Abstract
Endothelin-1 (ET-1) plays a key role in the regulation of endothelial nitric oxide synthase (eNOS) activation in liver sinusoidal endothelial cells (LSECs). In the presence of endotoxin, an increase in caveolin-1 (Cav-1) expression impairs ET-1/eNOS signaling; however, the molecular mechanism is unknown. The objective of this study was to investigate the molecular mechanism of Cav-1 in the regulation of LPS suppression of ET-1-mediated eNOS activation in LSECs by examining the effect of caveolae disruption using methyl-β-cyclodextrin (CD) and filipin. Treatment with 5 mM CD for 30 min increased eNOS activity (+255%, P < 0.05). A dose (0.25 μg/ml) of filipin for 30 min produced a similar effect (+111%, P < 0.05). CD induced the perinuclear localization of Cav-1 and eNOS and stimulated NO production in the same region. Readdition of 0.5 mM cholesterol to saturate CD reversed these effects. Both the combined treatment with CD and ET-1 (CD + ET-1) and with filipin and ET-1 stimulated eNOS activity; however, pretreatment with endotoxin (LPS) abrogated these effects. Following LPS pretreatment, CD + ET-1 failed to stimulate eNOS activity (+51%, P > 0.05), which contributed to the reduced levels of eNOS-Ser1177 phosphorylation and eNOS-Thr495 dephosphorylation, the LPS/CD-induced overexpression and translocation of Cav-1 in the perinuclear region, and the increased perinuclear colocalization of eNOS with Cav-1. These results supported the hypothesis that Cav-1 mediates the action of endotoxin in suppressing ET-1-mediated eNOS activation and demonstrated that the manipulation of caveolae produces significant effects on ET-1-mediated eNOS activity in LSECs.
Keywords: lipopolysaccharide
septic shock is a critical systemic inflammatory process caused by decreased tissue perfusion and oxygen delivery as a result of Gram-negative bacterial infection and sepsis. A major component of the outer membrane of Gram-negative bacteria is endotoxin (lipopolysaccharide, LPS). Endotoxemia causes hepatic microcirculatory disturbance, and this impaired hepatic microcirculation leads to areas of focal ischemia and subsequently causes hepatocellular damage (14). The major reason for this hepatic microcirculatory failure during endotoxemia is the imbalanced production of vasoactive substances (vasoconstrictors and vasodilators) from the parenchymal and nonparenchymal cells of the liver. This regional imbalance in the production of vasoactive factors causes heterogeneity of perfusion in the liver (23), leading to focal ischemia.
Sinusoidal perfusion is maintained by a multitude of vasoactive substances. Upstream resistance vessels in the splanchnic viscera control blood flow in the liver; in addition, regional regulation of the hepatic sinusoids by vasoactive factors further adjusts blood flow in the liver (6, 7, 9, 14). Two of the most potent vasoactive substances in the liver are endothelin-1 (ET-1) that primarily induces vasoconstriction and nitric oxide (NO) that causes vasodilatation in the hepatic sinusoids. The balance of the vasoactive effects between ET-1 and NO is crucial to maintain a normal hepatic microcirculation. In the liver, ET-1 is predominantly produced by liver sinusoidal endothelial cells (LSECs) (39). Although the net effect is vasoconstriction when ET-1 binds to endothelin-A (ETA) receptors on hepatic stellate cells or vascular smooth muscle cells, ET-1 binding to endothelin-B (ETB) receptors on LSECs causes vasodilatation by mediating endothelial nitric oxide synthase (eNOS) activation. LSECs represent an excellent model for ET-1/eNOS signaling because these cells express only ETB receptors (20).
Inflammatory and oxidative stress conditions sensitize the hepatic sinusoids to become hyperresponsive to the vasoconstrictive effects of ET-1 that ultimately lead to intrahepatic portal hypertension and liver injury (2, 4, 5, 8, 24, 37). The degree of dysregulation of the liver microcirculation is correlated with the severity of the stress conditions (25, 26). Recent studies demonstrate that the cause of the increased constrictor response is the disruption of the signaling pathways that couple between ETB receptor and eNOS. However, the molecular mechanism underlying this stress-induced eNOS dysfunction in the liver is not well understood.
One key signaling molecule that may mediate the stress-induced disruption of ET-1/eNOS signaling pathway is caveolin-1 (Cav-1). Cav-1 interacts eNOS to inhibit its activity ostensibly via inhibition of calmodulin binding. Using LPS as a stress model, we previously demonstrated that endotoxin inhibited ET-1-mediated eNOS activation by 1) increasing Cav-1 protein expression, 2) enhancing eNOS/Cav-1 association, 3) abolishing the ET-1-mediated eNOS translocation to the plasma membrane, 4) reducing the stimulatory phosphorylation of eNOS at Serine-1177 residue (eNOS-Ser1177), while 5) increasing the inhibitory phosphorylation of eNOS at Threonine-495 residue (eNOS-Thr495) in LSECs (22, 31). These observations suggested that Cav-1 mediates the effect of endotoxin in the suppression of ET-1-mediated eNOS activation. In this study, we investigated the molecular mechanism of Cav-1 in the regulation of ET-1/eNOS signaling in LSECs after LPS pretreatment by manipulation of the caveolae domains using a pharmacological approach with methyl-β-cyclodextrin (CD) and filipin.
MATERIALS AND METHODS
Materials.
ET-1 was obtained from American Peptide (Palo Alto, CA). CD and LPS (Escherichia coli O26:B6) were acquired from Sigma-Aldrich (St. Louis, MO). Filipin was purchased from Cayman Chemical (Ann Arbor, MI). Polyclonal antibodies for phosphorylated forms of eNOS-Ser1177 and eNOS-Thr495 were purchased from Cell Signaling Technology (Beverly, MA). Mouse eNOS antibody was acquired from BD Transduction Laboratories (San Diego, CA). Rabbit Cav-1 antibody was purchased from Sigma-Aldrich. Live/Dead viability assay was purchased from Molecular Probes (Eugene, OR). All other chemicals and regents used in the described experiments were purchased from Sigma-Aldrich, unless otherwise specified.
Animals.
Male Sprague-Dawley rats (Charles River Laboratories, Fayetteville, NC) weighing 250–450 g were housed in a temperature-controlled animal facility with alternating 12-h:12-h light/dark cycles and were fed standard laboratory chow with free access to water.
Hepatic sinusoidal endothelial cell isolation.
All studies were performed under a protocol approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Charlotte and adhere to the Guide for the Care and Use of Laboratory Animals (NIH publication 86-23, revised 1985). LSECs were isolated using a protocol as previously described (22). Each of the wells on a 12-well plate was precoated with type-VI collagen and was then loaded with 8 × 106 freshly isolated LSECs. After overnight incubation, LSECs (100% confluent) were either treated with culture media or with 100 ng/ml LPS dissolved in the culture media for 6 h.
Cell treatments.
The isolated LSECs were pretreated in either cell media or 100 ng/ml LPS for 5 h and then with either a vehicle, 5 mM CD, or 0.25 μg/ml filipin for 30 min. The cells were then treated with either a vehicle or 10 nM ET-1 for another 30 min.
Live/dead cell viability assay.
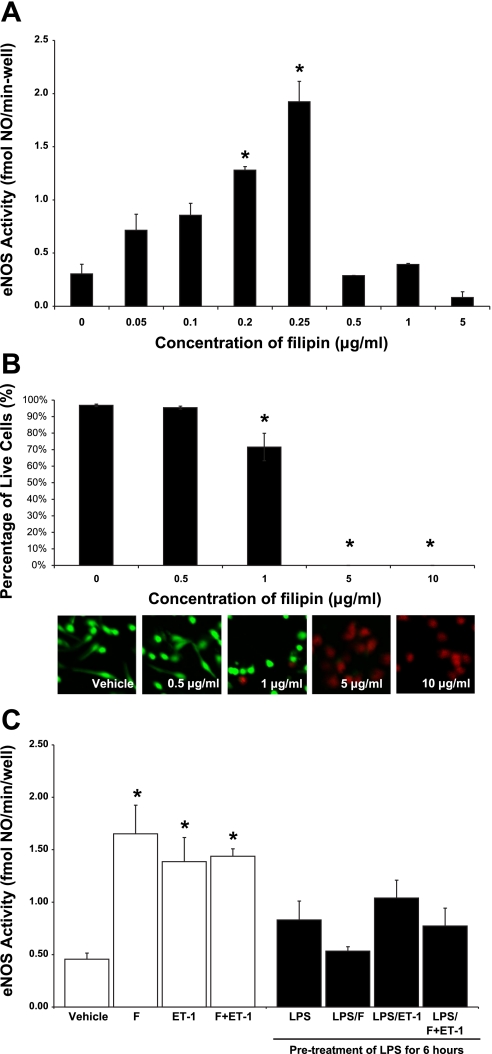
After the cell treatments, LSECs were washed twice with PBS and were then incubated with 2 μM calcein AM (CalAM) and 4 μM ethidium homodimer-1 (EthD-1). Cells treated with 70% methanol were used as a positive control for dead cells. After 30-min incubation, the cells were examined under a Fluoview confocal laser scanning microscope (Olympus), which allowed a concurrent view of live cells from CalAM at excitation/emission wavelength of 495 nm/515 nm (green) and dead cells from EthD-1 at 495 nm/635 nm (red). The percentage of viable cells was calculated after manually counting the number of live and dead cells.
Caspase assay.
Caspase-Glo 3/7 Assay was acquired from Promega (Madison, WI) for evaluating live cells that are undergoing apoptosis. Experimental procedure was designed according to the manufacturer's instructions. Briefly, a Caspase-Glo 3/7 substrate (Ac-DEVD-pNA) and a Caspase-Glo 3/7 buffer solution were mixed for the preparation of a Caspase-Glo 3/7 reagent. Following cell treatments, a cell lysate solution is collected. Equal volume (100 μl) of the Caspase-Glo 3/7 reagent was added to the cell lysate solution (total final volume of 200 μl) on a 96-well plate. The samples were incubated at room temperature for 1 h to allow a caspase reaction to occur. Following caspase cleavage of the Caspase-Glo 3/7 substrate, a luciferase substrate is released in this assay. The luminescence of each sample was measured at 485/527 nm in an uQuant microplate spectrophotometer with KCjunior software (Bio-Tek Instruments, Winooski, VT).
eNOS activity assay.
Rate of NO production was evaluated by eNOS activity assay as previously described (22). Briefly, 1 μCi/μl [3H]-l-arginine was added after the cell treatments. After 2 min, either a vehicle or 10 nM ET-1 was added for 30 min. The LSECs were then washed twice with arginine-free HEPES media on ice, and the reaction was terminated by ice-cold PBS. The cells were lysed, and cell lysates were then collected. Following a centrifugation at 10,000 g for 5 min, samples were run through glass columns filled with a cation exchange chromatography resin (Bio-Rad, Hercules, CA) that was previously equilibrated with a stop buffer. The flow through of each sample was collected in a glass vial, and a scintillation cocktail fluid (Fisher Scientific, Pittsburgh, PA) was then added. Radioactivity of each sample was quantified in a liquid scintillation counter (Beckman-Coulter, Fullerton, CA). Negative control cells were treated with an arginine-free HEPES media containing 10 μM NG-nitro-l-arginine methyl ester (l-NAME) and 10 mM EGTA, from which background radiation was evaluated. To convert radioactivity (disintegration/min; dpm) into the actual rate of NO production in each well, we designed this equation: eNOS activity = (x dpm/well) (1 Ci/2.2 × 1012 dpm) (1 mmol/53.4 Ci) (1 mol/1,000 mmol) (1015 fmol/1 mol) (1/30 min), where x is the measured radioactivity data in dpm.
Protein extraction and Western blots.
After the cell treatments, the LSECs (8 × 106 cells per sample) were rinsed twice with PBS and were lysed with a lysis master-mix. Cell lysates were collected and were centrifuged at 15,800 g for 10 min to remove insoluble fractions. Protein concentration of each sample was determined by Micro BCA protein assay kit (Pierce Biotechnology, Rockford, IL), and the volume of protein loading in Western blot was adjusted accordingly. Five micrograms of each protein sample were mixed with Laemmli loading buffer (Bio-Rad) and were then boiled for 7 min at 100°C. Standard Western blotting procedures were then followed as previously described (22).
Confocal fluorescence microscopy.
After the cell treatments, the LSECs (1 × 106 cells per well) were rinsed twice with PBS, fixed with 2% paraformaldehyde for 10 min, permeabilized with 0.1% Triton X-100 for 2 min, and were then blocked with 5% BSA (dissolved in PBS) for 60 min at room temperature. The LSECs were incubated overnight with rabbit Cav-1 (1:50) and mouse eNOS (1:50) primary antibodies in the 5% BSA at 4°C. The cells were then incubated in goat anti-rabbit Texas red-conjugated (1:500) and goat anti-mouse FITC-conjugated (1:250) secondary antibodies at 37°C for 1 h. Cell nuclei were stained with 2.5 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature. Confocal images were acquired with the Fluoview confocal laser scanning microscope (Olympus).
Visualization of NO with DAF-2 DA.
After the pretreatments with LPS, CD, or filipin, 5 μM 4,5-diaminofluorescein diacetate (DAF-2 DA) was added. The LSECs were then treated with either a vehicle or 10 nM ET-1 for 30 min. DAF-2 DA penetrates cell membrane and is hydrolyzed by intracellular esterase to membrane impermeable 4,5-diaminofluorescein (DAF-2), which then reacts with NO to form a fluorescent triazolofluorescein (DAF-2T). After the incubation, the cells were rinsed twice with PBS and fixed with 2% paraformaldehyde for 10 min. Cell nuclei were stained with 2.5 μg/ml DAPI for 5 min at room temperature. Confocal images were acquired at excitation/emission wavelengths of 495/515 nm with the Fluoview confocal laser scanning microscope (Olympus).
Statistical analysis.
Data are presented as means ± SE. Statistical differences between the media control and treatment groups were determined using one-way ANOVA followed by post hoc Dunnett's test. Statistical significance was set at P < 0.05. All statistical significances were calculated with Sigma Stat (version 2.03; SPSS, Chicago, IL).
RESULTS
Effect of CD on eNOS activity.
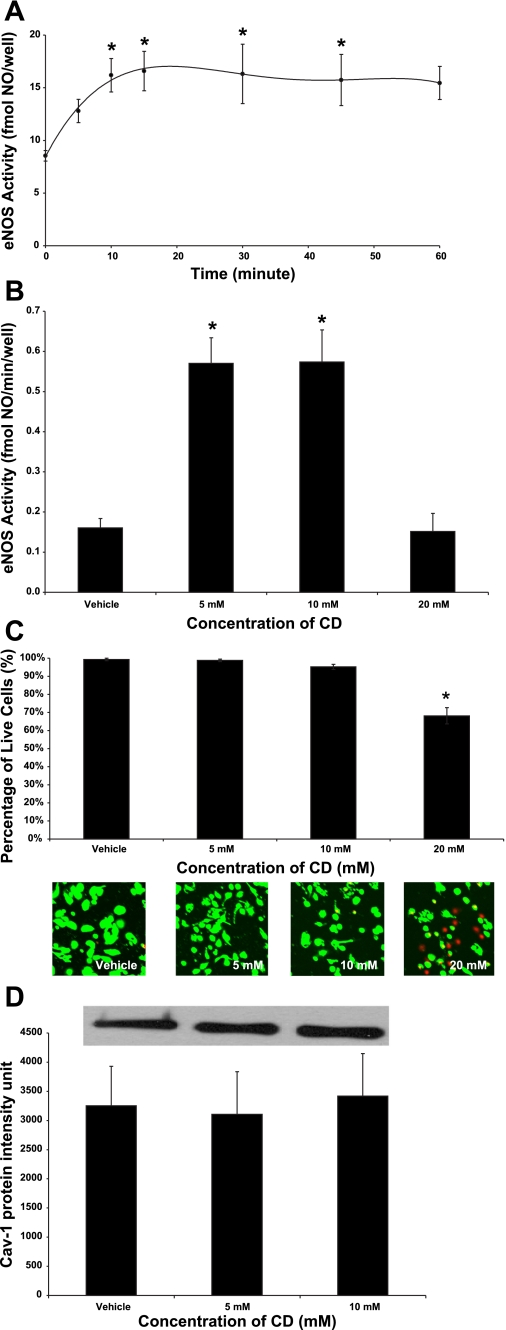
To determine whether disruption of caveolae minimizes Cav-1 inhibition of eNOS activity, we treated primary LSECs isolated from rats with CD, a cholesterol-binding agent that disrupts caveolae. In control media, LSECs produced 0.16 ± 0.05 fmol of NO per minute, per well (fmol·min−1·well−1). Dose response and time course experiments showed that the treatment of LSECs with 5 mM CD for 30 min produced an optimal increase in basal eNOS activity to 0.57 ± 0.13 fmol·min−1·well−1 (Fig. 1, A and B). Treatment with 10 mM CD for 30 min produced a similar response (Fig. 1B); however, eNOS activity was unchanged after incubating with 20 mM CD (Fig. 1B), which appeared to be the consequence of cytotoxicity (Fig. 1C). Cell viability studies showed that more than 95% of LSECs were viable after incubating in control media, 5 mM CD, or 10 mM CD; however, less than 70% of the cells remained viable after treating with 20 mM CD for 30 min (Fig. 1C). The observed increase in basal eNOS activity after caveolae disruption was not the result of Cav-1 degradation because treatment with 5 mM or 10 mM CD for 30 min did not alter Cav-1 protein expressions in LSECs (Fig. 1D).
Fig. 1.
Basal endothelial nitric oxide synthase (eNOS) activity and caveolin-1 (Cav-1) expression after cyclodextrin (CD) treatments. A: time course experiment showing measurement of eNOS activity after treating liver sinusoidal endothelial cells (LSECs) with 5 mM CD for 0, 5, 10, 15, 30, 45, and 60 min. *P < 0.05 vs. 0 min (n = 5). Following treatment of LSECs with vehicle, 5 mM CD, 10 mM CD, and 20 mM CD for 30 min, eNOS activity (B), cell viability (C), and Cav-1 protein (D) expression were evaluated. *P < 0.05 vs. vehicle (n = 5). Statistical significance was evaluated by one-way ANOVA with post hoc Dunnett's test to compare with the 0-min treatment or the vehicle.
Effects of CD on ET-1-mediated eNOS activity and NO production.
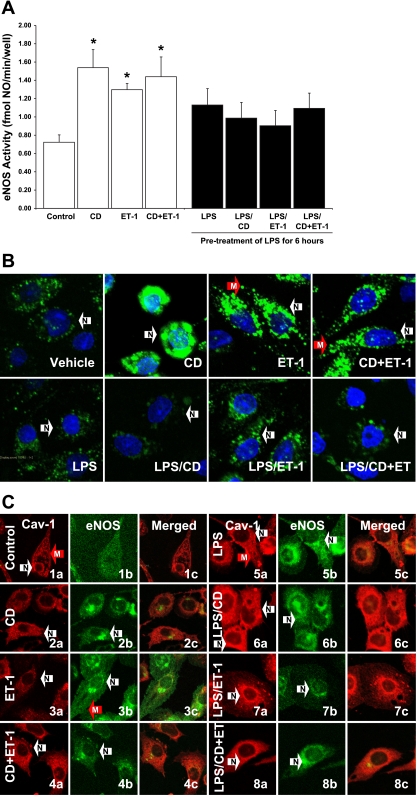
ET-1 induces eNOS activation, whereas endotoxin inhibits this ET-1-mediated eNOS response by increasing Cav-1 protein levels and by stimulating Cav-1 and eNOS interaction (22). We hypothesized that CD disrupts the caveolae and interferes with the interaction between Cav-1 and eNOS, which may affect the LPS suppression of ET-1-mediated eNOS activity. Results showed that treatment with CD or ET-1 alone for 30 min increased eNOS activity; furthermore, treatment with both CD and ET-1 (CD + ET-1) produced a response similar to either of these treatments alone (Fig. 2A). In the presence of LPS, neither CD nor ET-1 treatment induced eNOS activity (Fig. 2A). The combined treatment with both CD and ET-1 also failed to stimulate eNOS activity after the LPS pretreatment (Fig. 2A). Examination of NO production using the DAF-2 DA fluorescence technique confirmed the eNOS activity data (Fig. 2B). Treatment with CD induced NO production in the perinuclear region (Fig. 2B, arrow N). The addition of ET-1 stimulated NO production in the plasma membrane (Fig. 2B, arrow M). LPS pretreatment inhibited NO production in LSECs, even with the addition of CD and ET-1 (Fig. 2B).
Fig. 2.
Effect of CD on endothelial-1 (ET-1)-mediated eNOS activity and NO production. LSECs were pretreated with either a vehicle or 100 ng/ml LPS for 6 h. The cells were then treated with 5 mM CD and/or 10 nM ET-1 for 30 min. After the cell treatments, eNOS activity was measured (A). *P < 0.05 vs. vehicle (n = 5). Statistical significance was evaluated by one-way ANOVA with post hoc Dunnett's test to compare with the vehicle control. NO production (B) and the subcellular localizations (C) of Cav-1 (Texas-red) (a) and eNOS (FITC) (b) were determined by confocal microscopy after treating the LSECs with vehicle (1), 5 mM CD (2), 10 nM ET-1 (3), both CD and ET-1 (4), 100 ng/ml LPS (5), LPS and CD (6), LPS and ET-1 (7), and both CD and ET-1 (8) following LPS pretreatment. The images were merged (c) to evaluate the molecular interaction between Cav-1 and eNOS. Arrows indicate the perinuclear region (N, white arrows) and the plasma membrane (M, red arrows). The nuclei of the cells in (B) were stained with DAPI (n = 5).
Effect of CD and LPS on the subcellular localization and interaction of Cav-1 and eNOS.
To examine whether caveolae disruption increased eNOS activity by interfering with the Cav-1 and eNOS interactions in LSECs, we evaluated the subcellular localization of Cav-1 and eNOS after the cell treatments. In control media, Cav-1 was localized on the plasma membrane (arrow M) and in the perinuclear region (arrow N) while eNOS was dispersedly distributed throughout the cytoplasm (Fig. 2C, 1). Treatment with CD stimulated the translocation of both Cav-1 and eNOS to the perinuclear region (Fig. 2C, 2, arrows N). ET-1 treatment had no effect on Cav-1 subcellular localization; however, it induced eNOS translocation to the plasma membrane (Fig. 2C, 3). The combined treatment with CD and ET-1 (CD + ET-1) also induced the perinuclear colocalization of Cav-1 and eNOS (Fig. 2C, 4).
LPS increased (qualitatively) the expression of Cav-1 (Fig. 2C, 5). eNOS primarily showed perinuclear localizations following the endotoxin treatment (Fig. 2C, 5). Together with the effect of LPS on Cav-1 overexpression, CD induced an overwhelming amount (qualitatively) of Cav-1 in the perinuclear region (Fig. 2C, 6). CD remained effective in stimulating the subcellular localization of eNOS following the LPS pretreatment (Fig. 2C, 6). ET-1 treatment had no effect on Cav-1 localization and failed to induce eNOS translocation to the plasma membrane in the presence of LPS (Fig. 2C, 7). Following the LPS pretreatment, CD + ET-1 also failed to induce eNOS translocation to the plasma membrane after LPS pretreatment; however, the treatment was effective in stimulating the localization of a large amount of Cav-1 in the perinuclear region (Fig. 2C, 8).
Effect of caveolae disruption on eNOS phosphorylation.
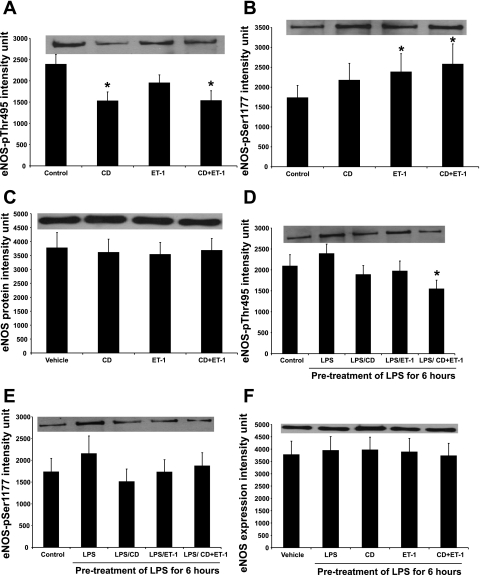
Activation state of eNOS is regulated and determined by the phosphorylation sites of eNOS. For instance, ET-1 induces eNOS activation by stimulating eNOS-Ser1177 phosphorylation (29); on the other hand, phosphorylation of eNOS-Thr495 residue is inhibitory to eNOS activity (17). To investigate the molecular mechanism of CD-induced and LPS-suppressed eNOS activation in primary LSECs, we evaluated eNOS phosphorylation after caveolae disruption with CD. Treatment with CD increased eNOS-Thr495 dephosphorylation (Fig. 3A) but had no effect on eNOS-Ser1177 phosphorylation (Fig. 3B). ET-1 stimulated eNOS-Ser1177 phosphorylation (Fig. 3B) but did not exert any effect on eNOS-Thr495 residue (Fig. 3B). CD + ET-1 increased eNOS-Thr495 dephosphorylation and eNOS-Ser1177 phosphorylation (Fig. 3, A and B). Treatment with CD, ET-1, or both had no effect on the expressions of β-actin (data not shown) and eNOS (Fig. 3C). These data suggested that caveolae disruption and ET-1 increased eNOS activity through two distinct mechanisms, CD by eNOS deinhibition through a reduction in eNOS-Thr495 phosphorylation and ET-1 by direct eNOS activation through an induction of eNOS-Ser1177 phosphorylation.
Fig. 3.
Effect of caveolae disruption and LPS on eNOS-Thr495 and eNOS-Ser1177 phosphorylations. Following the treatment with 5 mM CD and/or 10 nM ET-1 for 30 min, we collected the cell lysates from LSECs and evaluated eNOS-Thr495 phosphorylation (A), eNOS-Ser1177 phosphorylation (B), and eNOS protein expression using Western blotting (C). Following the LPS pretreatment, the LSECs were treated with 5 mM CD and/or 10 nM ET-1 for 30 min, and then eNOS-Thr495 phosphorylation (D), eNOS-Ser1177 phosphorylation (E), and eNOS protein expression (F) were evaluated. Statistical significance was evaluated by one-way repeated-measures ANOVA with Dunnett's test. *P < 0.05 vs. vehicle (n = 5).
LPS inhibits ET-1-mediated eNOS activation by inhibiting eNOS-Ser1177 phosphorylation and stimulating eNOS-Thr495 phosphorylation (22). To better understand why CD failed to reverse the inhibitory effect of LPS on ET-1-mediated eNOS activation, we also evaluated the phosphorylation of eNOS after the cell treatments. Treatment with CD or ET-1 alone following the LPS pretreatment had no effect on eNOS-Thr495 and eNOS-Ser1177 phosphorylations (Fig. 3, D and E). In contrast, the combined treatment with CD and ET-1 following the LPS pretreatment significantly reduced eNOS-Thr495 phosphorylation (Fig. 3D); however, this treatment failed to induce eNOS-Ser1177 phosphorylation (Fig. 3E). These treatments also produced no significant changes on the expressions of β-actin (data not shown) and eNOS (Fig. 3F). These data suggested that caveolae disruption stimulated eNOS deinhibition through a decrease in eNOS-Thr495 phosphorylation; however, the combined treatment with CD and ET-1 failed to rescue eNOS activity since ET-1 was ineffective in inducing eNOS-Ser1177 phosphorylation following the LPS pretreatment.
Effect of cholesterol readdition in saturating CD.
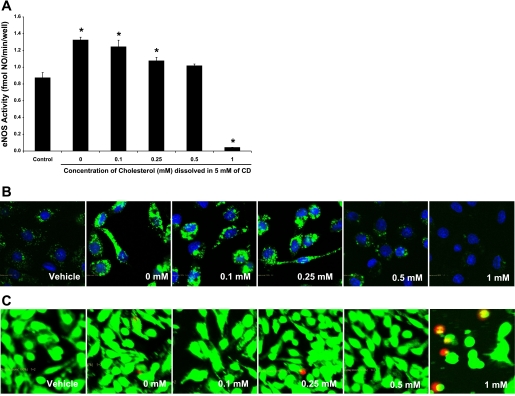
CD specifically binds cholesterol to disrupt the caveolae and the Cav-1/eNOS interaction to increase eNOS activity. If the reduction in caveolae-associated cholesterol was indeed responsible for the decrease in Cav-1 and eNOS molecular interactions and for the increase in eNOS activity, then readding cholesterol should maintain caveolae integrity and restore the Cav-1 inhibition of eNOS. To test this hypothesis, we added cholesterol to the 5 mM CD solution (Chol-CD) and measured eNOS activity. Adding 0.1 mM or 0.25 mM cholesterol to the CD solution was ineffective in abolishing the CD-induced eNOS activity; however, the reduction became significant when 0.5 mM cholesterol was added to the CD solution (Fig. 4A). Treatment with 1 mM Chol-CD decreased cell viability (Fig. 4C), which explained the dramatic decrease in eNOS activity (Fig. 4A). Evaluation of NO production using a DAF-2 DA fluorescence technique was consistent with these results (Fig. 4B).
Fig. 4.
eNOS activity and NO production after treating cells with cholesterol-saturated CD. LSECs were treated with vehicle, 0 mM, 0.1 mM, 0.25 mM, 0.5 mM, or 1 mM cholesterol, dissolved in 5 mM CD for 30 min. eNOS activity (A), NO production (B), and cell viability (C) were evaluated (n = 5).
Effect of filipin on eNOS activity.
Apart from using CD as a cholesterol sequester, we also investigated whether the same affect could be obtained with an alternative pharmacologic agent, filipin. This set of experiments is significant because CD may be nonspecific. Dose response experiment showed that the treatment of LSECs with 0.2 μg/ml filipin for 30 min produced an optimal increase in basal eNOS activity from 0.30 ± 0.09 fmol·min−1·well−1 in the control to 1.28 ± 0.03 fmol·min−1·well−1 (Fig. 5A). Treatment with 0.25 μg/ml filipin for 30 min produced a similar effect (Fig. 5A). However, eNOS activity was unaffected after treating the cells with 0.5, 1, and 5 μg/ml filipin (Fig. 5A), which appeared to be the result of an increasing level of cytotoxicity (Fig. 5B). Although 0.5 μg/ml filipin did not decrease LSEC viability in Fig. 5B, a caspase assay showed that the dosage induced Caspase 3/7 activity indicating the LSECs were undergoing apoptosis (data not shown). At 5 and 10 μg/ml filipin, none of the cells was viable (Fig. 5B).
Fig. 5.
eNOS activity after filipin treatments. Following treatment of LSECs with vehicle, 0.05, 0.1, 0.2, 0.25, 0.5, 1, and 5 μg/ml filipin for 30 min, basal eNOS activity (A) was measured. *P < 0.05 vs. vehicle (n = 3). B: to determine whether the failure of eNOS stimulation after the treatment with 0.5, 1, and 5 μg/ml filipin was attributable to cytotoxicity, we evaluated cell viability after treating the cells at these concentrations for 30 min. *P < 0.05 vs. vehicle (n = 5). C: effect of filipin (F) on ET-1-mediated eNOS activity. *P < 0.05 vs. vehicle (n = 5). Statistical significance was evaluated by one-way ANOVA with post hoc Dunnett's test to compare with the vehicle.
Effects of filipin on ET-1-mediated eNOS activity.
To investigate whether filipin can reverse the LPS inhibition of ET-1-mediated eNOS activity, we pretreated either the cell media or LSECs with 100 ng/ml for 6 h and then with 0.2 μg/ml filipin for 30 min, before the treatment of either a vehicle or 10 nM ET-1 for 30 min. Results showed that treatment with filipin or ET-1 alone for 30 min increased eNOS activity (Fig. 5C). In addition, the combined treatment with both filipin and ET-1 (F + ET-1) produced a similar effect (Fig. 5C). Following LPS pretreatment, neither the treatment of filipin nor ET-1 induced eNOS activity (Fig. 5C). Also, the combined treatment with both filipin and ET-1 failed to stimulate eNOS activity after LPS pretreatment (Fig. 5C). These results were consistent with the observations in LSECs treated with CD and suggested that the reported effects on eNOS activation were specific following the disruption of the caveolae with these pharmacological reagents.
DISCUSSION
In this study, we hypothesized that Cav-1 mediates the molecular action of LPS to disrupt the ET-1/eNOS signaling in LSECs. In the liver, nonparenchymal cells express higher level of Cav-1 proteins than hepatocytes, and, among these cells, LSECs have the highest level of Cav-1 expression (41). Cav-1 binds to many signaling molecules including G protein α-subunits, G protein-coupled receptor kinase, H-Ras, and PI3K and modulates their intracellular activities (27). In addition, Cav-1 binds to and inhibits eNOS activity (21). Using endotoxin as a stress model, we previously demonstrated that LPS increases Cav-1 protein expression and facilitates the interaction between eNOS and Cav-1 (22). Sessa et al. (12) also used a Cav-1 peptide transfection model and showed that the overexpression of Cav-1 attenuated ACh-mediated NO production (12).
To test our hypothesis, we disrupted the components of the caveolae using a pharmacological approach with both CD and filipin. The use of CD and filipin to manipulate cholesterol-containing domains is accepted in a wide range of fields. For instance, CD is effective in removing low-density lipoprotein-derived cholesterol from the plasma membrane and prevents the internalization of cholesterol to the endoplasmic reticulum (ER) for esterification (33). CD extracts more than 50% of the membrane cholesterol (38), and the extracted cholesterol leaves the cells, as demonstrated by a [3H]-cholesterol technique that measures cholesterol efflux (32).
A number of studies have addressed the effect of caveolae disruption on eNOS activity, but the results varied and were somewhat contradictory in different cell types. For instance, CD increases NO production in bovine aortic endothelial cells (BAECs) (3), human umbilical vein endothelial cells (HUVECs) (34), and cardiac myocytes (36), but it lowers NO production in cerebral blood vessels (42), islet β-cells (43), rat lung microvascular endothelial cells (30), and bovine pulmonary artery endothelial cells (44). In addition, the effects of caveolae disruption on a number of agonist-mediated eNOS activations were reported, but the results were dependent on the agonists used in those experiments. For example, CD reduces the ET-1-mediated contractile response in endothelium-denuded caudal artery (10). In contrast, although CD impairs ACh-induced relaxation in BAECs, it neither affects the contractile response to phenylephrine, nor the relaxant response to calcium ionophore A23187 or NO donor sodium nitroprusside in these cells (16). In this study, we showed that the caveolae disruption with either CD or filipin specifically induces basal eNOS activity without affecting Cav-1 protein expression, and the ET-1-mediated eNOS activation remained effective after CD treatment in LSECs. These results suggest the complicated role of caveolae in the regulation of eNOS activity. eNOS is inactive when it associates with Cav-1 in the caveolae (21); in contrast, binding of eNOS to calcium/calmodulin (40), Hsp90 (19), and dynamin-2 (13) in the caveolae increases eNOS activity.
Our confocal images of LSECs are consistent with previous findings that cholesterol depletion causes the redistribution of Cav-1 and eNOS to the perinuclear region. Cav-1 is expressed on the plasma membrane and in the trans-Golgi in untreated LSECs (35). When porcine pulmonary artery endothelial cells are treated with CD, Cav-1 and eNOS translocate from a subcellular caveolae membrane fraction to the intracellular membrane fraction (11); however, the exact locations of Cav-1 and eNOS were unknown in this study because the isolated intracellular membrane fraction contains ER, Golgi, mitochondria, and many other intracellular organelles (11). Treatment with CD also induced the disappearance of Cav-1 and eNOS from the plasma membrane and their redistribution to the perinuclear region in HUVECs (34).
Examination of eNOS localization represents an invaluable tool in determining the molecular mechanisms that activate eNOS. Specifically, the binding of eNOS to calcium/calmodulin, Hsp90, and dynamin-2 is predominant in the caveolae; in contrast, Akt-mediated eNOS activation is most pronounced at the cis-Golgi (18). The confocal images of Cav-1/eNOS as well as the DAF-2 DA images in this report suggest that CD and ET-1 stimulated eNOS activity via distinct mechanisms. On the one hand, CD-mediated redistribution of eNOS to Golgi indicates that the CD-induced eNOS activation is mediated through the molecular action of Akt. On the other hand, ET-1 stimulated the translocation of eNOS to the plasma membrane, suggesting that ET-1 increased eNOS activity by inducing eNOS binding to calcium/calmodulin and Hsp90. In addition, although CD results in movement of both Cav-1 and eNOS to the Golgi, it should be specified that CD stimulates the redistribution of Cav-1 to the trans-Golgi and eNOS to the cis-Golgi; therefore, the probability of Cav-1/eNOS interaction is minimized.
The molecular mechanisms and effects of eNOS phosphorylation are complex. Phosphorylation of eNOS-Thr495 negatively regulates eNOS activity, and phosphorylation of eNOS-Ser1177 residue has the opposite effect. The phosphorylation of eNOS-Ser1177 in LSECs is mediated through the molecular action of Akt (29). However, the exact molecular mechanism of eNOS-Thr495 dephosphorylation in LSECs is yet to be determined. It is known that three classes of serine/threonine protein phosphatases (PP1, PP2A, PP2B/calcineurin) are involved in the regulation of eNOS dephosphorylation. However, because only PP1 and PP2B/calcineurin have been shown to mediate the dephosphorylation of eNOS-Thr495 (17) and only PP1 and PP2A were found to interact with the Cav-1 scaffolding domain (28), we speculated that PP1 mediated the molecular effect of CD in the dephosphorylation of eNOS-Thr495 residue.
Only a very limited number of studies have examined the effects of caveolae disruption on endotoxin-induced signaling. In particular, Uekama et al. (1) investigated the potential use of CD and its derivatives in inhibiting LPS-induced signaling in macrophages, and Cuschieri et al. (22) examined the effect of CD on LPS-modulated MAPK activation in human promonocytic THP-1 cells. Consistent with our previous results of Cav-1 overexpression (22), our confocal images showed qualitatively that LPS increased the fluorescence intensity of Cav-1, and the combined CD/ET-1 treatment induced an excessive amount of Cav-1 to the perinuclear region relative to eNOS after addition of endotoxin. The increased Cav-1/eNOS colocalization in Golgi might partially explain why the CD/ET-1 combined treatment was insufficient to reverse the LPS suppression on NO production. Furthermore, the fact that the CD/ET-1 combined treatment did not completely restore LPS-attenuated eNOS activity suggests that additional signaling mechanisms may be involved in the regulation of endotoxin inhibition on ET-1-mediated eNOS activation.
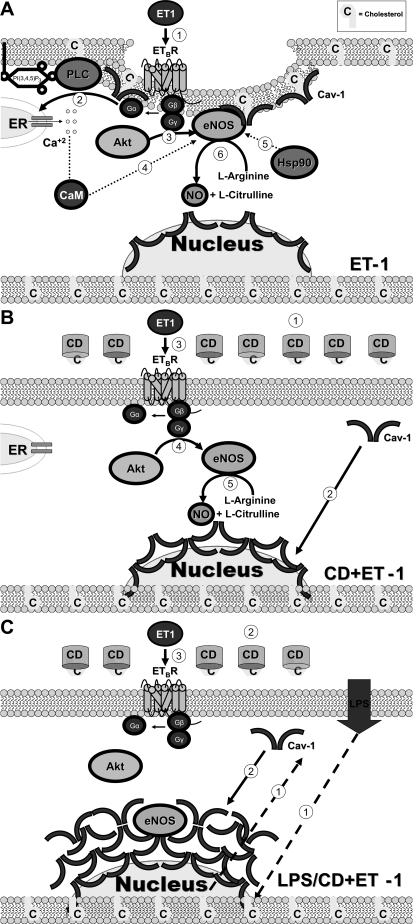
In this study, we hypothesized that Cav-1 mediates the molecular action of LPS in disrupting the ET-1-mediated eNOS activation in LSECs. We tested this hypothesis using CD to manipulate the components of caveolae, and we found that the combined treatment with CD/ET-1 induced the redistribution of Cav-1 and eNOS to the perinuclear region and stimulated eNOS-Ser1177 phosphorylation as well as eNOS-Thr495 dephosphorylation to increase eNOS activity. We also demonstrated that endotoxin induced Cav-1 expression, and the combined treatment with CD/ET-1 failed to reverse the LPS suppression of eNOS activity. This study suggests, for the first time, that CD and ET-1 activate NO production through distinct molecular mechanisms (Fig. 6). Moreover, Cav-1 appears to be functionally important in mediating suppression of ET-1-induced eNOS activation in endotoxemia.
Fig. 6.
Schematic representation of CD and ET-1-induced eNOS activity in LSECs. A: ET-1 induces eNOS translocation to the plasma membrane and stimulates heterotrimeric G protein dissociation; subsequently, the G protein βγ-subunit activates Akt while G protein α-subunit stimulates phospholipase C (PLC) and inositol trisphosphate to induce Ca+2 release from the endoplasmic reticulum (ER). Ca+2 flux induces CaM binding to eNOS, and this Ca+2/calmodulin binding displaces eNOS from Cav-1. Hsp90 further increases eNOS activity by facilitating the Ca+2/CaM-induced displacement of Cav-1 from eNOS. ETBR, endothelin-B receptor. B: CD extracts cholesterol from the plasma membrane and disrupts the caveolae, which leads to the translocation of Cav-1 to the perinuclear region. ET-1 stimulates heterotrimeric G protein dissociation. In the perinuclear region, Akt-mediated eNOS activation predominates. C: LPS induces Cav-1 overexpression. CD stimulates Cav-1 translocation to the perinuclear region. The ET-1-induced Akt fails to stimulate eNOS activity attributable to the increasing amount of Cav-1 binding to eNOS in the perinuclear region.
GRANTS
Current research and reported research has been made possible by the support of National Institute of Health (R01 DK38201-19). Kwok W. received fellowship support from the National Science Foundation.
ACKNOWLEDGMENTS
The authors recognize the superior technical assistance and supports of Dr. Didier Dréau and Mr. David L. Gray from the University of North Carolina at Charlotte.
REFERENCES
- 1.Arima H, Motoyama K, Matsukawa A, Nishimoto Y, Hirayama F, Uekama K. Inhibitory effects of dimethylacetyl-beta-cyclodextrin on lipopolysaccharide-induced macrophage activation and endotoxin shock in mice. Biochem Pharmacol 70: 1506–1517, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Ashburn JH, Baveja R, Kresge N, Korneszczuk K, Keller S, Karaa A, Yokoyama Y, Zhang JX, Huynh T, Clemens MG. Remote trauma sensitizes hepatic microcirculation to endothelin via caveolin inhibition of eNOS activity. Shock 22: 120–130, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, de la Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Invest 115: 969–977, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer I, Bauer M, Pannen BH, Leinwand MJ, Zhang JX, Clemens MG. Chronic ethanol consumption exacerbates liver injury following hemorrhagic shock: role of sinusoidal perfusion failure. Shock 4: 324–331, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Bauer M, Paquette NC, Zhang JX, Bauer I, Pannen BH, Kleeberger SR, Clemens MG. Chronic ethanol consumption increases hepatic sinusoidal contractile response to endothelin-1 in the rat. Hepatology 22: 1565–1576, 1995 [PubMed] [Google Scholar]
- 6.Bauer M, Zhang JX, Bauer I, Clemens MG. Endothelin-1 as a regulator of hepatic microcirculation: sublobular distribution of effects and impact on hepatocellular secretory function. Shock 1: 457–465, 1994 [PubMed] [Google Scholar]
- 7.Bauer M, Zhang JX, Bauer I, Clemens MG. ET-1 induced alterations of hepatic microcirculation: sinusoidal and extrasinusoidal sites of action. Am J Physiol Gastrointest Liver Physiol 267: G143–G149, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Baveja R, Kresge N, Ashburn JH, Keller S, Yokoyama Y, Sonin N, Zhang JX, Huynh T, Clemens MG. Potentiated hepatic microcirculatory response to endothelin-1 during polymicrobial sepsis. Shock 18: 415–422, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Baveja R, Zhang JX, Clemens MG. In vivo assessment of endothelin-induced heterogeneity of hepatic tissue perfusion. Shock 15: 186–192, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res 93: 839–847, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem 274: 32512–32519, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6: 1362–1367, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Cao S, Yao J, Shah V. The proline-rich domain of dynamin-2 is responsible for dynamin-dependent in vitro potentiation of endothelial nitric-oxide synthase activity via selective effects on reductase domain function. J Biol Chem 278: 5894–5901, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Chun K, Zhang J, Biewer J, Ferguson D, Clemens MG. Microcirculatory failure determines lethal hepatocyte injury in ischemic/reperfused rat livers. Shock 1: 3–9, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Cuschieri J. Implications of lipid raft disintegration: enhanced anti-inflammatory macrophage phenotype. Surgery 136: 169–175, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Darblade B, Caillaud D, Poirot M, Fouque M, Thiers JC, Rami J, Bayard F, Arnal JF. Alteration of plasmalemmal caveolae mimics endothelial dysfunction observed in atheromatous rabbit aorta. Cardiovasc Res 50: 566–576, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Fulton D, Babbitt R, Zoellner S, Fontana J, Acevedo L, McCabe TJ, Iwakiri Y, Sessa WC. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J Biol Chem 279: 30349–30357, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392: 821–824, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Housset CN, Rockey DC, Friedman SL, Bissell DM. Hepatic lipocytes: a major target for endothelin-1. J Hepatol 22: 55–60, 1995 [PubMed] [Google Scholar]
- 21.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 272: 18522–18525, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Kamoun WS, Karaa A, Kresge N, Merkel SM, Korneszczuk K, Clemens MG. LPS inhibits endothelin-1-induced endothelial NOS activation in hepatic sinusoidal cells through a negative feedback involving caveolin-1. Hepatology 43: 182–190, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kamoun WS, Shin MC, Keller S, Karaa A, Huynh T, Clemens MG. Induction of biphasic changes in perfusion heterogeneity of rat liver after sequential stress in vivo. Shock 24: 324–331, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Karaa A, Kamoun WS, Clemens MG. Oxidative stress disrupts nitric oxide synthase activation in liver endothelial cells. Free Radic Biol Med 39: 1320–1331, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Keller SA, Paxian M, Ashburn JH, Clemens MG, Huynh T. Kupffer cell ablation improves hepatic microcirculation after trauma and sepsis. J Trauma 58: 740–749; discussion 749–751, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Keller SA, Paxian M, Lee SM, Clemens MG, Huynh T. Kupffer cell ablation attenuates cyclooxygenase-2 expression after trauma and sepsis. J Surg Res 124: 126–133, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Krajewska WM, Maslowska I. Caveolins: structure and function in signal transduction. Cell Mol Biol Lett 9: 195–220, 2004 [PubMed] [Google Scholar]
- 28.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol 23: 9389–9404, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Premont RT, Kontos CD, Huang J, Rockey DC. Endothelin-1 activates endothelial cell nitric-oxide synthase via heterotrimeric G-protein betagamma subunit signaling to protein kinase B/Akt. J Biol Chem 278: 49929–49935, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Maniatis NA, Brovkovych V, Allen SE, John TA, Shajahan AN, Tiruppathi C, Vogel SM, Skidgel RA, Malik AB, Minshall RD. Novel mechanism of endothelial nitric oxide synthase activation mediated by caveolae internalization in endothelial cells. Circ Res 99: 870–877, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Merkel SM, Kamoun W, Karaa A, Korneszczuk K, Schrum LW, Clemens MG. LPS inhibits endothelin-1-mediated eNOS translocation to the cell membrane in sinusoidal endothelial cells. Microcirculation 12: 433–442, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Motoyama K, Arima H, Nishimoto Y, Miyake K, Hirayama F, Uekama K. Involvement of CD14 in the inhibitory effects of dimethyl-alpha-cyclodextrin on lipopolysaccharide signaling in macrophages. FEBS Lett 579: 1707–1714, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem 271: 21604–21613, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Nuszkowski A, Grabner R, Marsche G, Unbehaun A, Malle E, Heller R. Hypochlorite-modified low density lipoprotein inhibits nitric oxide synthesis in endothelial cells via an intracellular dislocalization of endothelial nitric-oxide synthase. J Biol Chem 276: 14212–14221, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Ogi M, Yokomori H, Oda M, Yoshimura K, Nomura M, Ohshima S, Akita M, Toda K, Ishii H. Distribution and localization of caveolin-1 in sinusoidal cells in rat liver. Med Electron Microsc 36: 33–40, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Ostrom RS, Bundey RA, Insel PA. Nitric oxide inhibition of adenylyl cyclase type 6 activity is dependent upon lipid rafts and caveolin signaling complexes. J Biol Chem 279: 19846–19853, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Pannen BH, Bauer M, Zhang JX, Robotham JL, Clemens MG. Endotoxin pretreatment enhances portal venous contractile response to endothelin-1. Am J Physiol Heart Circ Physiol 270: H7–H15, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Parpal S, Karlsson M, Thorn H, Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J Biol Chem 276: 9670–9678, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Rieder H, Ramadori G, Meyer zum Buschenfelde KH. Sinusoidal endothelial liver cells in vitro release endothelin—augmentation by transforming growth factor beta and Kupffer cell-conditioned media. Klin Wochenschr 69: 387–391, 1991 [DOI] [PubMed] [Google Scholar]
- 40.Sessa W. Regulation of endothelial derived nitric oxide in health and disease. Mem Inst Oswaldo Cruz 100, Suppl 1: 15–18, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Shah V, Cao S, Hendrickson H, Yao J, Katusic ZS. Regulation of hepatic eNOS by caveolin and calmodulin after bile duct ligation in rats. Am J Physiol Gastrointest Liver Physiol 280: G1209–G1216, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Stirone C, Boroujerdi A, Duckles SP, Krause DN. Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Mol Pharmacol 67: 105–113, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Veluthakal R, Chvyrkova I, Tannous M, McDonald P, Amin R, Hadden T, Thurmond DC, Quon MJ, Kowluru A. Essential role for membrane lipid rafts in interleukin-1beta-induced nitric oxide release from insulin-secreting cells: potential regulation by caveolin-1+. Diabetes 54: 2576–2585, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Wei Z, Al-Mehdi AB, Fisher AB. Signaling pathway for nitric oxide generation with simulated ischemia in flow-adapted endothelial cells. Am J Physiol Heart Circ Physiol 281: H2226–H2232, 2001 [DOI] [PubMed] [Google Scholar]