Abstract
Neonatal necrotizing enterocolitis (NEC) is a major cause of morbidity and mortality in premature infants. Oral administration of probiotics has been suggested as a promising strategy for prevention of NEC. However, little is known about the mechanism(s) of probiotic-mediated protection against NEC. The aim of this study was to evaluate the effects of Bifidobacterium bifidum treatment on development of NEC, cytokine regulation, and intestinal integrity in a rat model of NEC. Premature rats were divided into three groups: dam fed (DF), hand fed with formula (NEC), or hand fed with formula supplemented with 5 × 106 CFU B. bifidum per day (B. bifidum). All groups were exposed to asphyxia and cold stress to develop NEC. Intestinal injury, mucin and trefoil factor 3 (Tff3) production, cytokine levels, and composition of tight junction (TJ) and adherens junction (AJ) proteins were evaluated in the terminal ileum. B. bifidum decreased the incidence of NEC from 57 to 17%. Increased levels of IL-6, mucin-3, and Tff3 in the ileum of NEC rats was normalized in B. bifidum treated rats. Reduced mucin-2 production in the NEC rats was not affected by B. bifidum. Administration of B. bifidum normalized the expression and localization of TJ and AJ proteins in the ileum compared with animals with NEC. In conclusion, administration of B. bifidum protects against NEC in the neonatal rat model. This protective effect is associated with reduction of inflammatory reaction in the ileum, regulation of main components of mucus layer, and improvement of intestinal integrity.
Keywords: adherens junctions, intestinal barrier function, mucins, tight junctions
necrotizing enterocolitis (NEC) is a major cause of morbidity and mortality in premature infants. The risk factors to develop NEC are prematurity, enteral feeding, intestinal hypoxia-ischemia, and bacterial colonization (36). The precise contribution of each of these factors to NEC is unknown, but a critical role of intestinal bacterial flora in NEC pathogenesis has been shown (33, 68). NEC affects several thousand newborns in the United States every year, with death occurring in up to 50% of affected individuals (30). Currently, no predictive diagnostic tests, prevention, or effective treatments are available.
Normal healthy gut microbiota plays a vital role in human health and performs important metabolic functions supporting the digestive system (42). A number of studies suggest that initial intestinal colonization plays a pivotal role in the development of neonatal NEC (4, 10, 31). In fact, NEC in animal models does not occur prior to colonization of the intestine by bacteria or in germ-free animal models (6, 57).
Probiotics are living, nonpathogenic microorganisms that colonize the intestine and provide benefit to the host (28). Probiotics currently investigated in neonatal clinical practice are enterally fed normal commensals that do not translocate or cause mucosal injury to the host (21). Among probiotic organisms, Bifidobacterium predominates in the intestinal flora of breast-fed infants; other obligate anaerobes are rarely present (45). Recent clinical studies with NEC patients suggest that oral administration of probiotics is beneficial in the prevention of this disease (3, 39). However, selection of the optimal strain or a mixture of probiotic bacteria is critical to obtain desired protective effects, and the mechanisms of probiotic reduction of NEC are not well understood.
Cytokines are key regulators in inflammation in NEC, and several inflammatory and immune regulatory cytokines are dysregulated in this disease (16, 58). Among them, TNF-α, IL-10, and IL-6 are thought to have a diagnostic value in sepsis and in NEC (8, 14, 47). In vitro and in vivo studies with probiotics suggest their ability to decrease proinflammatory cytokines and activate the production of anti-inflammatory cytokines. Interestingly, not all probiotic strains have the same effect on the immune system, with differing immunological effects even within the same species of bacteria (18, 29).
It has been suggested that inflammation may initiate mucosal damage during NEC pathogenesis (49). There are several factors that contribute to intestinal barrier integrity, such as the mucus coat, secretion of antimicrobial factors, and enterocyte cell junctions (24, 69). The intestinal epithelium protects tissue against oxidative stress and invasion by microbes through the production of mucins and trefoil factors (TFF). There are two main classes of mucins: 1) secreted gel-forming mucins and 2) membrane-bound mucins (54). In the rat small intestine, Muc2 is the predominant secretory mucin produced by goblet cells, whereas Muc3 is the major membrane-bound mucin detected in goblet cells and enterocytes (1, 2). Mucins are cosecreted with TFFs, small peptides exerting multiple biological effects on epithelium. Trefoil factor 3 (TFF3) is the most abundant in the intestine (65) contributing to the viscoelastic properties of the mucus layer (66) and modulating epithelial healing processes (64). Impaired production of MUC2 and TFF3 has been reported in clinical and experimental NEC (12, 67), and intraperitoneal administration of TFF3 reduced NEC-like injury in neonatal rats (59, 70).
Studies using intestinal epithelial cells (IEC) have suggested that some probiotic strains may stimulate protective responses, including enhancement of epithelial barrier functions (56), mucin secretion (43), and stabilization of tight junction (TJ) structure (51). Formation of functional TJs and adherens junctions (AJs) is critical for the maintenance of gut permeability and intestinal barrier function. TJs form continuous intercellular contacts between epithelial cells and create a dynamic barrier to the paracellular movement of water, solutes, and immune cells (17). Several TJ proteins have been identified; among them the transmembrane proteins occludin and claudins are considered crucial for creating functional TJs in neonatal intestine (12).
AJs are another type of cellular connection anchoring cells one to another and attaching to components of the intracellular matrix. AJs are composed of transmembrane and cytosolic components. Cadherins and catenins are the two major families of proteins involved in AJs structure (5).
The aims of this study were to determine whether oral administration of Bifidobacterium bifidum OLB6378 (NITE BP-31, Meiji Dairies, Odawara, Japan) protects against experimental NEC and what the possible mechanisms involved in this process are. In a rat model of NEC, we evaluated the efficacy of B. bifidum treatment on disease development, the effect on mucins and TFF3 expression, and inflammatory cytokine production. Intestinal protein expression and histological localization of the TJ and AJ proteins were also determined.
MATERIALS AND METHODS
Experimental design.
This protocol was approved by the Animal Care and Use Committee of the University of Arizona (A-324801-95081). Seventy-six neonatal Sprague-Dawley rats (Charles River Laboratories, Pontage, MI) were collected by caesarian section 24 h before their scheduled birth, and the first feeding started 2 h after delivery. Animals were hand fed six times daily with a total volume of 850 μl of rat milk substitute per day (34). Experimental NEC was induced by asphyxia (breathing 100% nitrogen gas for 60 s) and cold stress (4°C for 10 min) twice daily (15). Caesarian section-delivered pups were divided into the following experimental groups: neonatal rats hand fed with formula (NEC; n = 30), neonatal rats hand fed with formula containing 5 × 106 CFU per day of Bifidobacterium bifidum OLB6378 (B. bifidum; n = 30), and dam-fed littermates fed by surrogate mothers as a baseline control (DF; n = 16). After 96 h, all surviving animals were terminated via decapitation. Animals that developed signs of distress or imminent death before 96 h were terminated and included in the study.
NEC evaluation.
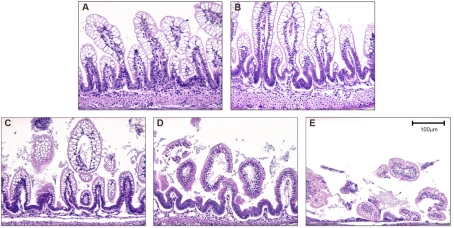
After termination, a 2-cm piece of distal ileum was removed and fixed in 70% ethanol, paraffin embedded, sectioned at 4–6 μm, and stained with hematoxylin and eosin for histological evaluation of NEC. Pathological changes in intestinal architecture were evaluated by use of our previously published NEC scoring system (15). Histological changes in the ileum were scored by a blinded evaluator and graded as follows: 0 (normal), no damage; 1 (mild), slight submucosal and/or lamina propria separation; 2 (moderate), moderate separation of submucosa and/or lamina propria, and/or edema in submucosal and muscular layers; 3 (severe), severe separation of submucosa and/or lamina propria, and/or severe edema in submucosa and muscular layers, region villous sloughing; 4 (necrosis), loss of villi and necrosis (Fig. 1). Intermediate scores of 0.5, 1.5, 2.5, and 3.5 were also utilized to more accurately assess levels of ileal damage when necessary (15, 26). To determine incidence of NEC, animals with histological scores of less than 2 have not developed NEC; animals with histological scores of 2 or greater have developed NEC (Fig. 1).
Fig. 1.
Histological scoring of the terminal ileum of neonatal rats. The ileum stained with hematoxylin and eosin showing representative sections for each morphological grading score. A: necrotizing enterocolitis (NEC) score 0, normal ileum. B: NEC score 1, mild damage with slight submucosal and/or lamina propria separation. C: NEC score 2, moderate to severe separation of submucosa and/or lamina propria, and/or edema in submucosal and muscular layers, partial villous sloughing. D: NEC score 3, severe separation of submucosa and/or lamina propria, region villous sloughing and initial villus necrosis. E: NEC score 4, necrosis and loss of villi structure and/or transmural necrosis. Magnification: ×200.
RNA preparation.
Total RNA was isolated from ileal tissue (snap frozen in liquid N2) using the RNeasy Plus Mini Kit (Qiagen, Santa Clarita, CA) as described in the manufacturer's protocol. RNA concentration was quantified by ultraviolet spectrophotometry at 260 nm, and the purity was determined by the A260/A280 ratio (SPECTRAmax PLUS; Molecular Devices, Sunnydale, CA). The integrity of RNA was verified by electrophoresis on a 1.2% agarose gel (12).
RT and real-time PCR.
RT real-time PCR assays were performed to quantify steady-state mRNA levels of Muc2, Muc3, Tff3, and selected cytokines (IL-1β, IL-6, IL-10, IL-12, IL-18, and TNF-α). cDNA was synthesized from 0.5 μg of total RNA. Real-time PCR amplification was performed using Primer Express Software (Applied Biosystems). Target probe was labeled with fluorescent reporter dye FAM. The following sequences were used: Muc2 (GenBank BC036170): sense primer 5′-actgggaatgtgactgctactg-3′, antisense primer 5′-accctggtaactgtagtaaagtccat-3′, and probe 5′-acaaagtgtgggtcccc-3′; TNF-α (GenBank X66539): sense primer 5′-gtgatcggtcccaacaagga-3′, antisense primer 5′-gggccatggaactgatgaga-3′, and probe 5′-cccatttgggaacttc-3′.
Predeveloped TaqMan primers and probes were used for the detection of Muc 3, Tff3, IL-1β, IL-6, IL-10, IL-12, and IL-18. Reporter dye emission was detected by an automated sequence detector combined with ABI Prism 7700 Sequence Detection System software (Applied Biosystems). Real-time PCR quantification was then performed with TaqMan 18S controls.
Immunohistology and enumeration of Muc2- and Tff3-positive cells.
A 2-cm section of distal ileum was collected from each animal and fixed overnight in 70% ethanol, paraffin embedded, and sectioned at 4–6 μm. Serial sections were stained for either Muc2 or Tff3. Briefly, after deparaffinization and rehydration, sections were blocked with 1.5% goat serum (Vector Laboratories, Burlingame, CA) in PBS for 30 min, then incubated with either rabbit anti-Muc2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit polyclonal Tff3 antibody (63) for 30 min, washed with PBS three times, and incubated with a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) for 30 min. Vectastain Elite ABC reagent (Vector Laboratories) was then applied, followed by diaminobenzidine as a substrate. Sections were counterstained with hematoxylin, dehydrated, and mounted on coverslips. Muc2 and Tff3-positive cells were counted from nine animals per experimental group, and the total number of epithelial cells per crypt-villus unit was also enumerated. Ten crypt-villus units were counted for each animal.
There is currently no commercially available anti-rat Muc3 antibody. Anti-human Muc3 antibody (Santa Cruz Biotechnology) was unsuccessfully tested on our rat intestinal tissue samples.
Western blot.
Individual frozen ileal samples were homogenized with a hand-held homogenizer (Pellet Pestle, Kimble/Kontes, Vineland, NJ) in a 5 × volume of ice-cold homogenization buffer (50 mM Tris·HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 0.1% SDS; 1% Na-deoxycholic acid; 1% Triton X-100; 50 mM DTT; 50 μg/ml aprotinin; 50 μg/ml leupeptin; 5 mM PMSF). The homogenates were centrifuged at 10,000 rpm for 5 min at 4°C and the supernatant was collected. Total protein concentration was quantified by use of the Bradford protein assay (7). For protein analysis, 40 μg of protein was added to an equal volume of 2× Laemmli sample buffer and boiled for 5 min. The samples were run on a 10 or 12% polyacrylamide gel (Bio-Rad, Hercules, CA) at 110 V for 1.5 h. Protein was transferred to Immuno-Blot PVDF membranes (Bio-Rad) at 50–60 V for 1 h. Membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (Sigma, St. Louis, MO) for 1 h at room temperature and then incubated with one of the following rabbit polyclonal antibodies: anti-occludin, anti-claudin-1, anti-claudin-2, anti-claudin-3, anti-β-catenin (Zymed Laboratories, San Francisco, CA) or mouse monoclonal antibodies: anti-E-cadherin (BD Biosciences, San Jose, CA), anti-α-catenin (Zymed Laboratories) antibody overnight at 4°C. After extensive washing, the membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated anti-rabbit IgG (Santa Cruz Biotechnology). Proteins were visualized with a chemiluminescent system (Pierce, Rockford, IL) and exposed to X-ray film.
Immunostaining of TJ and AJ proteins.
After deparaffinization and rehydration, sections were blocked in 5% BSA to prevent nonspecific staining and incubated with one of the following rabbit polyclonal antibodies: anti-occludin, anti-claudin-1, anti-claudin-2, anti-claudin-3, anti-β-catenin (Zymed Laboratories) or mouse monoclonal antibodies: anti-E-cadherin (BD Biosciences), anti-α-catenin (Zymed Laboratories), followed by incubation with Alexa-594 conjugated anti-rabbit or anti-mouse secondary antibody (Molecular Probes, Eugene, OR) and mounted with Vectashield Hard Set Mounting Medium containing DAPI as a nuclear counterstain (Vector Laboratories). Negative control sections were treated with the same procedure in the absence of primary antibody; no immunostaining was observed in the controls (not shown). Sections from each experimental group were immunostained for a specific antigen at the same time. Confocal laser scanning microscope (Zeiss LSM 510 NLO/META) was used for imaging TJ proteins. Imaging of AJ proteins was performed with an inverted fluorescence microscope (Nikon TE-300).
Statistics.
Statistical analyses between DF, NEC, and B. bifidum groups were performed by ANOVA followed by Fisher paired least significant difference test. The χ2 test was utilized to analyze difference in incidence of disease. All statistical analyses were conducted by use of the statistical program StatView for Macintosh computers (Abacus Concepts, Berkeley, CA). All numerical data are expressed as means ± SE.
RESULTS
Oral administration of B. bifidum reduces the severity and incidence of NEC.
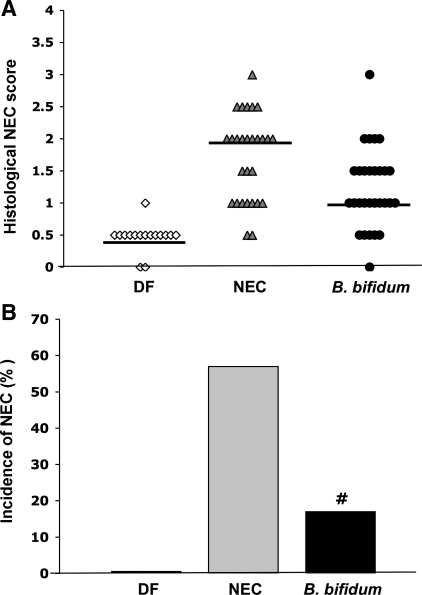
The degree of intestinal injury and the incidence of NEC were evaluated in prematurely born rats treated with or without B. bifidum. Ileal damage in rats administered B. bifidum was significantly reduced (P ≤ 0.01) to a median histological NEC score of 1.0 compared with 2.0 in the NEC group (Fig. 2A). The incidence of NEC (Fig. 2B) was markedly decreased to 17% (5/29) in the B. bifidum group compared with the NEC group with NEC incidence of 57% (16/28). In DF rats, the median histological score was 0.5 and incidence of NEC was 0% (0/16). The survival rates for these studies were as follows: DF, 16/16; NEC, 28/30; and B. bifidum, 29/30.
Fig. 2.
Severity and incidence of NEC in neonatal rat model. A: histological NEC score in the dam-fed (DF; n = 16), NEC (n = 28), and Bifidobacterium bifidum (n = 29) groups are shown. Ileal damage was assessed by the histological scoring system described in Fig. 1. Bars indicate median. B: incidence of NEC in the neonatal rat model of NEC. Animals with scores ≥2 are considered NEC positive; animals with ileal damage <2 do not have NEC. #P ≤ 0.01 vs. NEC, χ2 analysis.
Evaluation of inflammatory response in the ileum.
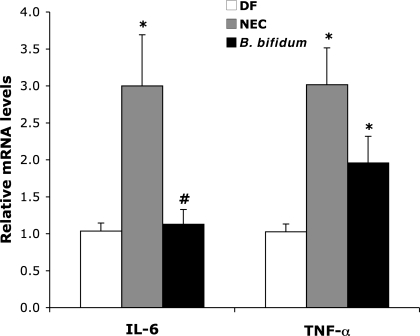
Cytokines are key regulators in inflammation and several cytokines are dysregulated in this disease (58). Gene expression of selected inflammatory cytokines in the terminal ileum was determined by RT-PCR. Proinflammatory IL-1β, IL-6, IL-12, IL-18, and TNF-α, and anti-inflammatory IL-10 are the major cytokines associated with NEC pathogenesis and neonatal sepsis (25, 58). IL-6 expression was significantly increased in animals with NEC and decreased in the B. bifidum group to levels found in DF animals (Fig. 3). TNF-α expression was significantly increased in NEC animals compare to DF. There was no statistically significant change in TNF-α expression between the NEC and B. bifidum groups. Oral administration of B. bifidum did not have any significant effect on gene expression of IL-1β, IL-10, IL-12, and IL-18 (not shown) in the site of injury.
Fig. 3.
IL-6 and TNF-α mRNA levels in neonatal rat ileum. The mean steady-state mRNA level for the DF group was assigned a value of 1.0, and mean mRNA levels for the NEC and B. bifidum groups were determined relative to this number. Values are means ± SE; n = 10–12 animals/experimental group. *P ≤ 0.01 vs. DF. #P ≤ 0.01 vs. NEC.
Changes in gene expression of mucins and Tff3 in the ileum.
The mucus layer is an essential part of intestinal barrier function. Ileal gene expression of two major mucins (Muc2 and Muc3) and Tff3 was evaluated by real-time PCR (Table 1). Muc2 mRNA levels in the ileum were significantly decreased in both the NEC and B. bifidum groups compared with the DF group (P ≤ 0.01). In contrast, Muc3 mRNA levels were significantly increased in the NEC (P ≤ 0.01) and B. bifidum (P ≤ 0.01) groups compared with the DF group. B. bifidum treatment significantly decreased (P ≤ 0.05) Muc3 gene expression compared with animals with NEC. Gene expression of Tff3 did not show any significant differences between studied groups.
Table 1.
Effect of enteral Bidobacterium bifidum on Muc2, Muc3, and Tff3 mRNA expression
| DF | NEC | B. bifidum | |
|---|---|---|---|
| Muc2 | 1.00±0.11 | 0.70±0.07* | 0.38±0.05*† |
| Muc3 | 1.00±0.08 | 2.51±0.33* | 1.69±0.14*† |
| Tff3 | 1.00±0.12 | 1.06±0.06 | 0.85±0.05 |
Values are means ± SE; n = 8–10 animals/experimental group. The mean steady-state mRNA level for the dam-fed (DF) group was assigned a value of 1.00, and mean mRNA levels from the necrotizing enterocolitis (NEC) and B. bifidum groups were determined relative to this number.
P ≤ 0.01 vs. DF.
P ≤ 0.05 vs. NEC.
Evaluation of Muc2 and Tff3 production.
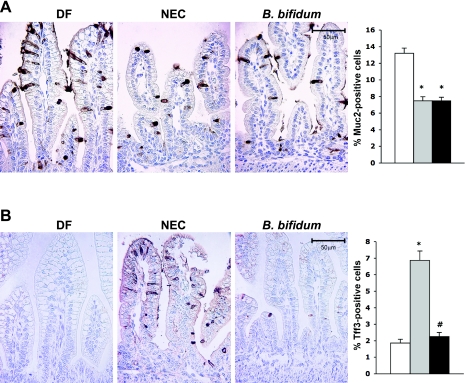
Ileal Muc2 production was evaluated by immunohistochemistry, and enumeration of Muc2-positive cells in the ileum was compared among all experimental groups (Fig. 4). Muc2 staining was significantly reduced (P ≤ 0.01) in both the NEC and B. bifidum groups compared with DF controls, but there was no difference between the NEC and B. bifidum groups (Fig. 4).
Fig. 4.
Muc2- and Tff3-positive cells in the ileum of neonatal rats. A: Muc2-stained representative slides from DF, NEC, and B. bifidum groups are shown. Magnification: ×400. Enumeration of Muc2-positive cells in neonatal rat ileum is shown in the graph (n = 9 animals/experimental group). Data are expressed as mean Muc2-positive cells/100 epithelial cells ± SE. *P ≤ 0.01 vs. DF. B: Tff3-stained representative slides from DF, NEC, and B. bifidum groups are shown. Magnification: ×400. Enumeration of TFF3-positive cells in neonatal rat ileum is shown in the graph (n = 9 animals/experimental group). Numbers are expressed as mean Tff3-positive cells/100 epithelial cells ± SE. *P ≤ 0.01 vs. DF, #P ≤ 0.01 vs. DF.
The production of ileal Tff3 was evaluated by enumeration of Tff3 positively stained cells. There was a significant increase of Tff3-positive cells in the ileal tissue from NEC animals compared with DF and B. bifidum-treated animals (P ≤ 0.01).
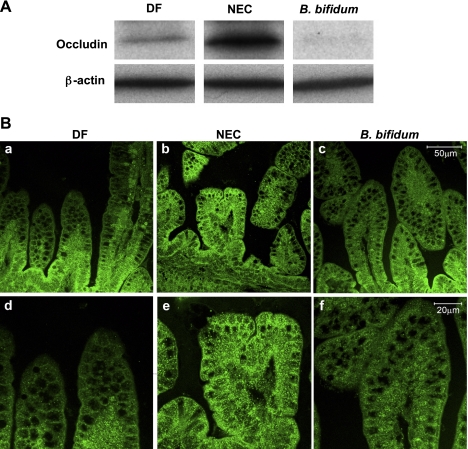
Occludin and claudin-3 expression and localization in the ileum.
Major changes in the distribution and content of TJ proteins, such as occludin and claudin-3 in the ileum of NEC rats, correspond with increased intestinal permeability in these animals (12). To determine whether B. bifidum treatment of experimental NEC normalizes these changes, we quantified protein expression of occludin, claudin-1, -2, and -3 by Western blot analysis and evaluated their histological localization by immunofluorescence microscopy.
Western blot analysis of occludin showed significantly higher levels in the NEC group compare to the DF and B. bifidum groups (Fig. 5A). Confocal microscopy revealed that in NEC animals, occludin was localized in the cytoplasm of ileal enterocytes as well as associated with the membrane. Occludin was expressed at higher intensity throughout the entire length of villi (Fig. 5B, b and e). B. bifidum treatment decreased the intensity of the signal and cellular distribution was mainly in the crypt epithelial cells similar to that seen in DF animals (Fig. 5B, a and d).
Fig. 5.
Effect of oral administration of B. bifidum on expression and localization of occludin. A: representative 65-kDa occludin bands from Western blot analyses are shown for the DF, NEC, and B. bifidum groups. B: representative slides from DF, NEC, and B. bifidum groups evaluated by confocal laser scanning microscopy (n = 6 animals/experimental group). No signal was observed in negative control sections (not shown). Magnification: ×400 (a, b, c), ×800 (d, e, f).
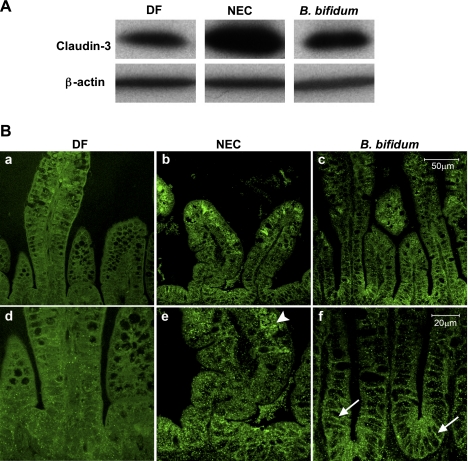
Previous studies from our laboratory revealed that among the claudin family members, expression of claudin-3 was markedly altered during NEC pathogenesis (12). Western blot analysis revealed a significant increase of claudin-3 in NEC animals compare to DF and B. bifidum treated rats (Fig. 6A). In the ileal tissue of NEC animals, claudin-3 was localized mainly in the cytoplasm of the enterocytes (Fig. 6B, e; arrowhead). The signal was more pronounced at the top of the villi compared with the crypts. B. bifidum treatment resulted in reduction of claudin-3 signal as well as its association with the membrane of enterocytes in the crypts (Fig. 6B, f; arrows) compared with NEC animals. Interestingly, in the DF group, intensity of the claudin-3 staining was very low (Fig. 6B, a and d). These data suggest the influence of stress and hand feeding on the early formation of TJs in the NEC and B. bifidum animals. On the basis of the distribution pattern of claudin-3 in these two groups, B. bifidum treatment appears to enhance the development and formation of functional TJs. There were no noticeable differences between experimental groups in tissue content and histological localization of claudin-1 and -2 (not shown).
Fig. 6.
Effect of oral administration of B. bifidum on expression and localization of claudin-3. A: representative 22-kDa claudin-3 bands from Western blot analyses are shown for DF, NEC, and B. bifidum groups. B: representative slides from DF, NEC, and B. bifidum groups were evaluated by confocal laser scanning microscopy (n = 6 animals/experimental group). In the NEC group, claudin-3 signal was dispersed throughout the cytoplasm (e; arrowhead). In the B. bifidum group, claudin-3 signal was associated with enterocyte membrane (f; arrows). No signal was observed in negative control sections (not shown). Magnification: ×400 (a, b, c), ×800 (d, e, f).
Changes in expression and localization of AJs in the ileum.
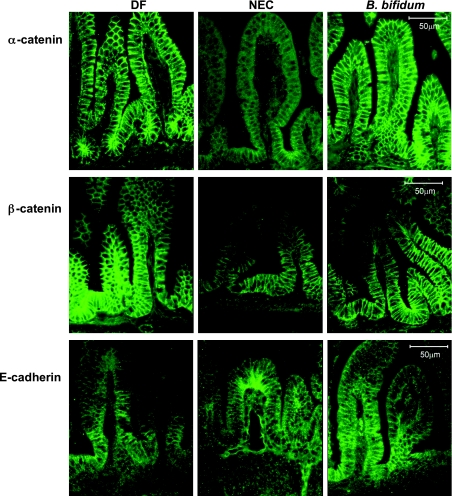
Cadherins and catenins are the two major families of proteins involved in AJ structure (5). Among AJs, E-cadherin, α-catenin, and β-catenin have the most influence on the adherent function of a cell. In this study we evaluated the distribution and changes in the localization of E-cadherin, α-catenin, and β-catenin in the distal ileum by immunofluorescent microscopy. Western blot analysis showed no statistically significant differences in the amount of these three proteins between experimental groups (not shown).
α-Catenin was distributed evenly throughout the entire villi and was clearly associated with the intestinal epithelial cell membrane in both DF controls and animals treated with B. bifidum. In comparison, the NEC group showed weak signal, which was mostly dispersed within the cytoplasm (Fig. 7).
Fig. 7.
Localization of adherens junction proteins (α-catenin, β-catenin, and E-cadherin) evaluated by inverted fluorescence microscopy. Representative slides from DF, NEC, and B. bifidum groups are shown (n = 6 animals/experimental group). No signal was observed in negative control sections (not shown). Magnification: ×400.
β-Catenin was associated with the enterocyte membrane in all groups, but there were obvious differences in the distribution throughout the villi. In the DF group, β-catenin was localized in the crypts, along most of the length of the villi, and at the tip of the villi. In contrast, β-catenin was found only in the crypts of the ileum of NEC animals; no signal was detected at the tip of the villi. In the B. bifidum group, a strong signal was observed in the crypts and villi, but only weak or no signal was detected at the tip of the villi (Fig. 7).
Immunohistochemical localization of E-cadherin revealed significant differences between NEC animals and the DF controls and rats receiving B. bifidum treatment. In the NEC group, E-cadherin was localized at the tip of the villi, mostly dispersed in the cytoplasm and partially in the lamina propria. This was in contrast with the DF and B. bifidum groups, in which the signal was localized mainly in the crypts and associated with the membrane (Fig. 7).
DISCUSSION
Our study shows that oral administration of B. bifidum OLB6378 reduces the incidence and severity of NEC in a neonatal rat model. Mechanisms of bifidobacterial-mediated reduction of experimental NEC include reduced cytokine expression in the site of injury and improved development of cellular junctional proteins in the intestinal epithelium. Interestingly, treatment with B. bifidum does not affect ileal Muc2 production but normalizes the production of Tff3 in the site of injury.
NEC is a multifactorial disease involving three major risk factors: prematurity, enteral feeding, and bacterial colonization. Inappropriate intestinal colonization with unfavorable bacteria is likely a critical factor in NEC pathogenesis (13). Both clinical and experimental studies have shown that oral administration of probiotics has beneficial effects and results in decreased incidence of NEC (3, 10, 39, 61). However, not all probiotics have the same protective effects and the safety of administering live bacteria to premature babies must be considered. Selection of the right probiotic strain seems to be critical to achieve the desirable protective effect.
Bifidobacterium is the predominant organism among the intestinal flora found in breastfed infants (45). However, bifidobacterium and lactobacilii in the stool from extremely low-birth-weight infants during the first month of life represent only 5% of detected bacterial species (20). Thus low levels of these commensal bacteria may allow for colonization of the intestinal tract with other bacterial species, including pathogenic bacteria (45). Bifidobacterium bifidum OLB6378 (NITE BP-31, Meiji Dairies) was originally isolated from human neonates. This strain induces strong IgA production in mouse Peyer's patches and polymeric Ig receptor production in the human colon carcinoma cell line HT-29 (48). In addition, this strain is resistant to low gastric pH and bile acids and has anti-inflammatory properties (unpublished data). Therefore, we selected this probiotic strain for further evaluation of protective effects against intestinal injury in our neonatal rat model of NEC. Our results demonstrate that oral administration of live B. bifidum OLB6378 significantly reduced the incidence of NEC (from 57 to 17%) and severity of ileal damage in the rat NEC model.
The mechanisms underlying the protective effects of probiotics on the developing intestine are not fully understood. However, it has been suggested that bacterial endotoxin can trigger an innate immune response characterized by cytokine production (60). Our and other laboratories have shown that proinflammatory cytokines are important factors contributing to NEC pathogenesis (25, 27, 58). Clinical studies have reported increased serum levels of TNF-α and IL-6 in NEC patients (58). Interestingly, experimental studies indicate that administration of probiotics (such as Bifidobacterium) may alter intestinal production of proinflammatory cytokines (18, 29, 50) via the reduction of intestinal luminal pH, elevated production of antimicrobial substances, and consequently the inhibition of growth of pathogenic bacteria (29). In the present study, we have found that ileal gene expression of TNF-α and IL-6 was significantly increased in the ileum of NEC rats compared with healthy controls. In animals fed with formula supplemented with B. bifidum, ileal IL-6 levels but not TNF-α were normalized to values seen in controls. Thus bifidobacterium-mediated protection against NEC may be regulated via inflammatory mediators, such as IL-6.
Some probiotic strains may moderate a non-immune-related protection mechanism such as acting on barrier integrity (38). Studies using intestinal epithelial cells have suggested that probiotic treatment of IEC stimulates mucin secretion (43) and the enhancement of epithelial barrier functions (56), reduces enterocyte apoptosis (32, 40), and stabilizes formation of cellular TJs (51, 52). Despite recent advances in understanding probiotic actions on IEC, little is known about the mechanisms of protection from in vivo models of gastrointestinal diseases. Both in vitro and in vivo studies showed increased Muc2 and Muc3 gene expression in these cells and tissues after exposure to VSL#3 (a clinically tested probiotic formula consisting of three bacterial groups: Lactobacilli, Bifidobacteria, and Streptococci). Among these groups, only the Lactobacilli species were responsible for increased Muc2 secretion (not Bifidobacteria or Streptococci) (9).
Intestinal mucins and trefoil factors are part of a complex regulatory network that generates the first line of host defense against enteric pathogens. Alterations of this complex may play a role in the pathogenesis of NEC (46, 67). TFF3 is a key peptide in mucosal protection and repair, and its overproduction is observed in a variety of gastrointestinal inflammatory conditions (53). Intestinal MUC2 is secreted solely by goblet cells, whereas membrane-bound MUC3 is expressed in both goblet cells and enterocytes (11). Previously, we have reported the reduction of goblet cell density and abnormalities in goblet cell morphology in the ileum of rats with NEC (12). Results from the present study further elucidate major components of intestinal mucus layer. In the NEC group, a decrease in ileal Muc2 expression is concomitant with an increase in Muc3 expression and Tff3 production. A similar pattern has been recently shown in rats with colitis where in both the small and large intestine, Muc2 expression was significantly decreased and Muc3 expression was increased (1). The authors concluded that colitis-induced injury causes mucosal barrier dysfunction not only in the colon, but also in the adjoining ileum (1). In our study, we speculate that alteration in goblet cell function leads to the reduction of Muc2 layer that functions to protect the delicate neonatal epithelium against injury. Increased expression of nonsecretory Muc3 and Tff3 peptide suggest an activation of acute repair mechanisms in the site of injury: neonatal ileal epithelium.
The exposure to formula feeding, hypoxia, and stress leads to a delay in colonization by normal commensal bacteria and increases risk of colonization by pathogenic species (13). The presence of bacterial pathogens regulates mucin synthesis and secretion in adult intestine (41). However, little is known about the role of microbiota on goblet cell maturation, Tff3, and mucin production in neonatal gut. In our study, Muc2 gene expression and enumeration of Muc2-positive cells was reduced in the ileum of rats receiving B. bifidum compared with the controls. Muc3 mRNA levels were also significantly decreased in the B. bifidum group compared with the NEC group. In addition, B. bifidum treatment of NEC markedly reduced number of Tff3-positive cells to values seen in normal healthy controls. We speculate that oral administration of B. bifidum decreases colonization on neonatal gut by pathogenic bacterial species, leading to the reduction of inflammation and Tff3 to the levels seen in dam-fed healthy controls.
Probiotics were shown to maintain integrity of the mucosal barrier, reducing its permeability, and strengthening intestinal TJs (44, 62). Epithelial TJ proteins determine intestinal barrier function by creating a barrier to the paracellular movement of solutes. The primary proteins identified as TJ-specific integral transmembrane proteins are occludin and claudins. In our previous work, we showed that these TJs are altered during NEC pathogenesis and intestinal paracellular permeability is increased in NEC animals as a result of these changes at the TJ barrier (12). Occludin is considered to be the primary sealing and integral protein of the TJs, whereas claudins are thought to be the proteins regulating the size selectivity of the TJ barrier. Results from our study indicate that expression and localization of occludin in the ileum of B. bifidum-treated animals has a similar pattern as seen in healthy controls. The strongest signal is observed in crypt epithelial cells and is gradually decreased toward the tip of villi. In contrast, NEC animals have occludin distributed within the enterocyte cytoplasm throughout the villi. Similar distribution is observed with claudin-3. We speculate that altered distribution pattern of these two TJ proteins contributes to increased intestinal permeability and disassembly of TJs leading to a breach in mucosal barrier in NEC animals. Treatment with B. bifidum prevents these pathological changes by protecting intestinal barrier integrity, thus blocking translocation of luminal toxins into systemic circulation.
Although increased intestinal permeability is typically associated with decreased TJ protein expression, our data were obtained from neonatal intestinal epithelium in contrast to most of the studies done in adult intestinal tissue (19, 37, 55). In one study, using Bifidobacterium infantis as a treatment in neonatal rat model of NEC, there were no changes in mucosal permeability (10). However, there are no studies showing TJ distribution or their levels after probiotic treatment in this model. Our study is the first to describe the distribution pattern of epithelial TJ proteins in the intestine of neonatal rats after treatment with B. bifidum.
AJs are another type of cellular connection anchoring cells one to another and attaching to components of the intracellular matrix. AJs play an important role in organogenesis of epithelial tissue and are composed of transmembrane and cytosolic components. Cadherins and catenins are the two major families of proteins involved in AJ structure (5). The extracellular domain of E-cadherin is essential for connecting cells and the intracellular domain regulates cell-to-cell contact and is directly associated with the actin cytoskeleton via α-catenin and β-catenin (22, 35). The development of functional AJs during the early postnatal period is not fully understood. This study describes for the first time the localization of the major AJ proteins in the ileum of neonatal rats and shows dramatic changes between healthy DFs, animals with NEC, and rats treated with B. bifidum. When the intestinal epithelium is damaged, the process of immediate repair starts to preserve the epithelial barrier and involves cell migration and proliferation. During epithelial restitution, cell junctions must rapidly disassemble and reassemble, which means that catenin-cadherin-mediated cell-to-cell contacts are transiently dissociated (23). We found that AJ proteins are dispersed in the cytoplasm of ileal enterocytes of NEC animals compared with samples from healthy controls and animals treated with B. bifidum. Disassembled AJs, because of the ileal damage in the NEC group, can also contribute to the “leakiness” of the tissue. In the DF and B. bifidum groups, with no or much smaller damage to the tissue respectively, AJ proteins were clearly associated with the membrane of enterocytes.
In conclusion, this study shows the protective effect of orally administered live B. bifidum OLB6378 against NEC injury in the neonatal rat model. The molecular mechanisms associated with these protective effects include prevention of inflammation in the ileum and improvement of intestinal integrity. Alterations in the epithelial TJ and AJ structure may be a crucial factor by which probiotics protect intestinal barrier function. We are currently investigating the beneficial effects of various probiotic strains in this model.
GRANTS
This research was supported by National Institute of Child Health and Human Development Grant HD039657 (to B. Dvorak) and a gift from Meiji Dairies Corporation.
REFERENCES
- 1.Amit-Romach E, Reifen R, Uni Z. Mucosal function in rat jejunum and ileum is altered by induction of colitis. Int J Mol Med 18: 721–727, 2006 [PubMed] [Google Scholar]
- 2.Amit-Romach E, Uni Z, Cheled S, Berkovich Z, Reifen R. Bacterial population and innate immunity-related genes in rat gastrointestinal tract are altered by vitamin A-deficient diet. J Nutr Biochem 20: 70–77, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 147: 192–196, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Blakey JL, Lubitz L, Campbell NT, Gillam GL, Bishop RF, Barnes GL. Enteric colonization in sporadic neonatal necrotizing enterocolitis. J Pediatr Gastroenterol Nutr 4: 591–595, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Blaschuk OW, Rowlands TM. Plasma membrane components of adherens junctions (Review). Mol Membr Biol 19: 75–80, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bousseboua H, Le Coz Y, Dabard J, Szylit O, Raibaud P, Popoff MR, Ravisse P. Experimental cecitis in gnotobiotic quails monoassociated with Clostridium butyricum strains isolated from patients with neonatal necrotizing enterocolitis and from healthy newborns. Infect Immun 57: 932–936, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Buck C, Bundschu J, Gallati H, Bartmann P, Pohlandt F. Interleukin-6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics 93: 54–58, 1994 [PubMed] [Google Scholar]
- 9.Caballero-Franco C, Keller K, De Simone C, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 292: G315–G322, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R., Jr Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model [see comments]. Gastroenterology 117: 577–583, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Chang SK, Dohrman AF, Basbaum CB, Ho SB, Tsuda T, Toribara NW, Gum JR, Kim YS. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology 107: 28–36, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 291: G938–G949, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J 15: 1398–1403, 2001 [DOI] [PubMed] [Google Scholar]
- 14.De Bont ES, Martens A, van Raan J, Samson G, Fetter WP, Okken A, de Leij LH, Kimpen JL. Diagnostic value of plasma levels of tumor necrosis factor alpha (TNF alpha) and interleukin-6 (IL-6) in newborns with sepsis. Acta Paediatr 83: 696–699, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics 103: 766–771, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol 10: 1337–1345, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol 13: 236–243, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol 281: G216–G228, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed 80: F167–F173, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs K, Lin J, Holzman IR. Necrotising enterocolitis: the state of the science. Indian J Pediatr 74: 67–72, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol 148: 399–404, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo X, Rao JN, Liu L, Zou TT, Turner DJ, Bass BL, Wang JY. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol 285: C1174–C1187, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg 14: 49–57, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, Payne CM, McCuskey RS, Besselsen DG, Dvorak B. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res 51: 733–739, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Halpern MD, Holubec H, Saunders TA, Dvorak K, Clark JA, Doelle SM, Ballatori N, Dvorak B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology 130: 359–372, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halpern MD, Khailova L, Molla-Hosseini D, Arganbright K, Reynolds C, Yajima M, Hoshiba J, Dvorak B. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am J Physiol Gastrointest Liver Physiol 294: G20–G26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammerman C, Bin-Nun A, Kaplan M. Germ warfare: probiotics in defense of the premature gut. Clin Perinatol 31: 489–500, 2004 [DOI] [PubMed] [Google Scholar]
- 29.He F, Morita H, Hashimoto H, Hosoda M, Kurisaki J, Ouwehand AC, Isolauri E, Benno Y, Salminen S. Intestinal Bifidobacterium species induce varying cytokine production. J Allergy Clin Immunol 109: 1035–1036, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Henry MC, Lawrence Moss R. Surgical therapy for necrotizing enterocolitis: bringing evidence to the bedside. Semin Pediatr Surg 14: 181–190, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Hoy C, Millar MR, MacKay P, Godwin PG, Langdale V, Levene MI. Quantitative changes in faecal microflora preceding necrotising enterocolitis in premature neonates. Arch Dis Child 65: 1057–1059, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter CJ, Williams M, Petrosyan M, Guner Y, Mittal R, Mock D, Upperman JS, Ford HR, Prasadarao NV. Lactobacillus bulgaricus prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in vitro and in the newborn rat model of necrotizing enterocolitis. Infect Immun 77: 1031–1043, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 177: 3273–3282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanno T, Koyanagi N, Katoku Y, Yonekubo A, Yajima T, Kuwata T, Kitagawa H, Harada E. Simplified preparation of a refined milk formula comparable to rat's milk: influence of the formula on development of the gut and brain in artificially reared rat pups. J Pediatr Gastroenterol Nutr 24: 242–252, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet 9: 317–321, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Kliegman RM, Willoughby RE. Prevention of necrotizing enterocolitis with probiotics. Pediatrics 115: 171–172, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol 159: 2001–2009, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamine F, Eutamene H, Fioramonti J, Bueno L, Theodorou V. Colonic responses to Lactobacillus farciminis treatment in trinitrobenzene sulphonic acid-induced colitis in rats. Scand J Gastroenterol 39: 1250–1258, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, Tsao LY, Chen CH, Su BH. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 122: 693–700, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res 64: 511–516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linden SK, Florin TH, McGuckin MA. Mucin dynamics in intestinal bacterial infection. PLoS One 3: e3952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macfarlane GT, Macfarlane S. Human colonic microbiota: ecology, physiology and metabolic potential of intestinal bacteria. Scand J Gastroenterol Suppl 222: 3–9, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52: 827–833, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121: 580–591, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Martin CR, Walker WA. Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol 32: 127–137, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Mattar AF, Coran AG, Teitelbaum DH. MUC-2 mucin production in Hirschsprung's disease: possible association with enterocolitis development. J Pediatr Surg 38: 417–421, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Morecroft JA, Spitz L, Hamilton PA, Holmes SJ. Plasma cytokine levels in necrotizing enterocolitis. Acta Paediatr Suppl 396: 18–20, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Nakamura Y, Terahara M, Yajima M, Moro I. Effect of Bifidobacterium bifidum OLB 6377 and Bifidobacterium bifidum OLB 6378 on expression of human polymeric immunoglobulin receptor. Digestive Organ Mucosal Immunol 42: 53–56, 2005 [Google Scholar]
- 49.Neu J. The ‘myth’ of asphyxia and hypoxia-ischemia as primary causes of necrotizing enterocolitis. Biol Neonate 87: 97–98, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Nicaise P, Gleizes A, Forestier F, Quero AM, Labarre C. Influence of intestinal bacterial flora on cytokine (IL-1, IL-6 and TNF-alpha) production by mouse peritoneal macrophages. Eur Cytokine Netw 4: 133–138, 1993 [PubMed] [Google Scholar]
- 51.Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol 286: G613–G626, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Parassol N, Freitas M, Thoreux K, Dalmasso G, Bourdet-Sicard R, Rampal P. Lactobacillus casei DN-114 001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res Microbiol 156: 256–262, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Podolsky DK. Mechanisms of regulatory peptide action in the gastrointestinal tract: trefoil peptides. J Gastroenterol 35: 69–74, 2000 [PubMed] [Google Scholar]
- 54.Porchet N, Aubert JP. [MUC genes: mucin or not mucin? That is the question] Med Sci (Paris) 20: 569–574, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85: 1139–1162, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology 130: 731–746, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Rozenfeld RA, Liu X, DePlaen I, Hsueh W. Role of gut flora on intestinal group II phospholipase A2 activity and intestinal injury in shock. Am J Physiol Gastrointest Liver Physiol 281: G957–G963, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Sharma R, Tepas JJ, 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, Premachandra BR. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg 42: 454–461, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Shi L, Zhang BH, Yu HG, Yu JP, Xi JL. Intestinal trefoil factor in treatment of neonatal necrotizing enterocolitis in the rat model. J Perinat Med 35: 443–446, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Shirota K, LeDuy L, Yuan SY, Jothy S. Interleukin-6 and its receptor are expressed in human intestinal epithelial cells. Virchows Arch B Cell Pathol Incl Mol Pathol 58: 303–308, 1990 [DOI] [PubMed] [Google Scholar]
- 61.Siggers RH, Siggers J, Boye M, Thymann T, Molbak L, Leser T, Jensen BB, Sangild PT. Early administration of probiotics alters bacterial colonization and limits diet-induced gut dysfunction and severity of necrotizing enterocolitis in preterm pigs. J Nutr 138: 1437–1444, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Stratiki Z, Costalos C, Sevastiadou S, Kastanidou O, Skouroliakou M, Giakoumatou A, Petrohilou V. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 83: 575–579, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Suemori S, Lynch-Devaney K, Podolsky DK. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci USA 88: 11017–11021, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol 4: 721–732, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Thim L. Trefoil peptides: from structure to function. Cell Mol Life Sci 53: 888–903, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Invest 32: 519–527, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Vieten D, Corfield A, Carroll D, Ramani P, Spicer R. Impaired mucosal regeneration in neonatal necrotising enterocolitis. Pediatr Surg Int 21: 153–160, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Waligora-Dupriet AJ, Dugay A, Auzeil N, Huerre M, Butel MJ. Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr Res 58: 629–635, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Walker WA. Development of the intestinal mucosal barrier. J Pediatr Gastroenterol Nutr 34, Suppl 1: S33–S39, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Zhang BH, Yu HG, Sheng ZX, Luo HS, Yu JP. The therapeutic effect of recombinant human trefoil factor 3 on hypoxia-induced necrotizing enterocolitis in immature rat. Regul Pept 116: 53–60, 2003 [DOI] [PubMed] [Google Scholar]