Abstract
Human bone marrow mesenchymal stem cells (MSCs) are a potent source of growth factors, which are partly responsible for their beneficial paracrine effects. We reported previously that transforming growth factor-α (TGF-α), a putative mediator of wound healing and the injury response, increases the release of vascular endothelial growth factor (VEGF), augments tumor necrosis factor-α (TNF-α)-stimulated VEGF production, and activates mitogen-activated protein kinases and phosphatidylinositol 3-kinase (PI-3K) pathway in human MSCs. The experiments described in this report indicate that TGF-α increases MSC-derived hepatocyte growth factor (HGF) production. TGF-α-stimulated HGF production was abolished by inhibition of MEK, p38, PI-3K, or by small interfering RNA (siRNA) targeting TNF receptor 2 (TNFR2), but was not attenuated by siRNA targeting TNF receptor 1 (TNFR1). Ablation of TNFR1 significantly increased basal and stimulated HGF. A potent synergy between TGF-α and TNF-α was noted in MSC HGF production. This synergistic effect was abolished by MEK, P38, PI-3K inhibition, or by ablation of both TNF receptors using siRNA. We conclude that 1) novel cross talk occurs between tumor necrosis factor receptor and TGF-α/epidermal growth factor receptor in stimulating MSC HGF production; 2) this cross talk is mediated, at least partially, via activation of MEK, p38, and PI-3K; 3) TGF-α stimulates MSCs to produce HGF by MEK, p38, PI-3K, and TNFR2-dependent mechanisms; and 4) TNFR1 acts to decrease basal TGF-α and TNF-α-stimulated HGF.
Keywords: transforming growth factor-α, vascular endothelial growth factor, tumor necrosis factor-α, epidermal growth factor receptor, phosphatidylinositol 3-kinase, tumor necrosis factor receptor
mesenchymal stem cells (MSCs) hold great therapeutic potential for endogenous tissue repair and regeneration (40). The therapeutic effects of stem cells may be derived in part from the secretion of cytoprotective growth factors and antioxidants (10–12, 17, 24, 44). Although the particular growth factors contributing to these reparative effects remain to be defined, evidence suggests that hepatocyte growth factor (HGF) may be a crucial mediator. HGF is produced by MSCs and is a potent inducer of angiogenesis (25). Moreover, HGF is reported to suppress vascular endothelial growth factor (VEGF)-induced inflammation and works to promote tissue repair and wound healing (25–27,36). Recently, we and others have demonstrated that the release of HGF from MSCs is increased following stimulation with tumor necrosis factor-α (TNF-α), interferon-γ, and LPS (6, 32, 49). However, other factors present during the acute inflammatory response that may stimulate stem cell HGF production have not been elucidated.
Transforming growth factor-α (TGF-α) is a member of the epidermal growth factor (EGF) superfamily. TGF-α activates the EGF receptor (EGFR) and initiates EGF signaling, such as the activation of mitogen-activated protein kinase kinase (MAPKK/MEK), mitogen-activated protein kinase (MAPK) (8), and phosphatidylinositol 3-kinase (PI-3K) pathways. Evidence also suggests that TGF-α may play an important role in inflammation, the injury response, and wound healing (34). TGF-α is produced by several cells involved in wound healing, including platelets (34), epithelial cells (2, 19), keratinocytes (3), and activated macrophages (31). In addition, the expression of TGF-α was upregulated in response to injury and inflammation (4, 29, 39). Furthermore, ectopic expression of TGF-α increased the inflammatory response in the lung (18) and skin (46) of transgenic mice. These observations suggest that TGF-α may be released at the site of injury by macrophages, platelets, or other cell types and may play a crucial role during tissue inflammatory responses, wound healing, and repair. With the emerging appreciation for the potential use of stem cells in the repair of injured tissue, the understanding of how prominent wound cytokines, such as TGF-α, affect stem cell function may allow for maximal growth factor production before, and during, therapeutic use. Moreover, the cross talk between TGF-α and other proinflammatory cytokines, such as TNF-α, requires detailed mechanistic definition. We hypothesized that 1) TGF-α would increase MSC-produced HGF via MEK, p38, and PI-3K-dependent mechanisms; 2) TGF-α and TNF-α would work either additively or synergistically to increase MSC-derived HGF; and 3) ablation of TNF receptor 1 (TNFR1) or TNF receptor 2 (TNFR2) would abolish the additive or synergistic effects of TGF-α and TNF-α on MSC HGF production.
MATERIALS AND METHODS
Reagents.
TNF-α (Chemicon, Temecula, CA) was used at a final concentration of 50 ng/ml. TGF-α (at various doses from 0 pg/ml to 1 μg/ml) was obtained from Sigma (St. Louis, MO). U0126 and PD98059 (MEK inhibitors, EMD, San Diego, CA) were used at final concentrations of 10 and 50 μM, respectively. P38 inhibitors SB202190 and SB203580 (EMD) were used at a final concentration of 10 μM. PI-3K inhibitors (1 μM wortmannin and 10 μM LY294002) were obtained from Sigma.
Human mesenchymal stem cell culture.
Mesenchymal stem cells (MSCs; purchased from Lonza Walkersville) were tested for purity by flow cytometry and for their ability to differentiate into osteogenic, chondrogenic, and adipogenic lineages. Cells were positive for CD105, CD166, CD29, and CD44. Cells were negative for CD14, CD34, and CD45. The cells were thawed, and the culture process was initiated according to the manufacturer's instructions. MSCs were plated in tissue culture flasks (Corning, Corning, NY) and cultured with mesenchymal stem cell growth medium (Lonza) at 37°C in 5% CO2 and 90% humidity. The medium was changed every 3 days. After cells attained 70% confluence, MSCs were plated in 12-well plates (Corning) at 0.05 × 106 cells·well−1·ml−1.
Experimental groups.
MSCs were exposed to 1) TGF-α at various doses from 0 ng/ml (vehicle control) to 1 μg/ml; 2) stimulant alone: TGF-α (250 ng/ml), TNF-α (50 ng/ml), or TNF-α plus TGF-α; 3) inhibitor alone: MEK inhibitors (50 μM PD98059 or 10 μM U0126), p38 inhibitors (10 μM SB202190 and SB203580), PI-3K inhibitors (1 μM wortmannin or 10 μM LY294002); and 4) stimulant (250 ng/ml TGF-α and/or 50 ng/ml TNF-α) plus the aforementioned inhibitors for 24 h. The concentrations of the inhibitors were determined according to previous laboratory characterization and literature data (5, 7, 52). After 24 h of incubation, supernatant was collected and the number of cells was counted by using a cytometer; the number of cells treated with stimulants and/or inhibitors did not show significant difference compared with vehicle control groups.
HGF ELISA.
Supernatants were collected after 24 h of incubation and measured for HGF production via ELISA using a commercially available kit (R&D Systems, Minneapolis, MN).
ELISA was performed according to the manufacturer's instructions. Briefly, 96-well plates were coated by diluted capture antibody for human HGF overnight in room temperature. After washing three times, the plates were blocked by PBS containing 1% of BSA. Supernatant was added to the 96-well plates, and the captured HGF was detected using biotinylated goat anti-human HGF. After 30 min of incubation with streptavidin-horseradish peroxidase, substrate solutions (1:1 mixture of H2O2 and tetramethylbenzidine) were added to the wells for 20 min, and the reaction was stopped by adding 2 N H2SO4. The plates were read at 450 nm on a microtiter plate reader. All samples and standards were measured in duplicate. After supernatant collection, the cells were counted using a cytometer; values were normalized to cell number (pg/ml per 105 cells) and are expressed as means ± SE.
Small interfering RNA transfection.
Small interfering RNAs (siRNAs) that specifically target human TNFR1 and TNFR2 were designed according to the software provided by Dharmacon siDESIGN center (Dharmacon Research, Lafayette, CO). SiRNA sequences corresponding to residues 587–605 (CTCCAAATGCCGAAAGGAA) of the coding region of human TNFR1 mRNA and siRNA sequences corresponding to residues 1297–1315 (CCACAATGGGAGACACAGA) of the coding region of human TNFR2 mRNA were selected. MSCs were transfected with the aforementioned specific siRNAs targeting TNFR1 or TNFR2. MSCs transfected with siRNA targeting GAPDH (Silencer GAPDH siRNA; Ambion) were used as control. Transfection was performed with Lipofectamine 2000 (Invitrogen) per the manufacturer's instructions. Briefly, 24 h before siRNA transfection, cells were plated in 12-well plates at 0.05 × 106 cells·well−1·ml−1. On culture day 2, cells were washed once with Optimem media (Invitrogen) and the lipofectamine-siRNA complex (100 nM) was then added. After 1 day of transfection (day 3 in culture), the lipofectamine-siRNA complex was washed out and the mesenchymal stem cell growth medium containing antibiotics was added to the cells and allowed to incubate for an additional 2 days. Three days after transfection (day 5 in culture), MSCs were exposed to TGF-α and/or TNF-α stimulation for 24 h. After 4 days of transfection, the supernatant was collected for HGF analysis. Cell extracts were prepared for performing Western blot analysis.
Protein isolation and Western blot analysis.
Western blot analysis was performed to measure the expression of TNFR1 and TNFR2. Cell extracts were prepared by direct lysis of cells in cold RIPA buffer (Sigma) containing a protease inhibitor cocktail (Sigma) and a phosphatase inhibitor cocktail 2 (Sigma). Lysates were then centrifuged at 12,000 rpm (12,902 G-force) for 10 min. Protein extracts (10 μg/lane) were electrophoresed on a 4–12% Bis Tris gel (Invitrogen) and transferred to a nitrocellulose membrane, which was stained by naphthol blue-black to confirm equal protein loading. The membranes were incubated in 5% dry milk for 1 h and then incubated with the following primary antibodies for TNFR1, TNFR2, and actin (Santa Cruz Biotechnology), followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody and detection using supersignal west pico stable peroxide solution (Pierce, Rockford, IL). Band densities were compared using TotalLab software (Nonlinear USA, Durham NC).
5-Bromo-2′-deoxyuridine incorporation.
5-Bromo-2′-deoxyuridine (BrdU) incorporation was used to measure DNA synthesis in a colorimetric immunoassay. Briefly, MSCs between 6 and 8 passages were plated in 96-well microtiter plates (Corning) at 5,000 cells/well and allowed to adhere overnight in mesenchymal stem cell growth medium (Lonza) at 37°C in 5% CO2 and 90% humidity. Subsequently, the cells were incubated with stimulants (250 ng/ml TGF-α and/or 50 ng/ml TNF-α) and 10 μM BrdU for 24 h. The labeled cells were fixed with ethanol. Before incubation with a monoclonal antibody to BrdU, DNA was partially digested with nucleases to allow the antibody to access BrdU. Finally, the unbound anti-BrdU antibody was washed out and the peroxidase substrate 2,2′-azino-bis(3)ethylbenzthiazoline-6-sulfonic acid (ABTS) was added. Peroxides catalyzed the cleavage of ABTS, producing a colored reaction product. The absorbance of the samples was determined with a standard microplate (ELISA) reader at 405 nm with a reference wavelength at 490 nm.
Data analysis.
Values represent means ± SE. Statistical differences between the control groups and those obtained under various treatment conditions were determined by using a one-way ANOVA followed by a Holm-Sidak post hoc analysis. Values of P < 0.05 were judged to be statistically significant.
RESULTS
TGF-α increased the production of HGF in MSCs.
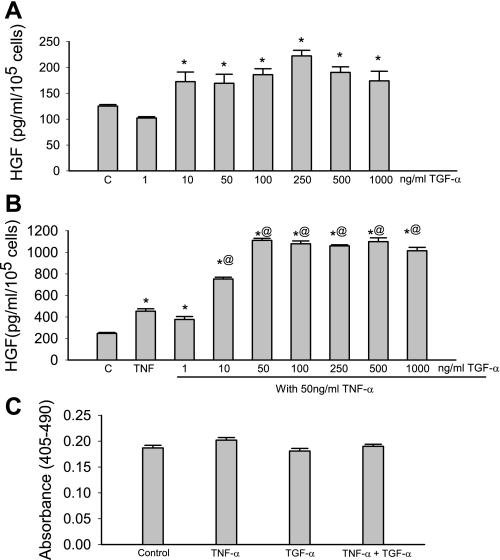
TGF-α significantly increased HGF production compared with control groups (Fig. 1A). For example, 250 ng/ml of TGF-α increased HGF production from 125 ± 3 pg/ml per 105 cells to 222 ± 11 pg/ml per 105 cells (n = 3). To examine whether there was cross talk between TGF-α and TNF-α, MSCs were treated with both TGF-α and TNF-α for a 24-h period. TNF-α alone (50 ng/ml) significantly increased HGF production. A potent synergy in HGF production was noted between TGF-α (10 ng/ml–1,000 ng/ml) and TNF-α (50 ng/ml) in MSCs (Fig. 1B). Interestingly, no significant change of cell proliferation was observed (Fig. 1C).
Fig. 1.
Transforming growth factor-α (TGF-α) increased hepatocyte growth factor (HGF) secretion from human mesenchymal stem cells (MSCs) and augmented TNF-stimulated HGF production. A: TGF-α (10–1,000 ng/ml) significantly increased HGF production. C, control. B: TNF-α (50 ng/ml) increased HGF production. TGF-α (10–1,000 ng/ml) significantly augmented TNF-stimulated HGF production. C: no significant change of cell proliferation was observed. Data are representative of three independent experiments. Results are means ± SE; n = 3–6 /group. *P < 0.05 vs. control; @P < 0.05, significantly increased vs. TNF as determined by 1-way ANOVA followed by a Holm-Sidak method post hoc analysis.
The effect of TNFR1 and TNFR2 siRNA on TGF-α and/or TNF-α-stimulated HGF production.
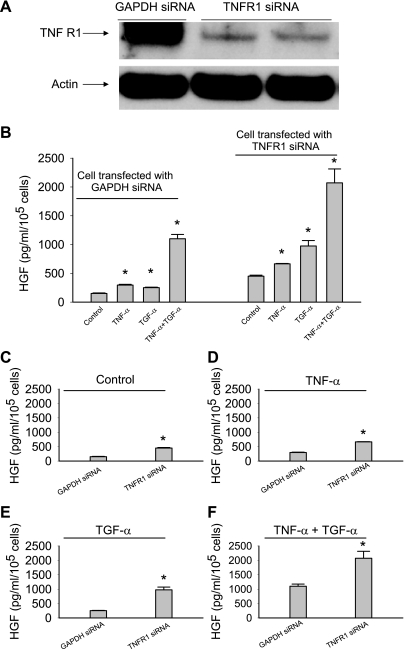
TNF-α acts through two structurally distinct receptors (TNFR1 and TNFR2), both of which are expressed on MSCs (Figs. 2A and 3A). Activation of the two receptors has been proposed to mediate distinct effects: TNFR1 contributes to cellular damage (38, 48), whereas TNFR2 is considered to be cytoprotective (14). It is unclear whether TNFR1 and TNFR2 play any role for TNF-α and TGF-α-stimulated HGF production in MSCs. To test this, MSCs were transfected with TNFR1 and/or TNFR2 siRNA. Three days after transfection, the expression of TNFR1 (55 kDa) and TNFR2 (75 kDa) was significantly decreased (Figs. 2A and 3A). There is no apparent attenuation or enhancement of TNFR2 expression in TNFR1-ablated cells and vice versa (data not shown). The transfected cells were then stimulated with TGF-α (250 ng/ml) and/or TNF-α (50 ng/ml). As shown in Fig. 2, B–F, MSCs transfected with TNFR1 siRNA significantly increased basal and stimulated HGF production compared with MSCs transfected with GAPDH siRNA.
Fig. 2.
Transfection of tumor necrosis factor (TNF) receptor 1 (TNFR1) small interfering RNA (siRNA), but not control GAPDH siRNA, caused a significant increase of both basal and TNF-α and/or TGF-α-stimulated release of HGF from MSCs. A: MSCs transfected with TNFR1 siRNA (100 nM) expressed less TNFR1 than MSCs transfected with GAPDH siRNA (100 nM). B–F: ablation of TNFR1 increased both the basal and stimulated release of HGF. Data are representative of at least two independent experiments. Results are means ± SE; n = 3/group. *P < 0.05 vs. control as determined by 1-way ANOVA followed by a Holm-Sidak method post hoc analysis.
Fig. 3.
Transfection of TNF receptor 2 (TNFR2) siRNA, but not control GAPDH siRNA, suppressed basal HGF production and ablated TGF-α stimulated HGF release. A: MSCs transfected with TNFR2 siRNA (100 nM) expressed less TNFR2 than MSCs transfected with GAPDH siRNA (100 nM). B–F: ablation of TNFR2 suppressed basal and TNF-α-stimulated HGF production and abolished the effects of TGF-α. Data are the combination of two sets of independent experiments and are expressed as means ± SE; n = 6/group. *P < 0.05 vs. control as determined by 1-way ANOVA followed by a Holm-Sidak method post hoc analysis.
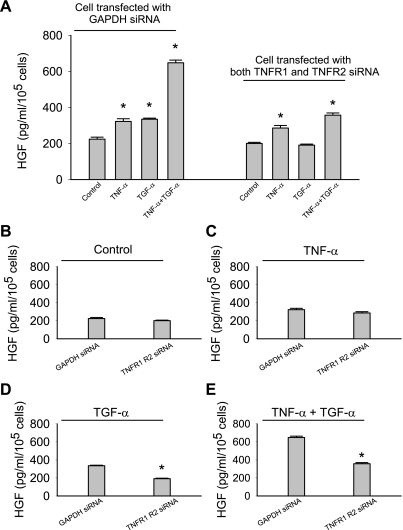
In contrast, knockdown of TNFR2 decreased both basal and stimulated HGF (Fig. 3, B–F). Additionally, these MSCs lost their response to TGF-α stimulation (Fig. 3B). Knockdown of either TNF receptor individually was not sufficient to abolish the synergistic response observed when TNF-α and TGF-α were added together (Figs. 2B and 3B). However, when both receptors were ablated, this synergetic response and TGF-α-stimulated HGF release were eliminated (Fig. 4, A, D, and E). Ablation of both TNF receptors restored the basal and TNF-α-stimulated HGF production to the level produced by MSCs with GAPDH ablation (Fig. 4, B and C).
Fig. 4.
MSCs with both TNFR1 and TNFR2 ablation exhibited normal basal and TNF-α-stimulated HGF production, but not TGF-α-stimulated HGF production. A–C: ablation of both TNFR1 and TNFR2 did not alter basal and TNF-α-stimulated HGF production. D and E: the stimulatory effect of TNF-α on MSC HGF production was significantly attenuated. Data are representative of at least two independent experiments. Results are means ± SE; n = 3/group. *P < 0.05 vs. control as determined by 1-way ANOVA followed by a Holm-Sidak method post hoc analysis.
Regulation of HGF secretion by MEK, p38, and PI-3K.
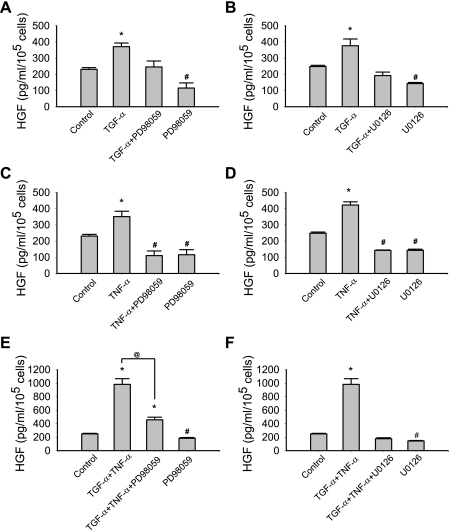
As a potent activator of EGFR, TGF-α activates a network of signaling cascades (8, 13, 30, 50). Our previous study demonstrated that TGF-α activated MEK-MAPK and PI-3K in MSCs (49). However, it was still unclear as to whether TGF-α would increase HGF production by MEK-, p38-, and PI-3K-dependent mechanisms. To study this, MSCs were exposed to TGF-α and/or TNF-α with or without MEK, p38, and PI-3K inhibition. Although the effects of these kinase inhibitors are relatively specific, these chemical inhibitors have been reported to produce “off-target” effects (7). To minimize the possibility that the data described in this report are due to the off-target effects of these kinase inhibitors, two pharmacologic inhibitors with different mechanisms of inhibition were used to inhibit one protein kinase. As demonstrated in Fig. 5, basal and TNF-α or TGF-α-stimulated HGF production were noted to be attenuated by MEK inhibition (PD98059 and U0126, Fig. 5, A–D). MEK inhibitors also suppressed the “synergy” effects between TNF-α and TGF-α on HGF production in MSCs (Fig. 5, E and F).
Fig. 5.
MEK inhibition [PD98059 (A, C, and E) and U0126 (B, D, and F)] suppressed the production of HGF in response to TNF-α and TGF-α. Data are representative of three independent experiments. Results are means ± SE; n = 3/group. *P < 0.05, significantly increased vs. control; #P < 0.05, significantly suppressed vs. control; @P < 0.05, TGF-α + TNF-α vs. TGF-α + TNF-α + PD98059 as determined by 1-way ANOVA followed by a Holm-Sidak method post hoc analysis. TNF-α: 50 ng/ml; TGF-α: 250 ng/ml; PD98059: 50 μM; U0126: 10 μM.
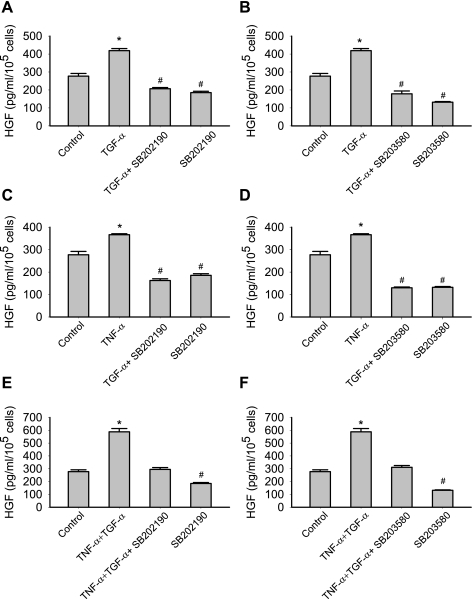
To further investigate whether p38 mediated the effect of TGF-α on HGF production, p38 inhibitor I (SB202190) and p38 inhibitor II (SB203580) were used. As demonstrated in Fig. 6, A–F, both p38 inhibitors abolished the TGF-α and/or TNF-α-induced production of HGF.
Fig. 6.
p38 inhibition [SB202190 (A, C, and E) and SB203580 (B, D, and F)] suppressed the production of HGF in response to TNF-α and TGF-α. Data are representative of three independent experiments. Results are means ± SE; n = 3/group. *P < 0.05, significantly increased vs. control; @P < 0.05, significantly suppressed vs. control as determined by 1-way ANOVA followed by a Holm-Sidak method post hoc analysis. TNF-α: 50 ng/ml; TGF-α: 250 ng/ml; SB202190: 10 μM; SB203580: 10 μM.
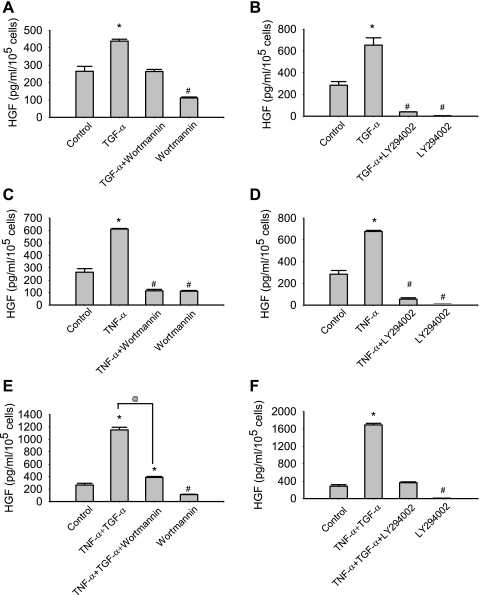
PI-3K inhibition was noted to suppress HGF production. As demonstrated in Fig. 7, A–D, LY294002 and wortmannin significantly suppressed both basal and TGF-α or TNF-α-stimulated HGF treatment. PI-3K inhibition also abolished the synergistic effects of TNF-α and TGF-α on HGF production in MSCs (Fig. 7, E and F).
Fig. 7.
PI-3K inhibition [wortmannin (A, C, and E) and LY294002 (B, D, and F)] suppressed the secretion of HGF in response to TNF-α and TGF-α. Data are representative of three independent experiments. Results are means ± SE, n = 3/group. *P < 0.05, significantly increased vs. control; #P < 0.05, significantly suppressed vs. control; @P < 0.05 TNF + TGF vs. TNF + TGF + wortmannin as determined by 1-way ANOVA followed by a Holm-Sidak method post hoc analysis. TNF-α: 50 ng/ml; TGF-α: 250 ng/ml; wortmannin: 1 μM; LY294002: 10 μM.
DISCUSSION
Adult progenitor cells have tremendous therapeutic potential in treating a variety of diseases. The release of soluble factors by these cells may be important mechanisms underlying tissue repair and functional improvement (11, 33). Herein, we demonstrated that 1) TGF-α (10–1,000 ng/ml) or TNF-α (50 ng/ml) significantly increased HGF production; 2) the TGF-α or TNF-α-stimulated HGF production was abolished by MEK, p38, and PI-3K inhibition; 3) MEK, p38, and PI-3K inhibition decreased basal HGF production; 4) a potent synergistic effect existed between TGF-α (10–1,000 ng/ml) and TNF-α (50 ng/ml)-stimulated HGF production that was abolished by MEK, p38, and PI-3K inhibition; 5) TNFR1 ablation significantly enhanced both basal and stimulated HGF secretion, whereas TNFR2 ablation attenuated both basal and stimulated HGF production; and 6) ablation of both TNF receptors restored the basal and TNF-α-stimulated HGF production, but not TGF-α-stimulated HGF production.
TGF-α is a potent mitogen of the EGF superfamily. TGF-α possesses sequence homology with EGF and binds to EGFR, thereby stimulating a network of intracellular signaling cascades that include the MAPK and PI-3K pathways (8). Our previous study demonstrated that TGF-α activated MAPK and PI-3K in MSCs (49). These results are consistent with the effects of TGF-α on other cell types, such as various tumor cell lines (28, 37, 41) and primary cells such as smooth muscle (16, 41, 51). Activation of these downstream mediators modulates the production of HGF from stem cells and may promote stem cell and native tissue survival after injury. Indeed, HGF seems to be a promising factor in stem cell-mediated repair, because HGF was demonstrated to facilitate an adhesive microenvironment for transplanted stem cells, thereby promoting cell adhesion, survival, proliferation, and repair of MSCs in injured tissue (9, 45).
This study sought to elucidate whether TGF-α increased stem cell-derived HGF, and if so, by what mechanisms this occurred. Our study using pharmacological inhibitors suggests that MEK, p38, and PI-3K are involved in basal and stimulated HGF production. To minimize the possibility that the data described in this report are due to the off-target effect of these kinase inhibitors, two pharmacologic inhibitors with different mechanisms of inhibition were used to inhibit one protein kinase. The effect of two inhibitors produced similar suppression on MSC-secreted HGF. We believe that the system is relatively clean and it is not likely that the experiments described in this report are due to the off-target effect of these inhibitors.
Knockdown of TNFR1 was found to increase both basal and stimulated HGF production, whereas knockdown of TNFR2 decreased both basal and stimulated HGF secretion. This observation indicates that the activation of TNFR1 may inhibit HGF production, thereby attenuating the therapeutic potential of MSCs. The activation of TNFR1 has been reported to suppress neural progenitor proliferation (15), to decrease the secretion of VEGF and insulin-like growth factor I from mouse MSCs (23), to generate reactive oxygen species, and to induce apoptosis (35). In contrast to TNFR1, TNFR2 does not contain a death domain and therefore does not transmit proapoptotic signals (35). The activation of TNFR2 leads to a persistent, PI3K-dependent NF-κB activation that may be essential for cell survival, proliferation, and growth factor production (1, 6, 21, 22, 42, 43). Our study demonstrated that the ablation of TNFR2 attenuated both basal and stimulated HGF production, indicating that TNFR2 acts to increase basal and stimulated HGF in human MSCs. Selective suppression of TNFR1 and enhancement of TNFR2 signaling could be a novel strategy to optimize the therapeutic potential of stem cells. Knockdown of either TNF receptor alone was not able to abolish the synergistic response as observed when TNF-α and TGF-α were added together. However, when both receptors were ablated, the synergetic response was abolished. This may indicate that a common signaling component downstream of both TNFR1 and TNFR2 mediates the synergistic HGF production. Ablation of both TNF receptors restored the basal HGF production to the level produced by MSCs with GAPDH ablation. Because there may still have functional TNFR1 and TNFR2 even after siRNA ablation, the observation may suggest that a proper “balance” between TNFR1 signaling and TNFR2 signaling determines the basal HGF production from human MSCs. Interestingly, TNF-α was still able to increase MSC-produced HGF while both receptors were ablated. This observation may suggest that 1) there is still functional TNFR1 and TNFR2; and 2) other members of the TNF receptor superfamily, such as CD40 or Fas, may be responsible for TNF-stimulated HGF production when both TNFR1 and TNFR2 were ablated (20).
It remains to be elucidated at what level TGF-α and TNF-α are affecting HGF production. It may occur at multiple levels, including the transcriptional level (regulated by transcription factors), the posttranscriptional level (regulated by RNA binding proteins or microRNAs), and the translational level (regulated by translation factors). This is an interesting question that warrants further investigation.
This study constitutes an initial report regarding the effect of TGF-α on stem cell HGF production. MSCs are a potent source of HGF, which may not only facilitate angiogenesis, but also suppress inflammation during ischemia and wound healing, and promote stem cell self survival, mobilization, proliferation, and homing during cellular therapy (47). Understanding how stem cells function is important for designing novel strategies to increase their ability to repair and regenerate injured tissue. Further in vivo studies must address the long-term behavior of the implanted, pharmacologically or genetically modified stem cells before widespread human application.
GRANTS
This work was supported in part by National Institutes of Health Grants R01GM070628, NIHR01HL085595, NIH K99/R00 HL0876077-01, NIH F32 HL092718-01, NIH F32 HL092719-01, and F32 HL093987-01A1.
ACKNOWLEDGMENTS:
We give our thanks to Dr. Chao-Hung Lee for technical assistance.
REFERENCES
- 1.Ait-Ali D, Turquier V, Tanguy Y, Thouennon E, Ghzili H, Mounien L, Derambure C, Jegou S, Salier JP, Vaudry H, Eiden LE, Anouar Y.Tumor necrosis factor (TNF)-alpha persistently activates nuclear factor-kappaB signaling through the type 2 TNF receptor in chromaffin cells: implications for long-term regulation of neuropeptide gene expression in inflammation. Endocrinology 149: 2840–2852, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bade EG, Feindler S.Liver epithelial cell migration induced by epidermal growth factor or transforming growth factor alpha is associated with changes in the gene expression of secreted proteins. In Vitro Cell Dev Biol 24: 149–154, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Barrandon Y, Green H.Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell 50: 1131–1137, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Brasken P, Renvall S, Sandberg M.Expression of epidermal growth factor and epidermal growth factor receptor genes in healing colonic anastomoses in rats. Eur J Surg 157: 607–611, 1991 [PubMed] [Google Scholar]
- 5.Chiariello M, Gomez E, Gutkind JS.Regulation of cyclin-dependent kinase (Cdk) 2 Thr-160 phosphorylation and activity by mitogen-activated protein kinase in late G1 phase. Biochem J 349: 869–876, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR.Human mesenchymal stem cells stimulated by TNF-α, LPS, or hypoxia produce growth factors by an NFκB- but not JNK-dependent mechanism. Am J Physiol Cell Physiol 294: C675–C682, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Davies SP, Reddy H, Caivano M, Cohen P.Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derynck R.Transforming growth factor alpha. Cell 54: 593–595, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, Zhang QW, Wang H, Jia XX, Wang LS.Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther 8: 467–474, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ.Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11: 367–368, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ.Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20: 661–669, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Haider H, Ashraf M.Bone marrow stem cell transplantation for cardiac repair. Am J Physiol Heart Circ Physiol 288: H2557–H2567, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M, Wolfman A.Oncogenic Ha-Ras-dependent mitogen-activated protein kinase activity requires signaling through the epidermal growth factor receptor. J Biol Chem 273: 28155–28162, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Higuchi Y, McTiernan CF, Frye CB, McGowan BS, Chan TO, Feldman AM.Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation 109: 1892–1897, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O.Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci 26: 9703–9712, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamura K, Kawamura N, Kumagai J, Fukuda J, Tanaka T.Tumor necrosis factor regulation of apoptosis in mouse preimplantation embryos and its antagonism by transforming growth factor alpha/phosphatidylionsitol 3-kinase signaling system. Biol Reprod 76: 611–618, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kudo M, Wang Y, Wani MA, Xu M, Ayub A, Ashraf M.Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol 35: 1113–1119, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Le Cras TD, Hardie WD, Deutsch GH, Albertine KH, Ikegami M, Whitsett JA, Korfhagen TR.Transient induction of TGF-α disrupts lung morphogenesis, causing pulmonary disease in adulthood. Am J Physiol Lung Cell Mol Physiol 287: L718–L729, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Lemarie CA, Tharaux PL, Esposito B, Tedgui A, Lehoux S.Transforming growth factor-alpha mediates nuclear factor kappaB activation in strained arteries. Circ Res 99: 434–441, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Locksley RM, Killeen N, Lenardo MJ.The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104: 487–501, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL.Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-d-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem 279: 32869–32881, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL.Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-d-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem 279: 32869–32881, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Markel TA, Crisostomo PR, Wang M, Herring CM, Meldrum DR.Activation of individual tumor necrosis factor receptors differentially affects stem cell growth factor and cytokine production. Am J Physiol Gastrointest Liver Physiol 293: G657–G662, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, Herring CM, Tan J, Lahm T, Meldrum DR.VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol 295: H2308–H2314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min JK, Lee YM, Kim JH, Kim YM, Kim SW, Lee SY, Gho YS, Oh GT, Kwon YG.Hepatocyte growth factor suppresses vascular endothelial growth factor-induced expression of endothelial ICAM-1 and VCAM-1 by inhibiting the nuclear factor-kappaB pathway. Circ Res 96: 300–307, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T.Structure and function of hepatocyte growth factor. Prog Growth Factor Res 3: 67–85, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Neuss S, Becher E, Woltje M, Tietze L, Jahnen-Dechent W.Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 22: 405–414, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Pino MS, Shrader M, Baker CH, Cognetti F, Xiong HQ, Abbruzzese JL, McConkey DJ.Transforming growth factor alpha expression drives constitutive epidermal growth factor receptor pathway activation and sensitivity to gefitinib (Iressa) in human pancreatic cancer cell lines. Cancer Res 66: 3802–3812, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Polk WH Jr, Dempsey PJ, Russell WE, Brown PI, Beauchamp RD, Barnard JA, Coffey RJ Jr.Increased production of transforming growth factor alpha following acute gastric injury. Gastroenterology 102: 1467–1474, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Porfiri E, McCormick F.Regulation of epidermal growth factor receptor signaling by phosphorylation of the ras exchange factor hSOS1. J Biol Chem 271: 5871–5877, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Rappolee DA, Mark D, Banda MJ, Werb Z.Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science 241: 708–712, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Ryan JM, Barry F, Murphy JM, Mahon BP.Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol 149: 353–363, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scherschel JA, Soonpaa MH, Srour EF, Field LJ, Rubart M.Adult bone marrow-derived cells do not acquire functional attributes of cardiomyocytes when transplanted into peri-infarct myocardium. Mol Ther 16: 1129–1137, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz G, Rotatori DS, Clark W.EGF and TGF-alpha in wound healing and repair. J Cell Biochem 45: 346–352, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Schutze S, Tchikov V, Schneider-Brachert W.Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol 9: 655–662, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Sengupta S, Gherardi E, Sellers LA, Wood JM, Sasisekharan R, Fan TP.Hepatocyte growth factor/scatter factor can induce angiogenesis independently of vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 23: 69–75, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Sewell JM, Smyth JF, Langdon SP.Role of TGF alpha stimulation of the ERK, PI3 kinase and PLC gamma pathways in ovarian cancer growth and migration. Exp Cell Res 304: 305–316, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Shen HM, Pervaiz S.TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J 20: 1589–1598, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Sottili M, Sternini C, Reinshagen M, Brecha NC, Nast CC, Walsh JH, Eysselein VE.Up-regulation of transforming growth factor alpha binding sites in experimental rabbit colitis. Gastroenterology 109: 24–31, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Springer ML, Brazelton TR, Blau HM.Not the usual suspects: the unexpected sources of tissue regeneration. J Clin Invest 107: 1355–1356, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stockhausen MT, Sjolund J, Axelson H.Regulation of the Notch target gene Hes-1 by TGFalpha induced Ras/MAPK signaling in human neuroblastoma cells. Exp Cell Res 310: 218–228, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Theiss AL, Simmons JG, Jobin C, Lund PK.Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem 280: 36099–36109, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Tian B, Nowak DE, Jamaluddin M, Wang S, Brasier AR.Identification of direct genomic targets downstream of the nuclear factor-kappaB transcription factor mediating tumor necrosis factor signaling. J Biol Chem 280: 17435–17448, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C.Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Vandervelde S, van Luyn MJ, Tio RA, Harmsen MC.Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol 39: 363–376, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Vassar R, Fuchs E.Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev 5: 714–727, 1991 [DOI] [PubMed] [Google Scholar]
- 47.Wang M, Tsai BM, Crisostomo PR, Meldrum DR.Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock 25: 454–459, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Wang M, Tsai BM, Crisostomo PR, Meldrum DR.Tumor necrosis factor receptor 1 signaling resistance in the female myocardium during ischemia. Circulation 114: I282–I289, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Crisostomo PR, Wang M, Markel TA, Novotny NM, Meldrum DR.TGF-α increases human mesenchymal stem cell-secreted VEGF by MEK- and PI3-K- but not JNK- or ERK-dependent mechanisms. Am J Physiol Regul Integr Comp Physiol 295: R1115–R1123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams R, Sanghera J, Wu F, Carbonaro-Hall D, Campbell DL, Warburton D, Pelech S, Hall F.Identification of a human epidermal growth factor receptor-associated protein kinase as a new member of the mitogen-activated protein kinase/extracellular signal-regulated protein kinase family. J Biol Chem 268: 18213–18217, 1993 [PubMed] [Google Scholar]
- 51.Yamanaka Y, Hayashi K, Komurasaki T, Morimoto S, Ogihara T, Sobue K.EGF family ligand-dependent phenotypic modulation of smooth muscle cells through EGF receptor. Biochem Biophys Res Commun 281: 373–377, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Zheng WH, Quirion R.Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci 7: 51, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]