Abstract
SecB, a remarkable chaperone involved in protein export, binds diverse ligands rapidly with high affinity and low specificity. Site-directed spin labeling and electron paramagnetic resonance spectroscopy were used to investigate the surface of interaction on the export chaperone SecB. We examined SecB in complex with the unfolded precursor form of outer membrane protein OmpA as well as with a truncated version of OmpA that includes the transmembrane domain and lacks both the signal peptide and the periplasmic domain. In addition, we studied the binding of SecB to the unfolded mature form of galactose-binding protein, a soluble periplasmic protein. We have previously used the same strategy to map the binding surface for the precursor of galactose-binding protein. We show that for all ligands tested the patterns of contact are the same.
Keywords: chaperone, export, SecB, site-directed spin labeling, EPR, protein–protein interaction, galactose-binding protein, OmpA
Introduction
Export of protein to the periplasmic space or to the outer membrane of the Gram-negative bacterium Escherichia coli is mediated by the general secretory, Sec system.1 The Sec system can translocate proteins only if they are devoid of stable tertiary structure. Therefore, in addition to a pathway through the membrane, provided by the translocon SecYEG, the Sec system includes a soluble chaperone SecB that serves to capture polypeptides destined for export before they acquire a stable, folded structure.2 SecB and its bound ligand form a ternary complex with SecA, an ATPase which itself has affinity for SecY. When the complex binds the translocon, SecA is stimulated to undergo cycles of ATP binding and hydrolysis,3,4 resulting in the transfer of the ligand from SecB to SecA and subsequently through the translocon.
A remarkable feature of the binding of SecB to its ligands is that there is no apparent consensus in primary, secondary, or tertiary structure among the polypeptides that this chaperone has been shown to bind. SecB selects its ligands by virtue of their non-native state.5 The presence of the leader peptide in precursors is crucial in later steps of export, but in the initial binding of SecB the role of the leader is indirect. It retards the folding of the precursor allowing SecB to bind the polypeptide before it acquires its native state; SecB will not bind a stable, folded protein.6,7 Therefore, the rate of folding relative to the rate of binding SecB determines a kinetic partitioning between the pathway of export and the nonproductive pathway of folding in the cytosol.
SecB is a tetramer of four identical subunits (17 kDa monomeric mass) organized as a dimer of dimers.8,9 It has been shown that the region of contact between ligands and SecB is large, comprising minimally 150 aminoacyl residues in the mature portion of three ligands tested: maltose-binding protein,10 galactose-binding protein,11 and the oligopeptide-binding protein.12
The aminoacyl residues of SecB that are involved in contacts with precursor galactose-binding protein were identified using site-directed spin labeling and electron paramagnetic resonance (EPR) spectroscopy.13 The sites of contact are distributed over all surfaces of the chaperone (see Fig. 1). There are contacts in the channel formed at the interface of the dimers, which was the binding site proposed by Xu et al.9 based on examination of the crystal structure of SecB. Contacts identified on the ends of SecB would allow passage of a polypeptide from one channel to the symmetrically related channel on the opposite side. There are additional contacts on the flat sides, which are made up of the eight-stranded β-sheets of the dimers. Several possible routes that a polypeptide might traverse as it wraps around the chaperone from the channel on one side to the other are indicated by arrows in Figure 1.
Figure 1.

Possible pathways around SecB. Contact sites are shown in green. The residues that demonstrated no contact are shown in gray. The arrows indicate possible routes around the chaperone. (A), (B), and (C) are each related to the previous structure by a 90° rotation about the vertical axis to the left. (D) is related to (A) by a 90° rotation about the horizontal axis toward the viewer. (A) Front view of the deep channel; the interface of the dimers that form the tetramer is aligned with the vertical axis. (B) Flat 8-stranded β-sheet that is the side of the tetramer. (C) Channel of the dimer interface at the face opposite that shown in (A). (D) End view of the tetramer. Reproduced by permission of Crane JM, Suo Y, Lilly AA, Mao C, Hubbell WL, Randall LL, J Mol Biol 363: 63–74, 2006, © 2006 Elsevier.
During the process of protein export, SecB binds to many different unfolded ligands including outer membrane proteins as well as soluble periplasmic proteins. Here we used the strategy that was successfully used to map contacts of the precursor form of the periplasmic galactose-binding protein to investigate the binding interactions between SecB and other ligands. We show that the four ligands use the same interactive surface.
Results
SecB binds diverse unfolded polypeptides whether or not they carry a leader. It also binds to both integral membrane proteins and soluble proteins. Therefore, it is of interest to know whether the different ligands have affinity for different surfaces on SecB. To address this question, we compared the sites of interaction on SecB that bind precursor galactose-binding protein to those sites that are in contact with the mature, unfolded form lacking the leader as well as to sites of interaction with an integral outer membrane protein OmpA in its full length precursor form (proOmpA) and in a truncated version comprising only the transmembrane domain (OmpA-TM).
Studies using size-exclusion chromatography and calorimetry show that both precursor and mature forms of galactose-binding protein interact with SecB, if the polypeptides are presented in an unfolded state.11,14,15 Size-exclusion chromatography was used here to demonstrate complexes between SecB and proOmpA and between SecB and OmpA-TM. Each of the ligands was unfolded in 4M urea. The denaturant was diluted from the proteins in the presence of SecB and the mixture was subjected to size-exclusion chromatography. In both cases, complexes were resolved that eluted ahead of the free SecB (see Fig. 2). Dilution of the urea from proOmpA in the absence of SecB resulted in elution of the protein in the void volume of the column indicating that the proOmpA was in an aggregated state (data not shown). When OmpA-TM was diluted from urea and applied to the size-exclusion column, no protein was recovered. This can be explained if the aggregates that formed were sufficiently large to be trapped by the guard column. The molar mass of the complexes was determined by use of a multiangle static light scatter detector. The mass observed for complexes containing proOmpA and SecB was approximately 110 kDa, consistent with the SecB tetramer (69 kDa) bound to one proOmpA (37 kDa). The mass of the complex of SecB with OmpA-TM, 96 kDa, was in accord with that expected for one OmpA-TM (19 kDa) bound to the chaperone.
Figure 2.

Protein mixtures were subjected to size-exclusion chromatography and the eluent was monitored to determine protein concentration by change in refractive index and molar mass by static light scatter. The traces represent the concentrations and the symbols represent the molar masses. The protein samples were applied at 12 μM each in 100 μL. The samples were SecB tetramer (blue); SecB tetramer and OmpA-TM (red); and SecB tetramer and proOmpA (green).
Contact sites between SecB and each of the four ligands discussed here were identified by comparative analyses of the spectra of spin-labeled variants of SecB free in solution and in complex with each of the polypeptide ligands. We examined a set of 40 spin-labeled variants of SecB that were used to map the binding interface for precursor galactose-binding protein.13 Each of the variants used here was analyzed in that study by size-exclusion chromatography and was shown to be properly folded and active in binding the precursor.
The line shape of an EPR spectrum reflects the mobility of the nitroxide on the nanosecond time scale,16,17 including both the rotation around bonds within the nitroxide side chain and local fluctuations of the backbone. The term “mobility” includes both the amplitude of the movement of the nitroxide as well as the rate of the movement. A line shape that reflects high mobility can arise from either large amplitude, slow movement or rapid movement of small amplitude. As the mobility of the nitroxide is constrained, either in amplitude or in rate, the spectrum broadens. All spectra are normalized, therefore, this broadening is readily visible as a decrease in the intensity of the central line. In addition, the overall spectral breadth increases, which is observed as an increase of intensity in the hyperfine extrema (Fig. 3, arrows). Changes in spectral line shape that are observed by visual inspection were used to identify residues that become constrained within a complex. We previously classified the changes we observed on formation of complexes between SecB and precursor galactose-binding protein into three qualitative groups: high degree of constraint, that is, those that show a high degree of visible change; significant constraint, that is, those that show changes that are less readily seen; and no change.13 We maintain those three categories in this work.
Figure 3.

Positions on SecB that show a high degree of constraint. The black traces are spectra of spin-labeled SecB alone and the red traces are a mixture of spin-labeled SecB and the unfolded ligand indicated. The arrows indicate the increase in intensity at the low field hyperfine extrema.
The simplest interpretation of a constraint is that the position carrying the spin label is involved in a contact or is very close to a residue that is. It is a formal possibility that constraints on the nitroxide result not from a direct contact but from a conformational change. In the case of a conformational change, both constraints and increases in mobility should be observed. For that reason, it is crucial to examine many sites so that patterns will emerge. In the 40 sites examined, only constraints were observed.
All spin-labeled variants were examined in complex with proOmpA and in all cases the degree of constraint was assigned to the same category as that observed in complexes with precursor galactose-binding protein (Table I). As examples, we show four of the 12 residues assigned to the category high constraint (see Fig. 3). The constraints broadened the spectra as reflected in the decreased amplitudes of the center lines and the increase in intensity at the low field hyperfine extrema (Fig. 3, indicated by arrows). Figure 4 shows, as examples, spin-labeled variants SecBK34 and SecBT92 representative of the category significant constraint and a variant, SecBS2, that showed no change in spectral line shape when in complex with ligands.
Table I.
Residues Tested for Constraint by Ligand Binding
| GBP |
OmpA |
|||
|---|---|---|---|---|
| pa | mb | p | TMc | |
| Strongd | ||||
| Q14 | + | + | + | + |
| Q33 | + | + | + | + |
| K41 | + | + | + | + |
| L42 | + | + | + | + |
| D43 | + | + | + | + |
| D45 | + | + | + | + |
| T65 | + | + | + | + |
| S85 | + | + | + | + |
| A87 | + | + | + | + |
| L126 | + | + | ||
| N127 | + | + | ||
| P130 | + | + | ||
| Significantd | ||||
| T10 | + | + | ||
| D20 | + | + | ||
| E24 | + | + | ||
| K34 | + | + | + | + |
| D35 | + | + | ||
| T46 | + | + | + | * |
| T63 | + | + | ||
| S67 | + | + | ||
| E77 | + | + | + | + |
| Q79 | + | + | ||
| T92 | + | + | + | + |
| C97 | + | + | ||
| L136 | + | + | + | * |
| L141 | + | + | + | + |
| Q144 | + | + | ||
| No Changed | ||||
| S2 | * | * | * | * |
| M9 | * | * | ||
| Q50 | * | * | * | * |
| V59 | * | * | * | * |
| L75 | * | * | * | * |
| N104 | * | * | ||
| Q143 | * | * | * | * |
| G146 | * | * | * | * |
| E147 | * | * | * | |
| G148 | * | * | ||
| T149 | * | * | ||
| E150 | * | * | ||
| A155 | * | * | ||
A change in spectral line shape observed that is consistent with the category is indicated with a plus symbol (+). An asterisk (*) indicates no change in spectral line shape was observed. The data included for precursor galactose-binding protein were previously published.13
p indicates the precursor form of either galactose-binding protein (GBP) or OmpA.
m indicates the mature form of galactose-binding protein (GBP).
TM indicates the truncated OmpA, OmpA-TM.
The residues are divided according to the degree of constraint.
Figure 4.

Positions on SecB that show either significant constraint or no change. The black traces are spectra of spin-labeled SecB alone and the red traces are a mixture of spin-labeled SecB and the unfolded ligand indicated. SecBK34 and SecBT92 show significant constraint and SecBS2 shows no significant change in spectral line shape when in complex with ligands.
The mature form of galactose-binding protein does not contain a leader sequence and yet for all of the variants tested the same pattern of constraints was observed as that observed for SecB in complex with the precursor. The tested species (see Table I and Figs. 3 and 4) included nine of the 12 strong constraints, six of the significant constraints and six of the variants showing no change (see Table I and Figs. 3 and 4). These observations indicate that the leader does not play a role in selection of the surface of interaction.
The outer membrane OmpA protein has two domains, one an integral membrane domain organized as an eight-stranded β-barrel (Residues 1–176) and a C-terminal domain which extends into the aqueous periplasmic space (Residues 177–325). It is possible that when OmpA engages SecB it is bound through the periplasmic domain. This would account for the similarity of contact sites observed in the complexes containing either precursor galactose-binding protein or proOmpA. To investigate this possibility, we used a truncated form of OmpA (OmpA-TM) that has both the leader sequence and the periplasmic domain deleted leaving only the 176 aminoacyl residues of the β-barrel.18
OmpA-TM showed a high degree of constraint with all nine residues tested in the strong constraint category (Table I, Fig. 3) indicating that the membrane domain can bind a surface of SecB similar to that bound by soluble proteins. When OmpA-TM was examined in complex with variants classified as showing significant but small constraints two differences were noted. SecB variants with nitroxides substituted at residues T46 and L136 showed no change in line shape when in complex with OmpA-TM whereas they showed constraint with the other ligands tested (see Fig. 5). In complex with variants carrying substitutions at the other positions that were tested, the pattern for all ligands was the same.
Figure 5.

Positions on SecB that show no change in complex with OmpA-TM. The black traces are spectra of spin-labeled SecB alone and the red traces are a mixture of spin-labeled SecB and the unfolded ligand indicated.
To ensure that the observed changes in line shape reflect selective binding of unfolded proteins to SecB, a small folded protein, CheB (37 kDa), was added to eight spin-labeled SecB variants and was shown to cause no change in spectral line shape. Figure 6 shows this control carried out with four variants, which showed strong constraint when in complex with unfolded protein. There was also no change in line shape observed when CheB was mixed with the SecB variant carrying a spin label at position Q79, which showed significant constraint with unfolded ligands (data not shown). Three variants (S2, N104, and Q143) that showed no change in line shape with unfolded ligands also showed no change when mixed with CheB (data not shown). Our previous work13 presented further evidence that the constraints reflect the binding of unfolded polypeptides in that the constraints seen with precursor galactose-binding protein were abolished by the inclusion of 3 mM CaCl2 in the reaction mixture. The CaCl2 causes galactose-binding protein to partition to the folded state, thereby eliminating binding to SecB.14
Figure 6.

Constraints on SecB require unfolded ligands. The black traces are spectra of spin-labeled SecB alone and the red traces are a mixture of spin-labeled SecB and the folded protein CheB.
The spectrum of a complex is a composite of the spectrum of the free nitroxide residues and the spectrum of the residues that are constrained. Thus one can reveal the spectrum of the constrained sites by examining the difference between the composite spectrum of the complex and the spectrum of SecB alone reduced incrementally until the difference spectrum observed has the features of a reasonable line shape. Figure 7 shows titrated spectra for E24 and K41 as examples. Other sites that showed changes large enough to allow titration were Q33, D35, L42, D45, and A87 (data not shown). The amount subtracted to reveal the constrained line shapes was between 30 and 75% for all residues indicating that a considerable fraction of the spectra reflects nitroxide residues that were not constrained. The concentrations of SecB and of ligand (20 μM each) in these experiments were well above the dissociation constants and therefore the entire population of SecB should be in complex with ligand.
Figure 7.
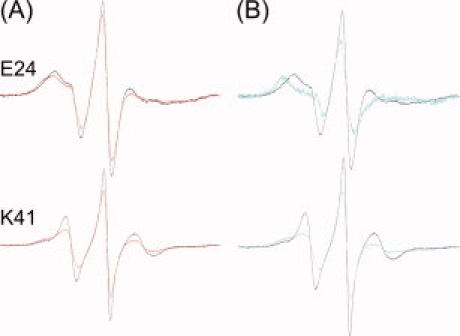
Titration of spectra to reveal the state of the nitroxide in contact sites. (A) The black traces are spectra of the indicated spin-labeled SecBs alone and the red traces are spectra of mixtures containing spin-labeled SecB and proOmpA. (B) The black traces are spectra of the indicated spin-labeled SecBs alone and the blue traces are the titrated spectra that represent the line shape of the constrained residues only. The titrated spectra were normalized to have the same total spin as the spectra of the complexes.
Discussion
SecB exhibits the remarkable ability to bind a diverse group of ligands recognizing them by virtue of the fact that they have non-native structure. The work presented here in combination with the previously published contact surface on SecB for precursor galactose-binding protein13 demonstrates that all ligands examined make contacts with SecB over the same large interactive surface. There is no selectivity based on the presence or absence of a leader sequence or on the nature of the polypeptide when it is matured and folded into its native state. The periplasmic binding protein exhibits the same contacts as does the intact outer membrane protein OmpA. The truncated form comprising only the transmembrane β-barrel showed only two minor differences (SecBT46 and SecBL136). Thus it appears that there is no selectivity by any ligand for particular areas on SecB.
Even though there are no specific sequences that mediate interaction with SecB, the binding to physiologic ligands is characterized by dissociation constants in the range of 5 nM to 30 nM.15 This high affinity coupled with low specificity has been attributed to the existence of multiple subsites of interaction. Studies with short peptide ligands19 showed that the SecB tetramer binds one peptide per monomer with micromolar affinity, whereas one long polypeptide ligand is bound to a tetramer with nanomolar affinity. The interaction of SecB with long stretches of polypeptides that simultaneously occupy all subsites would have an affinity reflecting the sum of the binding energies of the subsites. The large surface area of SecB identified as making contact with all of the ligands tested is consistent with the existence of multiple subsites of interaction. It should be noted that the residues constrained by ligands define the regions of contact, but provide no information as to which contacts contribute to the energy of stabilization of the complexes. Figure 8 displays the interactive surface on SecB with the residues colored to indicate the nature of the side chain that was substituted by the nitroxide (hydrophobic, gold; polar, blue; positively charged, bright blue; and negatively charged, red brown). Thus, consistent with the lack of specificity in binding, stabilization of the complex could arise from hydrophobic interactions, ionic bonds, and hydrogen bonds. It is not likely that the entire large surface is occupied in any one complex between SecB and a ligand. It reflects the population comprising many complexes in which ligands follow different routes around the SecB chaperone. Several possible routes are illustrated in Figure 1. If we consider any one complex it appears that a single unfolded polypeptide ligand does not make contact with all four symmetrically related residues as indicated by deconvolution of spectra into the contributions from the free residues and residues that are constrained.
Figure 8.

Chemical nature of the aminoacyl residues involved in contact. The contact sites displayed are those that have been tested with both periplasmic galactose-binding protein and the outer membrane protein OmpA. The CPK models are colored to indicate the nature of the aminoacyl residue (hydrophobic, gold; polar, blue; positively charged, bright blue; and negatively charged, red brown). The residues colored gray showed no contact when tested. The structure on the left is a view of the dimer interface. The view in the center was achieved by a 90° rotation about the vertical axis to show the flat β-sheet on the side of the tetramer. The view on the right is related to that on the left by a 90° rotation about the horizontal axis toward the viewer.
The Sec general secretory system cannot export folded proteins.2,20 SecB functions as a chaperone, holding ligands in a non-native state and delivering them to SecA for transfer through the SecYEG channel. To successfully compete with the irreversible processes of protein folding and aggregation the binding to SecB must be very rapid. The rate constant for association of the unfolded maltose-binding protein (mass, 41 kDa) has been estimated to be 108 M−1 s−1 or higher5 and that for a small model ligand, bovine pancreatic trypsin inhibitor (6.5 kDa), was determined to be diffusion limited (i.e., 5 × 109 M−1 s−1).21 The rate constants of association observed for most protein–protein complexes (105 to 107 M−1 s−1) are lower than the rate constants of collision. As binding interfaces usually cover only 5% to 20% of the surface of each of the interacting partners, only 1% of collisions would involve reactive surfaces.22 Approximately 50% of two ligands, galactose-binding protein and maltose-binding protein, are in contact with SecB10,11 and here we show that the sites of interaction are distributed over a very large surface of SecB. Thus it is likely that most encounters are productive accounting for the observed high rate constant of association.
In conclusion, the large interactive surface on SecB identified here is crucial to the remarkable ability of the chaperone to bind a wide diversity of ligands very rapidly, with high affinity and yet low specificity.
Materials and Methods
Mutagenesis and protein purification
To map the contact sites between SecB and each of the four ligands examined, we used a collection of SecB variants that each carried a single accessible cysteine13,23 that was substituted by reaction with a sulhydryl-specific nitroxide reagent. The construction of the cysteine mutants as well as the verification of the activity of the resultant single-accessible cysteine SecB variants were described in previous publications.13,23 The species of SecB were purified from the appropriate strains as described for wild-type SecB.24 The following ligands were purified as described: precursor galactose-binding protein,14 mature galactose-binding protein,11 and proOmpA.25 The ligand OmpA-TM was purified as described18 with the modifications given here. The cell pellet was suspended in 50 mM Tris (HCl), pH 9, and stored at −80°C. The cell suspension was thawed on ice and the following additions were made: dithiothreitol (DTT) to 2 mM, DNase to 10 μg mL−1, Mg(OAc)2 to 3 mM, and phenylmethylsulfonylfluoride (PMSF) to 1 mM final concentrations. The cells were mechanically disrupted by French Press at 8000 psi in two successive passes with DTT added (to 2 mM) between each pass. The lysate was incubated for 2 h on ice with 1 mM CaCl2 and 25 μg mL−1 micrococcal nuclease and then centrifuged three times using a Sorvall SS-34 at 1800g for 10 min at 4°C. The supernatant was discarded after each spin and after each of the first two spins the pellet was suspended in 50 mM Tris (HCl), pH 7. The final pellet was suspended in 10 mL of 8M urea, 20 mM Tris (HCl), pH 8, 2 mM DTT. An equal volume of isopropanol was added and the suspension was incubated at 55°C for 30 min and then centrifuged at 4°C for 8 min using a Beckman TL100.4 rotor at 417,000g. A HiTrap QFF column (GE Healthcare, Piscataway, NJ) was used to purify the protein. The equilibration buffer was equal parts (v/v) of 8M urea, 15 mM Tris (HCl), pH 8.5, 2 mM DTT and isopropanol. The wash buffer was 8M urea, 15 mM Tris (HCl), pH 8.5, 2 mM DTT. The protein was eluted using a NaCl gradient from 0–150 mM in 8M urea, 15 mM Tris (HCl), pH 8.5, 2 mM DTT. The purest fractions were pooled and concentrated using a Centriprep YM-10 (Millipore, Billerica, MA) concentrator. The protein was dialyzed against 4M urea, 10 mM Hepes (KOH), pH 7.6, 100 mM KOAc, and stored at −80°C.
Protein concentrations were determined spectrophotometrically at 280 nm using extinction coefficients of 47,600 M−1 cm−1 for SecB tetramer, 37,410 M−1 cm−1 for unfolded and mature forms of precursor galactose-binding protein, 52,955 M−1 cm−1 for proOmpA, 24,870 M−1 cm−1 for OmpA-TM, and 13,700 M−1 cm−1 for CheB.
Spin-labeling of SecB variants
The single-accessible SecB species used here were spin-labeled as described previously13,23 using the nitroxide reagent (1-oxyl-2,2,5,5-tetramethylpyrroline-3-methyl)-methanethiosulfonate (Toronto Research Chemicals, Canada). The methanethiosulfonate spin-label reagent and the nitroxide side chain it generates are shown in Figure 9.
Figure 9.
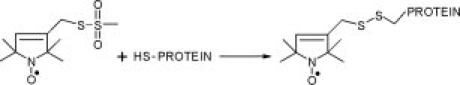
The methanethiosulfonate spin label and the side chain it generates.
EPR measurements
EPR spectroscopy was performed on a Bruker EMX X-band spectrometer with a high sensitivity resonator. Protein samples of 5 μL were loaded into synthetic silica capillaries (0.6 mm i.d. × 0.84 mm o.d., VitroCom, Mountain Lakes, NJ) sealed at one end. All spectra were acquired using incident microwave power at 20 mW, and a 100 kHz field modulation of 1 to 3 gauss as appropriate. Each capillary was scanned at 6°C using a scan width of 100 gauss centered at 3356 gauss. Fifteen scans were accumulated for each spectral line. All spectra were normalized and further analyzed using the Labview programs written by Christian Altenbach (UCLA).
For each experiment, spin-labeled SecB was used at a final concentration of approximately 20 μM tetramer (80 μM spin label), which was sufficient to generate a strong EPR signal. The polypeptide ligand, unfolded in denaturant (1M GnHCl for galactose-binding protein or 4M urea for OmpA), was added at 1.4-fold to 2-fold molar excess by rapid dilution into a solution held on ice containing spin-labeled SecB. The final concentration of denaturant was 0.17M for GnHCl or 0.4M for urea. The basic buffer was 10 mM Hepes (HAc), pH 6.7, 300 mM KOAc, 5 mM Mg(OAc)2. Additional components that were introduced with the ligands were also added to the solution of SecB alone so that conditions with and without ligand were identical. The same conditions were used for experiments with CheB except that the CheB was added in its native state to a solution of spin-labeled SecB that contained either 0.17M GnHCl or 0.4M urea. The presence of either denaturant did not cause a change in line shape of spin-labeled SecB in the absence of ligand.
Analyses of EPR spectral line shapes
The molecular tumbling of SecB (τc is 60 ns) is too slow to be averaged into the spectra. Therefore, the line shapes were interpreted in terms of mobility of the nitroxide. The timescale of tumbling (τc) for SecB was calculated from the rotational diffusion constant (Dr) of the protein as follows:
where k is Boltzmann's constant, T is temperature, η is the viscosity of the solvent, and a is the radius of hydration. We have determined the radius of hydration of SecB to be 3.3 nm by quasi-elastic light scatter using an in-line detector (QELS, Wyatt Technology, Santa Barbara, CA) following chromatography on a BioSep-SEC-S4000 size-exclusion column (7.8 mm i.d. × 30 cm, Phenomenex, Torrance, CA) in 10 mM Hepes (KOH), 300 mM KOAc, 5 mM Mg(OAc)2, pH 7.6 at 7°C. The ASTRA software provided with the instrument was used to calculate the hydrodynamic radius from the diffusion coefficient measured for material at the apex of the chromatographic peak.
In the course of this work, SecBT65 was reclassified to the strong constraint category for all ligands. In the original work, the small constraint observed was the result of a low signal to noise ratio in addition to the presence of free spin. The nitroxide free in solution gives three very sharp lines. The sharp signal of free nitroxide overlaps the line shape of the spin-labeled protein and makes it difficult to see changes in the line shape. As the free spin has three sharp lines and a nitroxide on a protein has a much broader spectrum, a small percentage of free spin dominates the line shape. The Labview software from Christian Altenbach provides a means to subtract free spin up to approximately 2–3%. Free spin in excess of 5% interferes with interpretation of the data.
Size-exclusion chromatography and molar mass determination
High-performance liquid chromatography was performed on a TSK 3000SWXL column (7.5 mm i.d. × 30 cm, Tosoh, Japan) in 0.35M NH4Ac, pH 7.0. The absolute molar mass of proteins was determined directly using static light scatter by passing the eluent through a multiangle laser light scatter detector followed by a differential refractometer (DAWN-EOS and Optilab rEX, respectively; Wyatt Technology, Santa Barbara, CA). The molar mass was determined using a specific refractive index increment (dn/dc) of 0.19 mL gm−1 and the Debye plotting formalism of the Astra software supplied with the instrument. The relationship between the weight average molar mass (Mw) and the excess Rayleigh ratio R(θ) at the low protein concentrations used here is given by:
where R(θ) is the light scattered by the solution at angle θ in excess of that scattered by pure solvent divided by the incident light intensity, c is the concentration of protein, P(θ) is the form factor that describes the angular dependence of the scatter, and K* is a constant dependent on the parameters of the system used in the study.
Acknowledgments
The authors thank Lukas K. Tamm for plasmid pET111, which expresses a variant of the transmembrane domain of OmpA that has a single tryptophan at position 7 and has phenylalanine substituted for the remaining four tryptophans. Purified CheB was the generous gift from Gerald L. Hazelbauer.
Glossary
Abbreviations:
- EPR
electron paramagnetic resonance
- proOmpA
precursor outer membrane protein A
- OmpA-TM
outer membrane protein A transmembrane domain
- GBP
galactose binding protein
- PMSF
phenylmethylsulfonylfluoride
- CaCl2
calcium chloride
- Mg(OAc)2
magnesium acetate
- DTT
dithiotreitol
- KAOc
potassium acetate
- GnHCl
guanidine hydrochloride.
References
- 1.Papanikou E, Karamanou S, Economou A. Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol. 2007;5:839–851. doi: 10.1038/nrmicro1771. [DOI] [PubMed] [Google Scholar]
- 2.Randall LL, Hardy SJS. SecB, one small chaperone in the complex milieu of the cell. Cell Mol Life Sci. 2002;59:1617–1623. doi: 10.1007/PL00012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 4.Economou A, Pogliano JA, Beckwith J, Oliver DB, Wickner W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell. 1995;83:1171–1181. doi: 10.1016/0092-8674(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 5.Randall LL, Hardy SJS. High selectivity with low specificity: how SecB has solved the paradox of chaperone binding. Trends Biochem Sci. 1995;20:65–69. doi: 10.1016/s0968-0004(00)88959-8. [DOI] [PubMed] [Google Scholar]
- 6.Liu GP, Topping TB, Cover WH, Randall LL. Retardation of folding as a possible means of suppression of a mutation in the leader sequence of an exported protein. J Biol Chem. 1988;263:14790–14793. [PubMed] [Google Scholar]
- 7.Diamond DL, Randall LL. Kinetic partitioning. Poising SecB to favor association with a rapidly folding ligand. J Biol Chem. 1997;272:28994–28998. doi: 10.1074/jbc.272.46.28994. [DOI] [PubMed] [Google Scholar]
- 8.Múren EM, Suciu D, Topping TB, Kumamoto CA, Randall LL. Mutational alterations in the homotetrameric chaperone SecB that implicate the structure as dimer of dimers. J Biol Chem. 1999;274:19397–19402. doi: 10.1074/jbc.274.27.19397. [DOI] [PubMed] [Google Scholar]
- 9.Xu Z, Knafels JD, Yoshino K. Crystal structure of the bacterial protein export chaperone SecB. Nat Struct Biol. 2000;7:1172–1177. doi: 10.1038/82040. [DOI] [PubMed] [Google Scholar]
- 10.Topping TB, Randall LL. Determination of the binding frame within a physiological ligand for the chaperone SecB. Protein Sci. 1994;3:730–736. doi: 10.1002/pro.5560030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khisty VJ, Munske GR, Randall LL. Mapping of the binding frame for the chaperone SecB within a natural ligand, galactose-binding protein. J Biol Chem. 1995;270:25920–25927. doi: 10.1074/jbc.270.43.25920. [DOI] [PubMed] [Google Scholar]
- 12.Smith VF, Hardy SJS, Randall LL. Determination of the binding frame of the chaperone SecB within the physiological ligand oligopeptide-binding protein. Protein Sci. 1997;6:1746–1755. doi: 10.1002/pro.5560060815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crane JM, Suo Y, Lilly AA, Mao C, Hubbell WL, Randall LL. Sites of interaction of a precursor polypeptide on the export chaperone SecB mapped by site-directed spin labeling. J Mol Biol. 2006;363:63–74. doi: 10.1016/j.jmb.2006.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topping TB, Randall LL. Chaperone SecB from Escherichia coli mediates kinetic partitioning via a dynamic equilibrium with its ligands. J Biol Chem. 1997;272:19314–19318. doi: 10.1074/jbc.272.31.19314. [DOI] [PubMed] [Google Scholar]
- 15.Randall LL, Topping TB, Suciu D, Hardy SJS. Calorimetric analyses of the interaction between SecB and its ligands. Protein Sci. 1998;7:1195–1200. doi: 10.1002/pro.5560070514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider D, Freed J. Calculating slow motional resonance spectra: a user's guide. In: Berliner L, Reuben J, editors. Spin labeling: Theory and application, biological magnetic resonance. New York: Plenum; 1989. pp. 1–76. [Google Scholar]
- 17.Fajer PG. Electron spin resonance spectroscopy labeling in peptide and protein analysis. In: Meyers RA, editor. Encyclopedia of analytical chemistry. London: Wiley; 2000. pp. 5725–5761. [Google Scholar]
- 18.Arora A, Abildgaard F, Bushweller JH, Tamm LK. Structure of outer membrane protein A transmembrane domain by NMR spectroscopy. Nat Struct Biol. 2001;8:334–338. doi: 10.1038/86214. [DOI] [PubMed] [Google Scholar]
- 19.Randall LL. Peptide binding by chaperone SecB: implications for recognition of nonnative structure. Science. 1992;257:241–245. doi: 10.1126/science.1631545. [DOI] [PubMed] [Google Scholar]
- 20.Hardy SJS, Randall LL. A kinetic partitioning model of selective binding of nonnative proteins by the bacterial chaperone SecB. Science. 1991;251:439–443. doi: 10.1126/science.1989077. [DOI] [PubMed] [Google Scholar]
- 21.Fekkes P, den Blaauwen T, Driessen AJ. Diffusion-limited interaction between unfolded polypeptides and the Escherichia coli chaperone SecB. Biochemistry. 1995;34:10078–10085. doi: 10.1021/bi00031a032. [DOI] [PubMed] [Google Scholar]
- 22.Janin J, Chothia C. The structure of protein-protein recognition sites. J Biol Chem. 1990;265:16027–16030. [PubMed] [Google Scholar]
- 23.Crane JM, Mao C, Lilly AA, Smith VF, Suo Y, Hubbell WL, Randall LL. Mapping of the docking of SecA onto the chaperone SecB by site-directed spin labeling: insight into the mechanism of ligand transfer during protein export. J Mol Biol. 2005;353:295–307. doi: 10.1016/j.jmb.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Randall LL, Crane JM, Lilly AA, Liu G, Mao C, Patel CN, Hardy SJS. Asymmetric binding between SecA and SecB two symmetric proteins: implications for function in export. J Mol Biol. 2005;348:479–489. doi: 10.1016/j.jmb.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 25.van der Does C, de Keyzer J, van der Laan M, Driessen AJ. Reconstitution of purified bacterial preprotein translocase in liposomes. Methods Enzymol. 2003;372:86–98. doi: 10.1016/s0076-6879(03)72005-9. [DOI] [PubMed] [Google Scholar]


