (Stoyanovsky et al., 1997; Cheong et al., 2005), we suggest that an action on these receptors is the most likely cause of the Ca2+-mobilising abilities of NO and GSNO in human sperm. Consistent with convergence of the actions of progesterone and NO, pre-treatment of cells with spermine NONOate prolonged significantly the [Ca2+]i transient induced by 3 μM progesterone (Fig. 7A,B). This effect was dependent upon the continued presence of NO, no synergism being observed when the NO donor was washed off simultaneously with introduction of progesterone. In effect this means that the actions of NO are reversed within 2.5 minutes (duration of the ‘control’ action of progesterone), consistent with the rapid reversibility of protein S-nitrosylation in sperm.
Though potential sources of NO are present throughout the female tract, it is probable that NO encountered by sperm in the fallopian tube and upon approaching and entering the cumulus oophorus provides a particularly potent stimulus (Rosselli et al., 1996; Ekerhovd et al., 1999; Hattori et al., 2001; Reyes et al., 2004; Tao et al., 2004; Lapointe et al,, 2006). Human cumulus samples expressed constitutive forms of NOS (as did COV434 human granulosa cells) and all three NOS isoforms were present in the oviduct, (Figs. 1, 6, S1, S2). Co-incubation of human sperm with human oviductal explants was at least as effective in inducing S-nitrosylation as was exposure of sperm to spermine NONOate or GSNO (Fig.6). Thus the reversible NO-induced mobilisation of Ca2+ stored in the neck region of the sperm, that we describe here, can occur in vivo. The recent observation that NO induces chemotaxis (Miraglia et al, 2007), though of great interest, is most unlikely to relate to our findings. The effect was at a dose of GSNO 500-1000x lower than that used here. Chemotactic effects are highly concentration specific, being lost when the concentration of the attractant is increased above the effective dose. Furthermore, chemotaxis was exerted through activation of sGC (mimicked by cGMP and sensitive to ODQ). The effect described here is seen with 50-100 uM GSNO (and 100 uM NONOate) and is exerted through protein S-nitrosylation (not mimicked by cGMP, insensitive to ODQ).
Our observation that progesterone, which is also present in the female tract and is synthesised by cells of the cumulus (Chian et al., 1999; Mingoti et al., 2002; Yamashita et al., 2003), can act synergistically with NO to mobilise Ca2+ is intriguing. Progesterone has been reported to have a weak hyperactivating effect on human sperm (Uhler et al., 1992; Yang et al., 1994; Jaiswal et al., 1999). In the presence of NO this effect might be expected to be enhanced, reflecting the increased duration of [Ca2+]i elevation that occurs under these circumstances. Examination of cells exposed to progesterone in the presence of NO confirmed that, though 100 μM spermine NONOate alone had little effect on activity of the flagellum, the transient action of progesterone on flagellar beating was transformed into a prolonged enhancement characterised by increased excursion of the midpiece (Fig. 7D). We propose that, within the oviduct, the synergistic actions of NO (by S-nitrosylation) and progesterone to mobilise stored Ca2+ in the sperm neck/midpiece (probably by activation of RyRs) will modulate flagellar activity, particularly bending in the midpiece (Bedu-Addo et al., 2008), contributing to the hyperactivation that is vital for penetration of the egg vestments.
Supplementary Material
Stimulation of flagellar activity by progesterone in the presence of NO•. Movie shows 4 cells imaged in the presence of 100 μM spermine NONOate (added approximately 8 minutes previously). Cells were imaged at 1 Hz and total length of recording is 5.1 minutes. Yellow marker (top left) shows point at which progesterone (3 μM) was added (approximately 1 minute into recording). After progesterone application the excursion of the flagellum increases, resulting in noticeable movement of the head in some of the cells. This increase in flagellar activity is prolonged (see fig 6 D), consistent with the prolonged/oscillatory effect of progesterone on [Ca2+]i under these conditions (fig 6A, B).
Stimulation of flagellar activity by progesterone without pretreatment with NO. Movie shows 5 cells, imaged at 1 Hz and total length of recording is 8.33 minutes. Yellow marker (top left) shows point at which progesterone (3 μM) was added. After progesterone application the excursion of the flagellum increases in some transiently in some cells, but the amplitude and (more particularly) the duration of this effect are brief compared to that seen in the presence of 100 μM NONOate.
Acknowledgments
Our thanks to Dr JT Hancock, University of the West of England at Bristol, for his great help with obtaining images of DAF-stained mouse oocytes, Dr Fleur Moseley for preparation of human cumulus and Clinical Oncology, LUMC, the Netherlands for kindly supplying COV434 cells. We thank the donors who contributed to our work and the staff at the Assisted Conception Unit (ACU), Birmingham Women's Hospital, for their help. This work was supported by The Wellcome Trust (078905). GM-O was supported by Fundação para a Cência e Tecnologia (FCT) Portugal (SFRH/BD/17780/2004) and TJC was supported by the Infertility Research Trust, UK.
References
- Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends. Neurosci. 2002;25:510–517. doi: 10.1016/s0166-2236(02)02254-3. [DOI] [PubMed] [Google Scholar]
- Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. USA. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedu-Addo K, Barratt CL, Kirkman-Brown JC, Publicover SJ. Patterns of [Ca2+](i) mobilization and cell response in human spermatozoa exposed to progesterone. Dev. Biol. 2007;302:324–332. doi: 10.1016/j.ydbio.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Bedu-Addo K, Costello S, Harper C, Machado-Oliveira G, Lefièvre L, Ford C, Barratt C, Publicover S. Mobilisation of stored Ca2+ in the neck region of human sperm – a mechanism for regulation of flagellar activity. Int. J. Dev. Biol. 2008 doi: 10.1387/ijdb.072535kb. in press. [DOI] [PubMed] [Google Scholar]
- Braughler JM, Mittal CK, Murad F. Effects of thiols, sugars, and proteins on nitric oxide activation of guanylate cyclase. J. Biol. Chem. 1979;254:12450–12454. [PubMed] [Google Scholar]
- Calabrese EJ. Nitric oxide: biphasic dose responses. Crit. Rev. Toxicol. 2001;31:489–501. doi: 10.1080/20014091111776. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Cheong E, Tumbev V, Abramson J, Salama G, Stoyanovsky DA. Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium. 2005;37:87–96. doi: 10.1016/j.ceca.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Chian RC, Ao A, Clarke HJ, Tulandi T, Tan SL. Production of steroids from human cumulus cells treated with different concentrations of gonadotropins during culture in vitro. Fertil. Steril. 1999;71:61–66. doi: 10.1016/s0015-0282(98)00416-6. [DOI] [PubMed] [Google Scholar]
- Creech MM, Arnold EV, Boyle B, Muzinich MC, Montville C, Bohle DS, Atherton RW. Sperm motility enhancement by nitric oxide produced by the oocytes of fathead minnows, Pimephelas promelas. J. Androl. 1998;19:667–674. [PubMed] [Google Scholar]
- Darszon A, Treviño CL, Wood C, Galindo B, Rodráguez-Miranda E, Acevedo JJ, Hernandez-González EO, Beltrán C, Martínez-López P, Nishigaki T. Ion channels in sperm motility and capacitation. Soc. Reprod. Fertil. Suppl. 2007;65:229–244. [PubMed] [Google Scholar]
- Davis KL, Martin E, Turko IV, Murad F. Novel effects of nitric oxide. Annu. Rev. Pharmacol. Toxicol. 2001;41:203–236. doi: 10.1146/annurev.pharmtox.41.1.203. [DOI] [PubMed] [Google Scholar]
- Foster MW, Stamler JS. New insights into protein S-nitrosylation. Mitochondria as a model system. J. Biol. Chem. 2004;279:25891–25897. doi: 10.1074/jbc.M313853200. [DOI] [PubMed] [Google Scholar]
- Ekerhovd E, Brännström M, WeijdegÅrd B, Norström A. Localization of nitric oxide synthase and effects of nitric oxide donors on the human Fallopian tube. Mol Hum Reprod. 1999;5:1040–1047. doi: 10.1093/molehr/5.11.1040. [DOI] [PubMed] [Google Scholar]
- Funahashi H. Induction of capacitation and the acrosome reaction of boar spermatozoa by L-arginine and nitric oxide synthesis associated with the anion transport system. Reproduction. 2002;124:857–864. [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide sensitive-guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J. Biol. Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- Harper CV, Publicover SJ. Reassessing the role of progesterone in fertilization--compartmentalized calcium signalling in human spermatozoa? Hum. Reprod. 2005;20:2675–2680. doi: 10.1093/humrep/dei158. [DOI] [PubMed] [Google Scholar]
- Harper CV, Barratt CL, Publicover SJ. Stimulation of human spermatozoa with progesterone gradients to stimulate approach to the oocyte. Induction of [Ca(2+)](i) oscillations and cyclical transitions in flagellar beating. J. Biol. Chem. 2004;279:46315–46325. doi: 10.1074/jbc.M401194200. [DOI] [PubMed] [Google Scholar]
- Harper C, Wootton L, Michelangeli F, Lefièvre L, Barratt C, Publicover S. Secretory pathway Ca(2+)-ATPase (SPCA1) Ca(2+) pumps, not SERCAs, regulate complex [Ca(2+)](i) signals in human spermatozoa. J. Cell Sci. 2005;118:1673–1685. doi: 10.1242/jcs.02297. [DOI] [PubMed] [Google Scholar]
- Hattori MA, Takesue K, Kato Y, Fugihara N. Expression of endothelial nitric oxide synthase in the porcine oocyte and its possible function. Mol. Cell Biochem. 2001;219:121–126. doi: 10.1023/a:1010830507846. [DOI] [PubMed] [Google Scholar]
- Herrero MB, Cebral E, Boquet M, Viggiano JM, Vitullo A, Gimeno MA. Effect of nitric oxide on mouse sperm hyperactivation. Acta Physiol. Pharmacol. Ther. Latinoam. 1994;44:65–69. [PubMed] [Google Scholar]
- Herrero MB, Cebral E, Franchi A, Motta A, Gimeno MF. Progesterone enhances prostaglandin E2 production via interaction with nitric oxide in the mouse acrosome reaction. Biochem. Biophys. Res. Commun. 1998;252:324–328. doi: 10.1006/bbrc.1998.9638. [DOI] [PubMed] [Google Scholar]
- Herrero MB, de Lamirande E, Gagnon C. Nitric oxide is a signaling molecule in spermatozoa. Curr. Pharm. Des. 2003;9:419–25. doi: 10.2174/1381612033391720. [DOI] [PubMed] [Google Scholar]
- Jaiswal BS, Tur-Kaspa I, Dor J, Mashiach S, Eisenbach M. Human sperm chemotaxis: is progesterone a chemoattractant? Biol. Reprod. 1999;60:1314–1319. doi: 10.1095/biolreprod60.6.1314. [DOI] [PubMed] [Google Scholar]
- Jarboe LR, Hyduke DR, Tran LM, Chou KJ, Liao JC. Determination of the Escherichia coli S-Nitrosoglutathione Response Network Using Integrated Biochemical and Systems Analysis. J. Biol. Chem. 2008;283:5148–5157. doi: 10.1074/jbc.M706018200. [DOI] [PubMed] [Google Scholar]
- Ji Y, Akerboom TP, Sies H, Thomas JA. S-nitrosylation and S-glutathiolation of protein sulfhydryls by S-nitroso glutathione. Arch. Biochem. Biophys. 1999;362:67–78. doi: 10.1006/abbi.1998.1013. [DOI] [PubMed] [Google Scholar]
- Joo BS, Park SH, Park SJ, Kang HS, Moon HS, Kim HD. The effect of nitric oxide on sperm cell function and embryo development. Am. J. Reprod. Immunol. 1999;42:327–334. doi: 10.1111/j.1600-0897.1999.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Kim BH, Kim CH, Jung KY, Jeon BH, Ju EJ, Choo YK. Involvement of nitric oxide during in vitro fertilization and early embryonic development in mice. Arch. Pharm. Res. 2004;27:86–93. doi: 10.1007/BF02980052. [DOI] [PubMed] [Google Scholar]
- Kirkman-Brown JC, Bray C, Stewart PM, Barratt CL, Publicover SJ. Biphasic elevation of [Ca(2+)](i) in individual human spermatozoa exposed to progesterone. Dev. Biol. 2000;222:326–335. doi: 10.1006/dbio.2000.9729. [DOI] [PubMed] [Google Scholar]
- Kirkman-Brown JC, Barratt CL, Publicover SJ. Slow calcium oscillations in human spermatozoa. Biochem. J. 2004;378:827–832. doi: 10.1042/BJ20031368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konji V, Montag A, Sandri G, Nordenbrand K, Ernster L. Transport of Ca2+ and Mn2+ by mitochondria from rat liver, heart and brain. Biochimie. 1985;67:1241–1250. doi: 10.1016/s0300-9084(85)80133-4. [DOI] [PubMed] [Google Scholar]
- Lancaster JR., Jr. A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide. 1997;1:18–30. doi: 10.1006/niox.1996.0112. [DOI] [PubMed] [Google Scholar]
- Lapointe J, Roy M, St-Pierre I, Kimmins S, Gauvreau D1, MacLaren LA, Bilodeau JF. Hormonal and spatial regulation of nitric oxide synthases (NOS) (neuronal NOS, inducible NOS, and endothelial NOS) in the oviducts. Endocrinology. 2006;147:5600–5610. doi: 10.1210/en.2005-1548. [DOI] [PubMed] [Google Scholar]
- Lefièvre L, Chen Y, Conner SJ, Scott JL, Publicover SJ, Ford WC, Barratt CL. Human spermatozoa contain multiple targets for protein S-nitrosylation: an alternative mechanism of the modulation of sperm function by nitric oxide? Proteomics. 2007;7:3066–3084. doi: 10.1002/pmic.200700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Moore PK. An overview of the biological significance of endogenous gases: new roles for old molecules. Biochem. Soc. Trans. 2007;35:1138–1141. doi: 10.1042/BST0351138. [DOI] [PubMed] [Google Scholar]
- McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem. J. 2002;367:541–548. doi: 10.1042/BJ20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium. 2004;35:621–628. doi: 10.1016/j.ceca.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Miki N, Kawabe Y, Kuriyama K. Activation of cerebral guanylate cyclase by nitric oxide. Biochem. Biophys. Res. Commun. 1977;75:851–856. doi: 10.1016/0006-291x(77)91460-7. [DOI] [PubMed] [Google Scholar]
- Mingoti GZ, Garcia JM, Rosa-e-Silva AA. Steroidogenesis in cumulus cells of bovine cumulus-oocyte-complexes matured in vitro with BSA and different concentrations of steroids. Anim. Reprod. Sci. 2002;69:175–186. doi: 10.1016/s0378-4320(01)00187-7. [DOI] [PubMed] [Google Scholar]
- Miraglia E, Rullo ML, Bosia A, Massobrio M, Revelli A, Ghigo D. Stimulation of the nitric oxide/cyclic guanosine monophosphate signaling pathway elicits human sperm chemotaxis in vitro. Fertil. Steril. 2007;87:1059–1063. doi: 10.1016/j.fertnstert.2006.07.1540. [DOI] [PubMed] [Google Scholar]
- O'Flaherty C, Rodriguez P, Srivastava S. L-arginine promotes capacitation and acrosome reaction in cryopreserved bovine spermatozoa. Biochim. Biophys. Acta. 2004;1674:215–221. doi: 10.1016/j.bbagen.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Pariente JA, Camello C, Camello PJ, Salido GM. Release of calcium from mitochondrial and nonmitochondrial intracellular stores in mouse pancreatic acinar cells by hydrogen peroxide. J. Membr. Biol. 2001;179:27–35. doi: 10.1007/s002320010034. [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm--making the most of what you've got. Nat. Cell Biol. 2007;9:235–242. doi: 10.1038/ncb0307-235. [DOI] [PubMed] [Google Scholar]
- Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, Losano G. Nitric oxide and cardiac function. Life Sci. 2007;81:779–793. doi: 10.1016/j.lfs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Revelli A, Costamagna C, Moffa F, Aldieri E, Ochetti S, Bosia A, Massobrio M, Lindblom B, Ghigo D. Signaling pathway of nitric oxide-induced acrosome reaction in human spermatozoa. Biol. Reprod. 2001;64:1708–1712. doi: 10.1095/biolreprod64.6.1708. [DOI] [PubMed] [Google Scholar]
- Reyes R, Vásquez ML, Delgado NM. Detection and bioimaging of nitric oxide in bovine oocytes and sperm cells. Arch. Androl. 2004;50:303–309. doi: 10.1080/01485010490448471. [DOI] [PubMed] [Google Scholar]
- Rosselli M, Dubey RK, Rosselli MA, Macas E, Fink D, Lauper U, Keller PJ, Imthurn B. Identification of nitric oxide synthase in human and bovine oviduct. Mol Hum Reprod. 1996;2:607–612. doi: 10.1093/molehr/2.8.607. [DOI] [PubMed] [Google Scholar]
- Rosselli M, Keller PJ, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum. Reprod. Update. 1998;4:3–24. doi: 10.1093/humupd/4.1.3. [DOI] [PubMed] [Google Scholar]
- Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- Singh SP, Wishnok JS, Keshive M, Deen WM, Tannenbaum SR. The chemistry of the S-nitrosoglutathione/glutathione system. Proc. Natl. Acad. Sci. USA. 1996;93:14428–14433. doi: 10.1073/pnas.93.25.14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanovsky D, Murphy T, Anno PR, Kim YM, Salama G. Nitric oxide activates skeletal and cardiac ryanodine receptors. Cell Calcium. 1997;21:19–29. doi: 10.1016/s0143-4160(97)90093-2. [DOI] [PubMed] [Google Scholar]
- Tao Y, Fu Z, Zhang M, Xia G, Yang J, Xie H. Immunohistochemical localization of inducible and endothelial nitric oxide synthase in porcine ovaries and effects of NO on antrum formation and oocyte meiotic maturation. Mol. Cell Endocrinol. 2004;222:93–103. doi: 10.1016/j.mce.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Thaler CD, Epel D. Nitric oxide in oocyte maturation, ovulation, fertilization, cleavage and implantation: a little dab'll do ya. Curr. Pharm. Des. 2003;9:399–409. doi: 10.2174/1381612033391748. [DOI] [PubMed] [Google Scholar]
- Uhler ML, Leung A, Chan SY, Wang C. Direct effects of progesterone and antiprogesterone on human sperm hyperactivated motility and acrosome reaction. Fertil. Steril. 1992;58:1191–1198. [PubMed] [Google Scholar]
- Weinberg JB, Doty E, Bonaventura J, Haney AF. Nitric oxide inhibition of human sperm motility. Fertil. Steril. 1995;64:408–413. doi: 10.1016/s0015-0282(16)57743-7. [DOI] [PubMed] [Google Scholar]
- Wennemuth G, Babcock DF, Hille B. Calcium clearance mechanisms of mouse sperm. J. Gen. Physiol. 2003;122:115–128. doi: 10.1085/jgp.200308839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . “World Health Organisation Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction”. 4th ed. Cambridge Univ. Press; Cambridge, UK: 1999. [Google Scholar]
- Wu TP, Huang BM, Tsai HC, Lui MC, Liu MY. Effects of nitric oxide on human spermatozoa activity, fertilization and mouse embryonic development. Arch. Androl. 2004;50:173–179. doi: 10.1080/01485010490425494. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Shimada M, Okazaki T, Maeda T, Terada T. Production of progesterone from de novo-synthesized cholesterol in cumulus cells and its physiological role during meiotic resumption of porcine oocytes. Biol. Reprod. 2003;68:1193–1198. doi: 10.1095/biolreprod.102.010934. [DOI] [PubMed] [Google Scholar]
- Yang J, Serres C, Philibert D, Robel P, Baulieu EE, Jouannet P. Progesterone and RU486: opposing effects on human sperm. Proc. Natl. Acad. Sci. USA. 1994;91:529–533. doi: 10.1073/pnas.91.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Loscalzo J. S-nitrosoprotein formation and localization in endothelial cells. Proc. Natl. Acad. Sci. USA. 2005;102:117–122. doi: 10.1073/pnas.0405989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman K, Carraro S, Doherty J, Henderson EM, Lendermon E, Liu L, Verghese G, Zigler M, Ross M, Park E, et al. S-nitrosylating agents: a novel class of compounds that increase cystic fibrosis transmembrane conductance regulator expression and maturation in epithelial cells. Mol. Pharmacol. 2006;70:1435–1442. doi: 10.1124/mol.106.023242. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zheng RL. Possible role of nitric oxide on fertile and asthenozoospermic infertile human sperm functions. Free Radic. Res. 1996;25:347–354. doi: 10.3109/10715769609149057. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc. Natl. Acad. Sci. USA. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vollmer M, De Geyter M, Litzistorf Y, Ladewig A, Durrenberger M, Guggenheim R, Miny P, Holzgreve W, De Geyter C. Characterization of an immortalized human granulosa cell line (COV434) Mol. Hum. Reprod. 2000;6:146–153. doi: 10.1093/molehr/6.2.146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stimulation of flagellar activity by progesterone in the presence of NO•. Movie shows 4 cells imaged in the presence of 100 μM spermine NONOate (added approximately 8 minutes previously). Cells were imaged at 1 Hz and total length of recording is 5.1 minutes. Yellow marker (top left) shows point at which progesterone (3 μM) was added (approximately 1 minute into recording). After progesterone application the excursion of the flagellum increases, resulting in noticeable movement of the head in some of the cells. This increase in flagellar activity is prolonged (see fig 6 D), consistent with the prolonged/oscillatory effect of progesterone on [Ca2+]i under these conditions (fig 6A, B).
Stimulation of flagellar activity by progesterone without pretreatment with NO. Movie shows 5 cells, imaged at 1 Hz and total length of recording is 8.33 minutes. Yellow marker (top left) shows point at which progesterone (3 μM) was added. After progesterone application the excursion of the flagellum increases in some transiently in some cells, but the amplitude and (more particularly) the duration of this effect are brief compared to that seen in the presence of 100 μM NONOate.