Abstract
Elucidating the structures of membrane proteins is essential to our understanding of disease states and a critical component in the rational design of drugs. Structural characterization of a membrane protein begins with its detergent solubilization from the lipid bilayer and its purification within a functionally stable protein-detergent complex (PDC). Crystallization of the PDC typically occurs by changing the solution environment to decrease solubility and promote interactions between exposed hydrophilic surface residues. As membrane proteins have been observed to form crystals close to the phase separation boundaries of the detergent used to form the PDC, knowledge of these boundaries under different chemical conditions provides a foundation to rationally design crystallization screens. We have carried out dye-based detergent phase partitioning studies using different combinations of 10 polyethylene glycols (PEG), 11 salts, and 11 detergents to generate a significant amount of chemically diverse phase boundary data. The resulting curves were used to guide the formulation of a 1536-cocktail crystallization screen for membrane proteins. We are making both the experimentally derived phase boundary data and the 1536 membrane screen available through the high-throughput crystallization facility located at the Hauptman-Woodward Institute. The phase boundary data have been packaged into an interactive Excel spreadsheet that allows investigators to formulate grid screens near a given phase boundary for a particular detergent. The 1536 membrane screen has been applied to 12 membrane proteins of unknown structures supplied by the structural genomics and structural biology communities, with crystallization leads for 10/12 samples and verification of one crystal using X-ray diffraction.
Keywords: crystallization, high-throughput, microbatch, membrane proteins, detergent
Introduction
Membrane proteins carry out a staggering range of fundamentally important biological functions, which include the processes of signal and energy transduction, molecular transport, detoxification, and catalysis. It is estimated that more than 60% of all currently available drugs target membrane proteins.1 As such, the structural elucidation of membrane proteins at atomic resolution is a vital component in efforts to further understand the underlying biological processes behind many disease states and to provide a rational basis for the design of new and more effective drugs. Although known genomes devote upward of 30% of their genes to encode for membrane proteins,2 these proteins are significantly underrepresented in the Protein Data Bank when compared with that of soluble proteins.3,4 This observed lack of coverage is primarily due to inherent difficulties associated with the overproduction and solubilization of the target protein from the lipid bilayer using detergents such that the protein maintains its native conformation and activity for subsequent structural characterization (reviewed in Refs.5–8).
X-ray crystallography is currently the method of choice for the high-resolution structure determination of membrane proteins. Once a membrane protein is solubilized from the lipid bilayer, it exists as a water-soluble protein-detergent complex (PDC), which is the entity that one aims to crystallize. Early attempts at membrane protein crystallization focused on generating a PDC that was homogeneous and monodisperse, which followed the logic used for the crystallization of soluble proteins.9,10 Nonionic detergents were used to produce a PDC that was small, spherical in shape, and free of natural lipid extracted from the originating membrane.11,12 It was soon realized that detergent-dependent phase transitions significantly influenced crystallization attempts, with a poor choice of detergent causing unwanted detergent phase separation and protein denaturation.13–15 Indeed, commercially available crystallization screens, developed for soluble proteins, are generally not successful crystallization reagents for membrane proteins. These crystallization reagents were designed without taking into consideration the phase behavior of the detergent associated with the PDC.16,17 Therefore, it is predicable that many of these crystallization reagents, when utilized with a PDC, lead to the formation of drops containing phase-separated detergent and denatured protein. It was noted that crystals of membrane proteins often occurred at conditions at or near the phase boundary of the detergent component of the PDC.11,16,18–20 These early observations have led to further study of the relationships that exist between the detergent-dependent phase behavior, exhibited by the PDC during crystal growth, and the major variables involved in this process (detergent, salt, precipitant, pH).13,14,17–19,21–24 Understanding these variables and their correlations affecting detergent phase behavior enables their rational modulation.
Different approaches have been used in the development of optimal crystallization screens for membrane proteins. In one approach, Iwata16 cataloged the crystallization conditions of both α-helical and β-barrel membrane protein structures that were deposited in the PDB. Subsequent analyses generated trends for precipitants, salts, detergents, and pH that were utilized to formulate different variations of sparse-matrix crystallization screens, including MemGold and MemPlus.25,26 Other groups have taken a different approach, characterizing the phase boundaries for a single detergent at a fixed concentration in the presence of different salts and polyethylene glycols (PEGs). Sampling within a defined set of chemicals and their associated concentrations allows the PDC to embrace the phase behavior of the detergent. The resulting data guide the design of “tailored” membrane protein crystallization screens based on the phase behavior of a particular detergent, avoiding undesirable conditions that promote detergent phase separation. Song and Gouaux23 were the first to report such a strategy, developing sparse-matrix screens for the detergent tetraethylene glycol monooctyl ether (C8E4) to crystallize the α-hemolysin heptamer. A key assumption was made during the design process of these crystallization reagents: the phase transition of the detergent component will dominate the crystallization process for a PDC. To generate the tailored screens, they formulated solutions consisting of different concentrations of PEG, salt, buffer, and detergent, followed by manual setup and observation of vapor-diffusion experiments, at both 4 and 20°C, to characterize which cocktails led to phase-separated drops. Empirical knowledge of the phase separation boundaries for these cocktails guided the formulation of 120 tailored cocktails that produced novel crystal morphologies of α-hemolysin. Other groups have reported methods to map and apply the phase boundaries of detergents to membrane protein crystallization. Wiener and Snook24 coupled the use of a modified commercial crystallization robot with dye partitioning to generate detergent–cocktail mixtures and facilitate the identification of phase-separated drops, whereas Loll et al.22 used commercial crystallization screens and a fluorescent dye in a microplate assay to achieve a similar goal.
The Hauptman-Woodward Medical Research Institute (HWI) provides a high-throughput (HT) crystallization screening service for both the structural genomics and biological crystallographic communities.27 This core facility serves as the hub of the Center for High-Throughput Structural Biology (CHTSB; http://www.chtsb.org), whose mission is to develop novel technologies for the expression and crystallization of membrane proteins and other difficult to crystallize biological macromolecules within the National Institutes of General Medical Sciences Protein Structure Initiative. We report here empirically derived detergent phase data for an extensive chemical landscape encompassing 11 detergents, 11 salts, and 10 PEGs. This phase data is readily accessible; it can be used by any investigator interested in formulating crystallization cocktails containing PEGs and salts to specifically target regions near, but not beyond, the phase partitioning boundary of these 11 detergents. In addition to reporting this phase information, we have used the data to formulate a membrane protein crystallization screen. The screen uses 1536 cocktails to sample regions of detergent phase space particularly relevant to crystallization. The membrane protein screen is offered as an alternative to the set of 1536 cocktails in use by the HT screening laboratory for soluble proteins. Our study reinforces limited literature citing the successful application of microbatch-under-oil crystallization methods28 to membrane proteins.29,30 We also demonstrate the efficiency that can be achieved by adapting this method to HT, enabling us to identify crystallization conditions for 12 of a group of 14 membrane proteins, 10 of which have never been reported to crystallize.
Results
Our first step toward developing a crystallization screen for membrane proteins was surveying the literature to identify a subset of detergents and other chemicals reported to crystallize membrane proteins. We evaluated the Membrane Proteins of Known Structure website, curated by the White laboratory at UC-Irvine (http://blanco.biomol.uci.edu/Membrane_Proteins_xtal.html) and the results published by Iwata16 to identify the most widely utilized detergents, salts, and PEG components for the crystallization of membrane proteins. Although we recognize this is a biased starting set based upon only a few hundred samples, it represented all of the information available at that time. This data, while limited, offered a rational means to select from the hundreds of detergents and thousands of chemicals that could, at least in principle, be used to develop a crystallization screen. We selected the components listed in Table I to formulate solutions for the generation of phase boundary data. This data would ultimately be the basis behind the formulation of the cocktails used in the membrane protein crystallization screen. We chose the dye-based method to detect detergent partitioning.24 One hundred different biological dyes were dissolved in distilled water and tested for their ability to phase separate into a detergent-rich phase using n-octyl-β-d-glucoside (β-OG) and pentaethylene glycol monooctylether (C8E5) as control detergents. Drop solutions consisting of detergent, reservoir solution, and dye were equilibrated against reservoirs containing 4.3M NaCl in sitting-drop vapor diffusion plates. The plates were incubated at room temperature and observed over the course of 2 weeks, with the dyes scored on their ability to partition in the detergent phase. Of the dyes tested, 35 optimally phase-partitioned, that is, they formed small, colored spheroids in the drop. The 35 dyes were subsequently used in experiments to determine their ability to phase-partition the detergent β-OG in microbatch-under-oil experiments with cocktails consisting of PEG 5000 monomethylether (mme) and LiCl. Four of these 35 dyes provided sufficient contrast to observe detergent phase partitioning. From these, acid black-24 was selected for use in further studies because of the good contrast it provided during visual inspection (Supporting Information Fig. S1).
Table I.
The Detergents, Salts, and Polyethylene Glycols Used in the Construction of Cocktails to Generate Phase Boundary Data
| Detergent |
PEG |
Salt |
|||
|---|---|---|---|---|---|
| Type | Detergent (%) | Type | PEG (%) | Type | Salt (M) |
| C10M | 0.18, 0.4, 0.5 | 400 | 1.44–69.0 | CaCl2 | 0.05–2.5 |
| C12M | 0.05, 0.5, 1.0 | 1000 | 1.0–34.6 | KCl | 0.04–2.2 |
| β-HG | 3.8 | 2000 | 2.0–22.2 | LiCl | 0.05–7.2 |
| β-OG | 0.5, 1.0 | 2000 mme | 0.72–34.6 | Li2SO4 | 0.05–0.4 |
| β-NG | 0.4 | 3350 | 2.0–22.2 | MgCl2 | 0.02–2.2 |
| CHAPS | 0.5, 1.0 | 4000 | 0.72–34.6 | Na2C3H2O4 | 0.04–2.4 |
| LDAO | 0.08, 0.25, 0.5 | 5000 mme | 0.72–34.6 | NaCl | 0.05–3.6 |
| C12E8 | 0.5 | 6000 | 2.0–22.2 | NaH2PO4 | 0.05–0.4 |
| C8E4 | 0.25, 0.5, 1.0 | 8000 | 0.72–34.6 | (NH4)2SO4 | 0.11–2.5 |
| C8E5 | 0.5, 1.0 | 20,000 | 0.72–18.7 | NH4H2PO4 | 0.04–2.2 |
| FC-12 | 0.1, 0.25 | (NH4)2HPO4 | 0.05–0.4 | ||
Generation of phase boundaries: coarse screening
For each combination of salt and PEG, we generated 288 different solutions to coarsely sample the chemical space. The goal of this portion of the study was to identify the concentrations of salt and PEG defining the detergent phase partitioning border. This was accomplished by volumetric dilution of concentrated detergent, salt, and PEG stock solutions as summarized in Figure 1(A,B). Ideally, and in many cases, these experiments produced outcomes with clearly defined areas where detergent and phase separation occurred. A typical plate and subsequent graph are depicted in Figure 1(C,D), respectively. The plates were incubated first at 20°C for 1 week, the outcomes recorded, followed by incubation at 4°C for 1 week, and subsequent recording of the outcomes. Graphs were then generated using Microsoft Excel. The effect of temperature on detergent phase partitioning19,20 was observed for some, but not all, of the detergents used in this study (Fig. 2).
Figure 1.
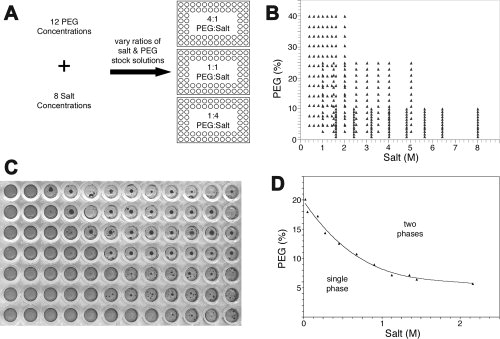
Experimental setup to generate phase boundary data. (A) Three 96-well plates were used to generate coarse phase boundary data for a given detergent:PEG:salt combination. Concentrated stock solutions of PEG and salt were prepared such that the solution concentrations were varied by row for salt and by column for PEG. Reaction ratios of PEG:salt were propagated into three 96-well plates, followed by the addition of detergent and acid black-24 dye. (B) Graph depicting the 288 different combinations of PEG:salt generated to sample the phase space for a given detergent. Example of (C) phase separation observed in a typical 96-well plate and (D) a typical phase boundary curve generated from the observed phase separation in the 96-well plate.
Figure 2.
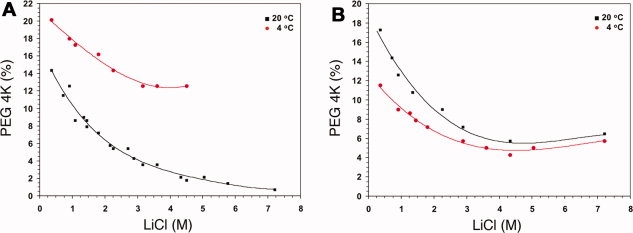
The effect of temperature on detergent phase partitioning. The phase separation for a particular detergent:PEG:salt combination was recorded 7 days post setup at 20°C, followed by transferring the plate to 4°C for an additional 7 days so that the effect of temperature on phase partitioning could be monitored. A shift in the phase boundary curve was observed for some, but not all of the detergent:PEG:salt combinations that were tested. Examples are depicted for the combination of: (A) 0.5% C8E4, PEG 4000, LiCl, and (B) 1% β-OG, PEG 4000, LiCl.
Generation of phase boundaries: fine screening
The coarse-grain phase data generated in the initial study were further refined. Specifically, regions bordering the phase partition curve were sampled in a second series of experiments using finer-grained increments of salt and PEG concentrations. For these experiments, salts were used at concentrations ranging from 0.05M to 0.4M and PEG concentrations varied from 2 to 22%.
Generation of cocktails for the membrane screen
Summary graphs that depicted phase boundaries as a function of PEG, salt, or detergent were generated; examples of which are represented in Figure 3. These graphs were used to formulate a grid screen31 by using concentrations of salt and PEG near the experimentally calculated phase boundary. From these summary curves, 1344 unique cocktails were generated. A complimentary group of 192 cocktails from the commercially available MemGold and Index crystallization screens were included to round out the screen to 1536, making it compatible with the HT screening facility at the HWI. The MemGold Screen25 is distributed by Molecular Dimensions and is composed of 96 cocktails formulated based on the most recent list of conditions from which membrane proteins have been successfully crystallized. The Index Screen is distributed by Hampton Research and also contains 96 cocktails formulated based on classes of precipitating agents that have effectively crystallized soluble proteins. In addition to the generation of the 1344 cocktails for the membrane screen, we created an Excel-based applet called “SlickSpot” that allows investigators to specifically target regions near the phase-partitioning boundaries of the 11 detergents tested through the formulation of their own grid screens (see Supporting Information Data and Supporting Information Fig. S2).
Figure 3.
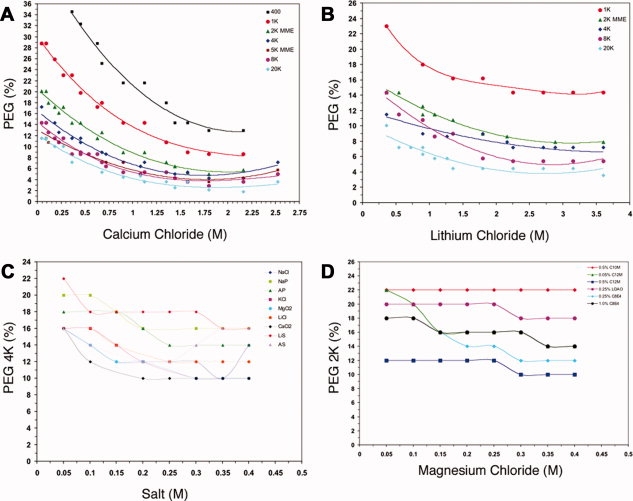
The effect of detergent phase partitioning as a function of PEG, salt, and detergent. Typical summary graphs depicting the effects on detergent phase partitioning as a function of the PEG, salt, and detergent used. The generation of summary graphs facilitates the design and formulation of grid screens for crystallization trials for membrane proteins in a particular detergent system. (A) The effect on the phase boundary curve of 0.5% C8E4 with calcium chloride as a function of PEG molecular weight at 20°C. (B) The effect on the phase boundary curve of 0.05% C12M with lithium chloride as a function of PEG molecular weight at 20°C. (C) The effect on the phase boundary curve of 0.05% C12M with PEG 4000 as a function of different salts at 20°C. (D) The effect on the phase boundary curve of PEG 2000 with magnesium chloride as a function of detergent at 20°C.
The Hampton Research Index screen is common to both the soluble protein and membrane protein 1536 cocktail screens utilized within HT screening facility at HWI. The inclusion of the Index screen in both sets of cocktails ensures a consistent set of reagent conditions for comparative analysis. If different preparations of a macromolecule undergo crystallization screening using both the standard and membrane screen, we can readily verify the sample batch consistency. In addition to cross-screen batch comparisons, the cocktails provide a basis to compare the relative solubility of macromolecules. The chemical diversity (inorganic salts, polyols, neutralized organic acids), range of pH values (3.1–8.6 at 25°C), and biased sparse matrix and grid screen approach used to formulate the Index screen's cocktails makes them an appropriate choice to generate a standard solubility profile for biochemically diverse macromolecules. The chemical coverage of the Index screen becomes a pointer to probable regions of interest in the accompanying chemical space sampled by the remaining cocktails, frequently identifying chemical classes that promote solubility (clear drops) or precipitation of the macromolecule.32
Validating the crystallization of a membrane protein near a generated phase boundary
We next wanted to validate that the generated phase boundary data could be utilized as a guide for the crystallization of membrane proteins. To this end, we obtained a sample of human aromatase, a membrane-bound cytochrome P450 enzyme, that catalyzes the biosynthesis of all oestrogens from androgens in vertebrates.33 Aromatase was purified from the human placenta using n-dodecyl-β-d-maltopyranoside (C12M) as both the solubilization and crystallization detergent and subsequently crystallized from cocktails containing PEG 4000 and NaCl. The phase boundary curve for 0.05% C12M with PEG 4000 and NaCl was generated [Fig. 4(A)] and a grid screen was subsequently formulated to sample the crystallization space near the predicted detergent phase boundary (Supporting Information Table SI). Indeed, aromatase crystals were observed to form just below the generated phase boundary curve [Fig. 4(B,C)].
Figure 4.
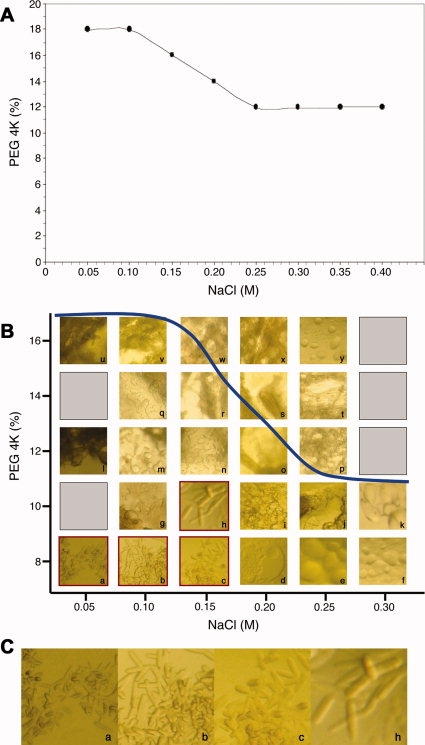
Crystallization of aromatase near the experimentally calculated phase boundary of 0.05% C12M with PEG 4000 and NaCl. (A) Experimentally determined phase boundary for 0.05% C12M, PEG 4000, NaCl. (B) Crystallization outcomes observed from a sitting-drop vapor diffusion grid screen whose cocktails were formulated based upon the experimentally determined phase boundary. The blue line represents the phase boundary for 0.05% C12M against PEG 4000 and NaCl and the lowercase letters correspond to the cocktails described in Supporting Information Table SI. Crystals of aromatase were observed in wells corresponding to cocktails a, b, c, and h (outlined in red). (C) Magnified images of the aromatase crystals in a, b, c, and h. These crystals appeared after 3 days and were less than 0.1 mm in size on their longest edge.
Formulation and dispensing
The 1536 cocktail Membrane Protein Crystallization Screen was designed to be compatible with the robotic liquid-handling systems used in the HT Crystallization Screening Laboratory at the HWI. Dispensing of the protein and crystallization cocktail solutions and crystallization under USP grade mineral oil were carried out as described by Luft et al.27 The robotic liquid-handling systems are used to improve efficiency; there is no underlying technical reason why the crystallization screening solutions could not be delivered using a manual pipette.
Testing the screen
We received membrane proteins from the structural genomics and structural biology communities to test the effectiveness of the membrane protein crystallization screen. Specifically, we set up crystallization trials for 12 membrane protein samples in varying detergent(s), which had not been previously crystallized (Table II). These proteins were from both the α-helical and β-barrel classes of membrane proteins. We also set up crystallization trials for two membrane proteins whose crystallization conditions and three-dimensional structures are known: murine cyclooxygenase-2 (COX-2)34 and the glycerol-3-phosphate transporter (GlpT) from E. coli.35,36 Conditions that produced crystals of both COX-2 and GlpT were identified in the 1536 membrane screen. These conditions were similar to those previously reported during the structural characterization of both proteins and would have been predicted from the phase boundary data used to generate the screen based upon the detergent system utilized to form the PDC. Of the 12 membrane proteins of unknown structure tested within the 1536 membrane screen, 10 gave at least one crystallization lead resulting from a cocktail formulated from the experimentally determined phase boundary curves based on microscopic inspection of the outcomes of each experimental set up over a 6-week period (Table II). The crystallization outcomes for these proteins ranged from needles and small clusters of plates to small single crystals. Representative images and their corresponding crystallization conditions are depicted in Figure 5.
Table II.
Crystallization Leads
| Sample | Detergent | MSC | HR INDEX | MemGold | Total |
|---|---|---|---|---|---|
| MP01 | FC-12 + C12M | 1 | 0 | 0 | 1 |
| MP02 | FC-12 + C12E8 | 0 | 0 | 1 | 1 |
| MP03 | C10M | 0 | 0 | 0 | 0 |
| MP04 | C12E8 + C12M | 9 | 0 | 0 | 9 |
| MP05 | β-OG | 138 | 3 | 3 | 144 |
| MP06 | CYMAL-3 | 44 | 2 | 0 | 46 |
| MP07 | CYMAL-3 | 6 | 0 | 0 | 6 |
| MP08 | LDAO | 63 | 5 | 1 | 69 |
| MP09 | LDAO | 19 | 0 | 3 | 22 |
| MP10 | FC-12 + C12M | 6 | 0 | 1 | 7 |
| MP11 | C12M | 1 | 1 | 0 | 2 |
| MP12 | C12M | 76 | 0 | 15 | 91 |
| COX-2 | β-OG | 13 | 1 | 3 | 17 |
| GlpT | C12M | 1 | 0 | 1 | 2 |
The number of crystallization leads identified for the 12 membrane proteins of unknown structure and positive controls (COX-2 and GlpT) broken down by cocktails derived from the phase boundary data (MSC), the Hampton Research Index Screen (HR INDEX), and the Molecular Dimensions MemGold Screen (MemGold). The crystallization detergent(s) for each sample is also listed.
Figure 5.
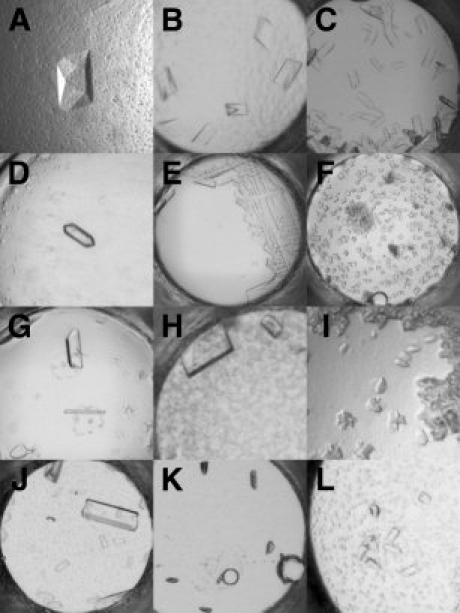
Crystals of membrane proteins identified using the 1536 membrane protein screen. Crystal image, with corresponding cocktail formulation, date of first appearance in the screen, and approximate size (longest edge) for: (A) MP01: 40% PEG 400, 0.1M HEPES, pH 7.0, 0.1M ammonium sulfate, 6 weeks, 0.2 mm; (B) MP04: 30% PEG 8000, 0.1M sodium citrate, pH 5.6, 0.2M NaCl, 4 weeks, 0.2 mm; (C) MP05: 30% PEG 3350, 0.1M sodium citrate, pH 5.6, 0.1M ammonium sulfate, 1 week, 0.1 mm; (D) MP06: 20% PEG 8000, 0.1M HEPES, pH 7.0, 0.1M ammonium sulfate, 4 weeks, 0.15 mm; (E) MP07: 50% PEG 400, 0.1M HEPES, pH 7.0, 0.2M ammonium sulfate, 5 weeks, 0.2 mm; (F) MP08: 20% PEG 3350, 0.1M TRIS, pH 8.5, 0.3M NaCl, 2 weeks, 0.05 mm; (G) MP09: 32% PEG 4000, 0.1M TRIS, pH 8.5, 0.3M lithium sulfate, 5 weeks, 0.2 mm; (H) MP10: 20% PEG 400, 0.1M HEPES, pH 7.0, 0.2M ammonium sulfate, 6 weeks, 0.2 mm; (I) MP11: 30% PEG 2000, 0.1M HEPES, pH 7.0, 0.2M magnesium chloride, 6 weeks, 0.05 mm; (J) MP12: 30% PEG 400, 0.1M HEPES, pH 7.0, 0.1M NaCl, 2 weeks, 0.4 mm; (K) COX-2: 8% PEG 8000, 0.1M TRIS, pH 8.5, 0.4M magnesium chloride, 5 weeks, 0.1 mm; (L) GlpT: 40% PEG 2000, 0.1M HEPES, pH 7.0, 0.05M lithium sulfate, 1 week, 0.1 mm.
An X-ray diffraction experiment is the absolute standard for determining whether or not crystals are protein or salt, and the only way to discern their feasibility for structure determination. Crystal retrieval from the narrow wells of the 1536 microassay plate for X-ray diffraction experiments is difficult given the small diameter of the conical wells (0.9 mm at the bottom of the well). As such, we chose to carry out optimization experiments in a container more suitable for crystal retrieval. Membrane protein sample 05 (MP05), which generated 138 leads in the cocktails tailored to the phase boundary data was chosen for optimization and scale-up to verify that the crystals were protein in nature. A single crystallization lead from our phase boundary-based screen [identified in Fig. 6(A)] was translated from microbatch-under-oil conditions and optimized using the sitting-drop vapor diffusion method. The crystals generated in Figure 6(B) appeared after 1 week at 20°C. One crystal from this well was cryopreserved using MPD and flash-frozen in a stream of nitrogen gas. Subsequent X-ray diffraction analysis indicated observable reflections beyond 2.1 Å resolution [Fig. 6(C)].
Figure 6.
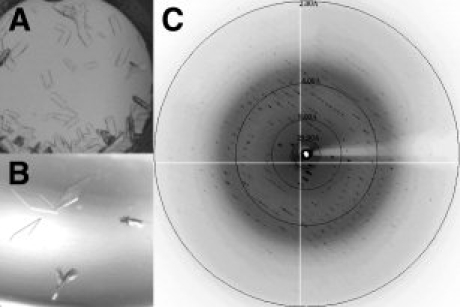
Crystallization optimization and diffraction of MP05. (A) Initial crystallization lead from 1536 membrane protein screen (30% PEG 3350, 0.1M sodium citrate, pH 5.6, 0.1M ammonium sulfate) generated using the microbatch-under-oil method. (B) Optimized crystallization lead (12% PEG 3350, 0.1M MES, pH 5.5, 0.1M magnesium chloride) generated using the sitting-drop vapor diffusion method. (C) Diffraction image of cryopreserved optimized crystal. Spots can be visualized out to 2.1 Å resolution.
Discussion
Detergent selection is a critical variable for membrane protein crystallization. The selection of an optimal detergent is a trial and error process that is complicated, and often contradictory. Not all membrane proteins maintain optimal stability during extraction and purification in detergents deemed best for crystallization. The most successful detergents used for crystallization have CMC values in the millimolar range and form small monodisperse micelles.10 For example, β-OG and n-decyl-N,N-dimethylamine-N-oxide (LDAO) are considered good crystallization detergents based on these properties and the fact that they will expose the largest possible polar surface for crystal contacts. Although optimization of micelle size is important to consider for crystallization, the chemical nature and role of the detergent head group must likewise be taken into consideration. Small, polar head groups are frequently an important factor in maximizing the polar accessibility of the PDC for crystal contact formation. Finally, small amphipathic molecules have been used to alter the properties of the PDC through their interaction with detergents.23 These small molecules are proposed to act by binding to and reducing the size of the detergent “belt” surrounding the membrane protein,13 and by forming mixed micelles which are more deformed in shape and thus may pack more advantageously into a crystal lattice.18 The addition of detergent-based variables to the already challenging process of macromolecular crystallization further highlights the importance of rationally designed targeted cocktails. The ability to draw on a vast collection of detergent phase separation behavior under different chemical conditions provides a solid foundation to systematically design crystallization screens for these especially challenging class of proteins.
We recognize that differences are likely to exist between phase behavior of pure detergent micelles and the detergent component forming a PDC. However, due to the scarcity of membrane protein samples, these differences cannot be measured and so far have not been accurately predicted. The standard practice in the membrane protein crystallization community is to assume that the solubility properties of the detergent will dominate the PDC. Thus, for crystallization purposes, the phase behavior of pure detergents provides a good estimate of how the PDC will behave with respect to phase separation under most solution conditions.13,18,24
Having a large number of chemically diverse crystallization cocktails is beneficial.27 Crystals produced from different chemical environments will exhibit unique physical properties. The same protein crystallized from different chemical cocktails is likely to produce very dissimilar crystals. As downstream structural work takes place, these differences can become readily apparent. The same protein crystallized from two different chemicals is likely to behave very differently with respect to soaking experiments, resistance to mechanical damage during mounting, ease of cryopreservation, and most importantly, in the quality and resolution limit of the resulting X-ray diffraction experiment.
In summary, we report here the generation and refinement of empirically derived detergent phase data that encompasses 11 detergents, 11 salts, and 10 PEGs. We have made this data readily accessible through the creation of an Excel-based applet called SlickSpot that allows an investigator interested in formulating cocktails for membrane protein crystallization trials to specifically target regions near, but not beyond, the phase partitioning boundary of the 11 detergents tested using grid-screening approaches. We have also used this data to formulate a 1536-cocktail crystallization screen compatible with the HT crystallization screening facility at the HWI. The screen is now offered as an alternative to the set of 1536 cocktails currently used for soluble proteins (http://www.hwi.buffalo.edu/High_Through/High_Through.html).
Materials and Methods
Biological dyes were purchased from Sigma (St. Louis, MO). n-Heptyl-β-d-glucopyranoside (β-HG), n-octyl-β-d-glucopyranoside (β-OG), n-nonyl-β-d-glucopyranoside (β-NG), n-decyl-β-d-maltopyranoside (C10M), n-dodecyl-β-d-maltopyranoside (C12M), tetraethylene glycol monooctyl ether (C8E4), pentaethylene glycol monooctyl ether (C8E5), octaethylene glycol monododecyl ether (C12E8), n-dodecyl-N,N-dimethylamine-N-oxide (LDAO), and CHAPS were purchased from Anatrace (Maumee, OH). Solid PEG 20,000, LiCl, KCl, NaCl, CaCl2·2H2O, MgCl2·6H2O, (NH4)2SO4, (NH4)2HPO4, NH4H2PO4, NaH2PO4, and Li2SO4·H2O were obtained from Sigma, and their corresponding stock solutions were made on site. 100% (v/v) PEG 400, 50% (w/v) PEG 1000, 50% (w/v) PEG 2000 mme, 50% (w/v) PEG 3350, 50% (w/v) PEG 4000, 50% (w/v) PEG 5000 mme, 50% (w/v) PEG 6000, 50% (w/v) PEG 8000, 3.4M sodium malonate pH 7.0, 1.0M sodium citrate tribasic dehydrate pH 5.6, 1.0M HEPES pH 7.0, 1.0M HEPES pH 7.5, and 1.0M Tris pH 8.5 solutions were purchased from Hampton Research (Aliso Viejo, CA), and 50% (w/v) PEG 2000 was purchased from Molecular Dimensions (Apopka, FL). These stocks were used to prepare all of the cocktail solutions. Corning 96-well plates, 24-well sitting-drop vapor diffusion plates and 1536-well plates were obtained from Sigma, Hampton Research, and Greiner BioOne (Frickenhausen, Germany), respectively. The Abgene Adhesive Crystallography seals and the Hampton Research ClearSeal film sealers were used to seal and prevent evaporation from the 96-well plates. The Hampton Research Index screen and the Molecular Dimensions MemGold screen were used as purchased and included as part of the 1536 membrane crystallization screen.
Dye selection
One hundred different biological dyes were initially tested for their ability to separate into detergent-rich and detergent-poor phases. Five hundred microliter of solution containing 4.3M NaCl and 0.1M HEPES (pH 7.5) were dispensed into the reservoirs of multiple 24-well sitting-drop vapor diffusion plates. Five microliter of either 1% (w/v) β-OG or 1% (v/v) C8E5, 5 μL reservoir solution, and 1 μL of 1% (w/v) dye were delivered to the posts of the crystallization trays, combining to form the experimental drops. The plates were incubated at room temperature for 2 weeks. The drops were analyzed to assess the impact of the dye on visualizing the detergent phase partition. Thirty-five of the dyes provided good visual contrast, allowing for the identification of phase separation when it occurred. Eight of the dyes with the best contrast, as judged visually through a microscope, were set up in a 96-well plate with LiCl and PEG 5000 mme. Solutions containing 0.03% (w/v) dye were combined with 1.0% (w/v) β-OG, 2.7M LiCl, and PEG 5000 mme ranging from 2.7 to 22.5% (w/v). Plates were incubated at 20°C for 2 h. Based upon visual inspection of these experiments, acid black was chosen as the dye best suited to observe detergent phase separation for the ensuing experiments.
96-Well microplate sealer assessment
About 60–100 μL of various PEG/salt mixtures were placed in three microplates, each covered with different sealing films, and incubated at 20°C for 7 days. After this time, the well volumes were measured and recorded. The Abgene Adhesive Crystallography seals had a negligible amount of evaporation and were further analyzed. Refractive indices of 4–48% PEG 5000 mme solutions were measured and 60 μL pipetted into a 96-well plate. The wells were covered with the Abgene sealer and incubated at 20°C for 2 weeks. The refractive indices were measured again to evaluate the amount of evaporation. There were only slight changes in the refractive indices of these PEG solutions indicating an increase in PEG concentration of ∼5% per well. Based upon this analysis, all phase boundary experiments were conducted using the Abgene plate sealers or the Hampton Research equivalent, the ClearSeal film sealers.
Phase boundary generation
For each phase boundary determination, 288 conditions were set up to encompass a wide range of PEG and salt conditions for each detergent. Experiments were conducted in three 96-well plates with a total reaction volume of 100 μL. Combinations of eight salts, seven PEGs, and nine detergents were used for the first round of phase boundary curve generation. Initially, eight stock solutions were made for each of the following salts: LiCl (2–10M), KCl (0.2–3M), NaCl (0.6–5M), CaCl2·2H20 (0.25–3.5M), MgCl2·6H2O (0.1–3M), (NH4)2SO4 (0.6–3.5M), NH4H2PO4 (0.2–3M), and sodium malonate (0.2–3.4M). Twelve stock solutions ranging from 8 to 96% for PEG 400, 4 to 48% for PEG 4000 and 8000, and 4 to 26% for PEG 20,000 were also prepared. Within a 96-well experiment plate, concentrations were varied by column for salt (8 conditions) and by row for PEG solutions (12 conditions). Reaction ratios for the salts and PEGs were as follows: Plate #1 1:4, Plate #2 1:1, and Plate #3 4:1. Once the salt and PEG stock solutions were added to the wells, 10 μL of 10× detergent/0.1% acid black 24 solution was introduced using a multichannel pipetter. The trays were covered with ClearSeal Film plate sealers and incubated for 7 days at 20°C. The plates were visually inspected for phase separation and the data were recorded. Following inspection, the plates were moved to 4°C for a period of 7 days. The plates were again visually inspected, recording the resulting phase behavior.
The coarse phase boundary curves determined using the three-plate protocol were refined. The fine screens were carried out using 0.05M increments of the following salts and 2% increments of PEG in a single 96-well plate. Eight stock solutions ranging in concentration from 0.25 to 2M were prepared for each of the following salts: LiCl, KCl, NaCl, CaCl2·2H2O, MgCl2·6H2O, (NH4)2SO4, (NH4)2HPO4, NH4H2PO4, NaH2PO4, and Li2SO4·H2O. Twelve stock solutions ranging in concentration from 3.3 to 37% were prepared for PEG 400, 2000, 3350, 4000, 6000, and 8000. Experiment drops in the 96-well plates were prepared by adding 10 μL of 0.1M Tris pH 8.0 to each well in the plate, followed by the addition of 20 μL of a single concentration of each of the eight salt stock solutions (added to each of the eight rows in the plate), followed by the addition of 60 μL of a single concentration of each of the 12 PEG solutions in each of the 12 columns of a plate. The experiment drop preparation was completed with the addition of 10 μL of the 10× detergent/0.1% acid black 24 solution to each of the 96 wells. Plates were sealed and left to incubate as described earlier.
Design of the 1536 screen
Phase boundary graphs (890) were generated using the different combinations of salts, PEGs, detergents, and temperature. One hundred eighty-four combinations of the salts, PEGs, and detergents showed no indications of phase separation based upon these dye-partitioning studies. The phase-separated detergents were analyzed using a graph to display the detergent phase behavior as it related to salt concentration versus PEG concentration for each of the chemically distinct salt and PEG combinations tested. The phase boundary graphs provided the data used to formulate 1344 cocktails. PEG and salt concentrations bordering the phase boundaries of the different detergents were selected to formulate cocktails. The frequency of sampling for concentrations of each PEG and salt combination were based on previously reported membrane protein crystallization conditions. The more frequently particular combinations of a PEG, salt, and detergent were used successfully to produce crystals of a membrane protein, the finer the sampling of that combination. The Hampton Research Index screen (96 cocktails) and the Molecular Dimensions MemGold screen (96 cocktails) were included in the screen to bring the final number of cocktails to 1536.
Construction of the 1536 microbatch under oil plates
The following salt stock solutions were prepared: 7.36M LiCl, 2.58M KCl, 4.98M NaCl, 3.97M CaCl2·2H2O, 4.4M MgCl2·6H2O, 3.8M (NH4)2SO4, 3.55M (NH4)2HPO4, 3.69M NaH2PO4, and 2.25M Li2SO4·H2O. Additionally, the following PEG and buffer stock solutions were prepared: 100% (v/v) PEG 400, 50% (w/v) PEG 3350, 50% (w/v) PEG 4000, 50% (w/v) PEG 6000, 50% (w/v) PEG 8000, 1.0M sodium citrate tribasic dehydrate pH 5.6, 1.0M HEPES pH 7.0, 1.0M Tris pH 8.5, and 50% (w/v) PEG 2000. The above salt, PEG, and buffer stock solutions were mixed volumetrically to prepare 5 mL of crystallization cocktail solutions. After preparing 5 mL stock solutions for each of the 1344 cocktails, the solutions were manually reformatted to prepare 96-well deep well cocktail source plates that were used as previously described to set up microbatch-under-oil crystallization experiments in 1536-well plates.27
Crystallization trials
For the aromatase sitting-drop vapor diffusion grid screening experiment, cocktails were designed based on the phase boundary curve generated for PEG 4000, NaCl, and C12M. Twenty-five cocktails with varying NaCl and PEG concentrations (Supporting Information Table SI) were made in a solution with 30 mM TRIS, pH 8.5. One hundred twenty-five microliter of each solution was pipetted into a specific reservoir of a Crystal Quick 96-well sitting drop plate (Hampton Research). One microliter cocktail and 1 μL protein were combined to form the drop solution. The plate was covered with a ClearSeal Film plate sealer and monitored for crystal growth at 4°C.
The HT crystallization facility at the HWI was used to set up 14 membrane proteins (COX-2, GlpT, and the 12 membrane proteins of unknown structure) with the 1536 cocktail membrane protein screen.27 The screening facility uses source/destination plate protocols with the TANGO liquid-handling system (Matrix Technologies Corp.) to set up micrtobatch-under-oil crystallization experiments. The experiments were set up in 1536-well plates (Greiner BioOne) using USP-grade mineral oil (Sigma). Upon completion, each experimental plate contained a single protein solution combined with an equal volume of 1536 different tailored crystallization cocktails under mineral oil (200 nL protein + 200 nL cocktail). An automated imaging system recoded the outcomes of the experiments immediately after the addition of protein solution, and weekly thereafter for 6 weeks. Plates were stored and recorded at 23°C. Outcomes were then reviewed to identify crystallization leads using the image-viewing program Macroscope, which is distributed by the HWI HT screening facility.
Crystallization optimization and diffraction analysis of MP05
For crystallization optimization of MP05, the sitting-drop vapor diffusion method was used. A standard grid screen was constructed that encompassed the following conditions: 7.5–16% PEG 3350, 0.1M MES, pH 5.5–7.0, and 0.025–0.1M magnesium chloride. Experimental drop volumes and reservoir solution volumes of 4 and 500 μL were used, respectively. Drops were prepared with a 1:1 (v/v) ratio of protein:precipitant solution, with crystallizations carried out and stored at 23°C. For cryopreservation, a crystal grown from 12% PEG 3350 was first transferred from the sitting drop to a solution consisting of 14% PEG 3350, 0.1M MES, pH 5.5, 0.1M magnesium chloride, and 5% MPD for 15 s, followed by transfer to the same solution containing 15% MPD for an additional 15 s. The crystal was then directly flash-frozen in a stream of cold nitrogen gas. Diffraction analysis was carried out on beamline A1 at the Cornell High Energy Synchrotron Source (Ithaca, NY). 1° oscillation images were collected with 15-s exposures.
Acknowledgments
The authors thank Dr. Debashis Ghosh at the HWI and Dr. Da-Neng Wang at the New York University School of Medicine for kindly supplying samples of aromatase and GlpT, respectively. They also thank Dr. Witek Kwiatkowski at the Salk Institute for supplying the detergent FC-12 used in this study. Diffraction experiments were conducted at the Cornell High Energy Synchrotron Source (CHESS), which is supported by the National Science Foundation under NSF award DMR-0225180, using the Macromolecular Diffraction at CHESS (MacCHESS) facility, which is supported by award RR-01646 from the National Institutes of Health, through its National Center for Research Resources.
References
- 1.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 2.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 3.Berman HM, Westbrook J, Feng Z, Gillil G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White SH. The progress of membrane protein structure determination. Protein Sci. 2004;13:1948–1949. doi: 10.1110/ps.04712004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter EP, Beis K, Cameron AD, Iwata S. Overcoming the challenges of membrane protein crystallography. Curr Opin Struct Biol. 2008;18:581–586. doi: 10.1016/j.sbi.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacapere JJ, Pebay-Peyroula E, Neumann JM, Etchebest C. Determining membrane protein structures: still a challenge! Trends Biochem Sci. 2007;32:259–270. doi: 10.1016/j.tibs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Loll PJ. Membrane protein structural biology: the high throughput challenge. J Struct Biol. 2003;142:144–153. doi: 10.1016/s1047-8477(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Wiener MC. A pedestrian guide to membrane protein crystallization. Methods. 2004;34:364–372. doi: 10.1016/j.ymeth.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 9.D'Arcy A. Crystallizing proteins—a rational approach? Acta Crystallogr D Biol Crystallogr. 1994;50:469–471. doi: 10.1107/S0907444993014362. [DOI] [PubMed] [Google Scholar]
- 10.Garavito RM, Ferguson-Miller S. Detergents as tools in membrane biochemistry. J Biol Chem. 2001;276:32403–32406. doi: 10.1074/jbc.R100031200. [DOI] [PubMed] [Google Scholar]
- 11.Garavito RM, Picot D, Loll PJ. Strategies for crystallizing membrane proteins. J Bioenerg Biomembr. 1995;28:13–27. [PubMed] [Google Scholar]
- 12.Kuhlbrandt W. Three-dimensional crystallization of membrane proteins. Q Rev Biophys. 1988;21:429–477. doi: 10.1017/s0033583500004625. [DOI] [PubMed] [Google Scholar]
- 13.Michel H. General and practical aspects of membrane protein crystallization. In: Michel H, editor. Crystallization of membrane proteins. Boca Raton: CRC Press; 1990. pp. 73–88. [Google Scholar]
- 14.Prive GG. Detergents for the stabilization and crystallization of membrane proteins. Methods. 2007;41:388–397. doi: 10.1016/j.ymeth.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Wiener MC. When worlds colloid. Protein Sci. 2006;15:2679–2681. doi: 10.1110/ps.062559306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwata S. Crystallization informatics of membrane proteins. In: Iwata S, editor. Methods and results in crystallization of membrane proteins. La Jolla: International University Line; 2003. pp. 281–297. [Google Scholar]
- 17.Scarborough GA. Large single crystals of the Neurospora crassa plasma membrane H+-ATPase: an approach to the crystallization of integral membrane proteins. Acta Crystallogr D Biol Crystallogr. 1994;50:643–649. doi: 10.1107/S0907444993014283. [DOI] [PubMed] [Google Scholar]
- 18.Garavito RM. Crystallizing membrane proteins: experiments on different systems. In: Michel H, editor. Crystallization of membrane proteins. Boca Raton: CRC Press; 1990. pp. 89–106. [Google Scholar]
- 19.Rummel G, Rosenbusch JP. Crystallization of bacterial outer membrane proteins from detergent solutions: porin as a model. In: Iwata S, editor. Methods and results in crystallization of membrane proteins. La Jolla: International University Line; 2003. pp. 101–129. [Google Scholar]
- 20.Zulauf M. Detergent phenomena in membrane protein crystallization. In: Michel H, editor. Crystallization of membrane proteins. Boca Raton: CRC Press; 1990. pp. 53–72. [Google Scholar]
- 21.Hitscherich C, Jr, Aseyev V, Wiencek J, Loll PJ. Effects of PEG on detergent micelles: implications for the crystallization of integral membrane proteins. Acta Crystallogr D Biol Crystallogr. 2001;57:1020–1029. doi: 10.1107/s0907444901006242. [DOI] [PubMed] [Google Scholar]
- 22.Loll PJ, Allaman M, Wiencek J. Assessing the role of detergent-detergent interactions in membrane protein crystallization. J Cryst Growth. 2001;232:432–438. [Google Scholar]
- 23.Song L, Gouaux JE. Membrane protein crystallization: application of sparse-matrices to the alpha-hemolysin heptamer. In: Carter CW Jr, Sweet RM, editors. Methods in enzymology. San Diego: Academic Press; 1997. pp. 60–73. [DOI] [PubMed] [Google Scholar]
- 24.Wiener MC, Snook CF. The development of membrane protein crystallization screens based upon detergent solution properties. J Cryst Growth. 2001;232:426–431. [Google Scholar]
- 25.Newstead S, Ferrandon S, Iwata S. Rationalizing alpha-helical membrane protein crystallization. Protein Sci. 2008;17:466–472. doi: 10.1110/ps.073263108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newstead S, Hobbs J, Jordan D, Carpenter EP, Iwata S. Insights into outer membrane protein crystallization. Mol Membr Biol. 2008;25:631–638. doi: 10.1080/09687680802526574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luft JR, Collins RJ, Fehrman NA, Lauricella AM, Veatch CK, DeTitta GT. A deliberate approach to screening for initial crystallization conditions of biological macromolecules. J Struct Biol. 2003;142:170–179. doi: 10.1016/s1047-8477(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 28.Chayen NE, Stewart PDS, Blow DM. Microbatch crystallization under oil: a new technique allowing many small-volume crystallization trials. J Cryst Growth. 1992;122:176–180. [Google Scholar]
- 29.D'Arcy A, Sweeney AM, Haber A. Practical aspects of using the microbatch method in screening conditions for protein crystallization. Methods. 2004;34:323–328. doi: 10.1016/j.ymeth.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Loll PJ, Tretiakova A, Soderblom E. Compatibility of detergents with the microbatch-under-oil crystallization method. Acta Crystallogr D Biol Crystallogr. 2003;59:1114–1116. doi: 10.1107/s0907444903008175. [DOI] [PubMed] [Google Scholar]
- 31.Cox MJ, Weber PC. An investigation of protein crystallization parameters using successive automated grid searches. J Cryst Growth. 1988;90:318–324. [Google Scholar]
- 32.Snell EH, Nagel RM, Wojtaszcyk A, O'Neill H, Wolfley JL, Luft JR. The application and use of chemical space mapping to interpret crystallization screening results. Acta Crystallogr D Biol Crystallogr. 2008;64:1240–1249. doi: 10.1107/S0907444908032411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh D, Griswold J, Erman M, Pangborn W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature. 2009;457:219–223. doi: 10.1038/nature07614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens AM, Pawlitz JL, Kurumbail RG, Gierse JK, Moreland KT, Stegeman RA, Loduca JY, Stallings WC. Crystallization of recombinant cyclo-oxygenase-2. J Cryst Growth. 1999;196:350–355. [Google Scholar]
- 35.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 36.Lemieux MJ, Song J, Kim MJ, Huang Y, Villa A, Auer M, Li XD, Wang DN. Three-dimensional crystallization of the Escherichia coli glycerol-3-phosphate transporter: a member of the major facilitator superfamily. Protein Sci. 2003;12:2748–2756. doi: 10.1110/ps.03276603. [DOI] [PMC free article] [PubMed] [Google Scholar]


