Abstract
Myostatin is a highly conserved negative regulator of skeletal muscle growth. Loss of functional myostatin in cattle, mice, sheep, dogs, and humans results in increased muscle mass. The molecular mechanisms responsible for this increase in muscle growth are not fully understood. Previously, we have reported that phenylephrine-induced cardiac muscle growth and Akt activation are enhanced in myostatin knockout mice compared with controls. Here we report that skeletal muscle from myostatin knockout mice show increased Akt protein expression and overall activity at baseline secondary to an increase in Akt mRNA. We examined the functional role of myostatin modulation of Akt in C2C12 myotubes, a well-established in vitro model of skeletal muscle hypertrophy. Adenoviral overexpression of myostatin attenuated the insulin-like growth factor-I (IGF-I)-mediated increase in myotube diameter, as well as IGF-I-stimulated Akt phosphorylation. Inhibition of myostatin by overexpression of the NH2-terminal portion of myostatin was sufficient to increase myotube diameter and Akt phosphorylation. Coexpression of myostatin and constitutively active Akt (myr-Akt) restored the increase in myotube diameter. Conversely, expression of dominant negative Akt (dn-Akt) with the inhibitory myostatin propeptide blocked the increase in myotube diameter. Of note, ribosomal protein S6 phosphorylation and atrogin-1/muscle atrophy F box mRNA were increased in skeletal muscle from myostain knockout mice. Together, these data suggest myostatin regulates muscle growth at least in part through regulation of Akt.
Keywords: growth differentiation factor-8, muscle growth, C2C12
myostatin, also known as growth differentiation factor-8 (GDF-8), is a member of the tumor growth factor-β (TGF-β) superfamily that negatively regulates skeletal muscle growth. The genetic loss of MSTN results in increased muscle mass in mice (19, 32), cattle (5, 10, 20), sheep (3), dogs (23), and humans (30), underscoring its well-conserved biological activity across species. Potential mechanisms for MSTN's ability to inhibit skeletal muscle hyperplasia, which primarily occurs during development, have been reported (9, 12, 34). However, little is known about how MSTN regulates skeletal muscle hypertrophy, which plays an important role in the muscle growth seen with genetic or pharmacological postnatal MSTN inhibition (6, 15, 37, 39, 41). MSTN has been shown to modulate in vivo skeletal muscle growth through the activin type IIa and IIb receptors (14, 15); however, the downstream signaling mechanisms responsible for MSTN's ability to regulate skeletal muscle hypertrophy are not well understood.
C2C12 cells, a myoblast cell line derived from murine satellite cells, have been used extensively as an in vitro model to study both muscle differentiation and hypertrophy. The withdrawal of serum from C2C12 myoblasts causes them to exit the cell cycle and fuse into myotubes. Differentiated myotubes exhibit a hypertrophic, as opposed to a hyperplastic, response to growth factors, such as insulin-like growth factor-I (IGF-I), characterized by an increase in myotube diameter and protein synthesis (13, 26, 27). Whereas these distinct processes may be interrelated, hypertrophy, or a growth in cell size may be more relevant for postnatal modulation of skeletal muscle.
C2C12 myotubes have been used as an in vitro model used to study IGF-I-mediated hypertrophic signaling pathways in skeletal muscle. Phosphatidylinositol 3 (PI3)-kinase/Akt/mammalian target of rapamycin (mTOR) activation downstream of IGF-I has been shown to induce hypertrophy both in C2C12 cells in vitro (26) as well as skeletal muscle in vivo (2). This cell line has also been used to model skeletal muscle atrophy in vitro. Dexamethasone-mediated decreases in myotube diameter and protein synthesis can be blocked by IGF-I (31). Akt, activated downstream of IGF-I, inhibits induction of atrogin-1/muscle atrophy F box (MAFbx) and muscle RING-finger protein 1 (MuRF1) ubiquitin-ligases by forkhead transcription factor FOXO1, thereby preventing muscle atrophy (31). Additionally a decrease in Akt phosphorylation was seen in myotubes after the addition of recombinant MSTN protein (18). Thus C2C12 cells provide a useful, well-characterized, in vitro model system to examine the effects of MSTN on inhibition of hypertrophy and/or induction of atrophy in skeletal muscle.
Previously, we have shown that MSTN inhibits phenylephrine (PE)-mediated hypertrophy in primary cardiomyocytes in vitro through inhibition of Akt (22). In vivo we found increased Akt activation and hypertrophy in response to PE stimulation in the hearts of MSTN knockout mice compared with controls. To test the hypothesis that Akt may play a role in skeletal muscle hypertrophy as well, we examined whether Akt was altered in these mice and used C2C12 myotubes to determine whether MSTN-mediated regulation of Akt signaling is functionally important for hypertrophy. Here we show a role for Akt in regulating MSTN-mediated inhibition of skeletal muscle hypertrophy.
METHODS
Mice.
All animal studies were carried out in accordance with approved IACUC protocols at BIDMC. MSTN null mice (19) were kindly provided by Dr. Se Jin Lee (Johns Hopkins University). Mice were backcrossed to C57Bl6 for ≥9 generations and littermate controls were used in all data presented. Mice aged 8–20 wk old were used for these studies.
Recombinant adenoviruses.
Construction of adenoviruses expressing cytomegalovirus-driven green fluorescent protein (GFP) and myostatin (MSTN), the inhibitory MSTN propeptide, which we term dominant negative MSTN (dnMSTN) (22), constitutively activated (myristoylated) Akt (Akt), and dominant negative (AA mutant) Akt (dnAkt) were described previously (24). Note that all constructs express GFP, which was used to confirm similar expression levels and permit visualization of transduced myotubes for measurement.
Cell culture.
C2C12 myoblasts (American Type Culture Collection, Rockville, MD) were grown to confluence in growth media (DMEM/15%FBS) on 60-mm tissue culture dishes and then switched to differentiation media (DMEM/2%HS) on day 0 (D0). Myotubes were infected with adenoviral constructs 48 h after being switched to differentiation media (day 2, D2), and stimulated with IGF-I (10 ng/ml, “Long-R3-IGF-I”, Sigma, St. Louis, MO) after an additional 24 h (day 3, D3). Myotubes were harvested for biochemical analysis or measured on day 4 (D4), 24 h after the addition of IGF-I (D4). Myotubes harvested for biochemical analysis on D4 were switched to serum-free DMEM for 2 h before 30-min stimulation with IGF-I, as indicated.
Myotube diameter measurements.
Microscopic images of live GFP-expressing myotubes (D4) were captured using a digital camera mounted on a Leica DM IRB microscope. Myotube diameter measurements were obtained using NIH Image software. Three short-axis measurements were taken along the length of a given myotube and averaged. At least five myotubes per plate were measured and replicated in three-to eight independent experiments.
Western blot analysis.
Heart and skeletal muscle tissue from mice were harvested, rinsed in cold PBS, snap frozen in liquid nitrogen, and homogenized in lysis buffer (Cell Signalling, Beverly, MA) plus 5 mM phenylmethylsulfonyl fluoride, followed by centrifugation at 20,000 g for 10 min at 4°C. Protein concentration was determined by Bradford Assay (Bio-Rad, Hercules, CA), and equal amounts of protein (∼100 μg) were loaded in each lane of a 4–15% precast Tris-glycine/sodium dodecyl sulfate-polyacrylamide gel (Bio-Rad). Proteins were separated by electrophoresis, transferred (semidry, Bio-Rad) to nitrocellulose membranes, and incubated overnight at 4°C with indicated antibody diluted (1:1,000) in 5% nonfat powdered milk/Tris-buffered saline/0.1% Tween. C2C12 myotubes were harvested on ice by scraping in lysis buffer and further processed as tissue samples. Antibodies were the following: anti-Akt, -phospho Akt (p473), -GSK3β, -phospho-S6, -S6, -p70S6K, -phospho-p70S6K (t389; t422/s424) (Cell Signaling, Beverly, MA), GAPDH (Abcam, Cambridge, MA).
Akt kinase assay.
Akt was immunoprecipitated from quadriceps lysates (400 μg) and incubated with substrate, and kinase activity was measured using an anti-phospho-GSK3α/β antibody to detect phosphorylation of a GSK-peptide (Akt substrate) by Western blot analysis, according to the manufacturer's instructions (Cell Signaling).
Real-time PCR.
Samples were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's directions. RNA was quantified using a Nanodrop spectrophotometer, and the quality of the RNA was confirmed with a Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). One-step SYBR PCR kit (Stratagene, La Jolla, CA) with predesigned, validated TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) to quantitate gene expression of Akt, MuRF-1, atrogin-1, and GAPDH. Analysis of change in gene expression was determined using the 2−ΔΔCt method (17), and a Student's t-test was performed on the normalized threshold cycle (Ct) values (ΔCt) for each group as this value demonstrated the most normally distributed dataset as determined by a Shapiro-Wilk test compared with the transformed Ct (2 −Ct or 2−ΔCt).
Statistics.
Data are represented as means ± SE and compared by two-tailed Student's t-test or ANOVA with Dunnett or Tukey post hoc analysis as appropriate using GraphPad Prism 4 software. The null hypothesis was rejected for P < 0.05.
RESULTS
Akt protein and activity are increased in skeletal muscle.
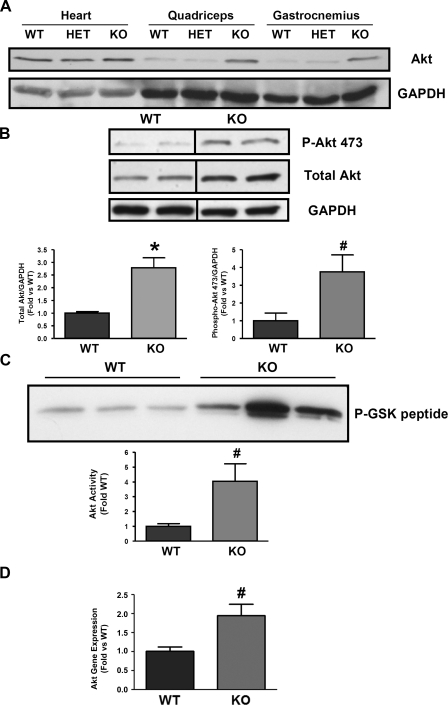
We have previously reported that MSTN inhibits cardiomyocyte hypertrophy by inhibiting p38 and Akt activation in a stimulus-specific manner without altering total Akt protein (22). To determine whether MSTN regulates Akt in skeletal muscle we examined Akt in skeletal muscle from MSTN-null mice (knockout, KO) (Fig. 1A). In contrast to heart muscle, total Akt expression was increased 2.8-fold in the quadriceps from KO mice (Fig. 1B) (n = 16, P < 0.001 vs. wild type, WT). Additionally, we found a approximately fourfold increase in baseline phosphorylation of Akt (Fig. 1B, n = 4, P < 0.05) that correlated with an overall increase in Akt kinase activity in the quadriceps muscle of KO mice (Fig. 1C, n = 4, P < 0.05). Interestingly, this increase in Akt activity appears predominantly due to an increase in protein levels of Akt rather than a change in the percent phosphorylated/total Akt. To further examine the mechanism responsible for the increase in Akt protein, we examined Akt mRNA and found a twofold increase in Akt mRNA in KO compared with WT quadriceps (P < 0.02), suggesting that MSTN deletion regulates Akt expression at the transcript level. We found no increase in total or phosphorylated p38 (data not shown).
Fig. 1.
Akt protein, activity, and mRNA are increased in myostatin knockout (KO) muscle. A: representative Western blot analysis of Akt from heart, quadriceps, and gastrocnemius muscle in myostatin wild-type (WT), heterozygous (HET), and homozygous (KO) myostatin KO mice. GAPDH is shown as a loading control. B: representative Western blot analysis from quadriceps of WT and KO mice was probed with anti-phospho-Akt (s437), Akt, and GAPDH antibodies. Bar graphs show quantification of blots from total Akt and phospho-Akt normalized to GAPDH. C: representative Akt kinase assay from WT and KO quadriceps. The graph shows quantification of Akt kinase assay results. Dividing lines on Western blot images depict where bands from the same blot have been juxtaposed. D: quantitative real-time RT-PCR using RNA samples from WT and KO quadriceps shows an increase in Akt gene expression in KO skeletal muscle. Gene expression was normalized to GAPDH. Total Akt, n = 16 WT:16 KO; p-Akt, n = 4 WT:4 KO; Akt kinase, n = 4 WT:4 KO; gene expression, n = 3 WT:4 KO. *P < 0.0001 vs. WT, #P < 0.05 vs. WT.
MSTN regulates Akt phosphorylation in myotubes.
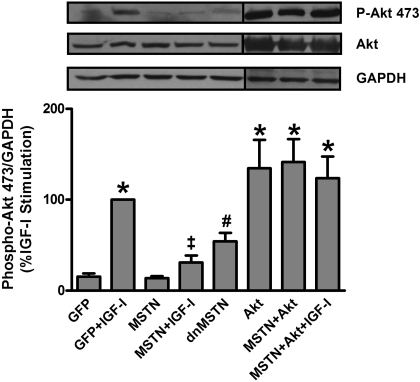
To determine whether MSTN inhibits Akt in myotubes, C2C12 myoblasts were grown to confluence in growth media (DMEM + 15% FBS) and then switched to differentiation media (DMEM + 2% HS) on D0. Fully differentiated myotubes were infected (D2) with adenoviral constructs expressing GFP only, MSTN, or dnMSTN, the NH2-terminal peptide of MSTN that has been previously shown to inhibit MSTN (7). Stimulation of GFP-infected myotubes for 30 min with IGF-I on D4 induced a 6.7-fold increase in Akt phosphorylation (n = 8, P < 0.001) that was blocked by MSTN expression (Fig. 2, n = 8, P < 0.001). dnMSTN overexpression was sufficient to increase Akt phosphorylation by 3.6-fold versus GFP (Fig. 2, n = 8, P < 0.05). Overexpression of membrane-targeted Akt (Akt) resulted in similar Akt phosphorylation (8.6-fold) compared with IGF-I stimulation (Fig. 2, n = 5, P < 0.001 vs. GFP, P = NS vs. GFP + IGF-I). Coexpression of MSTN and Akt with (n = 4) or without IGF-I (n = 3) did not significantly change Akt phosphorylation levels compared with GFP + IGF-I or Akt alone. No change in total Akt protein was observed with either MSTN or dnMSTN infection in myotubes.
Fig. 2.
Myostatin (MSTN) regulates Akt phosphorylation in myotubes. Representative Western blot analysis of phospho-Akt (s473), total Akt, and GAPDH from myotube lysates. Quantification of Western blot results from 4 to 9 independent experiments is shown as percent maximal insulin-like growth factor-I (IGF-I) stimulation. Dividing lines on Western blot images depict where bands from the same blot have been juxtaposed. dnMSTN, dominant negative MSTN. *P < 0.001 vs. GFP, ‡P < 0.001 vs. GFP+IGF-I, #P < 0.05 vs. GFP.
MSTN regulates Akt activity in myotubes.
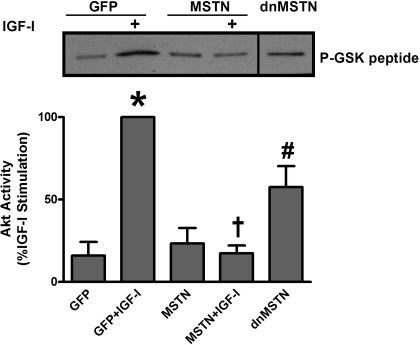
To confirm that changes in Akt phosphorylation mediated by MSTN and dnMSTN reflect actual Akt activity, we performed direct measurements of kinase activity. IGF-I increased Akt kinase activity 6.3-fold compared with GFP-infected control (n = 3, P < 0.001), and MSTN infection completely blocked activation (Fig. 3, n = 3, P < 0.001), whereas dnMSTN increased activity by 3.6-fold compared with GFP-infected myotubes (Fig. 3, n = 3, P < 0.05).
Fig. 3.
MSTN regulates Akt activity in myotubes. Representative blot of Akt kinase assay and quantification of 3 independent experiments are shown. Dividing lines on Western blot images depict where bands from the same blot have been juxtaposed. *P < 0.001 vs. GFP, †P < 0.01 vs. GFP+IGF-I, #P < 0.05 vs. GFP.
MSTN inhibits IGF-I-mediated myotube hypertrophy through Akt.
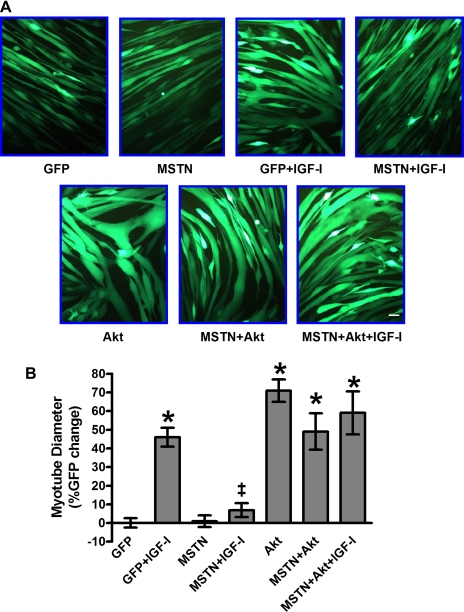
To determine whether the MSTN-mediated inhibition of Akt is functionally important for myotube hypertrophy, we examined the ability of MSTN to inhibit IGF-I-mediated hypertrophy of differentiated C2C12 myotubes. Myotubes were cultured and infected (D2) as described above, stimulated with IGF-I on D3, and photographed for measurement 24 h later. GFP-infected myotubes stimulated with IGF-I showed a 4.6-fold increase in myotube diameter (Fig. 4, P < 0.001). MSTN infection was sufficient to block IGF-I stimulated hypertrophy (Fig. 4, P < 0.001). Increasing Akt activity (Fig. 2) with overexpression of constitutively active Akt was sufficient to induce myotube hypertrophy (Fig. 4, P < 0.001 vs. GFP alone) that was not inhibited by MSTN overexpression. Additionally, the restoration of Akt activity rescued IGF-I-induced myotube hypertrophy that was blocked by MSTN (Fig. 4, P < 0.001 vs. GFP alone) suggest that MSTN inhibits myotube hypertrophy through inhibition of Akt.
Fig. 4.
MSTN inhibits IGF-I-mediated myotube hypertrophy through Akt. A: representative photographs of adenovirally infected, GFP-expressing myotubes. B: quantification of mean myotube diameter ± SE expressed as percent GFP control. A minimum of 5 myotubes per condition from 3 to 9 independent experiments were measured. (GFP, n = 104), (G+I, n = 53), (MSTN, n = 113), (M+I, n = 47), (Akt, n = 76), (MSTN+Akt+I, n = 58), (MSTN+Akt = IGF-I, n = 33) *P < 0.001 vs. GFP, #P < 0.05 vs. GFP, ‡P < 0.001 vs. GFP+IGF-I; scale bar, 50 μm (magnification: ×200).
Inhibition of MSTN results in Akt-dependent myotube hypertrophy.
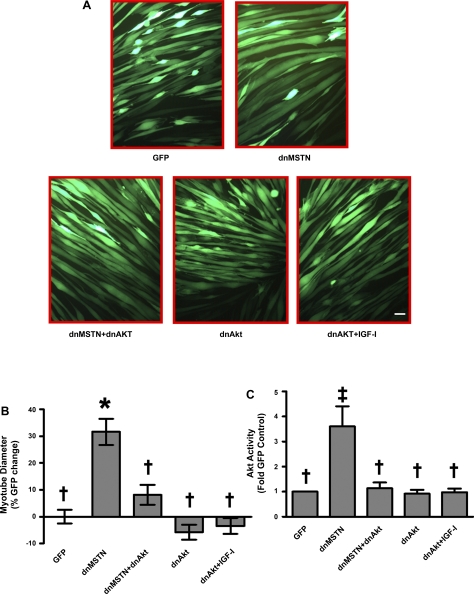
Adenoviral overexpression of dnMSTN was sufficient to induce a 3.2-fold increase in myotube diameter (Fig. 5, P < 0.001 vs. GFP). This hypertrophy was blocked by infection with dnAkt (Fig. 5, P < 0.01 vs. dnMSTN). IGF-I stimulated hypertrophy was similarly inhibited by dnAkt. Akt activity was reduced by dnAkt to levels seen at baseline in GFP-expressing control cells (Fig. 5, B and C). Thus inhibition of MSTN leads to Akt activation that appears necessary for the observed myotube hypertrophy in vitro.
Fig. 5.
Inhibition of MSTN results in Akt-dependent myotube hypertrophy. A: representative photographs showing adenovirally infected, GFP-expressing myotubes. B: quantitation of mean myotube diameter ± SE expressed as percent GFP change (GFP, n = 104, dnMSTN, n = 129, dnMSTN+dnAkt n = 100, dnAkt, n = 42, dnAkt+IGF-I, n = 49). C: quantification of Akt kinase assay Western blots (data from n = 3 independent experiments for all except dnAkt, n = 4). *P < 0.001, ‡P < 0.01 vs. GFP, †P < 0.01 vs. dnMSTN; scale bar, 50 μm (magnification: 200).
Downstream mediators of skeletal muscle mass increase.
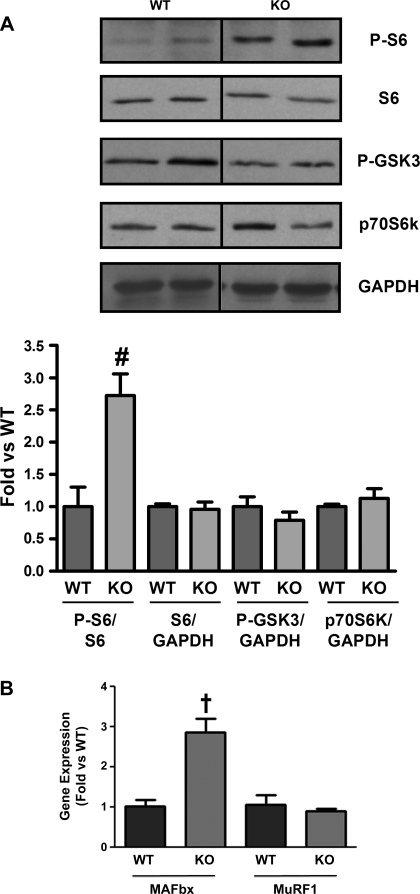
To determine possible downstream effectors of Akt that may mediate the observed increase in skeletal muscle mass seen in vivo, we examined changes in phosphorylation of p70S6 kinase, GSK3, S6, and mRNA for the E3 ligases MAFbx and MuRF1, which mediate atrophy (31). Interestingly, we found that only S6 kinase phosphorylation was increased (2.7-fold) in KO skeletal muscle compared with WT (Fig. 6, n = 4, P < 0.05). There was no difference in total p70S6 kinase (Fig. 6A), and baseline phosphorylation of p70S6 kinase was not detected in either group (data not shown). Phosphorylation of GSK3 was not increased in KO skeletal muscle (Fig. 6A, n = 4), suggesting it is not involved in mediating the increase in skeletal muscle mass downstream of Akt. We did not see a change in MuRF1 transcription; however, we found a surprising 2.8-fold increase in MAFbx transcription (Fig. 6B, n = 3, P < 0.01), which would be expected to increase atrophy. Thus modulation of atrophy is unlikely to contribute to the skeletal muscle hypertrophy with MSTN deletion, and the observed increase in MAFbx mRNA may represent a counter-regulatory anti-growth response. Together these data suggest that MSTN modulates myotube hypertrophy through Akt and that the increase in Akt activation seen in MSTN KO skeletal muscle may mediate, at least in part, the observed skeletal muscle hypertrophy without reducing atrophy.
Fig. 6.
Increased S6 phosphorylation and atrogin-1/muscle atrophy F box (MAFbx) mRNA in vivo. A: representative Western blots from quadriceps of WT and KO mice was probed with anti-phospho-S6, S6, phospho-GSK3, p70S6K, and GAPDH antibodies. Bar graphs show quantification of blots normalized to S6 or GAPDH. Dividing lines on Western blot images depict where bands from the same blot have been juxtaposed. Quantitative real-time RT-PCR using RNA samples from WT and KO quadriceps shows an increase in MAFbx gene expression in KO skeletal muscle. Gene expression was normalized to GAPDH. (Western blots; n = 3WT:4KO). MURF-1, muscle RING-finger protein-1. Gene expression, n = 3WT:3KO. †P < 0.01 vs. WT, #P < 0.05 vs. WT.
DISCUSSION
In this study, we demonstrate that the genetic loss of MSTN in vivo increased expression and overall activity of Akt in skeletal muscle. Furthermore, we show that in vitro overexpression of MSTN can block IGF-I-mediated myotube hypertrophy, whereas inhibition of MSTN results in hypertrophy in an Akt-dependent manner. Together these findings suggest a previously unappreciated mechanism by which MSTN modulates skeletal muscle growth:regulation of Akt signaling.
The current work extends our previous observations in cardiac muscle with some important differences. Previously, we reported that MSTN inhibits cardiomyocyte hypertrophy in response to PE, an α-adrenergic agonist, but not IGF-I (22). In contrast, in C2C12 myotubes, IGF-I-mediated hypertrophy is blocked by MSTN, suggesting MSTN may inhibit hypertrophy in a stimulus- and cell-type-specific manner. We previously demonstrated that PE-stimulated hypertrophy in the hearts of myostatin-null is enhanced in association with an increase in PE-stimulated Akt activity without a change in baseline heart size, total Akt, or Akt activity (22). In contrast, the MSTN null skeletal muscles are hypertrophied and show increased Akt expression and activity at baseline.
Using an in vitro model of skeletal muscle hypertrophy, we further demonstrated that the modulation of Akt activity was mechanistically important for MSTN's anti-hypertrophic effects. Although the use of adenoviral overexpression in C2C12 myotubes, like many other in vitro models, facilitates the understanding of signaling mechanism through ease of experimental manipulation, caution is warranted in direct extrapolation to the in vivo setting. Of note, in this case, loss of MSTN in vivo resulted in upregulation of total Akt and activity, whereas acute overexpression of dnMSTN or MSTN in C2C12 myotubes altered Akt activity without a change in overall levels of protein. Interestingly, we have previously observed that cardiac-specific overexpression of Akt in vivo increased MSTN expression (4, 22); however, adenoviral overexpression of Akt in cardiomyocytes in vitro did not. These situations may represent secondary adaptations of muscle to the chronic loss or enhancement of specific growth signaling pathways. The absence of increased p70S6 kinase and GSK3 phosphorylation in KO skeletal muscle, although shown to be increased by acute IGF-I/Akt stimulation in C2C12 myotubes (26), may also be subject to homeostatic regulation of chronic activation of growth signaling pathways (negative feedback) in response to chronic in vivo Akt activation. The increase in S6 phosphorylation observed in vivo is consistent with increased Akt activation and growth; however, it is not clear that Akt is directly responsible for this increase. Recent reports have provided further evidence for the importance of S6 regulation in controlling muscle growth downstream of MSTN. One report has shown that acute overexpression of MSTN in skeletal muscle via electroporation of DNA was sufficient to reduce skeletal muscle mass and was associated with a dramatic decrease in S6 phosphorylation (1). Interestingly, they did not observe an increase in mTOR phosphorylation despite the modulation of downstream targets. Acute inhibition of MSTN in vivo by administration of an inhibitory MSTN antibody (JA16) (38) or transfection of a dominant negative MSTN receptor (activin type IIB)(29) selectively into adult mouse skeletal muscle also increased muscle mass in association with increased phosphorylation of S6. These results are consistent with our findings that MSTN can regulate distal downstream effectors of Akt in the mTOR pathway to affect skeletal muscle hypertrophy (1). Surprisingly, we found an increase in MAFbx mRNA. Since Akt is able to inhibit atrophy by blocking FoxO-mediated transcriptional increases in E3 ligases including MAFbx (13, 28, 31), and MSTN has been reported to increase MAFbx transcription in C2C12 myotubes (18), we would have expected a decrease in transcription if the inhibition of atrophy secondary to increased Akt activity in MSTN KO skeletal muscle was involved. The observed increase in MAFbx transcription may represent another homeostatic counter-regulatory response to the increase in muscle mass or perhaps a direct effect of MSTN loss since it has recently been shown that MSTN decreases MAFbx transcription in human skeletal muscle myotubes (35). Overall our data are consistent with published data supporting the idea that MSTN predominantly modulates protein synthesis rather than protein degradation (33, 36, 38).
Our results are also consistent with recent work in C2C12 myoblasts, as opposed to the myotubes employed here, which demonstrated that MSTN modulates myoblast proliferation (hyperplasia) through inhibition of PI3 kinase-Akt and cyclin D1 (40). The current study demonstrates MSTN also modulates myotube growth or hypertrophy (as opposed to proliferation) through modulation of Akt signaling. Although the effects of PI3 kinase-Akt signaling in myoblasts and myotubes are different (27), together these studies place PI3 kinase-Akt signaling at the intersection of MSTN's effects in both stages of differentiation as well as on both muscle proliferation and growth. The latter effects are likely to provide insight into the postnatal muscle growth seen in the KO mice.
Interestingly, another report in skeletal muscle fibroblasts demonstrates an increase in Akt phosphorylation associated with exposure to exogenous MSTN (16). Although this is the opposite, and likely a cell-type-specific effect, it supports a connection between MSTN and Akt signaling.
In human myotubes MSTN administration was associated with a decrease in myotube diameter and Akt phosphorylation; however, MSTN did not inhibit IGF-1 stimulated Akt phosphorylation (35). Interestingly, pharmacological inhibition of a MSTN receptor (ALK) was sufficient to increase myotube diameter consistent with our own results using dnMSTN to directly inhibit endogenous baseline MSTN signaling. The differences between these studies could be explained by the use of different cell lines, since the human myotubes appear less responsive to IGF-I than C2C12 myotubes, or perhaps by the administration route of MSTN, since MSTN is thought to work via an autorcrine factor in vivo and exogenously added MSTN has different cellular effects than MSTN released from the cell in an autocrine fashion (25).
MSTN KO mice also display reduced adipose tissue (21). Interestingly, transgenic overexpression of Akt in skeletal muscle has also been reported to decrease adiposity in mice (8, 11), suggesting the increase in skeletal muscle Akt activity reported here may contribute to the decreased adiposity seen in MSTN KO mice. Similarly, the resistance to diabetes seen after MSTN deletion (21) could relate not only to the increase in muscle mass but the enhanced Akt signaling, which would be predicted to increase glucose uptake, although we have not directly examined this here.
In conclusion, we show that MSTN regulates Akt signaling in vitro and in vivo. In vitro studies suggest this regulation is necessary and sufficient for MSTN's effects on myotube hypertrophy in response to IGF-I. Moreover, we hypothesize that MSTN's modulation of Akt signaling could play an important role in other phenotypes seen with genetic manipulation of MSTN in vivo. Understanding the downstream mechanisms responsible for MSTN's effects on skeletal muscle growth, adipose tissue, and insulin resistance could help guide therapeutic strategies targeting this pathway in a wide variety of conditions.
GRANTS
This research was supported by a Leducq Foundation Network of Research Excellence (to S. A. Cook and A. Rosenzweig) and grants from the National Institutes of Health: National Heart, Lung, and Blood Institute, National Institute on Aging (to A. Rosenzweig and M. R. Morissette). A. Rosenzweig is a principal faculty member of the Harvard Stem Cell Institute.
ACKNOWLEDGMENTS
We thank Dr. Serafima Zaltsman for expertly managing the mouse colony.
Current address of M. R. Morissette: Division of Exercise Physiology, Center for Cardiovascular and Respiratory Sciences, West Virginia University School of Medicine, Morgantown, WV 26506.
REFERENCES
- 1.Amirouche A, Durieux AC, Banzet S, Koulmann N, Bonnefoy R, Mouret C, Bigard X, Peinnequin A, Freyssenet D. Down-regulation of akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology 150: 286–294, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibe B, Bouix J, Caiment F, Elsen JM, Eychenne F, Larzul C, Laville E, Meish F, Milenkovic D, Tobin J, Charlier C, Georges M. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet 38: 813–818, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem 277: 22528–22533, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset R, Georges M. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet 17: 71–74, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Grobet L, Pirottin D, Farnir F, Poncelet D, Royo LJ, Brouwers B, Christians E, Desmecht D, Coignoul F, Kahn R, Georges M. Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis 35: 227–238, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem 277: 40735–40741, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, Hamilton JA, Ouchi N, LeBrasseur NK, Walsh K. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab 7: 159–172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res 286: 263–275, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res 7: 910–916, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24: 9295–9304, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem 277: 49831–49840, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 280: 2737–2744, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98: 9306–9311, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, Wright JF, Barker C, Ehrmantraut G, Holmstrom J, Trowell B, Gertz B, Jiang MS, Sebald SM, Matzuk M, Li E, Liang LF, Quattlebaum E, Stotish RL, Wolfman NM. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA 102: 18117–18122, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem 283: 19371–19378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 18.McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 209: 501–514, 2006 [DOI] [PubMed] [Google Scholar]
- 19.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387: 83–90, 1997 [DOI] [PubMed] [Google Scholar]
- 20.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94: 12457–12461, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109: 595–601, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res 99: 15–24, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 3: e79, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings B, Kass DA, Champion HC, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest 115: 2128–2138, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rios R, Fernandez-Nocelos S, Carneiro I, Arce VM, Devesa J. Differential response to exogenous and endogenous myostatin in myoblasts suggests that myostatin acts as an autocrine factor in vivo. Endocrinology 145: 2795–2803, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3: 1009–1013, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 286: 1738–1741, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, Sandri M. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol 296: C1248–C1257, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350: 2682–2688, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Szabo G, Dallmann G, Muller G, Patthy L, Soller M, Varga L. A deletion in the myostatin gene causes the compact (Cmpt) hypermuscular mutation in mice. Mamm Genome 9: 671–672, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH, Jr, Kull FC, Jr, Gonzalez-Cadavid N. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab 280: E221–E228, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275: 40235–40243, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296: C1258–C1270, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Welle S, Bhatt K, Pinkert CA. Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab 290: E409–E415, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Welle S, Bhatt K, Pinkert CA, Tawil R, Thornton CA. Muscle growth after postdevelopmental myostatin gene knockout. Am J Physiol Endocrinol Metab 292: E985–E991, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Welle S, Burgess K, Mehta S. Stimulation of skeletal muscle myofibrillar protein synthesis, p70 S6 kinase phosphorylation, and ribosomal protein S6 phosphorylation by inhibition of myostatin in mature mice. Am J Physiol Endocrinol Metab 296: E567–E572, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci USA 100: 15482–15486, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang W, Zhang Y, Li Y, Wu Z, Zhu D. Myostatin induces cyclin D1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/AKT/GSK-3 beta pathway and is antagonized by insulin-like growth factor 1. J Biol Chem 282: 3799–3808, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, Hadhazy M, Wehling M, Tidball JG, McNally EM. Dominant negative myostatin produces hypertrophy without hyperplasia in muscle. FEBS Lett 474: 71–75, 2000 [DOI] [PubMed] [Google Scholar]