Abstract
Roux-en-Y gastric bypass (RYGB) has become the gold-standard bariatric procedure, partly because of the rapid resolution of accompanying diabetes. There is increasing evidence this is mediated by duodenal exclusion. We hypothesize that duodenal exclusion suppresses intestinal Na+/glucose cotransporter SGLT1-mediated glucose transport, improving glucose handling, and aimed to test this in a rodent RYGB model. Sprague-Dawley rats underwent sham procedure or duodenal exclusion by RYGB (10 cm Roux, 16 cm biliopancreatic limbs). Animals were maintained for 3 wk on a Western diet, before harvest at 10 AM, 4 PM, and 10 PM. Sections were taken from each limb for hematoxylin and eosin staining, and morphological assessment was performed. Functional glucose uptake studies, along with Western blotting and quantitative PCR, were performed on Roux limb. Histology showed morphometric changes in Roux and common limbs, with increase in villus height and crypt depth compared with BP and sham jejunum. Despite this, glucose transport was reduced by up to 68% (P < 0.001) in the Roux limb compared with sham jejunum. Normal diurnal rhythms in glucose uptake were ablated. This occurred at a posttranscriptional level, with little change in message but appearance of different weight species of Sglt1 on Western blotting. We have shown duodenal exclusion significantly influences both intestinal structure and glucose transport function, with glucose absorptive capacity reduced after RYGB. This provides a novel mechanistic explanation for some of the antidiabetic effects of RYGB.
Keywords: bariatric surgery, diabetes, sglt1
obesity, together with associated Type 2 diabetes mellitus (T2DM), has become a surgical disease (29). Bariatric surgery now represents the first-line treatment for morbid obesity, leading to resolution of obesity-related comorbidities such hypertension, sleep apnea, and T2DM and consequently improved life expectancy (1, 9, 35).
Roux-en-Y gastric bypass (RYGB) is regarded as the gold-standard therapy for weight loss, with 60–70% excess weight loss at two years (7). An important observation is the rapid associated resolution of T2DM, achieved prior to any significant weight loss; 84% of all patients had complete remission of T2DM, often within days after surgery (7). These impressive results have led many to regard RYGB as a metabolic operation. Unfortunately, 70% of T2DM patients do not fulfill the weight criteria to be eligible for bariatric surgery. Although there has been debate about the role of this procedure in T2DM in less obese patients, the invasive nature of the procedure has prevented widespread acceptance (10, 23, 34).
Unfortunately, the mechanisms underlying diabetes resolution in RYGB remain unclear, limiting development of less invasive alternatives. A major feature of T2DM is obesity-induced insulin resistance. However, the immediacy of T2DM resolution after RYGB suggests insulin sensitivity improves by a process independent of weight loss (41). Numerous studies have documented post-RYGB hormonal changes (11, 12, 28, 30). However, results often conflict and no clear pattern has emerged, so beneficial outcomes of surgery are generally not attributed solely to the hormonal changes (6).
A provocative aspect of RYGB is that duodenal isolation is critical to the success of this operation, by a mechanism not related to malabsorption. The critical role of proximal intestine in overall glucose homeostasis has recently been highlighted by several studies showing duodenal isolation resolves T2DM (31, 33). We contend that duodenal isolation alters the absorptive capacity of the remaining intestine to a state that ultimately improves overall metabolic control by moderation of the absorptive capacity of the intestine and delaying the temporal appearance of nutrients and gut hormones in the circulation. Thus the post-RYGB metabolic benefits arise because nutrient influx is slowed without being prevented.
To better understand this, we have focused on the role of proximal intestine in regulating the intestinal Na+/glucose cotransporter SGLT1, responsible for the major part of dietary glucose uptake under normal physiological conditions. There is accumulating evidence for disorders of intestinal glucose absorption and SGLT1 expression in T2DM and obesity. SGLT1 is overexpressed three- to fourfold in patients and animal models of T2DM (8, 13–15). Other studies have highlighted the importance of SGLT1 in overall glucose homeostasis and weight control: overexpression is associated with profound obesity in murine models (26), whereas intestinal glucose transport is increased in obese animals (5, 17, 25). We have recently shown that exposure of the proximal intestine to a glucose load leads to a rapid increase in intestinal SGLT1 expression (38) and hypothesized that isolation of this region following RYGB leads to downregulation of intestinal glucose transport. We believe that RYGB improves glucose homeostasis in part by altering intestinal glucose transport capacity.
This study aimed to assess SGLT1-mediated glucose transport capacity after RYGB, together with any changes in intestinal morphology that might influence nutrient transport capacity. In particular, since we have shown there is dramatic change in intestinal function throughout the circadian period (with peak function during feeding) (3), we aimed to assess nutrient transport capacity across the diurnal cycle. These studies aim to establish the role of proximal intestine in overall glucose homeostasis under normal physiological conditions and will be followed in the future by studies evaluating this hypothesis in T2DM disease models.
METHODS
Animal models.
All animal procedures were performed in accordance with protocols prospectively approved by Harvard Medical Area Standing Committee on Animals. Male Sprague-Dawley rats (300–325 g) were acquired from Harlan (Indianapolis, IN) and acclimatized for at least a week under a strict 12:12-h light-dark cycle [lights on at 7 AM, termed hereafter as hours after lights on (HALO)-0] with ad libitum access to chow and drinking water.
Under anesthesia, animals underwent either a vagal-sparing RYGB or a sham laparotomy (detailed in Supplemental Methods). Briefly, the stomach was transected to form a 1- to 2-ml gastric pouch, with care taken to preserve the vagus nerve (Fig. 1A). The pouch was drained through a 10-cm Roux limb and joined by a 16-cm biliopancreatic (BP) limb to form the common limb (Fig. 1B).
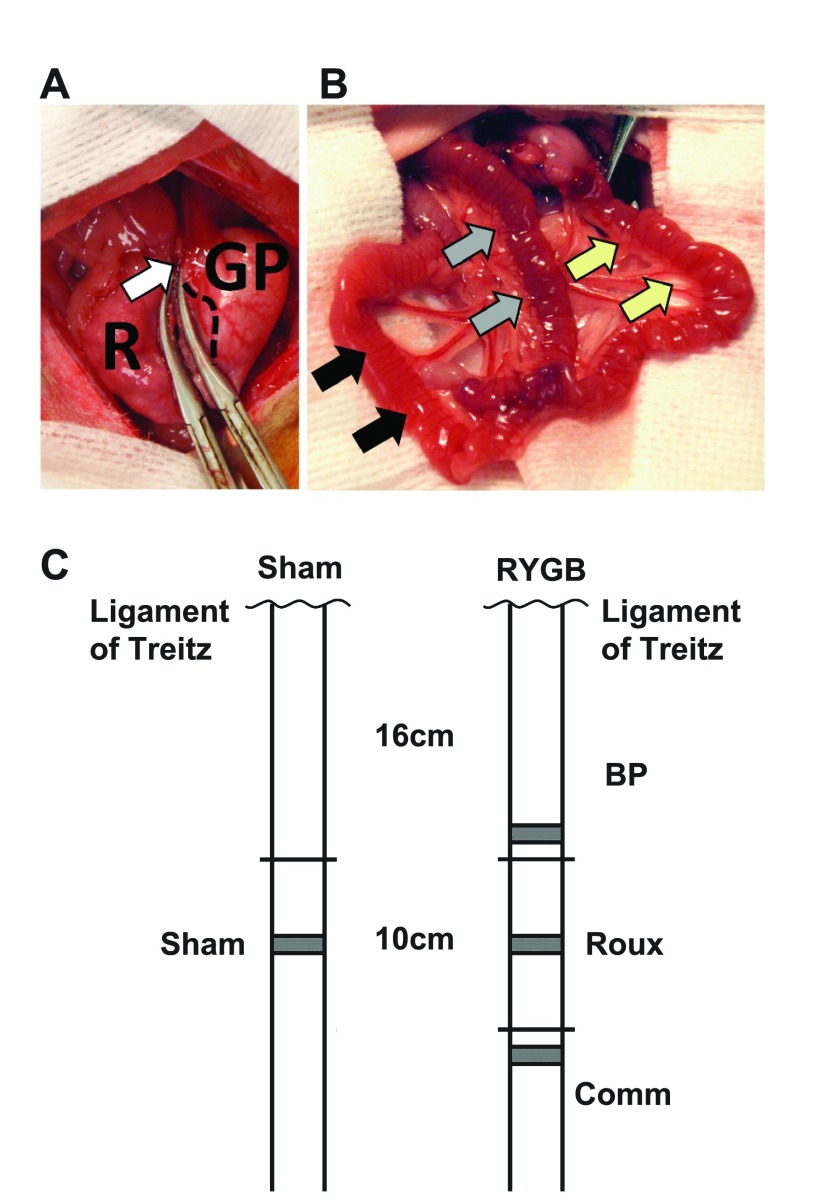
Fig. 1.
A: Transection of the stomach between hemostats, with the gastric pouch (GP) and gastric remnant (R). The gastric pouch includes glandular mucosa, with the junction between the glandular and squamous mucosa indicated with a dashed line. The vagus has been mobilized and preserved along with the left and right gastric vessels in the lesser curve (white arrow). B: final reconstruction, with a 10-cm Roux limb (yellow arrows) draining the gastric pouch before continuing as the common channel (black arrows). The Roux limb is joined by the 16-cm biliopancreatic (BP) limb, identified with gray arrows. C: schematic diagram showing the orthotopic position of BP, Roux, and common (Comm) limbs compared with the ligament of Trietz, and compared with sham animals. Sections for histology were taken from the gray shaded regions: these were all less than 8 cm from the orthotopic position of the sham jejunum.
Animals were maintained on liquid diet for 5 days postoperatively before being switched to a high-carbohydrate diet provided at nighttime only (43% carbohydrate, 41% fat). This was provided every night up to and including harvest.
Tissue harvest.
Animals were harvested at 3 wk postoperatively, at one of three times (HALO-3, HALO-9, HALO-15) to assess diurnal rhythms (n = 7–8). Animals were anesthetized with isoflurane 1–2%, and a midline laparotomy was performed. In RYGB models the Roux, BP, and common limbs were identified, and each was flushed with ice-cold mammalian Ringer's solution (3) on the mesenteric pedicle before rapid removal. A 6-cm length of the Roux limb was taken for functional glucose transport assays, and a further 1 cm of each of the three limbs was reserved for morphological analysis. Mucosa was scraped from the remaining Roux and BP limbs (36) and 10 cm of common limb. For sham animals, a 6-cm length of jejunum was harvested for functional studies from 16 cm distal to the ligament of Trietz (orthotopic position corresponding to the Roux limb). Mucosa was harvested by scraping from a further 10 cm, and a 1-cm length was reserved for histology.
Intestinal samples for histology from the RYGB animals were taken from the distal part of the BP limb, the distal the Roux limb proximal to the jejunojenunostomy, and from 1 cm distal to the jejunojejunal anastomosis for the common limb. For sham animals, histological sections were taken from the midpoint of the harvested length of midjejunum. Because of the surgical reorganization of the intestine after RYGB, this meant that none of the histological sections were within 8 cm from the orthotopic position of sections taken from sham animals (see Fig. 1C).
Functional glucose transport assays.
The everted sleeve method was used as previously described (3), using three sleeves from Roux limbs or the corresponding sham jejunum. Uptake experiments were performed with mammalian Ringer's containing 50 mm d-glucose and trace quantities of [14C]d-glucose and [3H]l-glucose (1 min, 38°C). A further single sleeve from each animal was incubated in uptake solution containing 20 μM phloridzin (Sigma, St. Louis, MO). Glucose uptake capacity was quantified by using [3H]l-glucose as a passive transport control.
Morphology.
Tissue was fixed overnight in 10% formalin before being orientated and embedded in paraffin blocks. Sections were cut at 4-μm thickness and mounted on Superfrost-plus slides before being stained with hematoxylin and eosin. Sections from each of the Roux, BP, and common limbs were assessed as were sections from sham jejunum. All assessments were performed on sections from animals harvested at HALO-3 and performed by a blinded investigator (A. T. Stearns). Villus height, crypt depth, crypt column count, and number of goblet cells per crypt column or per 250-μm length of midvillus were measured. Enterocyte width (nuclei/125 μm length) was recorded, as were circular muscularis thickness and submucosal depth. Goblet cells were defined as discrete rounded cells with colorless or slightly blue cytoplasm and were clearly distinguishable. All measurements were made under light microscopy (Olympus BX50; Center Valley, PA) at ×100 or ×400 magnification. Each assessment was made on villi or crypts where a single layer of enterocytes was identified, on villi where a central lacteal was identified. Measurements were made in at least triplicate per animal and segment.
Quantitative PCR.
Total cellular RNA was extracted and 2.5 ng mRNA was reverse transcribed (37). cDNA was added to SYBR Green Mastermix with appropriate primers for Sglt1 and Actin (Supplementary Methods) and quantified on an ABI7900HT thermal cycler. Transcriptional signal was expressed relative to Actin.
Western blotting.
Whole-cell protein lysates were extracted from frozen tissue aliquots harvested at HALO-15 and separated with Western blotting (39). Membranes were blotted for Sglt1 (Chemicon, Temecula, CA; 1:4,000) before being stripped and reblotted for actin (Neomarkers; 1:500).
Statistical analysis.
Statistical analysis was performed with GraphPad Prism V.5 and SPSS for Windows V.17. When comparing between times or between four intestinal sections, we used one-way ANOVA with Tukey post hoc analysis, with the exception of comparisons of goblet cell counts. This latter provides noncontinuous ordinal data, and therefore Kruskal-Wallis nonparametric ANOVA was used, with Dunn's post hoc testing between multiple groups. In cases in which only two measures were compared, Student's t-tests were performed.
RESULTS
Twenty-nine animals successfully underwent the RYGB procedure; of these, five animals died within the first 48 h after surgery. The cause of death was either anastomotic leak or, in most cases, hemoperitoneum from distraction of vessels on the lesser curve. One further animal continued to lose weight to the end of the study and was euthanized and excluded, leaving 23 animals in the RYGB group. Of 24 sham animals, two died perioperatively of respiratory complications. All remaining animals survived to harvest. Sham animals maintained their body weight postoperatively on liquid diet, losing 2.6 ± 0.4% body weight by the fifth day after surgery (Fig. 2A). On switching to Western Diet, animals rapidly gained weight, such that at the end of the study they had gained 37 ± 1.0% of starting body weight.
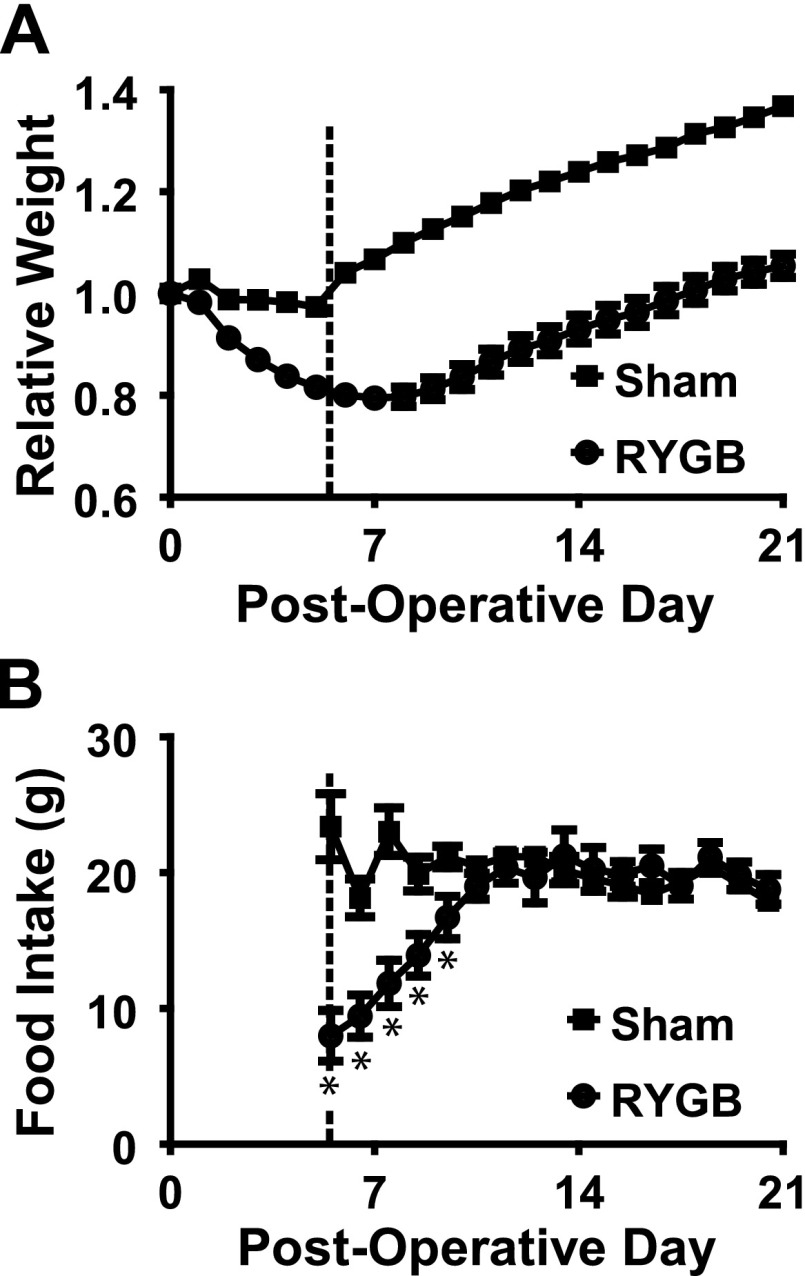
Fig. 2.
A: Daily weights for each of the sham and Roux-en-Y gastric bypass (RYGB) animals, where weight is given relative to the weight on the morning of surgery. Sham animals maintained their weight postoperatively until resumption of solid diet (indicated by the vertical dotted line); at this point, they rapidly gained weight. RYGB animals continued to lose weight until the 7th postoperative day; they remained significantly lighter (P < 0.05) than sham animals throughout the postoperative course. B: whereas sham animals ate relatively constant daily rations of food, RYGB animals initially ate very little. This rapidly increased, until by the 11th postoperative night food consumption was the same between operative arms. *P < 0.05 compared with shams.
In contrast, RYGB animals lost significant amounts of body weight on liquid diet, reaching a trough of 21 ± 1.6% body weight loss at 7 days postoperatively. Animals did not return to their preoperative weight until the 18th day after surgery, finishing the study with 5.4 ± 2.3% gain in body weight compared with preoperative weights. RYGB animals weighed significantly less than sham animals throughout the study (P < 0.001). Sham animals consumed constant quantities of rat chow after switching to the Western Diet (20.8 ± 0.2 g daily, Fig. 2B). RYGB animals gradually increased food intake for the first five nights after switching to rat chow, eating a mean of 20.0 ± 0.4 g thereafter. There was no significant difference in chow intake between arms after the first five nights (P > 0.3, Fig. 2B).
RYGB is characterized by structural changes in jejunal mucosa.
Striking changes were noted in both macroscopic and microscopic appearance. On harvest, the sham jejunum looked normal. However, in the RYGB animals, the intestine in enteric continuity was hypertrophic; the BP limb looked normal. These findings were replicated by the intestinal sleeve masses: sham jejunum weighed 90 ± 4 mg/cm, vs. 183 ± 6 mg/cm in Roux tissue (2.0-fold difference, P < 0.0001).
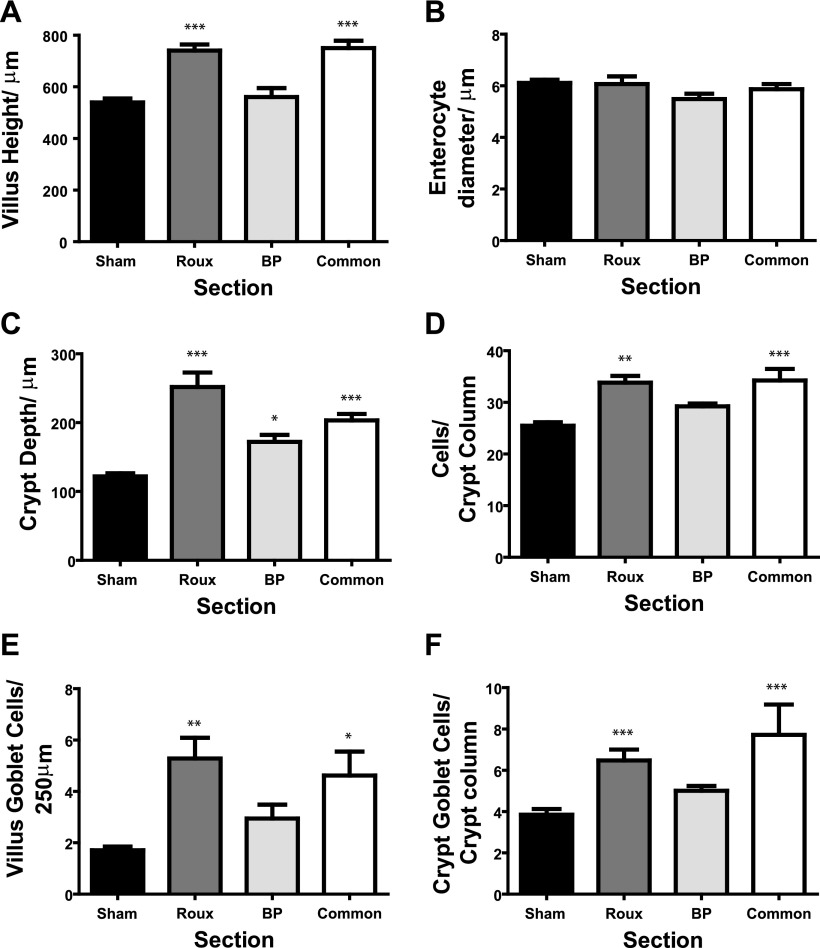
Microscopic changes were also seen. Broadly, the BP limb was similar to shams, with the exception of crypt depth. In contrast, the Roux and common limbs were similar to each other but different from shams and the BP limb. Of particular note, villus architecture changed (P < 0.0001). Villus length increased by ∼38% in the Roux limb compared with sham jejunum (741 ± 23 μm vs. 539 ± 16 μm; Fig. 3A, P < 0.001). This was due to increased numbers of enterocytes, rather than increased enterocyte diameter, which remained unchanged across all sections assessed (P = 0.18, Fig. 3B). Representative images are shown in Fig. 4A.
Fig. 3.
Summary of the morphological comparisons between sham jejunum and the differing limbs in RYGB. A: villus height, with Roux and common limbs significantly longer than both sham and BP limbs. B: mean enterocyte diameter, with no difference between the limbs, suggesting differences in length are due to enterocyte number rather than volume. C: crypt depth, increased depth in all limbs after RYGB compared with shams. This was partly due to an increase in crypt column cell count (D). Lastly, Roux and common limbs, but not BP limb, had increased numbers of goblet cells in both villus (E) and crypt (F) compared with shams. Compared with sham: *P < 0.05; **P < 0.01; ***P < 0.001.
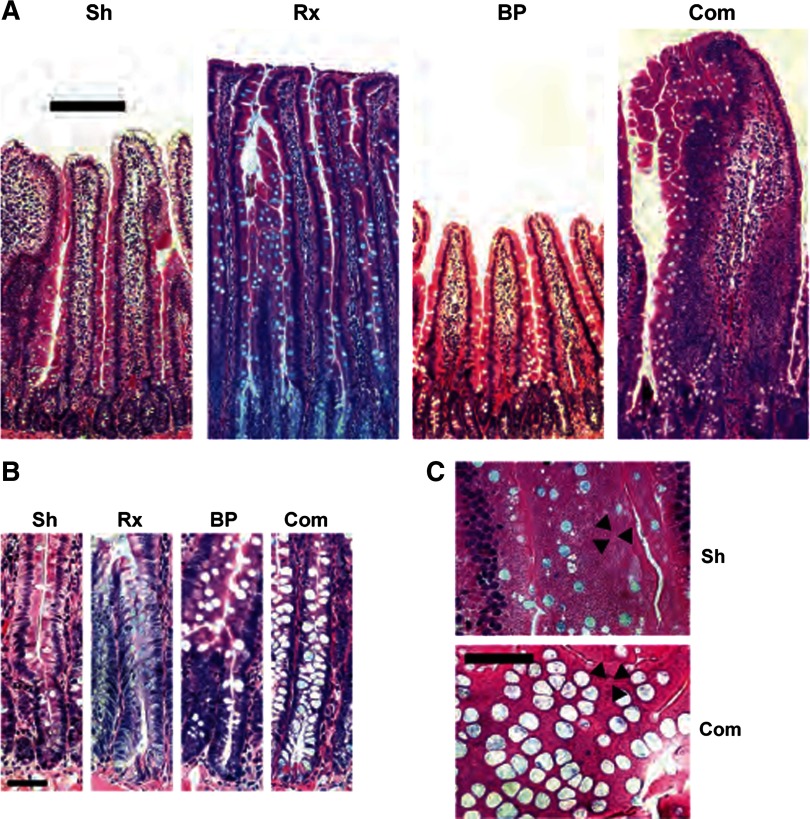
Fig. 4.
A: representative photomicrographs of intestinal sections of sham jejunum (Sh), Roux (Rx), BP, and common (Com) limb. The variations in villus height can be clearly seen, with Roux and common limb villi of greater length than those of sham jejunum and BP limb. B: representative crypts, again with deeper crypts in Roux and common limbs compared with sham jejunum and BP limbs. There was a shift in enterocyte lineage, with an increase in goblet cells in villi in Roux and common limbs compared with sham and BP limbs. This is seen in representative sections from common limb and sham jejunum cut perpendicular to sheets of midvillus enterocytes, as can be seen by the visible tight junctions between enterocytes (black arrowheads). In common limb villi, there is a much greater abundance of goblet cells than in sham jejunum. Scale bars: A, 200 μm; B and C, 50 μm.
Crypt depth increases were also seen after RYGB, with all three limbs showing deeper crypts compared with shams (P < 0.0001). For example, crypt depth in the Roux limb was 2.1-fold that of the shams (252 ± 21 μm vs. 122 ± 4 μm; P < 0.001, Fig. 3C). This was accompanied by an increase in crypt cell numbers in both Roux and common limbs, with cell count per column increasing from 25 ± 0.7 in jejunal crypts to 34 ± 1.3 in the Roux limb (P < 0.001). Representative photomicrographs are shown in Fig. 4A. There was an accompanying increase in muscularis circularis thickness, with Roux limb muscularis thicker than shams (26 ± 2.9 μm vs. 13 ± 1.8 μm, P < 0.001). Common limb muscularis thickness trended toward (but did not reach) a significant difference compared with sham jejunum (20 ± 2.5 μm, P = 0.08). Submucosal thickness did not vary across the four sections (P > 0.4, ANOVA; data not shown).
Furthermore, there appeared to be a shift in the cell lineages in animals after RYGB. In the Roux and common limbs, but not the BP limb, we noted an increase in goblet cell numbers in both the crypt and villus after bypass (P < 0.004). For example, the Roux limb had a mean of 5.2 ± 0.8 goblet cells per 250 μm midvillus length; this compared with 1.7 ± 0.1 for sham jejunum (P < 0.01, Fig. 3E). This was matched by changes in the number of goblet cells per crypt column (P < 0.0002), with increased numbers in Roux animals compared with shams (6.5 ± 0.5 vs. 3.8 ± 0.3; P < 0.001, Fig. 3F).
RYGB reduces intestinal glucose uptake capacity.
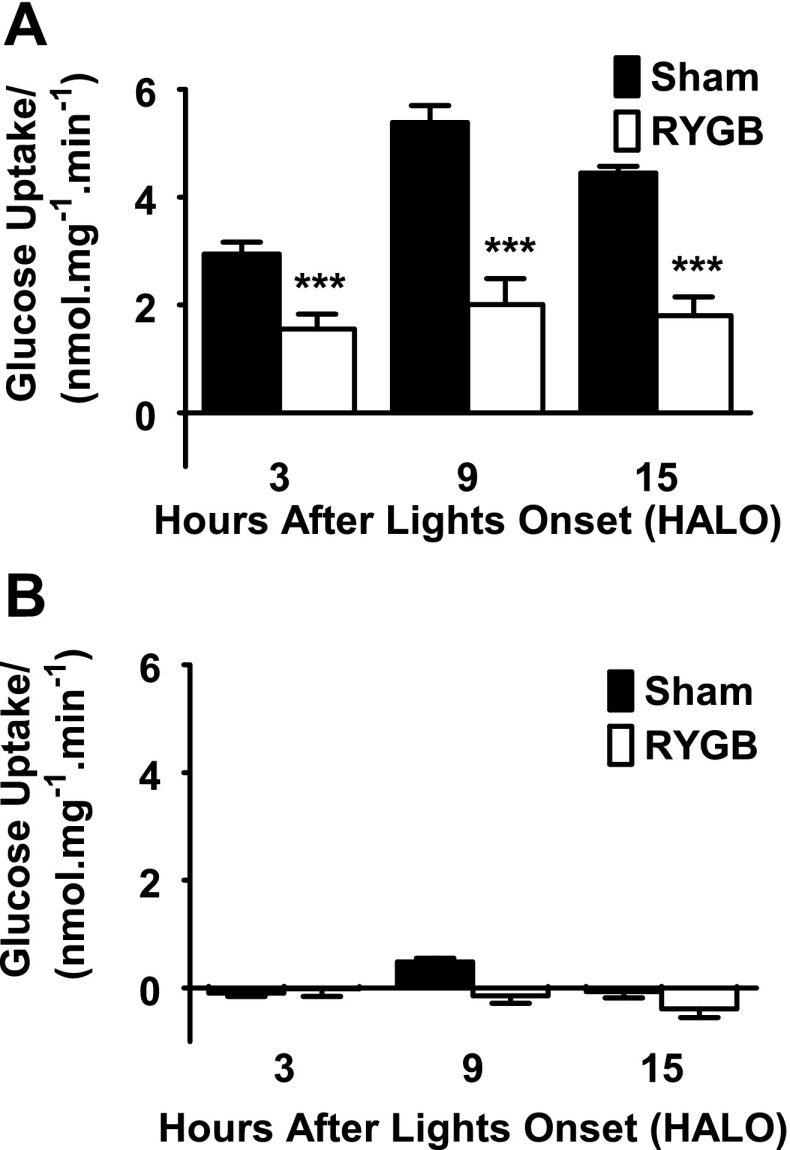
Sham animals showed the expected daily rhythm in glucose uptake capacity (P < 0.001, one-way ANOVA), with a peak glucose uptake capacity at the end of the light phase at HALO-9 (5.4 ± 0.3 nmol·mg−1·min−1), a 1.8 ± 0.1-fold increase compared with the trough at HALO-3 (2.9 ± 0.2 nmol·mg−1·min−1, Fig. 5A). Glucose transport was completely inhibitable at all time points by preincubation in 20 μM phloridzin (Fig. 5B), suggesting dependence on Sglt1. In contrast, in RYGB animals, there was no significant difference in glucose transport across the time points (P > 0.7, Fig. 5A). For example, peak glucose uptake at HALO-9 was 2.0 ± 0.5 nmol·mg−1·min−1, compared with trough capacity of 1.6 ± 0.3 nmol·mg−1·min−1 at HALO-3. Glucose transport capacity was 2.7 ± 0.2-fold higher in sham animals compared with RYGB animals at HALO-9 and significantly lower in RYGB animals compared with sham counterparts at all times (P < 0.002).
Fig. 5.
A: intestinal glucose transport capacity by time. Glucose uptake capacity in sham jejunum showed a normal diurnal rhythm, peaking at hours after lights on (HALO)-9 just prior to onset of feeding. This was completely abolished after RYGB. Glucose uptake capacity was significantly reduced in RYGB animals at all 3 times (***P < 0.001 compared with shams). B: the assay repeated in the presence of 20 μm phloridzin, confirming measured glucose transport represents Sglt1.
When considering glucose uptake per unit length, uptake was lower in the Roux limb compared with shams at HALO-15 (P = 0.05, 325 ± 63 nmol·min−1·cm−1 vs. 438 ± 18 nmol·min−1·cm−1), but not at other times (Table 1). As with uptake expressed per unit mass, in shams there was a normal diurnal variation between fasting and feeding times (P < 0.001); this was completely lost in the Roux limb (P > 0.7).
Table 1.
Glucose uptake on a per unit length basis for everted sleeves from sham or Roux animals
| Glucose Transport, nmol·min−1·cm−1 |
|||
|---|---|---|---|
| HALO-3 | HALO-9 | HALO-15 | |
| Sham | 253±19 | 436±40 | 438±18 |
| RYGB | 312±41 | 373±79 | 325±63* |
A normal diurnal rhythm was observed in sham animals, with expected variations between fasting [hours after lights on (HALO)-3] and feeding (HALO-15, P < 0.001). This was lost in the Roux limb (P > 0.7). Glucose transport was lower
(P = 0.05) in Roux compared to sham jejunum at HALO-15. RYGB, Roux-en-Y gastric bypass.
Changes in Sglt1-mediated glucose transport function are independent of transcriptional regulation.
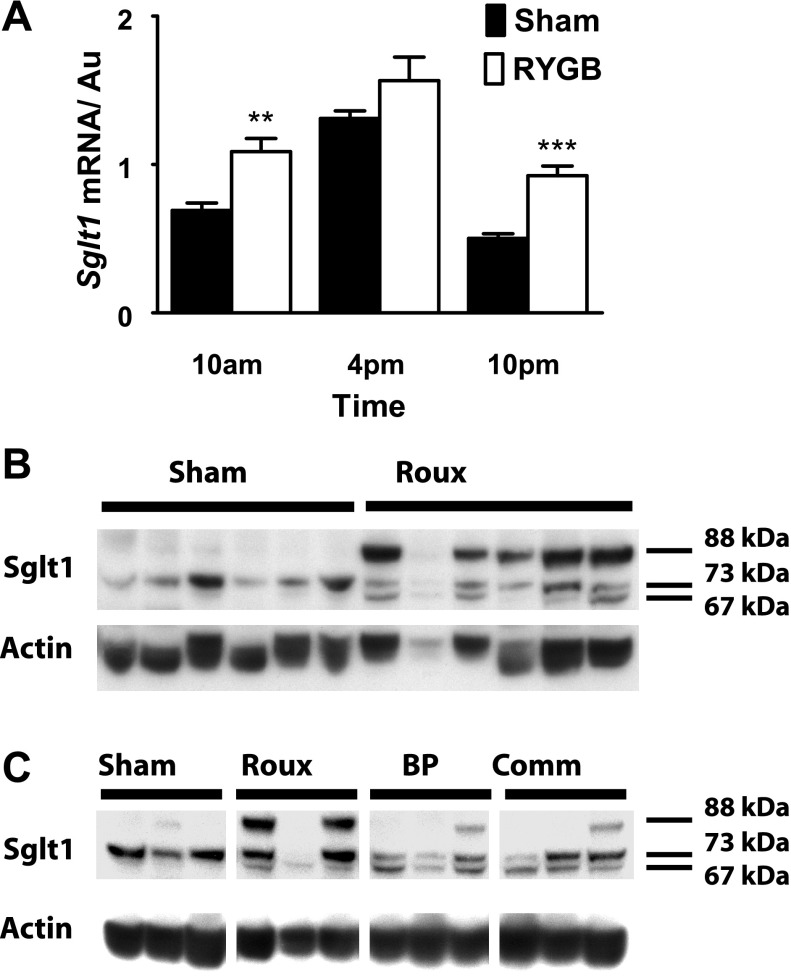
Having shown functional changes, we wondered whether Sglt1 transcription was suppressed, reducing Sglt1-mediated glucose transport. Shams showed the normal transcriptional profile for Sglt1, with peak message at HALO-9, shortly prior to the dark phase and onset of feeding. There was a significant difference in expression between the three time points, with expression at HALO-9 greater than at HALO-3 or HALO-15 (2.6-fold change, P < 0.0001).
Diurnal transcriptional rhythms in Sglt1 were maintained in the Roux limb, with peak expression at HALO-9 (P = 0.002, ANOVA) and a significant difference between expression at HALO-9 compared with other times (P < 0.05, Fig. 6A). The magnitude of the diurnal variation was blunted compared with shams, however, with 1.7-fold change between HALO-9 and HALO-15. This was due to increased basal expression of Sglt1 at HALO-3 and HALO-15 in Roux limb mucosa compared with shams (P < 0.002), rather than lower peak expression at HALO-9 (unchanged in RYGB vs. sham animals, P = 0.2).
Fig. 6.
A: Sglt1 transcriptional data, expressed relative to Actin and indexed to a standard. Both RYGB and sham animals show an expected diurnal rhythm, but it is blunted in RYGB, with increased basal expression (compared with shams: **P < 0.01; ***P < 0.001). B: a representative Western blot for Roux and sham tissue harvested at HALO-15; in sham tissue, there is a single band seen at 73 kDa. After RYGB, there are additional species seen at 67 and 88 kDa. C: a representative Western blot image for sham jejunum vs. Roux, BP, or common limbs. Note, the samples from RYGB animals are taken from the same 3 animals for each of the Roux, BP, or common channels. In RYGB animals, deglycosylated Sglt1 is seen in all 3 limbs, regardless of nutrient or BP exposure. However, the heavy species of Sglt1 at 88 kDa is more pronounced in the Roux limb compared with those exposed to BP secretions.
RYGB leads to apparent posttranscriptional modifications in Sglt1.
Western blotting of sham mucosa resulted in a single Sglt1 band, with an apparent mass of 73 kDa. This is consistent with our previous experience (36), with the native Sglt1 band running with an apparent molecular mass of 68–73 kDa (Fig. 6B). In contrast, blots from Roux limb mucosa showed three prominent bands in the 60- to 90-kDa range. Alongside the native Sglt1 band (73 kDa), was a second band forming a tight doublet, at ∼6 kDa lighter than the native species (67 kDa). This apparent difference in molecular weight is consistent with our previously described deglycosylated form (36). There was a third species also, apparently less mobile and with an estimated molecular mass at 88 kDa, 15 kDa heavier than the native band. The lighter-weight Sglt1 species was seen in all three limbs, regardless of nutrient or BP exposure (Fig. 6C). However, the heavy species of Sglt1 (88 kDa) was more pronounced in the Roux limb compared with those exposed to BP secretions (BP and common channels).
DISCUSSION
We have shown that in a rodent model of RYGB intestinal glucose absorptive function is suppressed. This was accompanied by posttranscriptional changes in Sglt1 and by morphological changes in the intestine. To our knowledge, this is the first report of changes in intestinal structure and SGLT1 function after gastric bypass.
The changes in intestinal morphological structure are an intriguing finding, particularly given that villus height is increased in both Roux and common but not BP limbs. The fact that morphological changes are confined to regions of mucosa exposed to luminal nutrients (but not necessarily pancreaticobiliary secretions) is interesting, and raises the possibility that luminal nutrient exposure is directly regulating the mucosa and crypt-villus progression. However, in contrast to intestinal adaptation to massive small bowel resection, the increase in villus surface area is not associated with an increase in intestinal glucose absorption, both on a per unit mass and per unit length basis (16, 22). Although crypt depth increases and muscularis hypertrophy will contribute slightly to the unit mass denominator, this is likely a relatively small component of the change, reinforced by the observation that, even on a per-unit-length basis, glucose transport decreases at HALO-15. This is despite the increased number of villus enterocytes and corresponding villus length. This strongly implies a change in enterocyte biology, likely because of either a relative reduction in the number of enterocytes capable of transporting glucose or a reduction in the glucose transport capacity for each individual cell. Other authors have identified a relative increase in secretory vs. absorptive cells after massive small bowel resection, with expansion of goblet and Paneth cell lines (18). Similarly, in this RYGB model, we observed a shift toward secretory cell lineages, with an increase in goblet cell numbers in both crypt and villus. This may represent an adaptive response, but it may also account for some of the reduction in glucose transport, because of a reduction in the proportion of absorptive cell lineages. The magnitude of the changes in glucose transport observed suggests that changes in enterocyte lineage are not the only pathway leading to reduction in glucose transport capacity after RYGB.
We focused our assessment on Sglt1, rather than other intestinal transporters, including facilitated basolateral glucose transporter member 2, Glut2. We believe that Sglt1, as well as being a key sugar transporter in disease, has significant therapeutic potential. In particular, in both rodent models of diabetes and T2DM patients, inhibition of Sglt1 by use of phloridzin and related compounds has been shown to improve plasma glucose and insulin profiles after a glucose challenge (20, 32). Remarkably, phloridzin also appeared to restore peripheral tissue sensitivity to insulin in a diabetic model (21). Furthermore, the role of Glut2 as a rate-limiting factor in the absorption of glucose under physiological conditions remains unclear, and we note the normal intestinal glucose absorption kinetics seen in Glut2-null mice (40). In our studies, glucose transport was almost completely inhibitable by phloridzin, suggesting further that under everted-sleeve assay conditions phloridzin-resistant Glut2-mediated uptake is minimal.
The underlying physiological pathways regulating Sglt1-mediated glucose transport, which are presumably disrupted after RYGB, remain unclear. Several human and animal studies have highlighted a critical role for the duodenum and proximal jejunum (“foregut hypothesis”) in glucose homeostasis (33). This is emphasized by recent studies showing that exclusion of this region of intestine from luminal exposure by an endoscopic sleeve resolves T2DM (2, 31). Recent studies have also highlighted a role in glucose homeostasis for intestinal sweet taste receptors, which are abundantly expressed in this region (4, 43). In previous work we have shown that proximal intestinal luminal glucose exposure leads to a rapid increase in intestinal Sglt1 expression, through a process likely mediated by the sweet taste receptors T1R2 and T1R3 (38). Others have demonstrated T1R3 regulates Sglt1 in the longer term (24). We therefore hypothesize that interruption of normal proximal intestinal sweet-taste regulation of Sglt1 may mediate some of the antidiabetic effects of RYGB.
We demonstrated that Sglt1-mediated function is downregulated after RYGB in a pathway independent of Sglt1 message and therefore likely posttranscriptional. This is perhaps not surprising; multiple reports suggest that regulation of intestinal Sglt1 function occurs predominantly at a posttranscriptional level (16). This is supported by the identification of additional species on Western blotting, with a more mobile band consistent with that previously identified as deglycosylated Sglt1 in earlier studies (36). There is an appearance of a further, less mobile species, which may represent further posttranscriptional modifications such as ubiquitination. It is furthermore remarkable that gastric bypass and duodenal exclusion abolished the normal diurnal rhythms in intestinal glucose transport (but not transcription), a finding we have shown to be replicated by vagal afferent blockade (39). This supports our earlier conjecture that vagal afferents signaling nutrient delivery to the proximal intestine may regulate intestinal Sglt1.
There were several aspects of the experimental design that needed particular emphasis. In this study, we used Sprague-Dawley rats, rather than obese or diabetic models. Interpretation of data from a disease model is difficult without an understanding of the impact of a surgical intervention on normal physiology. These studies provide the foundation for future studies in obese and diabetic animals or patients, which will be important because it is difficult to extrapolate the effect of RYGB procedure on the regulation of Sglt1 in the context of dysregulated expression under disease conditions.
In previous work, we have also shown the afferent vagus regulates Sglt1 (39), perhaps through detection of its substrate glucose. It is for this reason that we performed a vagal-sparing RYGB and were careful to exclude the vagal trunks from the gastric transection during the procedure. Although care was taken during this procedure to avoid vagal injury, there is the possibility of inadvertent nerve injury. This may have contributed to the loss of diurnal rhythms in Sglt1, which have been shown to be regulated by vagal afferents (39).
Although mucosa from Roux, BP, and common limbs were used for sglt1 message and protein measurements, performing functional studies from all segments simultaneously would have been technically demanding, making us chose one region only for the studies. We selected only the Roux limb for our functional studies because Sglt1 decreases along the jejunoileal axis; performing assays from the common limb would entail using relatively distal jejunum, which would be expected to express low glucose uptake capacity.
We allowed animals to have ad libitum access to food, albeit under night-restricted conditions imposed to minimize differences in feeding activity patterns between RYGB and sham animals. We considered this important, because the RYGB animals initially ate very little whereas shams ate regular rations of food. Under a pair-feeding schedule, the sham animals would therefore consume their daily rations very quickly, before undergoing a period of enforced fasting. Such a fast-feed model is then difficult to compare with an ad libitum fed model. Indeed, by the end of the study, food consumption was very similar between sham and RYGB animals, further justifying the choice of this feeding schedule. The animal models did show resumption of weight gain postoperatively. However, this is in the context of juvenile and nonobese animals: at surgery, animals were aged 8 wk and still growing. A parallel might be bariatric surgery performed in younger adolescent patients, in which, despite resolution of comorbidities, linear growth may take place, although data on the impact of RYGB on the linear growth of children are lacking (19). Alternatively, the resumption of linear growth and adaptation to consumption of similar levels of chow may reflect too large a gastric pouch. However, since the experiment particularly focused on the physiological effects of duodenal exclusion, this is of limited concern in the context of demonstrated changes in glucose transport capacity.
In our model, and in the context of a diet rich in simple carbohydrates, high SGLT1-mediated intestinal glucose transport capacity would be expected to lead to rapid uptake of dietary glucose with resulting portal hyperglycemia. In contrast, the RYGB, by suppressing intestinal glucose absorption would slow glucose uptake, blunting postprandial portal glycemic excursions. Furthermore, this would enhance ileal delivery of glucose, with consequential effects on ileal gut peptide hormone secretion and satiety (“hind gut” theory) (27, 42). Future studies in disease models will therefore need to evaluate hormonal changes that may occur with this surgery and contribute to the therapeutic benefit. Since we used a nonobese and nondiabetic model, we deferred any hormonal assessments, concentrating on the impact of this procedure on intestinal glucose absorption. However, once this is repeated in a disease model, in which therapeutic outcomes are of interest, hormonal measurements become critical to identify other factors that may have contributed to therapeutic benefit.
In summary, we here demonstrate for the first time that RYGB is accompanied by changes in intestinal glucose transport capacity, with a 63% reduction in Sglt1-mediated glucose uptake prior to onset of feeding. This is furthermore in the context of changes in intestinal morphology and structure, with proliferative changes in villi, and a shift from an absorptive to a secretory cell lineage. This provides a novel explanation for the resolution of impaired oral glucose handling after gastric bypass surgery.
GRANTS
Funding sources include Harvard Clinical Nutrition Center Grant P30-DK040561 (A. Tavakkolizadeh); Berkeley Fellowship and George Herbert Hunt Travelling Fellowship (A. T. Stearns); and Nutricia Foundation Fellowship (A. Balakrishnan).
Supplementary Material
ACKNOWLEDGMENTS
We thank Jan Rounds for invaluable managerial support. Dr. Stanley W. Ashley, Professor of Surgery at Brigham and Women's Hospital, Boston, and Dr. David B. Rhoads, Associate Professor in Pediatric Endocrinology at MassGeneral Hospital for Children, provided supervisory support and training for A. T. Stearns and A. Balakrishnan, as well as critical review of the manuscript.
This manuscript has been presented as an oral presentation at Academic Surgical Congress 2009, Fort Myers, FL (February 2009). It was published in abstract form only in a supplementary issue of Journal of Surgical Research as related to this meeting.
REFERENCES
- 1. Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med 357: 753–761, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Aguirre V, Stylopoulos N, Grinbaum R, Kaplan LM. An endoluminal sleeve induces substantial weight loss and normalizes glucose homeostasis in rats with diet-induced obesity. Obesity (Silver Spring) 16: 2585–2592, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balakrishnan A, Stearns AT, Rounds J, Irani J, Giuffrida M, Rhoads DB, Ashley SW, Tavakkolizadeh A. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter (SGLT1). Surgery 143: 813–818, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32: 41–49, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Bihler I, Freund N. Sugar transport in the small intestine of obese hyperglycemic, fed and fasted mice. Diabetologia 11: 387–393, 1975. [DOI] [PubMed] [Google Scholar]
- 6. Bose M, Olivan B, Teixeira J, Pi-Sunyer FX, Laferrere B. Do Incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: what are the evidence? Obes Surg 19: 217–229, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 292: 1724–1737, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Burant CF, Flink S, DePaoli AM, Chen J, Lee WS, Hediger MA, Buse JB, Chang EB. Small intestine hexose transport in experimental diabetes. Increased transporter mRNA and protein expression in enterocytes. J Clin Invest 93: 578–585, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 240: 416–423; discussion 423–414, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen R, Pinheiro JS, Correa JL, Schiavon CA. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m2: a tailored approach. Surg Obes Relat Dis 2: 401–404, discussion 404, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 33, Suppl 1: S33–S40, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623–1630, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Dyer J, Garner A, Wood IS, Sharma AK, Chandranath I, Shirazi-Beechey SP. Changes in the levels of intestinal Na+/glucose co-transporter (SGLT1) in experimental diabetes. Biochem Soc Trans 25: 479S, 1997. [DOI] [PubMed] [Google Scholar]
- 14. Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 282: G241–G248, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Fedorak RN, Cheeseman CI, Thomson AB, Porter VM. Altered glucose carrier expression: mechanism of intestinal adaptation during streptozocin-induced diabetes in rats. Am J Physiol Gastrointest Liver Physiol 261: G585–G591, 1991. [DOI] [PubMed] [Google Scholar]
- 16. Ferraris RP, Diamond J. Regulation of intestinal sugar transport. Physiol Rev 77: 257–302, 1997. [DOI] [PubMed] [Google Scholar]
- 17. Ferraris RP, Vinnakota RR. Intestinal nutrient transport in genetically obese mice. Am J Clin Nutr 62: 540–546, 1995. [DOI] [PubMed] [Google Scholar]
- 18. Helmrath MA, Fong JJ, Dekaney CM, Henning SJ. Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am J Physiol Gastrointest Liver Physiol 292: G215–G222, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Inge TH, Krebs NF, Garcia VF, Skelton JA, Guice KS, Strauss RS, Albanese CT, Brandt ML, Hammer LD, Harmon CM, Kane TD, Klish WJ, Oldham KT, Rudolph CD, Helmrath MA, Donovan E, Daniels SR. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics 114: 217–223, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Johnston KL, Clifford MN, Morgan LM. Possible role for apple juice phenolic, compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J Sci Food Agric 82: 1800–1805, 2002. [Google Scholar]
- 21. Kahn BB, Shulman GI, Defronzo RA, Cushman SW, Rossetti L. Normalization of blood-glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose-transport in adipose-cells without restoring glucose transporter gene-expression. J Clin Invest 87: 561–570, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwan WC, Quamme GA, Freeman HJ. Sodium-dependent d-glucose transport in brush-border membrane vesicles after massive distal small bowel resection in the rat. Gastroenterology 92: 1987–1993, 1987. [DOI] [PubMed] [Google Scholar]
- 23. Lee WJ, Wang W, Lee YC, Huang MT, Ser KH, Chen JC. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI>35 and <35 kg/m2. J Gastrointest Surg 12: 945–952, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morton AP, Hanson PJ. Monosaccharide transport by the small intestine of lean and genetically obese (ob/ob) mice. Q J Exp Physiol 69: 117–126, 1984. [DOI] [PubMed] [Google Scholar]
- 26. Osswald C, Baumgarten K, Stumpel F, Gorboulev V, Akimjanova M, Knobeloch KP, Horak I, Kluge R, Joost HG, Koepsell H. Mice without the regulator gene Rsc1A1 exhibit increased Na+-d-glucose cotransport in small intestine and develop obesity. Mol Cell Biol 25: 78–87, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patriti A, Aisa MC, Annetti C, Sidoni A, Galli F, Ferri I, Gulla N, Donini A. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery 142: 74–85, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Patriti A, Facchiano E, Gulla N, Aisa MC, Annetti C. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 245: 157–158, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, Dohm L. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 222: 339–350; discussion 350–332, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 16: 298–305, 2008. [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez-Grunert L, Galvao Neto MP, Alamo M, Ramos AC, Baez PB, Tarnoff M. First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis 4: 55–59, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79: 1510–1515, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 244: 741–749, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 239: 1–11, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741–752, 2007. [DOI] [PubMed] [Google Scholar]
- 36. Stearns AT, Balakrishnan A, Rhoads DB, Ashley SW, Tavakkolizadeh A. Diurnal expression of the rat intestinal sodium-glucose cotransporter 1 (SGLT1) is independent of local luminal factors. Surgery 145: 294–302, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stearns AT, Balakrishnan A, Rhoads DB, Ashley SW, Tavakkolizadeh A. Diurnal rhythmicity in the transcription of jejunal drug transporters. J Pharm Sci 108: 144–148, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stearns AT, Balakrishnan A, Rhoads DB, Ashley SW, Tavakkolizadeh A. Duodenal glucose sensing and regulation of intestinal glucose absorption after Roux-en-Y gastric bypass. J Surg Res 151: 226–227, 2009. [Google Scholar]
- 39. Stearns AT, Balakrishnan A, Rounds J, Rhoads DB, Ashley SW, Tavakkolizadeh A. Capsaicin-sensitive vagal afferents modulate posttranscriptional regulation of the rat Na+/glucose cotransporter SGLT1. Am J Physiol Gastrointest Liver Physiol 294: G1078–G1083, 2008. [DOI] [PubMed] [Google Scholar]
- 40. Stumpel F, Burcelin R, Jungermann K, Thorens B. Normal kinetics of intestinal glucose absorption in the absence of GLUT2: evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc Natl Acad Sci USA 98: 11330–11335, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 15: 474–481, 2005. [DOI] [PubMed] [Google Scholar]
- 42. Woltman T, Reidelberger R. Effects of duodenal and distal ileal infusions of glucose and oleic acid on meal patterns in rats. Am J Physiol Regul Integr Comp Physiol 269: R7–R14, 1995. [DOI] [PubMed] [Google Scholar]
- 43. Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK, Blackshaw LA. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut 58: 337–346, 2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.