Abstract
Kv4.3, with its complex open- and closed-state inactivation (CSI) characteristics, is a primary contributor to early cardiac repolarization. The two alternatively spliced forms, Kv4.3-short (Kv4.3-S) and Kv4.3-long (Kv4.3-L), differ by the presence of a 19-amino acid insert downstream from the sixth transmembrane segment. The isoforms are similar kinetically; however, the longer form has a unique PKC phosphorylation site. To test the possibility that inactivation is differentially regulated by phosphorylation, we expressed the Kv4.3 isoforms in Xenopus oocytes and examined changes in their inactivation properties after stimulation of PKC activity. Whereas there was no difference in open-state inactivation, there were profound differences in CSI. In Kv4.3-S, PMA reduced the magnitude of CSI by 24% after 14.4 s at −50 mV. In contrast, the magnitude of CSI in Kv4.3-L increased by 25% under the same conditions. Mutation of a putatively phosphorylated threonine (T504) to aspartic acid within a PKC consensus recognition sequence unique to Kv4.3-L eliminated the PMA response. The change in CSI was independent of the intervention used to increase PKC activity; identical results were obtained with either PMA or injected purified PKC. Our previously published 11-state model closely simulated our experimental data. Our data demonstrate isoform-specific regulation of CSI by PKC in Kv4.3 and show that the carboxy terminus of Kv4.3 plays an important role in regulation of CSI.
Keywords: recovery from closed-state inactivation, open-state inactivation, carboxy terminus
the rapidly inactivating, voltage-dependent K+ channels in cardiac and smooth muscle myocytes and neurons play important and different roles in these diverse cell types (2, 9, 10, 18, 20, 28, 30, 34, 47). In cardiac myocytes, the rapidly inactivating K+ current (Ito) contributes to the early and subsequent phases of repolarization and, as a result, to excitation-contraction coupling (16, 28, 42, 46). The pore-forming α-subunits Kv4.2 and/or Kv4.3 are the primary contributors to early repolarization in the heart of many animal species and humans (11, 31, 32, 39, 49, 50). Much has been learned about our understanding of Kv4.2 and Kv4.3 channel gating. However, Kv4 inactivation is very complex, and the underlying mechanisms are still incompletely resolved (5, 9, 17, 18, 20, 21, 22, 34, 49).
Kv4 inactivation mechanisms are different from those in other rapidly inactivating Kv channels, such as Shaker and Kv1.4. In these channels, the rapid and slow components of inactivation result from a ball and chain mechanism occluding the inner vestibule (N-type), and pore closure (C-type) (7, 12, 19, 23, 25, 30, 40, 41, 46, 52). In contrast, Kv4 inactivation is more complex; deletion of either the amphipathic region of the NH2 terminus of Kv4.1 or a largely hydrophilic region in the COOH terminus abolishes the rapid component of inactivation (20). In contrast, other data suggest that the fast component of inactivation is due to occlusion of the inner vestibule by the NH2 terminus (20). Furthermore, the slower components of inactivation in Kv4 differ from C-type inactivation reported for Shaker K+ channels, where pore closure plays a critical role (7, 17, 19, 23, 25, 40, 44, 52). In contrast to Shaker K+ channels, there is a substantial degree of inactivation in the Kv4 family that occurs from closed states (5, 6, 9, 17, 49). Finally, unlike conventional N- and C-type inactivation mechanisms, both of which have the same coupling to activation (36), Kv4 inactivation states are coupled to different states in the activation pathway (5, 9, 49).
There are two variants of Kv4.3, short (Kv4.3-S) and long (Kv4.3-L), in both rat and human hearts (36, 47). The long isoform contains a 19-amino acid insert after amino acid 487 in the COOH terminus, with a consensus protein kinase C (PKC) phosphorylation site (36, 47). Previous work showed that activation of PKC with phorbol esters had a selective effect on the isoform of human Kv4.3 expressed in mouse Ltk− fibroblasts, i.e., only the long form of hKv4.3 showed a decrease in peak current and a shift in the availability and recovery from open-state inactivation (36). The selectivity of effect seen with phorbol esters on open-state inactivation in Kv4.3-L suggested that a PKC phosphorylation site in the long isoform played an essential role in the modulation of open-state inactivation properties of Kv4.3 (36, 47). However, these experiments did not examine the effects of an increased PKC activity on closed-state inactivation in the two Kv4.3 isoforms, which could provide valuable insights into the mechanisms underlying channel inactivation.
In our previous work, we resolved different isochronal inactivation relationships in Kv4.3 using pulses with different durations (49). Our data indicated that Kv4.3 inactivation can occur from either the closed or open state, with identified multiple inactivation components. However, multiple inactivation pathways with different coupling to activation raise questions about the underlying mechanisms, i.e., are the different inactivation mechanisms coupled to each other, or are they independent? If the mechanisms are independent, we hypothesized that there would be interventions capable of modifying closed-state inactivation without changing open-state inactivation. If so, such an effect might be achieved by phosphorylation of a site(s) within the COOH terminus (36, 47). Such understanding of the modulation of closed-state inactivation would provide novel insight into the regulation of Kv4 current in smooth muscle myocytes, neurons, and myocytes in ischemic myocardium. Thus we set out to examine the effects of phorbol 12-myristate 13-acetate (PMA) and injected PKC on open- and closed-state inactivation to gain further insight into the mechanisms of inactivation gating in Kv4.3 channels.
We have shown that an increase in PKC activity has the same effects on peak current-voltage relationship (I-V), open-state inactivation, and recovery from open-state inactivation in both isoforms of Kv4.3. Surprisingly, an increase in PKC activity had opposite effects on closed-state inactivation and recovery from closed-state inactivation in the two isoforms. Application of PMA decreased the magnitude of closed-state inactivation in Kv4.3-S, whereas its magnitude was increased in Kv4.3-L. When the threonine in the primary consensus PKC phosphorylation site in Kv4.3-L was mutated to alanine (Kv4.3-L[T504A]), the effect of increased PKC activity was similar to that for the Kv4.3-S. More importantly, mutation of T504 to aspartic acid, a change that often mimics phosphorylation of a serine or threonine, did not respond to PMA. These observations provide important insights into the regulation of Kv4.3 gating. The differences in response of closed-state inactivation to increased PKC activity imply that the putative phosphorylation site within the 19-amino acid insert in the COOH terminus of Kv4.3-L plays an important role in closed-state inactivation of the channel. Our previously published 11-state Kv4.3 model (49) closely simulates our experimental data and, as a result, the effects of PMA on the gating of the two isoforms of Kv4.3. Our data show that increased PKC activity can modulate recovery from closed-state inactivation and, as a consequence, closed-state inactivation independently of activation and open-state inactivation.
MATERIALS AND METHODS
RNA preparation and channel expression.
Wild-type (WT) cDNA of rat Kv4.3 (short form) was a gift of Dr. David McKinnon (SUNY Stony Brook, Stony Brook, NY), and its use has been previously described (14, 38). Synthetic mRNA was prepared using a mMESSAGE mMACHINE T7 kit (Ambion, Austin, TX). Kv4.3-L and Kv4.3 mutant channels with mutated PKC consensus phosphorylation sites in the 19-amino acid insert 81 amino acids downstream from the sixth transmembrane segment in the COOH terminus (Kv4.3-L[T504A], Kv4.3-L[S503A], Kv4.3-L[S499A], and Kv4.3-L[T504D]) were prepared by performing PCR-based site-directed mutagenesis as previously described (13, 33).
Xenopus laevis were handled in compliance with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals and National Institutes of Health Guide for the Care and Use of Laboratory Animals, as well as with University at Buffalo Institutional Animal Care and Use Committee Guidelines. All protocols were approved by the University at Buffalo State University of New York Institutional Animal Care and Use Committee. Mature female X. laevis (Xenopus One, Ann Arbor, MI) were anesthetized by immersion in a solution of ethyl 3-aminobenzoate methane sulfonate salt (1.5 g/l; Sigma-Aldrich, St Louis, MO), and ovarian lobes were removed through a small incision in the abdominal wall as previously described (37, 38). The lobes were placed in a collagenase-containing, Ca2+-free OR2 solution [in mM: 82.5 NaCl, 2 KCl, 1 MgCl2, 5 HEPES, pH 7.4, with 1–2 mg/ml collagenase (type II; Sigma, St Louis, MO)] to remove the follicular layer. The solution containing the oocytes was gently agitated for about 1.5 h, and collagenase activity was then arrested by bovine albumin as previously described (14, 38). Defolliculated oocytes (stages V and VI) were then injected with synthetic mRNA (up to 25 ng) using a Nanoject microinjection system (Drummond Scientific, Broomall, PA) and incubated at 18°C for 24–72 h in an antibiotic-containing Barth's solution [in mM: 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.82 MgSO4, 0.33 Ca(NO3)2, 0.41 CaCl2, and 10 HEPES, pH 7.4, with 1% antibiotic-antimycotic solution (Invitrogen)].
Solution preparation.
Solutions containing 4α-phorbol 12,13-didecanoate (4α-PDD; Sigma-Aldrich) and PMA (Calbiochem, EMD Biosciences, La Jolla, CA) were prepared immediately before each experiment. Ten nanomolar PMA was selected as the concentration that produced a sustained and significant effect while minimizing nonspecific effects (1, 29, 36). PKC from rat brain (Calbiochem) was stored at −70°C and thawed immediately before use. Protein concentration was 35 μg/ml with specific activity >1,000 U/mg protein. A Nanoject II was used to inject 27.6 nl of PKC solution or 0.97 ng of PKC into each oocyte. An inactivated PKC solution was prepared by heating the solution to 50°C for 30 min and then cooling to room temperature before injection (51). The solution in which PKC was dissolved was prepared with 100 mM NaCl, 20 mM Tris·HCl, 1 mM DTT, 500 μM EDTA, 500 μM EGTA, and 10% glycerol, pH 7.5.
Electrophysiological techniques.
Oocytes were voltage-clamped using a two-microelectrode high-performance oocyte clamp amplifier (CA1-B; Dagan, Minneapolis, MN), as described in detail previously (14, 38). Microelectrodes were fabricated from 1.5-mm-outer diameter borosilicate glass tubing (TW150F-4; WPI) using a two-stage puller (L/M-3 P-A; Adams & List, Great Neck, NY) filled with 3 M KCl and having resistances of 0.6–1.5 MΩ. During recordings, oocytes were continuously bathed in control ND-96 solution (in mM: 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 10 HEPES, pH 7.4 adjusted with NaOH). Currents were recorded at room temperature (21–23°C) and filtered at 2.5 kHz.
Experimental protocols.
To evaluate the effects of PMA on heterologously expressed Kv4.3-S, Kv4.3-L, and Kv4.3-L[T504A] with a mutated consensus PKC phosphorylation site in the 19-amino acid insert, we performed voltage-clamp protocols before and after exposure to PMA (30 min) in ND-96 solution. The voltage-clamp protocol for measurement of closed-state inactivation consisted of four pulses [holding potential (HP) = −100 mV]: a pulse P1 (+40 mV, 800 ms in duration), P2 (−100 mV, 5,000 ms), P3 (−50 mV, 800–14,400 ms), and P4 (+40 mV, 800 ms). The pulse protocol was repeated every 30 s. Normalized currents for closed-state inactivation were determined as the ratio of peak currents during P4 to those during P1; these values are shown as a function of P3 duration. Kinetics of closed-state inactivation were obtained by fitting the normalized peak amplitudes as a function of P3 duration (49). Steady-state inactivation relationships for the open state were determined using a two-pulse protocol: current traces were obtained during depolarizing P1 pulses between −120 and +50 mV in 10-mV voltage steps (HP = −90 mV) for 2,000 ms, followed by a P2 pulse to +50 mV for 1,000 ms. Steady-state inactivation relationships were determined as the ratio of the maximum of IKv4.3 during P2, Imax2,Kv4.3, for the depolarization voltage during P1 to the maximum value of Imax2,Kv4.3, Imax2,Kv4.3(V = −120 mV). Steady-state inactivation relationships were fitted by the Boltzmann function fi(V) = 1/{1 + exp[(V1/2,i − V)/ki]}, where V1/2,i and ki are the half-inactivation potential and the slope factor, respectively. Conductance G(V) at each voltage was calculated from the equation G(V) = Imax1,Kv4.3/(V − EK), where V is the depolarization voltage during P1, Imax1,Kv4.3 is the maximum IKv4.3 current during P1, and EK = −100 mV, which is the reversal potential in ND-96. The voltage dependence of G/Gmax was obtained by normalization of G(V) to the maximal conductance value Gmax, the latter obtained by fitting G(V) with a Boltzmann function fa(V) = Gmax/{1 + exp[(V1/2 − V)/k]}4, where V1/2 and k are the half-activation potential and the slope factor, respectively. Normalized conductance G/Gmax obtained in this way approaches 1 as V approaches infinity and slightly differs from the values reported when G(V) was normalized to G(V = +50 mV) (48). Recovery from closed-state inactivation was obtained using a five-pulse protocol (HP = −90 mV): a pulse P1 (+50 mV, 500 ms in duration), P2 (−120 mV, 5,000 ms), P3 (−50 mV, 10,000 ms), P4 (varied from −80 to −60 mV and from 10 to 13,610 ms), and P5 (set at +50 mV for 500 ms). A 5,000-ms pulse P2 was chosen to enable the channel to recover from inactivation during P1. Recovery from open-state inactivation was measured using a two-pulse protocol: pulses to +50 mV (P1) for 800 ms from HP = −90 mV were followed by a second 200-ms depolarization (P2) to +50 mV with a variable time gap (49).
Data analysis.
Digitized data were collected and analyzed using pCLAMP 9 software (Molecular Devices) and stored in a computer. Unless otherwise stated, raw data traces from two-microelectrode voltage-clamp recordings were not leakage or capacitance subtracted. The pulse protocols and the equations that best fit the data are presented. Data are shown as means ± SE. Confidence levels were calculated using Student's t-test.
Computer simulations.
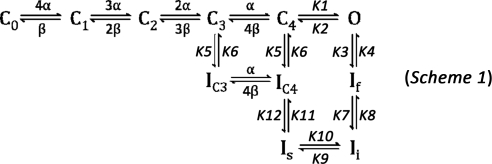
Our Markov model (49) of Kv4.3 gating was implemented using FORTRAN 90 program code that runs on a Dell XPS 1210 notebook (Intel Centrino Duo, 2.0 GHz, 2 GB RAM). The model includes five closed states (C0–C4), one open state (O), and five inactivated states (If, Ii, Is, IC3, and IC4) (see Scheme 1 in results). We assumed that initially (at −90 mV) all channels were in state C0 (C0 = 1.0). Rate constants were adjusted to fit experimental data on gating following exposure to PMA in the two isoforms of Kv4.3.
RESULTS
Effects of PMA on activation, open-state inactivation, and closed-state inactivation in the short and long isoforms of Kv4.3.
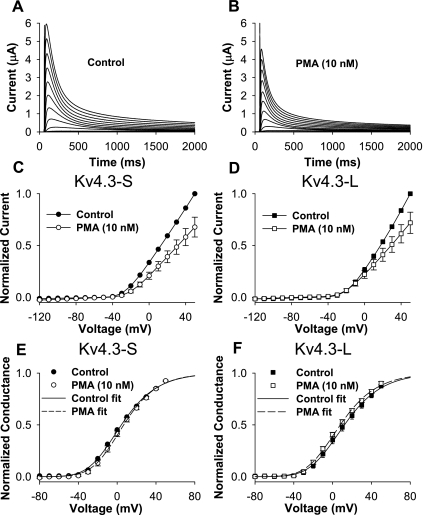
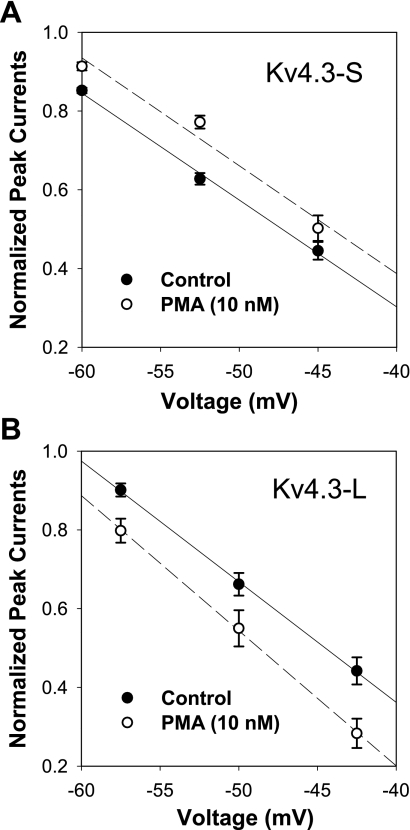
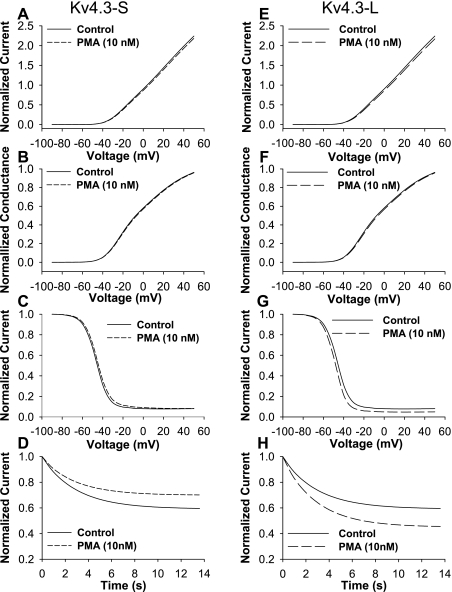
To establish the effects of a phorbol ester, PMA, on activation and inactivation, we evaluated the effects of different concentrations of PMA on normalized peak I-V. At 1 nM, normalized peak I-V was reduced to 0.79 ± 0.05 (P = 0.007, n = 5) without a significant change in open-state inactivation kinetics. We then selected 10 nM PMA to examine its effects on peak I-V, normalized conductance (G/Gmax), and open-state inactivation in short and long isoforms of Kv4.3 (Figs. 1 and 2). Our data show that 10 nM PMA caused a similar modest decrease in peak I-V in the short and long isoforms of Kv4.3 (0.68 ± 0.10 and 0.72 ± 0.10, respectively; Fig. 1, A–D). PMA (10 nM) had no significant effects on G/Gmax in Kv4.3-S and Kv4.3-L (Fig. 1, E and F, and Table 1) and did not change the steady-state inactivation relationship in either form of Kv4.3 (Fig. 2, A and B). However, 10 nM PMA accelerated open-state inactivation kinetics at +50 mV to a similar degree in both isoforms (Fig. 2, C–F, and Table 1). Our data indicate that although PMA reduced peak I-V and accelerated open-state inactivation kinetics, it had no effects on steady-state activation and inactivation (see Supplemental Table 1). (Supplemental data for this article is available online at the American Journal of Physiology-Cell Physiology website.) The presence of a 19-amino acid insert in the long isoform of Kv4.3 did not alter the effects of PMA on peak I-V, G/Gmax, and open-state inactivation.
Fig. 1.
PMA effects on Kv4.3 isoforms. A and B: current recordings for the long isoform of Kv4.3 (Kv4.3-L) during a 0.033-Hz pulse train consisting of 2,000-ms depolarizing pulses between −90 and + 50 mV [holding potential (HP) = −90 mV] in control (A) and in 10 nM PMA (B). PMA (10 nM) decreased peak normalized currents at potentials positive to −40 mV in both the short (Kv4.3-S) and Kv4.3-L isoforms (C and D, respectively). PMA (10 nM) had no significant effects on normalized conductance (G/Gmax) in both Kv4.3-S and Kv4.3-L (E and F, respectively).
Fig. 2.
Table 1.
Effects of PMA and injected PKC on peak I-V, G/Gmax, steady-state inactivation, kinetics of open-state inactivation, and relative magnitude of closed-state inactivation in Kv4.3-S, Kv4.3-L, and mutants Kv4.3-L[T504A] and Kv4.3-L[T504D]
| n | Peak I-V |
G/Gmax |
SSI |
OSI |
CSI, Normalized current | ||||
|---|---|---|---|---|---|---|---|---|---|
| V[1/2], mV | k, mV | V[1/2], mV | k, mV | τfast, ms | τslow, ms | ||||
| Kv4.3-S control | 9 | 1.00 | −33.6±0.8 | 22.8±0.2 | −42.3±1.5 | 6.1±0.2 | 113±12 | 590±66 | 0.58±0.03 |
| Kv4.3-S +10 nM PMA | 9 | 0.68±0.10† | −29.2±2.4 | 21.5±0.6 | −40.3±1.9 | 6.6±0.6 | 90±8† | 480±33* | 0.72±0.04† |
| Kv4.3-L control | 9 | 1.00 | −26.8±2.5 | 23.4±0.7 | −39.5±1.8 | 6.3±0.3 | 107±4 | 546±17 | 0.64±0.05 |
| Kv4.3-L +10 nM PMA | 9 | 0.72±0.10* | −30.8±1.5* | 23.3±0.8 | −41.9±1.0* | 6.4±0.3 | 87±3† | 441±12† | 0.48±0.05† |
| Kv4.3 L[T504A] control | 8 | 1.00 | −32.5±2.8 | 22.2±0.4 | −42.0±1.5 | 5.9±0.4 | 106±5 | 543±18 | 0.51±0.05 |
| Kv4.3 L[T504A] +10 nM PMA | 8 | 0.69±0.08† | −32.1±1.7 | 22.5±0.6 | −43.1±2.2 | 5.9±0.4 | 91±5† | 482±22† | 0.64±0.05† |
| Kv4.3-S control | 7 | 1.00 | −30.4±2.0 | 22.3±0.3 | −43.5±1.9 | 6.8±0.3 | 97±3 | 582±19 | 0.58±0.05 |
| Kv4.3-S +0.97 ng of PKC | 7 | 0.78±0.04† | −26.0±2.0* | 21.3±0.5 | −42.9±2.5 | 6.2±0.4 | 84±3† | 482±11† | 0.73±0.05† |
| Kv4.3-L control | 6 | 1.00 | −22.8±2.9 | 21.8±0.3 | −41.4±1.3 | 6.4±0.4 | 109±4 | 570±34 | 0.74±0.07 |
| Kv4.3-L +0.97 ng of PKC | 6 | 0.77±0.06* | −28.4±2.1 | 22.6±1.2 | −43.3±1.1 | 6.4±0.4 | 93±4† | 463±11† | 0.56±0.06† |
| Kv4.3 L[T504A] control | 6 | 1.0 | −28.4±0.8 | 21.0±0.2 | −41.4±1.1 | 6.4±0.3 | 83±3 | 500±24 | 0.62±0.05 |
| Kv4.3 L[T504A] +0.97 ng of PKC | 6 | 0.81±0.02† | −26.0±1.1 | 20.7±1.5 | −41.3±1.5 | 5.9±0.3 | 77±2* | 454±9* | 0.76±0.04† |
| Kv4.3 L[T504D] control | 11 | 1.0 | −30.3±2.2 | 23.5±0.6 | −40.8±1.8 | 5.6±0.2 | 108±8 | 570±40 | 0.54±0.07 |
| Kv4.3 L[T504D] +10 nM PMA | 11 | 0.89±0.03† | −29.9±1.9 | 23.2±0.5 | −38.4±1.4 | 5.6±0.1 | 104±7 | 539±35 | 0.53±0.06 |
Values are means ± SE; n = no. of cells. Kv4.3-S, short form of Kv4.3; Kv4.3-L, long form of Kv4.3. Peak I-V, peak current-voltage relationship; G/Gmax, normalized conductance; SSI, steady-state inactivation; OSI, open-state inactivation; CSI, closed-state inactivation; V[1/2], half-activation and half-inactivation potentials; k, slope factor; τf and τs, fast and slow components of inactivation. CSI values are normalized current at the end of 14,400 ms of CSI [ratio of the peak current (P4) at 14.4 s and the maximum peak current (P1)].
P < 0.05;
P < 0.01 vs. control.
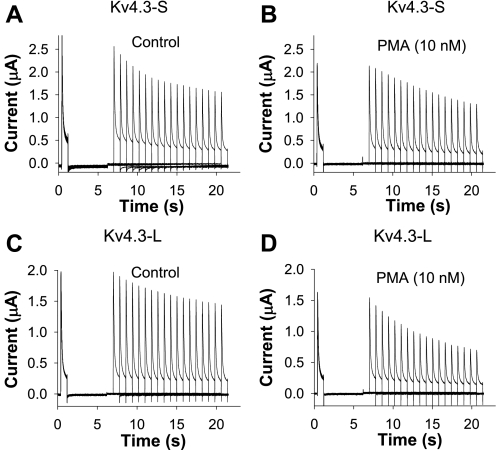
To determine whether the effects of PMA on closed-state inactivation were similar to its effects on open-state inactivation in the two Kv4.3 isoforms, we next examined the effects of 10 nM PMA on closed-state inactivation. In these experiments, we measured the effects of PMA on the normalized current at +50 mV, 14.4 s after depolarization from −100 to −50 mV (Fig. 3). The most significant difference between the responses to PMA was their opposing effects on closed-state inactivation at 14.4 s in the two isoforms of Kv4.3. After exposure to 10 nM PMA, the value of closed-state inactivation (at 14.4 s) changed from 0.58 ± 0.03 to 0.72 ± 0.04 in Kv4.3-S, representing a decrease in closed-state inactivation (Fig. 4A and Table 1). In contrast, 10 nM PMA decreased the magnitude of the normalized current representing the fraction of noninactivated channels (due to closed-state inactivation at 14.4 s) from 0.64 ± 0.05 to 0.48 ± 0.05 in Kv4.3-L. This change represented an increase in closed-state inactivation at 14.4 s (Fig. 4B and Table 1). The directionally opposite effects of PMA on closed-state inactivation in the short and long forms of Kv4.3 were unexpected, because they differed from the parallel effects on open-state inactivation. Hence, our data demonstrate that PMA can elicit independent responses in open- and closed-state inactivation in Kv4.3.
Fig. 3.
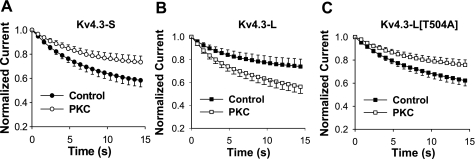
Effects of PMA on closed-state inactivation of Kv4.3-S (A and B) and Kv4.3-L (C and D). Voltage-clamp protocol consisted of 4 pulses (HP = −100 mV): a pulse P1 (+40 mV, 800 ms in duration), P2 (−100 mV, 5,000 ms), P3 (−50 mV, 800–14,400 ms), and P4 (+40 mV, 800 ms). Current is plotted as a function of time in control (A and C) and during exposure to PMA (B and D). Data show opposite effects of PMA on closed-state inactivation in Kv4.3-S and Kv4.3-L, which are plotted in Fig. 4, A and B.
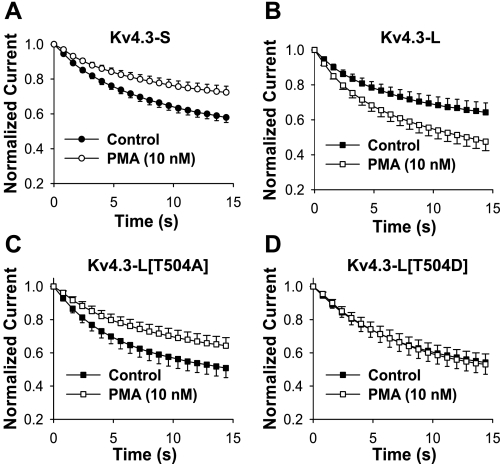
Fig. 4.
Effects of PMA on closed-state inactivation of Kv4.3-S (A), Kv4.3-L (B), Kv4.3-L[T504A] (C), and Kv4.3-L[T504D] (D). Voltage-clamp protocol is described in Fig. 3 legend. Normalized peak current (P4/P1 currents), before and after exposure to 10 nM PMA, are plotted as a function of P3 pulse duration. Note that PMA had opposite effects on the magnitude of closed-state inactivation in Kv4.3-S and Kv4.3-L. The effects of PMA on Kv4.3-S and Kv4.3-L[T504A] were similar, and no effect on closed-state inactivation in Kv4.3-L[T504D] was observed.
Bioinformatic analysis of the extra 19 amino acids in Kv4.3-L showed the presence of consensus phosphorylation sites for PKC not present in Kv4.3-S, suggesting a possible mechanism for the differing effects of PMA on closed-state inactivation. Alternatively, the insertion of 19 amino acids in Kv4.3-L could be modifying the effect of PMA at a remote site in the channel. To test the former possibility, we evaluated three different Kv4.3-L mutants, each converting a potentially phosphorylated serine or threonine in the 19-amino acid insert to an alanine (S499A, S503A, and T504A). In contrast to the WT Kv4.3-L, the effects of 10 nM PMA on the mutant channel Kv4.3-L[T504A] were similar to that seen in the WT Kv4.3-S channel, i.e., PMA decreased the magnitude of closed-state inactivation in the mutant channel (Fig. 4C). The effects of PMA on Kv4.3-L[S499A] and Kv4.3-L[S503A] were similar to those observed in WT Kv4.3-L but smaller in magnitude. The decrease in normalized current following PMA was 12% (0.73 ± 0.07 to 0.64 ± 0.05) for Kv4.3-L[S499A] and 19.6% (0.46 ± 0.10 to 0.37 ± 0.09) for Kv4.3-L[S503A], compared with 25% for WT Kv4.3-L (0.64 ± 0.05 to 0.48 ± 0.05). In contrast, the extent of closed-state inactivation with PMA was decreased by 25% (0.51 ± 0.05 to 0.64 ± 0.05) for Kv4.3-L[T504A]. These data suggest that the unique effect of PMA on Kv4.3-L is due to phosphorylation of T504.
To further test the potential role of phosphorylation of T504, we constructed Kv4.3-L[T504D], which adds a permanent negative charge at the putative phosphorylation site, mimicking the effect of phosphorylation (27). Closed-state inactivation in the T504D mutant failed to respond to 10 nM PMA; however, it reacted similarly to WT Kv4.3-L with respect to other gating parameters and reduction in peak current amplitude (Fig. 4D and Table 1). Our data show that PMA has opposite effects on closed-state inactivation in the short and long forms of Kv4.3 that are most likely mediated through the direct phosphorylation by PKC of a site only present in the 19-amino acid long form insert.
Each functional tetrameric Kv4.3-L channel has four 19-amino acid inserts compared with Kv4.3-S and, by extension, four PKC sites whose phosphorylation inhibits closed-state inactivation. In contrast to the rest of the channel, there is very little structural information about the COOH-terminal cytoplasmic region of rapidly inactivating K+ channels. What little is known has been derived from electron microscopic analysis of Kv4.2 (23) and suggests that the phosphorylation sites lie at the periphery of the channel complex and are unlikely to come in contact. If this is true, then each phosphorylated threonine at position 504 should be able to contribute to the enhancement of closed-state inactivation, regardless of the phosphorylation state of the other three. This model is reminiscent of Shaker N-type inactivation, where each NH2-terminal “ball” contributes to inactivation (26).
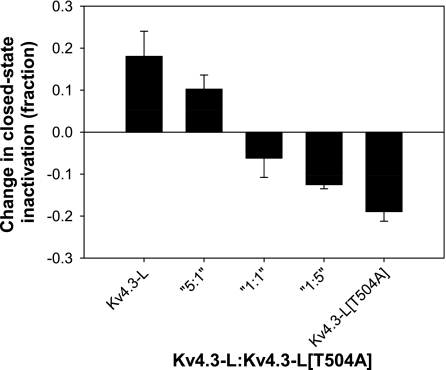
To test the relative contribution of each T504 phosphorylation to enhancement of closed-state inactivation, we coexpressed Kv4.3-L and Kv4.3-L[T504A] mRNA in ratios of 1:5, 1:1, and 5:1 and examined their responses to PMA (Fig. 5). Our assumption is that both the mutant and WT channels are translated and processed at the same efficiency and have an equal probability of being incorporated into a channel complex. The data in Fig. 5 suggest a model where each phosphorylation site acts independently; as the ratio of presumably phosphorylated WT Kv4.3 decreases, so does the effect on closed-state inactivation. At a 5:1 ratio of WT to T504A, where less than one-half of the channel complexes will be made up of four WT subunits, there is still substantial effect on closed-state inactivation. Likewise, at the 1:5 ratio, <1% of the tetramers will be all WT, and there is still measurable effect on closed-state inactivation. These data are consistent with the idea that phosphorylation at T504 on each of the four subunits independently influences closed-state inactivation.
Fig. 5.
Effects of PMA on the change in magnitude of closed-state inactivation at different ratios of injected Kv4.3-L in vitro-transcribed mRNA to injected Kv4.3-L[T504A] in vitro-transcribed mRNA.
To ascertain whether the effects of PMA on closed-state inactivation have similar voltage dependence in the two isoforms of Kv4.3, we examined the effects of 10 nM PMA on the magnitude of closed-state inactivation at different voltages in both isoforms (Fig. 6). In these experiments, we again measured the effects of PMA on the relative magnitude of the current resulting from closed-state inactivation at 14.4 s. The magnitude of closed-state inactivation had nearly identical voltage dependence in Kv4.3-S and Kv4.3-L. However, PMA had an opposite effect on closed-state inactivation at each voltage tested. As expected, PMA decreased the magnitude of closed-state inactivation in Kv4.3-S and increased the magnitude of closed-state inactivation in Kv4.3-L without altering the voltage dependence of closed-state inactivation in the two isoforms of Kv4.3.
Fig. 6.
Effects of PMA on the voltage dependence of closed-state inactivation at 14,400 ms (protocol is described in Fig. 3 legend) in Kv4.3-S and Kv4.3-L. Normalized peak current (V) = −aV + b, where a and b are the fitting constants. Despite the opposite effects of PMA on closed-state inactivation in Kv4.3-S and Kv4.3-L, the voltage dependence of the current amplitudes was similar.
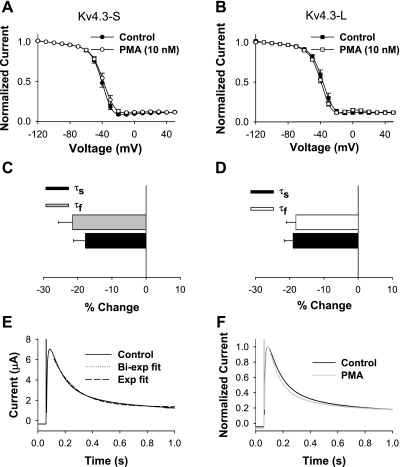
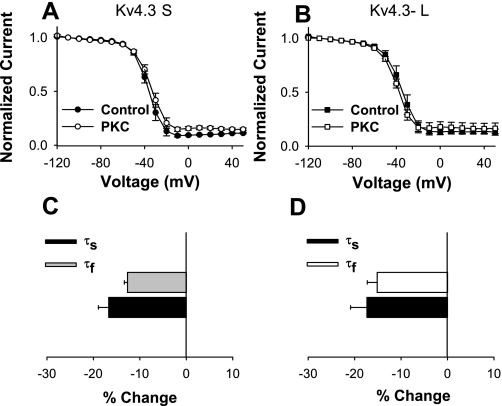
Differences in magnitude of closed-state inactivation could be attributed to changes in closed-state inactivation and/or recovery from closed-state inactivation. We first evaluated the effects of 10 nM PMA on closed-state inactivation in both isoforms. Whereas we showed that PMA had significant effects on the magnitude of closed-state inactivation in Kv4.3-S and Kv4.3-L, we observed no significant effects on the time constant of closed-state inactivation [Kv4.3-S: 6,680 ± 481 ms (control), 6,464 ± 498 ms (PMA), n = 9; Kv4.3-L: 5,700 ± 614 ms (control), 5,547 ± 318 ms (PMA), n = 9]. These observations suggested that the observed isoform-specific effect on closed-state inactivation could be due to PMA-induced differences in recovery from closed-state inactivation. We tested this possibility by examining the effects of PMA on the recovery from both open- and closed-state inactivation. PMA had no significant effect on recovery from open-state inactivation in the two isoforms of Kv4.3 (Fig. 7 and Table 2). On the other hand, recovery from closed-state inactivation at −80 mV was accelerated by PMA in Kv4.3-S and slowed in Kv4.3-L (Fig. 7 and Table 2). The time constant for recovery from closed-state inactivation decreased in Kv4.3-S at −80, −70, and −60 mV by 18.4, 20.5, and 34.0%, respectively. In contrast, the recovery time constant from closed-state inactivation in Kv4.3-L increased by 19.0, 23.2, and 30.5% at −80, −70, and −60 mV, respectively (Table 2). Thus our data show that the major isoform-specific effect of application of phorbol esters on Kv4.3 is to regulate the recovery from closed-state inactivation.
Fig. 7.
Experimental data on recovery from open- and closed-state inactivation of Kv4.3-S and Kv4.3-L. Recovery from closed-state inactivation was obtained using a 5-pulse protocol (HP = −90 mV): a pulse P1 (+50 mV, 500 ms in duration), P2 (−120 mV, 5,000 ms), P3 (−50 mV, 10,000 ms), P4 (−80 mV, with duration varied from 10 to 13,610 ms), and P5 (set at +50 mV for 500 ms). Representative current traces are shown for recovery from closed-state inactivation of Kv4.3-S (A and B) and Kv4.3-L (E and F) in control (A and E) and after application of PMA (B and F). Normalized currents for recovery from closed-state inactivation are plotted for Kv4.3-S (circles; C) and Kv4.3-L (squares; G) as functions of P4 duration before (filled symbols) and after application of PMA (open symbols). Recovery from open-state inactivation was measured using a 2-pulse protocol: pulses to +50 mV (P1) for 800 ms from HP = −90 mV were followed by a second 200-ms depolarization (P2) to +50 mV with a variable time gap. Normalized currents for recovery from open-state inactivation were plotted for Kv4.3-S (circles; D) and Kv4.3-L (squares; H) as functions of interpulse duration before (filled symbols) and after application of PMA (open symbols).
Table 2.
Effects of PMA on recovery from closed- and open-state inactivation in Kv4.3-S and Kv4.3-L
| Vm, mV | Kv4.3-S Recovery τrec − CSI, ms | Kv4.3-L Recovery τrec − CSI, ms | Kv4.3-S Recovery τrec − OSI, ms | Kv4.3-L Recovery τrec − OSI, ms |
|---|---|---|---|---|
| −90 | ||||
| Control | 283±13 | 307±26 | ||
| PMA (10 nM) | 276±16 | 307±27 | ||
| −80 | ||||
| Control | 713±49 | 684±27 | ||
| PMA (10 nM) | 582±54# | 814±40# | ||
| −70 | ||||
| Control | 1,368±96 | 1,251±6 | ||
| PMA (10 nM) | 1,088±74# | 1,540±22# | ||
| −60 | ||||
| Control | 3,060±220 | 3,020±150 | ||
| PMA (10 nM) | 2,020±260# | 3,950±220# |
Values are means ± SE; n = 5 cells. Vm, voltage potential; τrec, time constant of recovery from inactivation.
P < 0.01 vs. control.
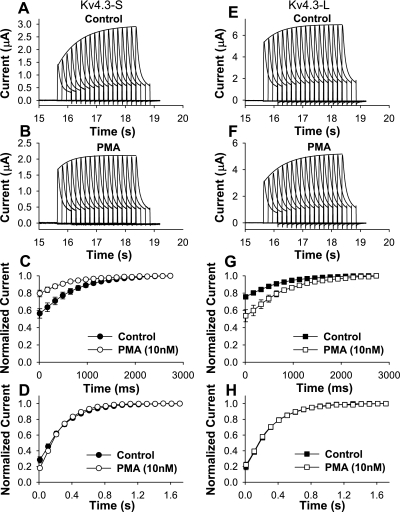
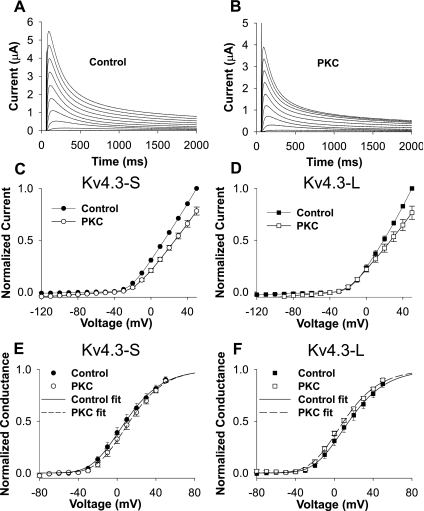
Two types of effects that could confound our results. One possibility is that PMA is somehow influencing Kv4.3 directly, instead of by its well-established activation of PKC. A second possibility is that PMA is influencing Kv4.3 activity though an intermediary protein present in oocytes. To exclude the former possibility, we examined the effects of the inactive stereoisomer of PMA, 4α-PDD. 4α-PDD had no significant effects on peak I-V and open- and closed-state inactivation in Kv4.3-S and Kv4.3-L (data not shown). To exclude the possibility that the effects of PMA were due to their effects on other proteins in the oocyte, we used PKC isolated from rat brain. Our data show that 0.97 ng of PKC caused a modest but similar decrease in peak I-V (0.78 ± 0.04 and 0.77 ± 0.06) in the short and long forms of Kv4.3. Injected PKC had no significant effects on G/Gmax in Kv4.3-S and Kv4.3-L (Fig. 8 and Table 1). Steady-state inactivation in the absence and presence of injected PKC was not significantly different between the short and long isoforms of Kv4.3 (Fig. 9 and Table 1). However, open-state inactivation kinetics at +50 mV were accelerated to a similar degree in both the short and long forms of Kv4.3. Heat-inactivated PKC and PKC carrying buffer alone had no effect (see Supplemental Figs. 1–4). Thus injected PKC had the same pattern of effects seen with PMA on peak I-V, G/Gmax, and open-state inactivation in the Kv4.3-S and Kv4.3-L isoforms.
Fig. 8.
Effects of injected PKC on Kv4.3 splice variants. A and B: current recordings for Kv4.3-L during a 0.033-Hz pulse train consisting of 2,000-ms depolarizing pulses between −90 and +50 mV (HP = −90 mV) in control (A) and after injection of PKC (B). Injected PKC (0.97 ng) decreased peak normalized currents at potentials positive to −40 mV in both Kv4.3-S (C) and Kv4.3-L (D). Injected PKC had no significant effects on G/Gmax in both Kv4.3-S (E) and Kv4.3-L (F). Data from two-microelectrode recordings were leakage subtracted.
Fig. 9.
Effects of injected PKC on steady-state inactivation (A and B) and kinetics of open-state inactivation (C and D). PKC had no significant effects on steady-state inactivation in both Kv4.3-S (A) and Kv4.3-L (B). However, PKC decreased the fast and slow time constants of open-state inactivation to a similar degree in both Kv4.3-S (C) and Kv4.3-L (D). Kinetics of open-state inactivation were measured at +50 mV. There were no significant differences in the relative amplitudes for the fast and slow inactivation.
We then examined the effects of injected PKC on closed-state inactivation in both isoforms of Kv4.3 and in the mutant Kv4.3-L[T504A] (Fig. 10). As was previously observed after treatment with PMA, injected PKC had opposite effects on closed-state inactivation in the two isoforms of Kv4.3. After injection of 0.97 ng of PKC, closed-state inactivation at 14.4 s changed from 0.58 ± 0.05 to 0.73 ± 0.05, representing a decrease in closed-state inactivation in Kv4.3-S. In contrast, 0.97 ng of PKC changed the magnitude of closed-state inactivation at 14.4 s from 0.74 ± 0.07 to 0.56 ± 0.06, representing an increase in closed-state inactivation in Kv4.3-L (Fig. 10 and Table 1). In the mutant Kv4.3-L[T504A], the effects of injected PKC on closed-state inactivation changed from 0.62 ± 0.05 to 0.76 ± 0.04, similar to that seen in the WT Kv4.3-S channel (Fig. 10). The similarities of the effects of PMA and injected PKC on the gating properties of Kv4.3 suggest that the effects of PMA are mediated via increased PKC activity.
Fig. 10.
Effects of injected PKC on closed-state inactivation of Kv4.3-S (A), Kv4.3-L (B), and Kv4.3-L[T504A] (C). Voltage-clamp protocol is described in Fig. 3 legend. Normalized peak currents, before and after injection of 0.97 ng of PKC, are plotted as a function of P3 pulse duration. Note that PKC had opposite effects on the magnitude of closed-state inactivation in Kv4.3-S and Kv4.3-L. The effects of PKC on Kv4.3-S and Kv4.3-L[T504A] were similar.
Simulations.
To gain mechanistic insights into the effects of PKC on Kv4.3-S and Kv4.3-L, we used our previously published 11-state model (Scheme 1) [Scheme 2 in Ref. 49], modified to account for the experimental findings in this study (summarized in Table 3). Our PMA experimental data on Kv4.3-S show the following: 1) a decrease in peak I-V, 2) acceleration of two components of open-state inactivation kinetics, 3) a decrease in magnitude of closed-state inactivation; and 4) acceleration of recovery from closed-state inactivation (Figs. 1–4 and Tables 1 and 2). No significant changes in voltage dependence of G/Gmax, steady-state inactivation relationships, closed-state inactivation kinetics, and recovery from open-state inactivation were observed. According to these data, rate constants K6 and K12 in the model (49) were increased by a factor of 2.3 to fit the experimental data on closed-state inactivation for Kv4.3-S and Kv4.3-L under control conditions. To simulate the effects of PMA on the Kv4.3-S open-state inactivation pathway, we increased rate constant K3 by a factor of 1.4 without changing the backward rate constants (K4, K8, and K10). This modification ensured an increase in open-state inactivation kinetics without causing a significant change in open-state recovery kinetics. In the closed-state inactivation pathway, the forward rate constant K6 was multiplied by 1.7 to fit the experimental data following exposure to PMA, which showed a decrease in magnitude of closed-state inactivation and acceleration of recovery from closed-state inactivation. In addition, rate constant K12 was multiplied by a factor of 2.4 (the product of 1.7 × 1.4) to ensure thermodynamic reversibility. The increase in K12 also avoided the change in recovery from open-state inactivation that is determined by K4, K8, and K10. Our simulations showing an acceleration of the two components of open-state inactivation kinetics, a decrease in magnitude of closed-state inactivation, and an acceleration of recovery from closed-state inactivation were associated with changes in rate constants that also led to an acceleration of closed-state inactivation kinetics that was not observed experimentally. To prevent such acceleration of closed-state inactivation kinetics, both K5 and K6 were multiplied by an additional factor of 0.75. The resulting simulation replicates the major effects of PMA on Kv4.3-S (Fig. 11). The only experimental data our model could not reproduce was the decrease in the normalized current amplitude in the presence of PMA (Fig. 1C). This discrepancy is addressed further in the discussion.
Table 3.
Rate constants for Kv4.3-S and Kv4.3-L Markov models in control conditions and after exposure to 10 nM PMA
| Rate Constants | Kv4.3-S and Kv4.3-L Control | Kv4.3-S + 10 nM PMA | Kv4.3-L + 10 nM PMA |
|---|---|---|---|
| K1 | 6 | 6 | 6 |
| K2 | 1.5 | 1.5 | 1.5 |
| K3 | 0.021 | 0.0294 | 0.0294 |
| K4 | 0.007 | 0.007 | 0.007 |
| K5 | 0.0052 | 0.0039 | 0.0065 |
| K6 | 0.0001196 | 0.0001525 | 0.00007475 |
| K7 | 0.0052 | 0.0052 | 0.00676 |
| K8 | 0.0036 | 0.0036 | 0.0036 |
| K9 | 0.0009 | 0.0009 | 0.0009 |
| K10 | 0.04 | 0.04 | 0.04 |
| K11 | 0.249941 | 0.249941 | 0.249941 |
| K12 | 0.0022425 | 0.005337 | 0.002041 |
Rate constants K1–K12 are expressed in s−1. Rate constants affected by activation of PKC are shown in bold type. The voltage-dependent rate constants α[V] and β[V] are the same as reported in Ref. 49 and are not shown.
Fig. 11.
Simulations of PMA effects on Kv4.3-S and Kv4.3-L. Simulations of peak current-voltage relationship (I-V; A and E), G/Gmax (B and F), steady-state inactivation (C and G), and closed-state inactivation (D and H) under experimental conditions described in Figs. 1, 2, and 4 are shown. PMA (10 nM) had no significant effects on simulations of peak I-V, G/Gmax, and steady-state inactivation. In contrast, PMA reduced the magnitude of closed-state inactivation in Kv4.3-S and increased the magnitude of closed-state inactivation in Kv4.3-L. These simulations closely reproduce the experimental data.
A similar strategy was applied to the modeling of PMA effects on Kv4.3-L: 1) a decrease in peak I-V, 2) acceleration of two components of open-state inactivation kinetics, 3) an increase in magnitude of closed-state inactivation, and 4) slowing recovery from closed-state inactivation (Figs. 1–4 and Tables 1 and 2). As in the case of Kv4.3-S, no significant changes in voltage dependence of G/Gmax, steady-state inactivation relationships, closed-state inactivation kinetics, and recovery from open-state inactivation were observed for Kv4.3-L. To simulate changes in Kv4.3-L gating due to effects of PMA, we increased rate constant K3 by a factor 1.4 to account for acceleration of open-state inactivation, multiplied K6 by 0.5 to account for slowing recovery from closed-state inactivation, and multiplied K12 by 0.7 (0.5 × 1.4) to ensure thermodynamic reversibility. However, with only these changes in rate constants we were unable to simulate acceleration of the second component of open-state inactivation and slowing recovery from closed-state inactivation. In addition, the model resulted in slowing of closed-state inactivation kinetics and acceleration of recovery from open-state inactivation, which were not observed in the experiments. Therefore, we increased K7 by a factor 1.3, K5 and K6 by an additional 1.25, and K12 by an additional 1.3. The resulting model and its simulations closely approximated experimental changes in Kv4.3-L gating in response to activation of PKC, however the deviation between simulated and experimental peak I-V data for Kv4.3-L was also observed.
The resulting rates for Markov models of Kv4.3-S and Kv4.3-L in response to exposure to PMA are shown in Table 3. The simulations confirmed experimental findings on PKC effects on different isoforms of Kv4.3. In both isoforms, the primary effect of PKC was on recovery from closed-state inactivation. In Kv4.3-S, the magnitude of closed-state inactivation in the model was decreased due to the acceleration of recovery from closed-state inactivation, whereas in Kv4.3-L, the recovery from closed-state inactivation was slowed. In addition, in both Kv4.3-S and Kv4.3-L the model demonstrated acceleration of open-state inactivation, without changes in the recovery from open-state inactivation. Opposing effects of PKC on the open- and closed-state inactivation supports our experimental findings on the independent regulation of these inactivation pathways by PKC.
DISCUSSION
Despite detailed biophysical studies of Kv4.3 channels, many unresolved questions remain (2, 5, 9, 10, 17, 18, 20–22, 44, 49). We recently showed that the complexity of gating in Kv4.3 channels is explained by multiple inactivation components accounting for both open- and closed-state inactivation (49). Multiple inactivation pathways suggest the possibility of independent modulation of open- and closed-state inactivation. Recent observations showed that phosphorylation of the COOH termini of voltage-gated K+ channels can alter channel gating (29, 36, 38, 43). In Kv4.3 channels, stimulation and inhibition of PKC have been shown to modify peak I-V, availability, and recovery from open-state inactivation (29, 44). However, these effects were noted in the long isoform of Kv4.3 but not in the short isoform. To ascertain whether parallel effects would be seen in closed- and open-state inactivation, we selected PMA and PKC isolated from rat brain to further examine the coupling of closed- and open-state inactivation during an increase in PKC activity.
Although it is widely accepted that open- and closed-state inactivation in Kv4 channels are coupled to activation, the nature of the coupling is uncertain. Skerritt and Campbell (45) recently showed that mutation of arginine to alanine in the Kv4.3 voltage-sensing transmembrane segment not only altered activation but also altered the development of closed-state inactivation, suggesting that the coupling between activation and closed-state inactivation might be altered. This study begs the question whether other interventions could alter the coupling between inactivation and activation (45) and provides compelling evidence to further explore the mechanisms that underlie the coupling between activation and open- and closed-state inactivation in Kv4.3 channels. A second recent study by Barghan and Bähring (6) suggested that amino acids critical for voltage-dependent gate opening were also critical for closed-state inactivation in Kv4.2, suggesting a dynamic coupling between the two gating events in Kv4.2. Together with the data presented in the current study, these findings suggest that closed-state inactivation is coupled with different aspects of channel gating and that at least parts of the process must be physically separable from open-state inactivation.
Our study demonstrated that stimulation of PKC had minimal effects on activation and steady-state inactivation and yet had substantial effects on closed-state inactivation. Whereas previous studies examined the effects of PKC stimulation and inhibition on activation and open-state inactivation and recovery, we are the first to show that increases in PKC activity can have large and different effects on CSI in the two isoforms. Indeed, when the consensus phosphorylation site was mutated, the effects of PMA on closed-state inactivation elicited either the same responses in Kv4.3-S and Kv4.3-L[T504A] or no responses, as was the case for Kv4.3-L[T504D], indicating that the unique consensus PKC phosphorylation site in Kv4.3-L plays a critical role in determining the magnitude of closed-state inactivation.
To ascertain whether the effects of PMA on closed-state inactivation were due to changes in the forward or reverse processes, we examined the effects of PMA on open- and closed-state inactivation and recovery. PMA had no significant effect on recovery from open-state inactivation in the two isoforms of Kv4.3; however, recovery from closed-state inactivation at −80 mV was accelerated by PMA in Kv4.3-S and slowed in Kv4.3-L (Table 2). Our simulations confirmed the experimental finding of the primary effect of PKC activation on the recovery from closed-state inactivation. Simulations closely reproduced the experimental data on G/Gmax, steady-state inactivation, development of closed-state inactivation, and recovery from open-state inactivation and closed-state inactivation. It is apparent that the changes in rate constants regulating closed-state inactivation and recovery are sufficient to reproduce the development of closed-state inactivation under experimental conditions, without any effect from the rate constant responsible for the open-state inactivation pathway. The changes in rate constants used to simulate our experimental data on closed-state inactivation support the view that closed-state inactivation is modulated directly by sites within the COOH terminus and that these changes have their most profound influence on the reverse process, recovery from closed-state inactivation.
Our simulations accounted for all of our experimental data except for the decrease in Kv4.3 current. Low concentrations of PMA are known to reduce the membrane surface density of several heterologously expressed ion channels and transporters in Xenopus oocytes, including epithelial Na+ channel (ENaC) (1). We showed that 1 nM PMA caused a significant decrease in peak current of both isoforms without any significant effect on the two components of open-state inactivation. Our data support our view that in Xenopus oocytes, part of the effect of PMA on peak current is due to membrane trafficking.
The change in voltage dependence of the time constant for recovery from closed-state inactivation during exposure to PMA was significant. In addition, the changes in recovery time constants were sufficiently large to account for the decrease in closed-state inactivation magnitude in Kv4.3-S and the increase in closed-state inactivation magnitude in Kv4.3-L during exposure to PMA (Table 2). The lack of concordance between the effects of PMA on open- and closed-state inactivation in conjunction with the increase in recovery from closed-state inactivation emphasizes the importance of amino acid 504 in the development of closed-state inactivation. In Kv4.3-L, the negative charges in T504 that appear during exposure to PKC are sufficient to slow recovery from inactivation and slow closed-state inactivation. Furthermore, this effect on closed-state inactivation is mediated via each channel subunit, as we have shown in the experiments with different ratios of WT Kv4.3-L and the mutant Kv4.3-L[T504A]. In fact, regulatory effects of protein phosphorylation by PKC on channel gating have been identified in other Kv channels (4, 8, 15). In addition, we can exclude the possibility that PMA, an activator of PKC activity, could have modulated other proteins within the oocytes, which in turn influence Kv4.3 closed-state inactivation. Our injected PKC data show striking similarities to those resulting from PMA. Hence, our data explain how PKC causes directionally opposite effects on closed-state inactivation, which is due to different changes in recovery from closed-state inactivation but not open-state inactivation.
In this study, we have linked a specific effect on closed-state inactivation to specific residue T504 within the COOH terminus of Kv4.3-L. However, a recent study on modulation of human ERG1 by PKC (13) raised concern about linkage of a particular phenotype to phosphorylation of a specific residue. That study showed that many sites in human ERG1 are phosphorylated under basal conditions, which raised concerns about the ability to assign phosphorylation of a specific amino acid to a particular modulation of the channel. Similar concerns could be expressed about our proposed linkage between a particular closed-state inactivation phenotype to a specific residue within the COOH terminus. Although the PKC-mediated decrease in Kv4.3-S closed-state inactivation suggests at least one other phosphorylation site is involved, our data show that the phosphorylation of amino acid 504 in Kv4.3-L by PKC accounts for the opposing responses in closed-state inactivation between the short and long isoforms of Kv4.3.
Isoform-specific effects of PKC on Kv4.3-L have been previously examined (36). In contrast to data presented in the current study, Po et al. (36) observed decreases in total current expression, a hyperpolarizing shift in steady-state inactivation, and slow recovery from inactivation only in Kv4.3-L; Kv4.3-S was relatively unaffected. These differences, like our changes in closed-state inactivation, were attributed to phosphorylation of a threonine in the unique 19-amino acid insert in Kv4.3-L [position T503 in the human clone used by Po et al. (36), position T504 in the rat clone we used]. The human and rat proteins differ by only 4 of 655 amino acids. Therefore, the major difference between these two studies appears to be the expression systems; Xenopus oocytes in our case compared with Ltk− fibroblasts (L cells) used by Po et al. (36). Kv4.3 associates with a variety of ancillary subunits, many of which are expressed in the absence of Kv4.3 and could be present to a greater or lesser extent in either cell type (3, 35, 37). In addition, there are 10 isoforms of PKC whose expression is likely different in oocytes and L cells (33). Given these differences, we cannot reconcile our results with those of previous investigations (36).
Regardless of earlier studies, our novel data on open- and closed-state inactivation sheds new and important information on the role of the consensus phosphorylation site in the COOH terminus in the long isoform of Kv4.3 and their differential effects on recovery from inactivation. Our data also show that the effects of PMA on closed-state inactivation are similar at all voltages tested and that the phosphorylation of the consensus phosphorylation site in splice variant insert in each of the subunits contributed to the effect of increased PKC activity on closed-state inactivation. Finally, we have shown that recovery from closed-state inactivation is a major determinant of the magnitude of closed-state inactivation during depolarization. The singular importance of this novel observation is of importance in understanding the mechanism played by the COOH terminus in response to PKC and its impact on the availability of channels for opening.
Physiological implications.
We showed that an increase in PKC activity altered the peak I-V and the magnitude of closed-state inactivation of IKv4.3. A decrease in peak current amplitude after application of PMA was observed in native Ito in human and rat cardiac myocytes (29, 36). If the change in magnitude of closed-state inactivation occurs in native channels, it would change the degree of inactivation of Ito. Hence, an increase in closed-state inactivation would decrease the fraction of channels that are available for opening in atrial and ventricular myocytes. The magnitude of increase in closed-state inactivation during stimulation by PKC in the long form of Kv4.3 would result in a decrease in peak I-V at potentials where closed-state inactivation occurs, such as −60 mV. This effect would be dominant in those cells expressing Kv4.3-L, in the sinoatrial node, in cardiac myocytes during ischemia, or in vascular smooth muscle and neurons under physiological conditions, where the resting potential is more depolarized than in normal cardiac myocytes.
Limitations.
This study showed that increased PKC activity has significant effects on closed-state inactivation that was dependent on the presence of the consensus phosphorylation site in the isoform insert of Kv4.3. However, we cannot exclude the possibility that these effects are not solely due to the phosphorylation of the consensus phosphorylation site in the isoform variant insert in the long isoform of Kv4.3 or a specific isoform of PKC (8, 13). Finally, we also cannot exclude the possibility that the kinase that phosphorylates Kv4.3-L is not PKC but an intermediary kinase that requires PKC activation. Regardless, our data indicate that Kv4.3-L is directly phosphorylated by a PKC-dependent mechanism (13).
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-52874.
Supplementary Material
ACKNOWLEDGMENTS
We thank Paul Morales for technical assistance.
REFERENCES
- 1.Awayda MS. Specific and nonspecific effects of protein kinase C on the epithelial Na+ channel. J Gen Physiol 115: 559–570, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol 284: C583–C595, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Anantharam A, Lewis A, Panaghie G, Gordon E, McCrossan ZA, Lerner DJ, Abbott GW. RNA interference reveals that endogenous Xenopus MinK-related peptides govern mammalian K+ channel function in oocyte expression studies. J Biol Chem 278: 11739–11745, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Antz C, Bauer T, Kalbacher H, Frank R, Covarrubias M, Kalbitzer HR, Ruppersberg JP, Baujrowitz T, Faker B. Control of K channel gating by protein phosphorylation: structural switches of the inactivation gate. Nat Struct Biol 6: 146–150, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bähring R, Boland LM, Varghese A, Gebauer M, Pongs O. Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels. J Physiol 535: 65–81, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barghaan J, Bähring R. Dynamic coupling of voltage sensor and gate involved in closed-state inactivation of Kv4.2 channels. J Gen Physiol 133: 205–224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron 15: 951–960, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Beck EJ, Sorensen RG, Slater SJ, Covarrubias M. Interactions between multiple phosphorylation sites in the inactivation particle of a K+ channel. Insights into the molecular mechanism of protein kinase C action. J Gen Physiol 112: 71–84, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck EJ, Bowlby M, An WF, Rhodes KJ, Covarrubias M. Remodelling inactivation gating of Kv4 channels by KChIP1, a small-molecular weight calcium-binding protein. J Physiol 538: 691–706, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev 84: 803–833, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Brahmajothi MV, Campbell DL, Rasmusson RL, Morales MJ, Trimmer JS, Nerbonne JM, Strauss HC. Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes. J Gen Physiol 113: 581–600, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claydon TW, Kehl SJ, Fedida D. Closed-state inactivation induced in KV1 channels by extracellular acidification. Channels (Austin) 2: 139–142, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Cockerill SL, Tobin AB, Torrecilla I, Willars GB, Standen NB, Mitcheson JS. Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits. J Physiol 581: 479–493, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comer MB, Campbell DL, Rasmusson RL, Lamson DR, Morales MJ, Zhang Y, Strauss HC. Cloning and characterization of an Ito-like potassium channel from ferret ventricle. Am J Physiol Heart Circ Physiol 267: H1383–H1395, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Desai R, Kronengold J, Mei J, Forman SA, Kaczmarek LK. Protein kinase C modulates inactivation of Kv3.3 channels. J Biol Chem 283: 22283–22294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D. Role of the Kv4.3 K+ channel in ventricular muscle A molecular correlate for the transient outward current. Circ Res 79: 659–668, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Dougherty K, De Santiago-Castillo JA, Covarrubias M. Gating charge immobilization in Kv4.2 channels: the basis of closed-state inactivation. J Gen Physiol 31: 257–273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebauer M, Isbrandt D, Sauter K, Callsen B, Nolting A, Pongs O, Bahring R. N-type inactivation features of Kv4.2 channel gating. Biophys J 86: 210–223, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshi T, Zagotta WN, Aldrich RW. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxyterminal region. Neuron 7: 547–556, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci 27: 343–369, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Jerng HH, Covarrubias M. K+ channel inactivation mediated by the concerted action of the cytoplasmic N- and C-terminal domains. Biophys J 72: 163–174, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jerng HH, Shahidullah M, Covarurubias M. Inactivation gating of Kv4 potassium channels. Molecular interactions involving the inner vestibule of the pore. J Gen Physiol 113: 641–659, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang X, Bett GCL, Li X, Bondarenko VE, Rasmusson RL. C-type inactivation involves a significant decrease in the intracellular aqueous pore volume of Kv1.4 K+ channels expressed in Xenopus oocytes. J Physiol 549: 683–695, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim LA, Furst J, Gutierrez D, Butler MH, Xu S, Goldstein SA, Grigorieff N. Three-dimensional structure of Ito: Kv4.2-KChIP2 ion channels by electron microscopy at 21 Å resolution. Neuron 41: 513–519, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Jurman ME, Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron 16: 859–867, 1996 [DOI] [PubMed] [Google Scholar]
- 26.MacKinnon R, Aldrich RW, Lee AW. Functional stoichiometry of Shaker potassium channel inactivation. Science 262: 757–759, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Marcus F, Rittenhouse J, Moberly L, Edelstein I, Hiller E, Rogers DT. Yeast (Saccharomyces cerevisiae) fructose-1,6-bisphosphatase. Properties of phospho and dephospho forms and of two mutants in which serine 11 has been changed by site-directed mutagenesis. J Biol Chem 263: 6058–6062, 1988 [PubMed] [Google Scholar]
- 28.Muraki K, Imaizumi Y, Watanabe M, Habuchi Y, Giles WR. Delayed rectifier K+ current in rabbit atrial myocytes. Am J Physiol Heart Circ Physiol 269: H524–H532, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Nakamura TY, Coetzee WA, Vega-Saenz de ME, Artman M, Rudy B. Modulation of Kv4 channels, key components of rat ventricular transient outward K+ current by PKC. Am J Physiol Heart Circ Physiol 273: H1775–H1786, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev 85: 1205–1253, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol 525: 285–298, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nerbonne JM, Nichols CG, Schwarz TL. Genetic manipulation of cardiac K+ channel function in mice. What have we learned, and where do we go from here? Circ Res 89: 944–956, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Parker PJ, Murray-Rust J. PKC at a glance. J Cell Sci 117: 131–132, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Patel SP, Campbell DL. Transient outward potassium current, “Ito,” phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. J Physiol 569: 7–39, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel SP, Campbell DL, Morales MJ, Strauss HC. Heterogeneous expression of KChIP2 isoforms in the ferret heart. J Physiol 539: 649–656, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Po SS, Wu RC, Juang GJ, Kong W, Tomaselli GF. Mechanism of alpha-adrenergic regulation of expressed hKv4.3 currents. Am J Physiol Heart Circ Physiol 281: H2518–H2527, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Pourrier M, Schram G, Nattel S. Properties, expression and potential roles of cardiac K+ channel accessory subunits: MinK, MiRPs, KChIP, and KChAP. J Membr Biol 194: 141–152, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Qu YJ, Bondarenko VE, Xie C, Wang S, Awayda MS, Strauss HC, Morales MJ. W-7 modulates Kv4.3: pore block and Ca2+-calmodulin inhibition. Am J Physiol Heart Circ Physiol 292: H2364–H2377, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Radicke S, Cotella D, Graf EM, Ravens U, Wettwer E. Expression and function of dipeptidyl-aminopeptidase-like protein 6 as a putative β-subunit of human cardiac transient outward current encoded by Kv4.3. J Physiol 565: 751–756, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmusson RL, Morales MJ, Castellino RC, Zhang Y, Campbell DL, Strauss HC. C-type inactivation controls recovery in a fast inactivating cardiac K+ channel (Kv1.4) expressed in Xenopus oocytes. J Physiol 489: 709–721, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasmusson RL, Morales MJ, Wang S, Liu S, Campbell DL, Brahmajothi MV, Strauss HC. Inactivation of voltage-gated cardiac K+ channels. Circ Res 82: 739–750, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH. Regulation of cardiac excitation contraction coupling by action potential repolarization: role of the transient outward potassium current (Ito). J Physiol 546: 5–18, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sergeant GP, Ohya S, Reihill JA, Perrino BA, Amberg GC, Imaizumi Y, Horowitz B, Sanders KM, Koh SD. Regulation of Kv4.3 currents by Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Cell Physiol 288: C304–C313, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Shahidullah M, Covarrubias M. The link between ion permeation and inactivation gating of Kv4 potassium channels. Biophys J 84: 928–941, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skerritt MR, Campbell DL. Role of S4 positively charged residues in the regulation of Kv4.3 inactivation and recovery. Am J Physiol Cell Physiol 293: C906–C914, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Strauss HC, Morales MJ, Wang S, Brahmajothi MV, Campbell DL. Voltage-dependent K+ channels. In: Heart Physiology and Pathophysiology, edited by Sperelakis N, Kurachi Y, Terzic A, Cohen MV. San Diego, CA: Academic, 2001, p. 259–280 [Google Scholar]
- 47.Takimoto K, Li D, Hershman KM, Li P, Jackson EK, Levitan ES. Decreased expression of Kv4.2 and novel Kv4.3 K+ channel subunit mRNA in ventricles of renovascular hypertensive rats. Circ Res 81: 533–539, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Bondarenko VE, Qu Y, Morales MJ, Rasmusson RL, Strauss HC. Activation properties of Kv4.3 channels: time, voltage and [K+]o dependence. J Physiol 557: 705–717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S, Bondarenko VE, Qu Y, Bett GCL, Morales MJ, Rasmusson RL, Strauss HC. Time- and voltage-dependent components of Kv4.3 inactivation. Biophys J 89: 3026–3041, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeola SW, Snyders DJ. Electrophysiological and pharmacological correspondence between Kv4.2 current and rat cardiac transient outward current. Cardiovasc Res 33: 540–547, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Yavin E, Inserte-Igual J, Gil S. Ischemia-triggered translocation and inactivation of protein kinase C isoforms in the fetal brain. J Neurochem 65: 2594–2602, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Zagotta WN, Hoshi T, Aldrich RW. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science 250: 568–571, 1990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.