Abstract
Somatostatin (SST), an important neuropeptide of the gastrointestinal tract has been shown to stimulate sodium chloride absorption and inhibit chloride secretion in the intestine. However, the effects of SST on luminal butyrate absorption in the human intestine have not been investigated. Earlier studies from our group and others have shown that monocarboxylate transporter (MCT1) plays an important role in the transport of butyrate in the human intestine. The present studies were undertaken to examine the effects of SST on butyrate uptake utilizing postconfluent human intestinal epithelial Caco2 cells. Apical SST treatment of Caco-2 cells for 30–60 min significantly increased butyrate uptake in a dose-dependent manner with maximal increase at 50 nM (∼60%, P < 0.05). SST receptor 2 agonist, seglitide, mimicked the effects of SST on butyrate uptake. SST-mediated stimulation of butyrate uptake involved the p38 MAP kinase-dependent pathway. Kinetic studies demonstrated that SST increased the maximal velocity (Vmax) of the transporter by approximately twofold without any change in apparent Michaelis-Menten constant (Km). The higher butyrate uptake in response to SST was associated with an increase in the apical membrane levels of MCT1 protein parallel to a decrease in the intracellular MCT1 pool. MCT1 has been shown to interact specifically with CD147 glycoprotein/chaperone to facilitate proper expression and function of MCT1 at the cell surface. SST significantly enhanced the membrane levels of CD147 as well as its association with MCT1. This association was completely abolished by the specific p38 MAP kinase inhibitor, SB203580. Our findings demonstrate that increased MCT1 association with CD147 at the apical membrane in response to SST is p38 MAP kinase dependent and underlies the stimulatory effects of SST on butyrate uptake.
Keywords: butyrate absorption, human intestine, CD147, p38 MAPK
short-chain fatty acids (SCFAs), butyrate, propionate, and acetate are present at high concentrations in the colonic lumen and are produced by anaerobic bacterial fermentation of dietary fiber in the large bowel (43). Among these, butyrate plays a key role in colonic epithelial homeostasis and represents an important fuel for colonocytes (37). Butyrate is also known to stimulate colonic electroneutral NaCl absorption and to inhibit Cl− secretion in the colon (2, 35). In addition, butyrate has been shown to prevent colonic mucosal inflammation (22), and butyrate enemas have been reported to be effective in the treatment of ulcerative colitis (39). Hence, decreased availability of butyrate in the colonocytes as a consequence of reduced butyrate uptake has been implicated in various inflammatory conditions and in several cases of acute diarrhea (41) and in colon carcinogenesis (25). Because butyrate absorption is a critical factor in determining colonocyte health, epithelial integrity, and electrolyte absorption, it is essential to understand the cellular and molecular mechanism(s) regulating absorption of butyrate in the human intestine.
Previous studies from our laboratory and others have demonstrated the involvement of monocarboxylate transporter (MCT1) in the luminal absorption of butyrate in human intestinal epithelial cells (6, 10, 21, 36). Furthermore, studies have shown that MCT1 is the major butyrate transporter in the large intestine (21, 36). Earlier studies have shown that MCT1 expression and function are regulated by its substrate butyrate (4, 9) and leptin (6) in intestinal epithelial cells. Also recently it has been shown that MCT1 expression and function are downregulated in patients with inflammatory bowel disease and in colonic epithelial HT29 cells in response to the proinflammatory cytokines TNF-α and IFN-γ (41). Furthermore, studies from our laboratory have shown the acute inhibition of MCT1 by enteropathogenic Escherichia coli (E. coli) infection, a food borne human pathogen associated with diarrhea (3). However, to date, almost no information is available with respect to the acute effects of the anti-inflammatory/proabsorptive peptide hormone, somatostatin (SST), on butyrate absorption and MCT1 expression in intestinal Caco2 cells.
SST is an important neuropeptide of the human gastrointestinal tract, which is known to act as a neurotransmitter and hormone (20, 26). SST has been shown to function as a proabsorptive/anti-inflammatory molecule, and its longer acting analog octreotide has long been utilized as an antidiarrheal agent (33). SST is known to stimulate NaCl absorption and inhibit chloride secretion, cell proliferation, and gut motility (8, 30) in the gastrointestinal tract. SST also inhibited secretion of proinflammatory mediators IL-8 and IL-1β upon TNF-α stimulation in HT29 and Caco2 cells (7). Previous studies have emphasized the role of SSTR1 and 2 receptor subtypes (G protein coupled) in mediating the inhibitory effects of somatostatin on colonic ion secretion in animal models (27, 45) and cultured human colon cancer cell lines (44). The present studies were undertaken to examine in detail the effects of SST on butyrate uptake and the potential involvement of MCT1.
One of the mechanisms by which MCT1 surface expression and activity might be regulated is through an interaction with a chaperone protein, CD147 (23, 34). CD147 protein is a cell surface glycoprotein and has been shown to colocalize with MCTs (MCT1, MCT3, and MCT4) in different cell types (17, 23, 32, 34, 48), and its mRNA has recently been shown to be expressed in the human small intestine and colon (42). Earlier studies have shown that the expression of MCT1 was reduced in CD147 knockout mice (31), indicating that CD147 is required for the proper targeting of MCT1 to the cell surface. Therefore, the potential role of CD147 in regulating MCT1 in Caco2 cells in response to SST was also examined.
Our studies demonstrated that SST stimulated butyrate uptake in human intestinal epithelial Caco2 cells via a SST receptor subtype 2 and involvement of p38 MAPK-mediated pathway. Moreover, this stimulation of butyrate uptake in response to SST was due to an increase in the membrane levels of MCT1 and CD147 as well as enhanced association of MCT1 with CD147 in Caco2 cells. We speculate that the anti-diarrheal effects of somatostatin on electrolyte absorption in the human intestine may also involve increased surface MCT1 levels to enhance SCFA absorption.
MATERIALS AND METHODS
Materials.
Caco2 cells were obtained from American Type Culture Collection (Manassas, VA). 14[C]Butyric acid (sodium salt) was procured from American Radiolabeled Chemicals (St. Louis, MO). SST and seglitide were obtained from Sigma (St. Louis, MO). p38 MAPK inhibitor, SB 203580, was obtained from Biomol (Plymouth Meeting, PA). Bisindolylmaleimide (BIM) was obtained from Calbiochem (San Diego, CA). Sulfo-NHS-SS-Biotin and streptavidin agarose were from Pierce, Rockford, IL. Affinity-purified mouse monoclonal antibody against CD147 was procured from AbCam (Cambridge, MA). Goat anti-mouse or goat anti-rabbit antibody conjugated to horseradish peroxidase and protein L agarose were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other chemicals were of at least reagent grade and were obtained from either Sigma Chemicals or Fisher Scientific (Pittsburgh, PA).
Cell culture.
Caco-2 cells were grown in Modified Eagle's medium supplemented with 4.5 g/l glucose, 2 mM glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 10 mM HEPES, 1% essential and nonessential amino acids, and 20% fetal bovine serum, pH 7.4 in 5% CO2-95% O2 at 37°C. For the uptake experiments, cells from passages between 20–45 were plated in 24-well plates (Falcon, Franklin Lakes, NJ) at a density of 2 × 104 cells/ml. Confluent monolayers were then used for transport experiments at days 10–12 postplating. To study the effect of SST on butyrate uptake, cells were acutely exposed to 25 and 50 nM SST in serum-free cell culture medium for 30–60 min. In another set of experiments, cells were plated in 12-well Transwell collagen-coated inserts at a density of 1 × 104 cells/ml, and SST (25 nM, 30 min) was added from either the apical or basolateral side.
In separate sets of experiments, cells were pretreated with specific p38 MAPK inhibitor, SB 203580 (30 μM), for 1 h before the addition of SST (50 nM). The inhibitor was also coincubated along with SST for another 30 min.
14C butyrate uptake.
Butyrate uptake was assessed in Caco-2 cells as described previously by us with minor modifications (1). Briefly, cells were incubated at room temperature for 20 min in tracer-free buffer containing (in mM) 259 mannitol and 20 HEPES, pH 7.4. Cells were then washed and incubated with buffer containing (in mM) 260 mannitol and 20 HEPES, pH 6.5, 5.0 14C butyrate (1 mCi/ml) for a time period of 5 min. The uptake was stopped by washing the cells twice, with ice-cold 1× PBS. Finally, cells were solubilized with 0.5 N NaOH for at least 4 h. The protein concentration was measured by the method of Bradford (5). The radioactivity was counted by a Tri-CARB 1600-TR liquid scintillation counter (Packard Instruments, Downers Grove, IL). The uptake was expressed as nmol/mg protein per 5 min. For the kinetic experiments, butyrate uptake was performed with increasing concentrations of the substrate butyrate ranging from 0.5–15 mM. The uptake values were analyzed for simple Michaelis-Menten kinetics using a nonlinear regression data analysis from a computerized model (GraphPad; PRISM, San Diego, CA).
Cloning of human CD147 for transfection in Caco2 cells.
Full-length cDNA of human CD147 was amplified from small intestine by RT-PCR. Briefly, 5 μg of total RNA was used for reverse transcription with random primers using SuperScript II reverse transcriptase kit (Invitrogen, Carlsbad, CA). The full-length cDNA of hCD147 was then amplified by PCR utilizing gene-specific primers and the proof-reading Elongase enzyme mix (Invitrogen) according to the manufacturer's instructions. The primer sequences (designed on the basis of gene bank accession number BC009040) are 5′primer: AAGGCCTCTGTCGACACCATGGCGGCTGCGCTGTTCGTGCTGC (Kozak sequence is underlined); 3′ primer: AGAATTCGCAAGCTTGGAAGAGTTCCTCTGGCGGACGTTCTTG.
PCR products were excised from 1% agarose gel and purified utilizing Sephaglas BandPrep Kit (Amersham Pharmacia Biotechnology, Piscataway, NJ). The amplified fragment was cloned into the pAcGFP1-N In Fusion expression mammalian vector (Clontech, Mountain View, CA) in frame with green fluorescent protein (GFP). The orientation and the sequence of the insert were confirmed by sequencing, and the expression of hCD147-GFP fusion protein was examined by Western blotting utilizing anti-GFP antibodies (AbCam).
Transient transfection.
For transfection studies, Caco2 cells were transfected utilizing Amaxa Nucleofector System (Amaxa, Cologne, Germany) according to manufacturer's instructions. Briefly, ∼10 × 106 cells were harvested and then were electroporated in 100 μl of solution T (supplied by Amaxa) along with 30 μg of hCD147-GFP cDNA construct. The cells were transferred to full media and plated onto a 24-well plate. After 24 h, transfected cells were used for cell surface biotinylation.
Biotinylation of cell surface proteins.
Cell surface biotinylation studies to measure surface protein expression of MCT1 and CD147 were done as described previously (38). It should be noted that CD147 is endogenously expressed in Caco2 cells; however, for determining CD147 expression in Caco2 cells, we used CD147-GFP-transfected cells because the commercially available anti-GFP antibody is more sensitive in detecting CD147 protein (GFP tagged to CD147) in biotinylated samples compared with the mouse antibody raised against the native human CD147 protein (Abcam). Briefly, untreated or SST-treated normal Caco2 or CD147/GFP cDNA-transfected cells were washed thrice with 1× PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 at 4°C. The apical surface of Caco-2 cells was then exposed to Sulfo-NHS-SS-Biotin (Pierce) at a concentration of 1.5 mg/ml in borate buffer, pH 9.0, by incubating for an hour at 40C in horizontal motion. Unbound NHS-SS-biotin was then quenched with PBS containing CaCl2, MgCl2 and 100 mM glycine for 20 min at 4°C. Cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris·HCl, pH 7.4, 5 mM EDTA, 1% Triton X-100, 0.1% SDS, and protease inhibitor cocktail) and sonicated for 20 s. After centrifugation at 4°C for 30 min, the cell debris was removed and equal amounts of supernatant containing 2 mg/ml protein were incubated overnight with streptavidin agarose at 4°C and then washed thrice with lysis buffer. The streptavidin agarose beads were spun down and boiled in Laemmli sample buffer containing DTT. Proteins were separated on 10–12% SDS-PAGE gels and probed with human anti MCT1 (Alpha Diagnostics International, San Antonio, TX) as previously described (1) or anti-GFP (CD147 tagged to GFP) antibody. CD147 expression was detected by incubating protein-bound nitrocellulose membranes in blocking buffer containing 1× TBS, 0.1% Tween 20, and 5% nonfat dry milk for 1 h at room temperature. Membranes were then incubated with the mouse monoclonal anti-GFP antibody (1: 500 dilution) in 1× TBS and 1% milk overnight at 4°C followed by washes for 45 min with wash buffer containing 1× TBS and 0.1% Tween 20. Bands were visualized with enhanced chemiluminescence reagent. The bands were quantified using densitometric analysis and expressed as arbitrary units indicating the relative surface expression of MCT1 or CD147 over the total MCT1 or CD147 (sum of apical and intracellular) expression.
Cell lysates, immunoprecipitation, and Western blotting.
Caco2 cells grown to confluence in six-well plates (10 × 104 cells/ml; Corning Costar, Cambridge, MA) were serum starved overnight and treated with SST (25 and 50 nM) for 30 and 60 min or p38 MAPK inhibitor, SB203580 (30 μM). Cells were washed with ice-cold PBS three times and lysed in 20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, and 1× complete protease inhibitor cocktail. The cells were homogenized by passing 10 times through 26-gauge needle. The lysate was centrifuged at 5000 g for 5 min at 4°C, and protein concentration was determined by the method of Bradford (5). To detect association of MCT1 with CD147, CD147 was immunoprecipitated by incubating cell lysates (500 μg) with monoclonal anti-CD147 overnight at 4°C, with mixing. Protein L agarose beads were added [40 μl of a 50% (wt/vol) solution] and mixed for an additional 4 h at 4°C. Immunoprecipitates were washed three times in lysis buffer, solubilized in SDS gel loading buffer, and boiled for 5 min. The samples obtained above were subjected to 10% SDS-PAGE and transferred to nitrocellulose membranes. MCT1 expression was detected utilizing human anti MCT1 antibody (Alpha Diagnostics) (1).
Statistical analysis.
Results are expressed as means ± SE. Each independent set represents means ± SE of data from at least nine wells used on three separate occasions. Student's t-test was used for statistical analysis. P < 0.05 or less was considered statistically significant.
RESULTS
SST increases butyrate uptake in Caco2 cells.
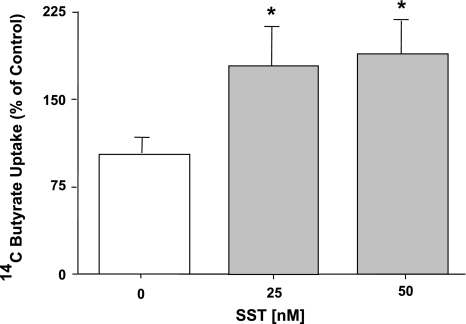
SST is known to function as a proabsorptive peptide by stimulating NaCl absorption (13, 18) and inhibiting Cl− secretion (15, 16, 45) in the intestine. Therefore, we examined the possible role of SST in the regulation of butyrate uptake in Caco2 cells. Confluent Caco-2 cells grown on 24-well plastic supports at 10–12 days postplating were treated with SST (25 nM or 50 nM) for 30 min, and pH-driven 14C butyrate uptake was measured as described in materials and methods. Figure 1 shows that SST treatment to Caco-2 cells significantly increased the butyrate uptake at 25 nM and 50 nM (∼60–70%; P < 0.05).
Fig. 1.
Effect of somatostatin (SST) on butyrate uptake in Caco2 cells. Overnight serum-starved postconfluent Caco2 cells were treated with 25 and 50 nM concentrations of SST in serum-free cell culture medium for 30 min. [14C]Butyrate uptake (5 mM) was measured as described in materials and methods. Results are expressed as a percentage of control value and represent means ± SE of 6 separate experiments performed in triplicate. *P < 0.05 compared with control.
Effect of luminal and serosal SST on butyrate uptake in Caco2 cells.
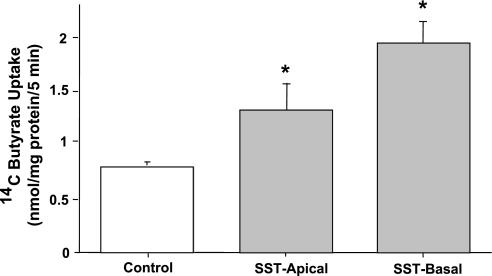
Our initial studies showed that SST1, 2, and 5 receptor mRNA is present in both human small intestine and colon as well as in differentiated Caco2 cells (data not shown). Because SST receptors 1, 2, and 5 in the intestine are expressed on both the apical and basolateral membranes (7, 45, 46), we examined the effects of luminal or serosal SST (25 nM, 30 min) on apical butyrate uptake in Caco2 cells grown on permeable supports. Figure 2 shows that luminal SST significantly stimulated apical butyrate uptake, whereas the basolateral addition of SST on butyrate uptake was slightly more (∼2.4- vs. 2.0-fold) compared with control. For all subsequent experiments the effects of only luminal SST were examined.
Fig. 2.
SST treatment from apical or basolateral side stimulates butyrate uptake in Caco2 cells. Overnight serum-starved postconfluent Caco2 cells grown on permeable supports were treated with 25 nM SST in serum-free cell culture medium from either the apical or basolateral side for 30 min. [14C]Butyrate uptake (5 mM) was measured as described in materials and methods. Results represent means ± SE of 3 separate experiments performed in triplicate. *P < 0.05 compared with control.
Effect of SST receptor agonists on butyrate uptake in Caco2 cells.
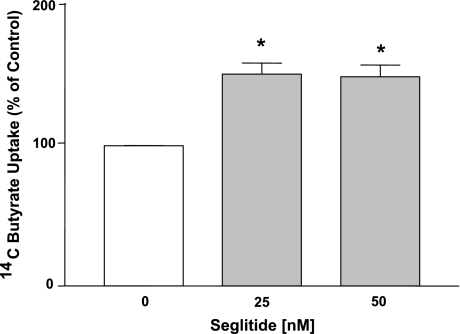
SSTR1 and 2 receptor subtypes have been shown to be involved in mediating the inhibitory effects of SST on ion secretion in animal and cell culture models (27, 44, 45). The involvement of SSTR1 and 2 receptors in SST-stimulated effects on butyrate uptake in Caco2 cells was examined. Because STR1 agonist L-797,591 was commercially unavailable, we used the selective SSTR2 receptor agonist, seglitide. As shown in Fig. 3, apical addition of the seglitide (25–50 nM) for 30 min resulted in a significant increase in butyrate uptake. These observations suggest that SSTR2 receptor agonist mimicked the effects of SST in stimulating butyrate uptake in Caco2 cells.
Fig. 3.
Seglitide mimics the effects of SST on butyrate uptake in Caco-2 cells. Overnight serum-starved postconfluent Caco2 cells were treated with 25–50 nM of seglitide (SSTR2 receptor agonist) in serum-free cell culture medium for 30 min. [14C]Butyrate uptake (5 mM) was measured as described in materials and methods. Results are expressed as percentage of control value and represent means ± SE of 4 separate experiments performed in triplicate. *P < 0.05 compared with control.
Role of p38 MAPK in SST-induced stimulation of butyrate uptake in Caco2 cells.
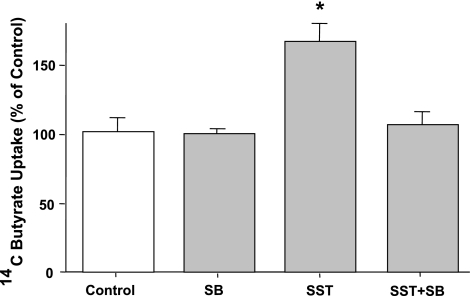
SST is well known to trigger a number of intracellular pathways including activation of MAPKs (24, 29). Therefore, we next determined the role of MAPKs in the SST-mediated stimulation of butyrate uptake in Caco2 cells. The effects of PD98059 (a specific inhibitor of Erk1/2, 30 μM, 1 h) and SB203580 (a specific p38 MAPK inhibitor, 30 μM, 1 h) on butyrate uptake in response to SST were examined. Specific p38 MAPK inhibitor, SB203580, blocked the SST-mediated stimulation of butyrate uptake in Caco2 cells (Fig. 4), suggesting the involvement of p38 MAPK on the effects of SST on butyrate uptake. However, PD98059 failed to show any effect indicating that Erk 1/2 are not involved (data not shown).
Fig. 4.
Effect of p38 MAPK inhibitor, SB203580 (SB), on SST-induced stimulation of butyrate uptake in Caco-2 cells. Overnight serum-starved postconfluent Caco2 cells were preincubated with SB203580 (30 μM), specific inhibitor of p38 MAPK for 60 min in the cell culture medium and then coincubated with 25 nM of SST for another 30 min. [14C]Butyrate uptake (5 mM) was measured as described in materials and methods. Results are expressed as percentage of control value and represent means ± SE of 4 separate experiments performed in triplicate. *P < 0.05 compared with control.
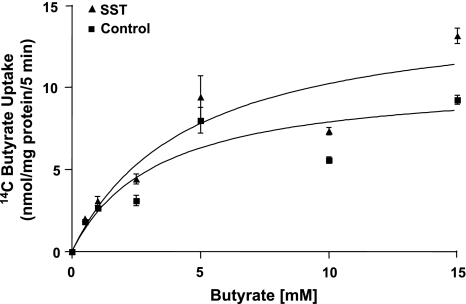
Effect of SST on kinetics of butyrate uptake in Caco2 cells.
To understand the mechanisms by which SST stimulates butyrate uptake, we evaluated its effect on the kinetic parameters of butyrate uptake in Caco2 cells. Butyrate uptake was measured at increasing concentrations of butyrate ranging from 0.5–15 mM in response to SST treatment to Caco-2 cells. MCT1 activity exhibited saturation with increasing butyrate concentrations in both control and SST-treated cells as analyzed by GraphPad Prism software (Fig. 5). Incubation with SST significantly increased the maximal velocity (Vmax) of the transport process (∼41%) compared with untreated cells (14.86 ± 1.14 compared with 10.52 ± 1.67 nmol/mg protein per 5 min, P < 0.05) with no significant changes in the apparent Michaelis-Menten constant (Km) for butyrate (control: 3.34 ± 0.95 mM compared with SST: 4.54 ± 1.33 mM). These results suggest that SST treatment alters either the number of active transporters on the membrane or the turnover rate of the transporter but not the affinity of the transporter for butyrate.
Fig. 5.
Effect of SST on the kinetics of butyrate uptake in Caco2 cells. Overnight serum-starved postconfluent Caco2 cells were treated with 50 nM SST in serum-free cell culture medium for 30 min. [14C]Butyrate uptake was measured for 5 min in the presence of increasing concentrations of the substrate, butyrate (0.5–15 mM). [14C]Butyrate uptake was expressed as nmol/mg protein per 5 min. Representative Michaelis-Menten plot of experiments performed on 3 different occasions is shown.
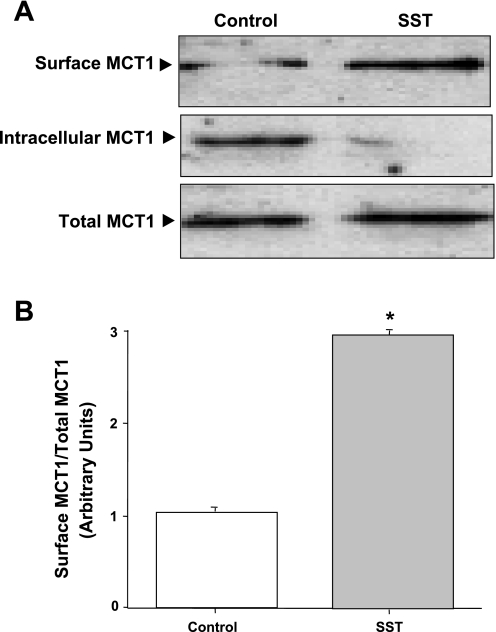
Surface levels of MCT1 are increased by SST.
The observed increase in the value of Vmax of MCT1 in response to SST suggested that SST might increase MCT1 activity via recycling of the transporter from the intracellular pools to the apical compartment. We, therefore, examined the effects of SST on the surface levels of MCT1 in Caco2 cell membranes. Cell surface biotinylation studies were performed to determine the changes in cell surface levels of MCT1 protein or the biotin-treated fraction of total cellular protein. Biotinylated proteins from control and SST-treated cells were separated from the cell lysate by avidin, and proteins were probed with human MCT1 antibody. As shown in Fig. 6A, cell surface biotinylation studies demonstrated an increase (2.8-fold increase compared with control, Fig. 6B) in the apical membrane levels of MCT1 parallel to a decrease in the intracellular pool.
Fig. 6.
Cell surface expression of monocarboxylate transporter 1 (MCT1). Overnight serum-starved postconfluent Caco2 cells were treated with 25 nM SST in serum-free cell culture medium for 30 min and subjected to biotinylation at 4°C using sulfo-NHS-SS-biotin. After solubilization, biotinylated proteins were extracted with streptavidin-agarose from equal amounts of total cellular protein. Surface and intracellular fractions were run on 12% SDS-polyacrylamide gel electrophoresis followed by transfer to nitrocellulose membrane. The blot was immunostained with a rabbit anti-hMCT1 (A). A representative blot of 3 different experiments is shown. The data were quantified by densitometric analysis and expressed as arbitrary units and represent means ± SE of 3 determinations (B). *P < 0.05 compared with untreated control.
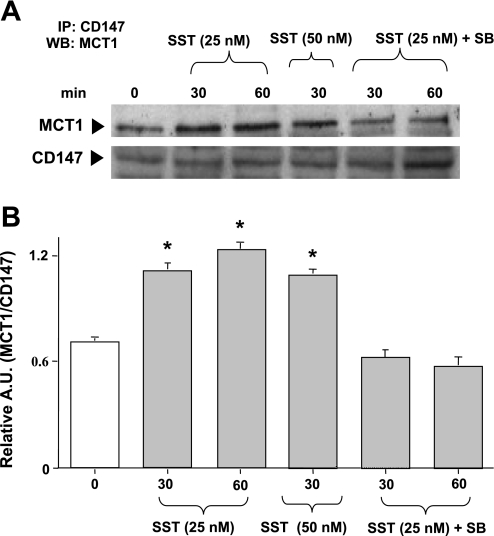
SST induces association of MCT1 with CD147.
Previous studies have demonstrated that coexpression of accessory protein, CD147 (also known as basigin) not only improved trafficking of MCT1 to the plasma membrane but also influenced its catalytic activity (47). The interaction between MCT1 and CD147 is required for proper targeting of MCT1 from the endoplasmic reticulum/Golgi apparatus to the plasma membrane (23). Moreover, studies have shown that luminal leptin enhanced butyrate uptake via an increased translocation of CD147/MCT1 to the apical plasma membrane of Caco2-brush border-expressing (BBE) cells (6). To elucidate the potential role of CD147 in the stimulatory effects of SST on butyrate uptake in Caco2 cells, we examined the association of MCT1 with CD147 in SST-treated Caco2 cells. Cells were treated with SST (25 and 50 nM) at 30 or 60 min, lysed and immunoprecipitated with anti-CD147-specific antibody, and probed with anti-MCT1 antibody. As shown in Fig. 7A, immunoprecipitation analysis showed an enhanced association of MCT1 with CD147 in response to SST (25 nM) at both 30 and 60 min (2.2-fold increase compared with control, Fig. 7B). Interestingly, the enhanced MCT1/CD147 association at both 30 and 60 min was abolished in the presence of SB203580. These results indicate that CD147 plays an important role in the regulation of MCT1 activity by SST.
Fig. 7.
SST induces MCT1/CD147 association in Caco-2 cells. Overnight serum-starved postconfluent Caco2 cells were treated with SST (25 and 50 nM) in serum-free cell culture medium for 30–60 min. Cells were also pretreated with the p38 MAPK inhibitor SB203580 (30 μM) for 60 min and then coincubated with SST (25 nM) for another 30–60 min. Cells were washed with 1× PBS, lysed, and immunoprecipitated (IP) with anti-CD147 antibody. Immunoprecipitates were analyzed by 10% SDS-polyacrylamide gel electrophoresis, followed by transfer of proteins to nitrocellulose and probed with anti-MCT1 antibody (MCT1). The Western blots (WB) were stripped and reprobed with the anti-CD147 antibody (CD147) to indicate equal loading of protein in each lane (A). A representative blot of 3 different experiments is shown. The data were quantified by densitometric analysis and expressed as arbitrary units (A.U.) and represent means ± SE of 3 determinations (B). *P < 0.05 compared with untreated control.
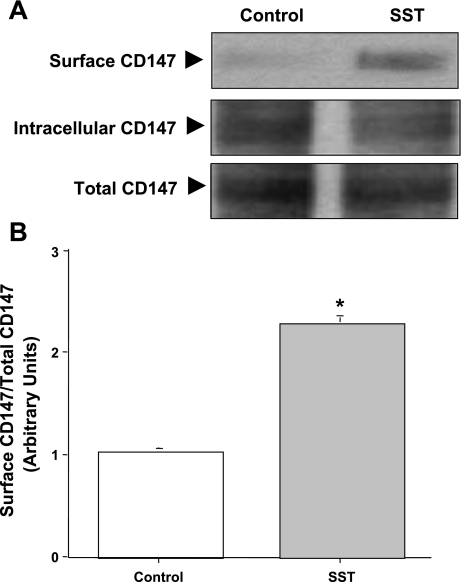
SST increases CD147 membrane levels.
As SST enhanced the association of MCT1 with CD147, we next determined whether SST also increases CD147 levels in membranes of Caco2 cells. Western blot analysis was performed in the biotinylated samples of untreated and SST-treated CD147-GFP transfected Caco2 cells using monoclonal mouse GFP antibody. Figure 8A shows that SST significantly increased the membrane levels of CD147 compared with control (2.2-fold increase compared with control, Fig. 8B). These results demonstrate that, similar to an increase in surface MCT1 levels, SST also increased the membrane levels of CD147 in Caco2 cells.
Fig. 8.
SST increases CD147 membrane expression in Caco2 cells. CD147-green fluorescent protein (GFP)-transfected Caco2 cells were treated with 25 nM of SST for 30 min. The membrane fractions were subjected to 10% SDS-PAGE and probed with specific mouse monoclonal GFP antibody to detect CD147 expression (tagged to GFP) (A). A representative blot of 3 different experiments is shown. The data were quantified by densitometric analysis and expressed as arbitrary units and represent means ± SE of 3 determinations (B). *P < 0.05 compared with untreated control.
DISCUSSION
We and others have previously shown the involvement of MCT1 in the luminal absorption of butyrate in human intestinal epithelial cells (6, 10, 21, 36). Butyrate is known to play an important role in maintaining colonic epithelial integrity and function (37). The present studies were designed to investigate the mechanisms underlying the regulation of butyrate uptake by the proabsorptive peptide hormone, SST, in the human intestine. SST has been shown to exert antisecretory effects in intestinal epithelial T84 and HT29–Cl19A cells (14, 44). The SST analog, lanreotide (BIM 23014), has been shown to reduce ion secretion in vivo under basal and PGE1-stimulated conditions in the human jejunum (40). Also, SST infusion has been reported to inhibit watery diarrhea in patients with carcinoid syndrome (11). Present studies for the first time demonstrated that luminal SST stimulated butyrate uptake in Caco2 cells. The concentration of SST used in our present study is in the physiological range (25–50 nM) and is in agreement with previous studies showing that SST at physiological nanomolar concentrations (1–100 nM) markedly inhibited both basal- and agonist-stimulated ion secretion in animal models (27, 45) and cultured intestinal epithelial cells (14, 44).
SST is known to exhibit diverse physiological functions via G protein-coupled receptors (SSTRs) (8, 30). Our receptor mRNA expression studies utilizing real-time PCR have shown that SSTR1, 2, and 5, but not 3 and 4, are expressed in the human small intestine, colon, and Caco2 cells (data not shown). Previous studies have shown that SST inhibits colonic ion secretion via SST receptor subtypes 1 and 2, respectively (27, 44, 45). We showed that seglitide (SSTR2 agonist) increased butyrate uptake in Caco2 cells, suggesting the involvement of SSTR2. SSTR2 activation has been shown to activate ERK1/2 and p38 MAPK in murine myeloid 32D cells (29). However, our studies showed that p38 MAPK, but not ERK1/2, is involved in mediating the effects of SST, as only the specific p38 MAPK inhibitor, SB203588, blocked SST-induced effects on butyrate uptake in Caco2 cells.
With respect to the mechanisms underlying modulation of butyrate uptake by SST, our results showed that SST increased the Vmax for butyrate uptake with no significant changes in Km. The increase in Vmax for butyrate uptake indicates an increase in the number of MCT1 molecules on the apical membrane attributable to increased exocytic insertion or decreased endocytic retrieval. We have previously shown that MCT1 may be modulated via a recycling mechanism in response to enteropathogenic E. coli infection (3) and serotonin (19) in Caco2 cells. Our present studies also demonstrated that SST-mediated upregulation of MCT1 occurs via a significant increase in the apical membrane levels of MCT1 parallel to a decrease in the intracellular pool.
Interestingly, parallel to an increase in membrane levels of MCT1 in Caco2 cells, we also found a similar increase in the membrane levels of CD147 by SST, further attesting to its role as a chaperone protein to target MCT1 to the cell surface. Similar observations were noted in Caco2-BBE and FRTL5 (rat thyroid) cells treated with leptin and thyroid-stimulating hormone, respectively (6, 17). Our coimmunoprecipitation studies showed that SST enhanced the association of MCT1 with CD147 in Caco2 cells. These data are in accordance with previous studies in murine heart plasma membranes (23), epithelial cells of kidney, retina (12), and thyroid (17) in which CD147 was found to be tightly associated with MCT1 to facilitate proper targeting of MCT1 to cell surface.
Previous studies have demonstrated that CD147 plays an important functional role in butyrate transport activity. For example, MCT1/CD147 association in Caco2-BBE cells was found to be critical for butyrate uptake as butyrate uptake was decreased in antisense CD147 transfected Caco2-BBE cells when compared with empty vector or nontransfected cells (6). Disruption of MCT1/CD147 association by covalent modification of CD147 with the cell impermeant organomercurial reagent, p-chloromercuribenzenesulfonate, inhibited transport activity (47). Moreover, enhanced association of MCT1 and CD147 by leptin was shown to increase butyrate uptake in Caco2-BBE cells (6). However, previous studies failed to address the possible signaling mechanisms involved in increasing MCT1/CD147 association. Our studies provided novel evidence on the involvement of p38 MAPK in SST-induced association of CD147 with MCT1, as this association was abolished in the presence of the specific p38 MAPK inhibitor, SB203580. Because SST-mediated effects on butyrate uptake are dependent on p38 MAPK pathway, these results further suggested that SST-induced MCT1/CD147 association may be crucial for butyrate transport in the human intestine. Also, our low stringent analysis of both MCT1 and CD147 using in sillico analysis by Scansite 2.0 (28) identified potential phosphorylation sites for p38 MAPK. We speculate that these sequence motifs may play an important role in the direct or indirect phosphorylation of MCT1 and CD147 that could result in the enhanced association of MCT1 and CD147. Further studies are needed to address this important issue.
In summary, our results demonstrated that SST stimulates butyrate uptake in Caco2 cells via the activation of p38 MAPK involving SST receptor 2, which subsequently results in an increase in the membrane levels of both MCT1 and CD147. Increased MCT1 and CD147 then associate at the membrane leading to the stimulation of apical butyrate uptake process in Caco2 cells. Our findings provide novel mechanistic insights into the hormonal regulation of MCT1 and CD147 in the human intestine. We speculate that SST secreted in the intestinal lumen during inflammation might play a protective role in maintaining colonic epithelial integrity by increasing the activity, surface levels, and association of MCT1/CD147 for enhanced SCFA absorption.
GRANTS
These studies were supported by the Department of Veterans Affairs and the NIDDK grants DK 54016 and DK 81858 (P. K. Dudeja), DK 33349 (K. Ramaswamy), DK 71596 (W. Alrefai), DK 74459 (R. K. Gill), P01 DK 067887 (P. K. Dudeja and K. Ramaswamy), and CCFA grant Ref no. 1942 (S. Saksena).
REFERENCES
- 1.Alrefai WA, Tyagi S, Gill R, Saksena S, Hadjiagapiou C, Mansour F, Ramaswamy K, Dudeja PK. Regulation of butyrate uptake in Caco-2 cells by phorbol 12-myristate 13-acetate. Am J Physiol Gastrointest Liver Physiol 286: G197–G203, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Binder HJ, Mehta P. Short-chain fatty acids stimulate active Na and Cl absorption in vitro in the rat distal colon. Gastroenterology 96: 989–996, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G, Dudeja PK. Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol 290: G30–G35, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-kappaB pathway. J Cell Biochem 103: 1452–1463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 6.Buyse M, Sitaraman SV, Liu X, Bado A, Merlin D. Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem 277: 28182–28190, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Chowers Y, Cahalon L, Lahav M, Schor H, Tal R, Bar-Meir S, Levite M. Somatostatin through its specific receptor inhibits spontaneous and TNF-alpha- and bacteria-induced IL-8 and IL-1 beta secretion from intestinal epithelial cells. J Immunol 165: 2955–2961, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Csaba Z, Dournaud P. Cellular biology of somatostatin receptors. Neuropeptides 35: 1–23, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Cuff MA, Lambert DW, Shirazi-Beechey SP. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. J Physiol 539: 361–371, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuff MA, Shirazi-Beechey SP. The human monocarboxylate transporter, MCT1: genomic organization and promoter analysis. Biochem Biophys Res Commun 292: 1048–1056, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Davis GR, Camp RC, Raskin P, Krejs GJ. Effect of somatostatin infusion on jejunal water and electrolyte transport in a patient with secretory diarrhea due to malignant carcinoid syndrome. Gastroenterology 78: 346–349, 1980 [PubMed] [Google Scholar]
- 12.Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc Natl Acad Sci USA 102: 16245–16250, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dharmsathaphorn K, Binder HJ, Dobbins JW. Somatostatin stimulates sodium and chloride absorption in the rabbit ileum. Gastroenterology 78: 1559–1565, 1980 [PubMed] [Google Scholar]
- 14.Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol Gastrointest Liver Physiol 246: G204–G208, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Dharmsathaphorn K, Sherwin RS, Cataland S, Jaffe B, Dobbins J. Somatostatin inhibits diarrhea in the carcinoid syndrome. Ann Intern Med 92: 68–69, 1980 [DOI] [PubMed] [Google Scholar]
- 16.Dharmsathaphorn K, Sherwin RS, Dobbins JW. Somatostatin inhibits fluid secretion in the rat jejunum. Gastroenterology 78: 1554–1558, 1980 [PubMed] [Google Scholar]
- 17.Fanelli A, Grollman EF, Wang D, Philp NJ. MCT1 and its accessory protein CD147 are differentially regulated by TSH in rat thyroid cells. Am J Physiol Endocrinol Metab 285: E1223–E1229, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Farthing MJ. The role of somatostatin analogues in the treatment of refractory diarrhoea. Digestion 57, Suppl 1: 107–113, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Gill R, Saksena S, Alrefai WA, Tyagi S, Ramaswamy K, Dudeja PK. Serotonin inhibits MCT1 activity by retrieval of the transporter from the apical membranes in Caco-2 cells. Gastroenterology 126: A145, 2004 [Google Scholar]
- 20.Gyr KE, Meier R. Pharmacodynamic effects of Sandostatin in the gastrointestinal tract. Digestion 54, Suppl 1: 14–19, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279: G775–G780, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 118: 724–734, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J 19: 3896–3904, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahlou H, Saint-Laurent N, Esteve JP, Eychene A, Pradayrol L, Pyronnet S, Susini C. sst2 Somatostatin receptor inhibits cell proliferation through Ras-, Rap1-, and B-Raf-dependent ERK2 activation. J Biol Chem 278: 39356–39371, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Lambert DW, Wood IS, Ellis A, Shirazi-Beechey SP. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br J Cancer 86: 1262–1269, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewin MJ. The somatostatin receptor in the GI tract. Annu Rev Physiol 54: 455–468, 1992 [DOI] [PubMed] [Google Scholar]
- 27.McKeen ES, Feniuk W, Humphrey PP. Somatostatin receptors mediating inhibition of basal and stimulated electrogenic ion transport in rat isolated distal colonic mucosa. Naunyn Schmiedebergs Arch Pharmacol 352: 402–411, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31: 3635–3641, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oomen SP, van Hennik PB, Antonissen C, Lichtenauer-Kaligis EG, Hofland LJ, Lamberts SW, Lowenberg B, Touw IP. Somatostatin is a selective chemoattractant for primitive (CD34(+)) hematopoietic progenitor cells. Exp Hematol 30: 116–125, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 20: 157–198, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci 44: 1305–1311, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Philp NJ, Wang D, Yoon H, Hjelmeland LM. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci 44: 1716–1721, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Pless J, Bauer W, Briner U, Doepfner W, Marbach P, Maurer R, Petcher TJ, Reubi JC, Vonderscher J. Chemistry and pharmacology of SMS 201–995, a long-acting octapeptide analogue of somatostatin. Scand J Gastroenterol Suppl 119: 54–64, 1986 [DOI] [PubMed] [Google Scholar]
- 34.Poole RC, Halestrap AP. Interaction of the erythrocyte lactate transporter (monocarboxylate transporter 1) with an integral 70-kDa membrane glycoprotein of the immunoglobulin superfamily. J Biol Chem 272: 14624–14628, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Resta-Lenert S, Truong F, Barrett KE, Eckmann L. Inhibition of epithelial chloride secretion by butyrate: role of reduced adenylyl cyclase expression and activity. Am J Physiol Cell Physiol 281: C1837–C1849, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification of a monocarboxylate transporter isoform type 1 (MCT1) on the luminal membrane of human and pig colon. Biochem Soc Trans 26: S120, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 83: 424–429, 1982 [PubMed] [Google Scholar]
- 38.Saksena S, Gill RK, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Role of Fyn and PI3K in H2O2-induced inhibition of apical Cl-/OH- exchange activity in human intestinal epithelial cells. Biochem J 416: 99–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheppach W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas. A placebo-controlled trial German-Austrian SCFA Study Group. Dig Dis Sci 41: 2254–2259, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Sobhani I, Rene E, Ramdani A, Bayod F, Sabbagh LC, Thomas F, Mignon M. Lanreotide inhibits human jejunal secretion induced by prostaglandin E1 in healthy volunteers. Br J Clin Pharmacol 41: 109–114, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, Mosnier JF, Galmiche JP, Shirazi-Beechey S, Segain JP. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology 133: 1916–1927, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Till A, Rosenstiel P, Brautigam K, Sina C, Jacobs G, Oberg HH, Seegert D, Chakraborty T, Schreiber S. A role for membrane-bound CD147 in NOD2-mediated recognition of bacterial cytoinvasion. J Cell Sci 121: 487–495, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81: 1031–1064, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Warhurst G, Barbezat GO, Higgs NB, Reyl-Desmars F, Lewin MJ, Coy DH, Ross I, Grigor MR. Expression of somatostatin receptor genes and their role in inhibiting Cl- secretion in HT-29cl.19A colonocytes. Am J Physiol Gastrointest Liver Physiol 269: G729–G736, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Warhurst G, Higgs NB, Fakhoury H, Warhurst AC, Garde J, Coy DH. Somatostatin receptor subtype 2 mediates somatostatin inhibition of ion secretion in rat distal colon. Gastroenterology 111: 325–333, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Warhurst G, Higgs NB, Grigor MR, Ross I, Barbezat GO. Expression of multiple somatostatin receptor genes in human colonic epithelial cells. Biochem Soc Trans 23: 18S, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Wilson MC, Meredith D, Fox JE, Manoharan C, Davies AJ, Halestrap AP. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J Biol Chem 280: 27213–27221, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Wilson MC, Meredith D, Halestrap AP. Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J Biol Chem 277: 3666–3672, 2002 [DOI] [PubMed] [Google Scholar]