Abstract
Progesterone (P4) inhibits the gastrointestinal muscle contraction by downregulating Gαq/11 proteins that mediate contraction, by upregulating Gαs proteins that mediate relaxation, and by altering the pattern of cyclooxygenase (COX) enzymes and prostaglandins. We aimed to examine whether P4 treatment of guinea pigs in vivo affects basal colon motility [basal motility index (MI)] by altering the levels and actions of PGF2α and PGE2. Guinea pigs were treated with intramuscular (IM) P4 for 4 days. The BASAL MI, the PGF2α-induced contraction, and PGE2-induced inhibition of contraction were examined in muscle strips and cells. The levels of PGF2α and PGE2 were measured by radioimmunoassay. Treatment with P4 reduced the basal MI, the levels of PGF2α, and PGF2α-induced contraction. P4 increased PGE2 levels, and PGE2 induced relaxation. Pretreatment with IM RU-486 (10 mg/kg per day), a P4 receptor antagonist, 1 h before P4 blocked the actions of P4. The PGF2α antagonist Al-1180 abolished basal MI and PGF2α-induced contraction. N-ethylmaleimide, which blocks unoccupied membrane receptors, blocked Ach and VIP actions but had no effect on PGF2α and PGE2 effects. A COX-1 inhibitor decreased and a COX-2 inhibitor increased PGF2α levels; GTPγS increased and GDPβS decreased the levels of PGF2α. Gαq/11 protein antibodies (Abs) reduced PGF2α levels, and Gαi3 Abs blocked its motor actions. Gαs Abs increased PGF2α but decreased PGE2 levels. We concluded that P4 decreases basal MI by reducing PGF2α levels caused by downregulation of Gαq/11 and that PGF2α-induced contraction was blocked by downregulating Gαi3. P4 also decreased the basal MI by increasing PGE2 levels, and PGE2 induced relaxation by upregulating Gαs proteins.
Keywords: colon motility, guinea pigs, PGF2α, PGE2, G proteins
we have shown that colon muscle cells from female patients with slow-transit constipation (STC) have signal transduction abnormalities that are reproduced in normal human colon muscle cells treated in vitro with progesterone (P4) (10, 31). P4 inhibits the contraction of colon and of other gastrointestinal smooth muscle cells (5, 8, 12, 23, 26). These muscle cells have an impaired response to agonists that are dependent on G protein-coupled receptors such as CCK-8, ACh, and GTPγS (8, 28). The motility abnormalities of female patients with STC and of P4-treated normal muscle cells may be due to downregulation of Gαq/11 and upregulation of Gαs proteins (10, 31). This conclusion is supported by findings that both types of colon muscle cells respond normally to receptor and G protein-independent agonists such as KCl and diacylglycerol. Similar G protein abnormalities have been reported in muscle cells treated with P4 and during gestation that are presumably induced by rising P4 levels in the last two trimesters of pregnancy (1, 14, 15, 19, 29, 30). Moreover, the basal motility index (MI) of colon muscle strips from patients with STC is lower than that of muscle strips from control females without constipation (10). The reduction of basal MI abnormalities in patients with STC is associated with changes in cyclooxygenase (COX) enzymes and prostaglandin levels. In the muscle strips from these patients, the levels of COX-1 enzyme and of excitatory prostaglandins such as thromboxane A2 (TxA2) are reduced. In contrast, the levels of COX-2 enzymes and of inhibitory PGE2 levels are increased. Prostaglandins such as TxA2 and PGF2α contract muscle cells in the colon circular muscle layer generated by COX-1, and PGE2 relaxes muscle cells generated by COX-2. These COX enzymes and prostaglandin abnormalities are reproduced in normal muscle cells treated in vitro with P4 (10−5 M) for 6 h. However, it is not known whether changes in prostaglandins contribute to the genesis of the basal MI and whether these changes induced by P4 affect the contractility of the colon. The role of prostaglandins in the genesis of tonic and phasic contractions has been reported in different gastrointestinal muscle cells (4, 11).
The present studies were therefore aimed at determining the effects of the in vivo P4 treatment on basal MI of guinea pig colon and examining whether these actions are mediated by prostaglandins because of their role in the genesis of the colon motor functions.
MATERIALS AND METHODS
Animals.
Male guinea pigs (weighing 450–500 g) were purchased from the Charles River Laboratory (Wilmington, MA). The animal Welfare Committee of Rhode Island Hospital approved their use. Animals were housed in thermoregulated rooms with free access to food and water. For the studies on the effects of P4, guinea pigs were selected at random and injected with P4 (2 mg/kg body wt) or equivalent volume of saline daily for 4 days. They were euthanized on the fifth day. After an overnight fast, the guinea pigs were sedated with an intramuscular injection of ketamine hydrochloride (30 mg/kg) and euthanized by pentobarbital (30 mg/kg ip). Guinea pig colons were promptly removed, rinsed with ice-cold and oxygenated modified Krebs solution, and placed in a dissecting pan containing the same solution continuously aerated with 95% O2-5% CO2. The colon was kept in ice-cold oxygenated Krebs's solution (116.6 mM NaCl, 3.4 mM KCl, 21.9 mM NaHCO3, 1.2 mM NaH2PO4, 2.5 mM CaCl2, 1.2 mM MgCl2, and 5.4 mM glucose). The mucosa and serosa were carefully peeled off under a dissecting microscope. The circular muscle layer of the colon was further cleaned by gently removing the remaining connective tissue.
Isolation and permeabilization of colon muscle cells.
Muscle cells were isolated, and in some experiments they were permeabilized using methods described previously (3, 5, 7, 9, 17, 33). The circular muscle layer was cut into 2-mm-wide strips and digested in HEPES buffer containing 0.5 mg/ml type F collagenase and 2 mg/ml papain (activity of 13.9 U/mg protein) for 20 min at 35°C in a shaking water bath. The buffer was gently gassed with 100% O2 during the tissue digestion. At the end of the digestive process, the tissue was filtered through Nitex mesh 200 (Tetko, Elmsford, NY) and rinsed with 20 ml HEPES. The tissue remaining on the filter was collected and incubated in HEPES buffer at 35°C for 15 min to allow free dispersion of cells. For preparation of permeable cells, the partly digested muscle cells were washed with “cytosolic buffer,” a medium with the following composition (in mM): 20 NaCl, 100 KCl, 25 NaHCO3, 0.96 NaH2PO4, 0.48 CaCl2, 5.0 MgSO4, and 1.0 EGTA, pH 7.2, 2% bovine serum albumin. The cells were maintained by equilibrating with 95% O2-5% CO2 at 31°C. The dispersed cells were exposed to saponin (75 μg/ml) and then centrifuged at 200 g for 3 min. Cells were washed once with modified cytosolic buffer by centrifugation and resuspended in modified cytosolic buffer and equilibrated at 31°C for 15 min before the experiment. The modified cytosolic buffer was prepared with cytosolic buffer plus 1.5 mM ATP, 5 mM creatine phosphate, 10 U/ml of creatine phosphokinase, and 10 μM antimycin A.
Studies of contraction and inhibition of contraction of dissociated muscle cells.
Briefly, muscle squares were incubated at 31°C for 30 min in HEPES-buffered medium containing 150 U/ml collagenase (type II) and 0.01% soybean trypsin inhibitor (3, 7, 18). The partly digested tissues were washed with enzyme-free medium, and muscle cells were allowed to disperse spontaneously for 30 min. Muscle cells were harvested by filtration through 450-mm Nitex. Muscle contraction was measured as previously described in intact and permeable cells. Permeable cells were used to study the effect of antibodies against G proteins (Gαq/11, Gαi3, Gαi1/2, Gαs) and then fixed in acrolein at 1% final concentration (20). The cell length was measured with a phase contrast microscope (Carl Zeiss, Jena, Germany) and a closed circuit television camera (Panasonic, Secaucus, NJ) connected to a Macintosh Computer with NIH Image software. The average length of 30 cells, measured in the absence of agonists, was taken as the control length and compared with length measured after addition of agonists. Shortening was defined as the percent decrease in the average length of 30 cells after treatment with agonists compared with the control length.
Inhibition of contraction.
Inhibition of contraction was measured in permeable muscle cells by determining the effect of inhibitors on cell length using a method previously reported (3, 7, 18). Single muscle cells were initially incubated with VIP 10−6 M, for 60 s followed by 10−6 M L-a-1.2-dioctanoyl glycerol (DOG) for 30 s after which the cells were fixed with 1% acrolein. DOG (10−6 M) causes maximal contraction in intact and permeable smooth muscle cells from guinea pig colon. Individual cell lengths were measured by scanning micrometry using phase contrast microscopy. Relaxation was expressed as percent inhibition of DOG-induced contraction.
Measurement of phasic contractions in colon muscle strips.
Strips were mounted in 1-ml muscle chambers as previously described in detail (6, 28). Briefly, circular muscle strips of the colon were obtained by removing the mucosa, longitudinal muscle layer, and serosa. They were initially stretched to 1.0 g of passive force and were equilibrated by continuous perfusion with oxygenated Krebs's solution at 37°C. After 1-h perfusion, basal spontaneous phasic contractions gradually developed and stabilized after another 30-min period of equilibration. The strips were then treated with tetrodotoxin 10−5 M and after 30 min before any studies. Stable phasic contractions of control and treated muscle strips were measured with Grass isometric force transducers and amplifiers connected to a Biopac data acquisition system. The combined tonic and phasic activity was determined by calculating the MI measured over a 30-min period. It was calculated as MI = [A(g) × D(s)] or area under the curve and expressed as mN/min (28).
Measurement of PGF2α and PGE2 content.
PGF2α and PGE2 were measured using an Eicosanoid Enzyme Immunoassay kit (Cayman Chemical, Ann Arbor, MI) (10, 17). Muscle strips or cells were homogenized in eicosanoid homogenization buffer [0.1 M phosphate buffer (pH 7.4) containing 1 mM EDTA and 20 μg/ml indomethacin] at 4°C according to the manufacturer's instructions. The homogenate was centrifuged at 15,000 g for 15 min at 4°C, and an aliquot of the supernatant was taken for protein measurement. The rest of the supernatant was used for PGF2α purification using a specific Affinity Column. The resulting extracts were dissolved in enzyme immunoassay buffer (1.0 M phosphate buffer pH 7.4 containing 0.01% NaN3, 0.037% EDTA, 0.1% BSA). The PGF2α and PGE2 concentration was quantified by using a PGF2α Competitive Enzyme Immunoassay kit expressed as ng/mg protein.
Chemicals.
P4, PGF2α, GTPγS, GDPβS, COX enzyme inhibitors, 8′bromo-c′AMP (8B-cAMP), cysteine alkylating agent N-ethylmaleimide (NEM) and pertussis toxin (PTX) and other reagents were purchased from Sigma Chemical (St. Louis, MO); AL-8810 (11β-fluoro analog of PGF2α from Cayman Laboratories, Ann Arbor, MI) (16); G protein subunit antibodies were purchased from CytoSignal (Irvine, CA).
Statistics.
Statistical analysis was performed using one- and two-factorial repeated ANOVA comparing values between controls and experimental and progesterone-treated guinea pigs or muscle cells treated with inhibitor or antibodies. P value of <0.05 was considered significant. Previous studies using similar treatments had shown that significance could be achieved using three to four samples of controls and experimental treated.
RESULTS
Effect of P4 on basal colonic motility (basal MI) and prostaglandins.
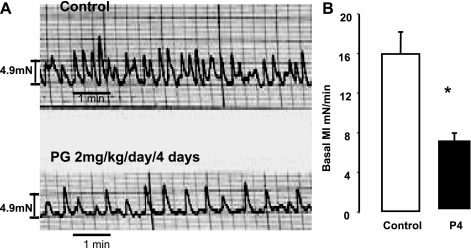
P4 treatment [2 mg/kg intramuscularly (IM)] daily for 4 days markedly reduced the basal MI of the phasic contractions of the descending sigmoid colon of guinea pigs compared with that of muscle strips from saline-treated guinea pigs (Fig. 1).
Fig. 1.
The basal motility index (MI) of colon muscle strips treated with progesterone (P4) (2 mg/kg per day) or saline intramuscular (IM) for 4 days. A: basal motility tracings of muscle strips from guinea pigs treated with saline (control) or P4. B: P4 decreased the basal MI of muscle strips compared with saline treatment (*P < 0.01 by ANOVA). Values are means ± SE of basal MI (n = 4).
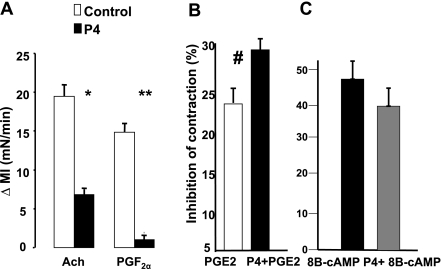
We then evaluated two prostaglandins, PGF2α that contracts and PGE2 that inhibits the phasic contractions of colon circular muscle strips. P4 reduced the contraction induced by PGF2α and by ACh (Fig. 2A). P4 does not affect the contraction of agonists that are G protein independent such as KCl (data are not shown). In contrast, P4 increased the inhibition of muscle contraction induced by PGE2 (Fig. 2B) but had no effect on the inhibition of contraction induced by 8B-cAMP, a second messenger that is receptor and G protein independent (Fig. 2C). These findings are in agreement with previous studies showing that P4 blocks excitatory agonists that act on G protein-coupled receptors.
Fig. 2.
A: P4 blocked the contraction (Δ MI) of ACh (10−6 M) and PGF2α (10−6 M) (*P < 0.001, **P < 0.005 by ANOVA). B: in contrast, P4 increased the percent inhibition of contraction induced by PGE2 (10−6 M) (#P < 0.05 by ANOVA). C: there were no differences in the percent inhibition of contraction of muscle cells induced by 8-bromo c′AMP (8B-cAMP) between P4- and saline-treated muscle cells. Values are means ± SE of n = 3.
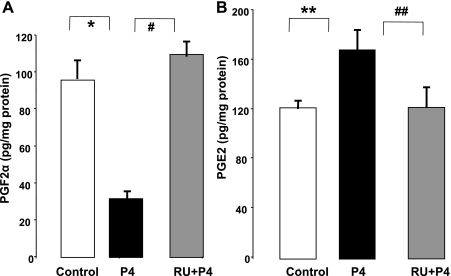
We next investigated the effect of P4 on PGF2α and PGE2 muscle levels. To examine whether these effects were specific, P4 was antagonized by pretreating guinea pigs with the P4 receptor antagonist RU-486 (10 mg/kg body wt per day) or saline for 4 days. Guinea pigs were treated with RU-486 or equivalent volumes of saline injections 1 h before the injection of P4. Consistent with previous studies, the IM administration of P4 alone decreased PGF2α (Fig. 3A) and increased PGE2 levels (Fig. 3B). Pretreatment with RU-486 blocked the changes in the levels of both prostaglandins induced by P4.
Fig. 3.
A: treatment with P4 in vivo decreased the levels of PGF2α (*P < 0.001 by ANOVA) and was blocked by pretreatment with RU-486 (RU) (#P < 0.001 by ANOVA, mg/kg body wt). B: P4 increased the levels of PGE2 (**P < 0.01 by ANOVA) and was blocked by pretreatment with RU-486 (##P < 0.01 by ANOVA). Values are means ± SE of n = 4.
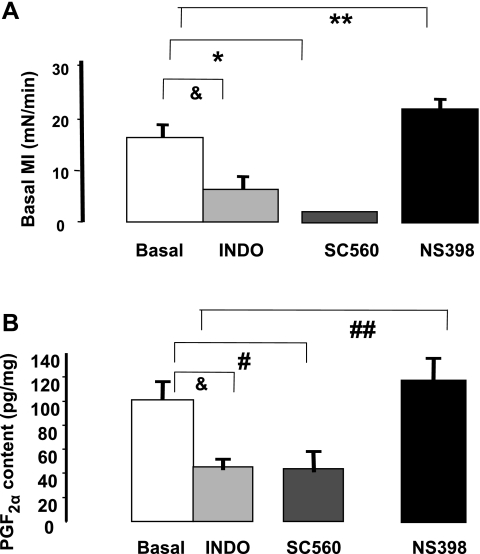
We next investigated the role of PGF2α in the genesis of the basal MI of the colon by examining the effects of COX enzyme inhibitors on the basal MI and levels of PGF2α. The levels of prostaglandins were determined in muscle strips treated with selective and nonselective inhibitors of COX enzymes. We have previously shown that COX-1 enzyme generates excitatory prostaglandins such as TxA2 and PGF2α and that COX-2 enzyme generates PGE2 (10). The COX-1 enzyme inhibitor SC560 (10−6 M) reduced the basal MI (Fig. 4A) and caused a concomitant reduction in the levels of PGF2α (Fig. 4B). In contrast, the COX-2 inhibitor NS398 (10−6 M) increased the basal MI (Fig. 4A) and the levels of PGF2α (Fig. 4B). The nonselective COX inhibitor indomethacin reduced both basal MI and lowered PGF2α levels (Fig. 4, A and B). Moreover, we also compared the percent increase in PGE2 levels in cells treated with P4 and COX-2 inhibitor NS398. No significant differences were found between P4-induced increase (31.4 ± 3%) and NS398-induced increased (33.8 ± 4%) in PGE2 levels.
Fig. 4.
Effects of selective and nonselective (indomethacin) inhibitors on basal MI (A) and on PGF2α levels (B) in cells from normal animals. SC560, a cyclooxygenase (COX)-1 inhibitor (10−5 M), decreased the basal MI (*P < 0.01 by ANOVA) and the levels of PGF2α (#P < 0.01 by ANOVA). In contrast, NS398 (10−5 M), a COX-2 inhibitor, increased the basal MI (**P < 0.01 by ANOVA) and the levels of PGF2α (##P < 0.05 by ANOVA). Indomethacin (10−5 M) lowered the basal MI and the PGF2α levels (&P < 0.01 by ANOVA). Values are means ± SE of n = 4.
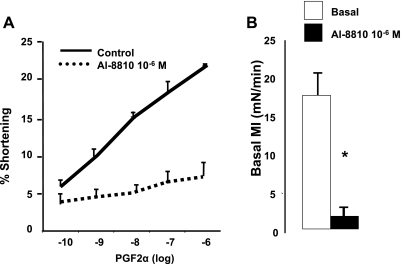
To further investigate the role of PGF2α in the genesis of the basal MI, we examined the effect of PGF2α receptor antagonist Al-8810 (16) on PGF2α (10−10-10−6 M)-induced contraction and on the basal MI. Al-8810 (10−6 M) blocked the contraction in dissociated muscle cells induced by increasing concentrations of PGF2α (Fig. 5A) and almost abolished the basal MI of colonic muscle strips (Fig. 5 B). These data taken together suggest that PGF2α contributes to the genesis of basal colonic motility.
Fig. 5.
A: Al-8810 (10−6 M) blocked the contraction of muscle cells induced by increasing concentrations of PGF2α (10−10–10−6 M) (P < 0.001, by ANOVA). B: Al-8810 (10−6 M) abolished the basal MI of colon muscle strips (*P < 0.001 by ANOVA). Values are means ± SE of n = 4.
Effect of the cysteine alkylating agent NEM on basal MI and agonist-induced contraction.
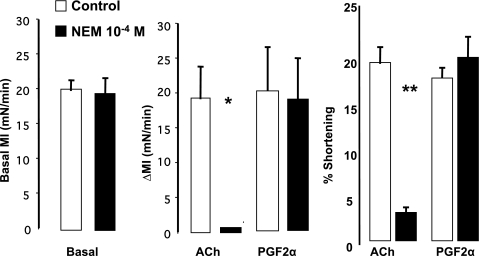
We then examined the cellular source of PGF2α and PGE2. Colon muscle strips and dissociated muscle cells were treated with NEM that inactivates membrane receptors when unoccupied by their endogenous ligands (13). NEM (10−4 M) had no effect on the basal MI of muscle strips pretreated with TTX (10−5 M). It also did not impair the PGF2α-induced contraction in muscle strips (Fig. 6B) or in muscle cells (Fig. 6C). However, NEM blocked the contraction induced by ACh in muscle strips (Fig. 6B) and in cells (Fig. 6C).
Fig. 6.
Effect of N-ethylmaleimide (NEM) (10−4 M) on the basal MI of colon muscle strips and on the contraction induced by exogenous agonists. Left: NEM 10−4 M had no effect on the basal MI. Middle: in muscle strips, NEM blocked the contraction induced by ACh (10−6 M) (*P < 0.001 by ANOVA) but had no effect on the contraction induced by PGF2α (10−6 M). Right: NEM blocked the contraction induced by ACh (10−6 M) in dissociated muscle cells (**P < 0.001 by ANOVA) but also had no effect on PGF2α-induced contraction. Values are means ± SE of n = 4.
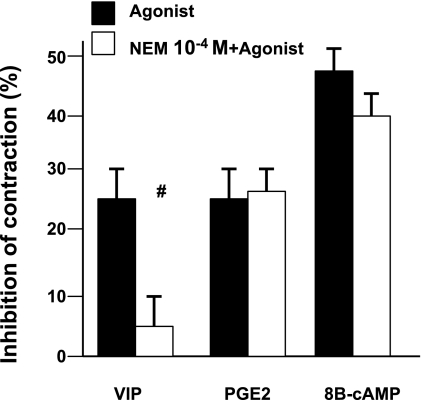
NEM also blocked the VIP inhibition of contraction in dissociated muscle cells (17) but did not antagonize the actions of PGE2 and 8B-cAMP, a receptor- and G protein-independent second messenger (Fig. 7). These results suggest that endogenous factors, among them PGF2α and PGE2, contribute to the genesis of the basal MI of the colon muscle strips. Thus prostaglandins by occupying their receptors protect them against the actions of NEM. These findings also suggest that colon muscle cells are the source of these prostaglandins that act as autacoids or paracrine factors. Taking all these findings together, they suggest that PGF2α and PGE2 may contribute to genesis of the colon basal MI.
Fig. 7.
Effects of NEM (10−4 M) on the inhibition of contraction induced by agonists. NEM blocked the effect of the exogenous inhibitor VIP (10−6 M) (#P < 0.001 by ANOVA) but had no effect on the inhibition of contraction induced by PGE2 (10−6 M) (an endogenous autacoid) and by 8B-cAMP (10−5 M) (receptor- and G protein-independent agonist). Values are means ± SE of n = 3.
P4 effects on PGF2α and PGE2 levels mediated by G proteins.
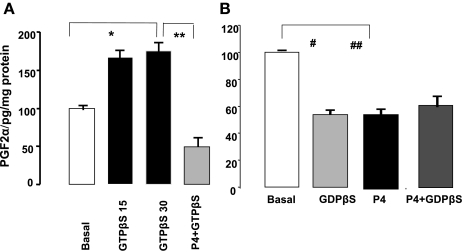
Because P4 alters the pattern of G proteins in smooth muscle cells, we examined in permeable muscle cells the role of G proteins in P4-induced changes in the levels of prostaglandins. Muscle cells were stimulated with GTPγS, a direct G protein activator (18), and with GDPβS, a nonspecific G protein blocker. GTPγS increased (Fig. 8A) and GDPβS reduced (Fig. 8B) the levels of PGF2α within 15 min (Fig. 8, A and B). Pretreatment of muscle cells with P4 (10−5 M) for 6 h prevented the GTPγS increase in PGF2α levels. In contrast, GDPβS lowered PGF2α levels and did not decrease them further in muscle cells pretreated with P4 for 6 h. These results suggest that P4 modulates the levels of PGF2α through its known effects on G protein patterns (10, 30). Like the results obtained with GDPβS, antibodies against Gq/11 proteins (1:400 titer) did not reduce the levels of PGF2α further in cells pretreated with P4 for 6 h.
Fig. 8.
A: GTPγS (10−4 M) increased the levels of PGF2α (*P < 0.02 by ANOVA). This increase was blocked by pretreating muscle cells with P4 (10−5 M) (**P < 0.02 by ANOVA). B: GDPβS (10−4 M) reduced the levels of PGF2α (#P < 0.01 by ANOVA). P4 (10−5 M) alone also reduced the levels of PGF2α (##P < 0.01 by ANOVA). However, the addition of GDPβS (10−4 M) to muscle cells pretreated with P4 had no additional effects (10−5 M). Values are means ± SE of n = 4.
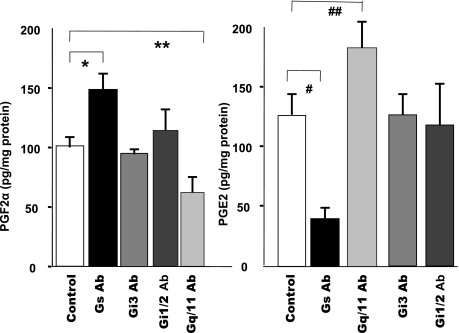
We next examined whether specific G proteins mediate the effects of P4 on the levels of PGF2α and PGE2. Permeable muscle cells were pretreated with antibodies (Abs) (1:400 titer) against specific G proteins Gαq/11, Gαi3, Gαi1/2, and Gαs for 60 min before their exposure to prostaglandins. Gαq/11 Abs reduced and Gαs Abs increased the basal levels of PGF2α (Fig. 9A). On the other hand, Abs against Gαs proteins decreased and Gαq/11 increased the levels of PGE2 (Fig. 9B). Abs against the proteins of Gαi had no effect on either prostaglandin.
Fig. 9.
The effects of G protein antibodies (Abs) on levels of PGF2α and PGE2 in permeable colon muscle cells from normal animals. Left: Abs against Gαq/11 decreased (*P < 0.01 by ANOVA) and Abs against Gαs increased the basal levels of PGF2α (**P < 0.01 by ANOVA). Right: Abs against Gαs lowered (#P < 0.005 by ANOVA) and Gαq/11 Abs increased (##P < 0.01 by ANOVA) the levels of PGE2. Values are means ± SE of n = 3.
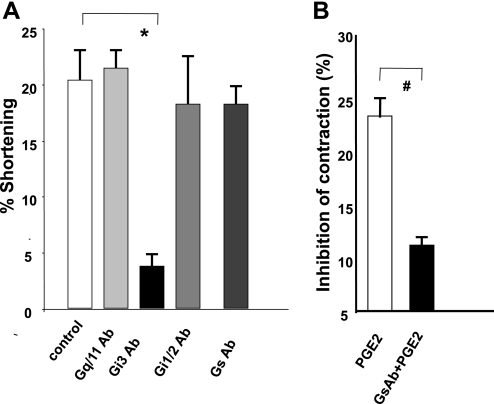
Moreover, Gαi3 Abs blocked the contraction induced by PGF2α, whereas Abs against Gαq/11, Gαs, and Gαi1/2 proteins had no effect (Fig. 10A). In contrast, Abs against Gs protein significantly reduced the PGE2-induced inhibition of contraction (10B). These results suggest that PGF2α generation is mediated by Gαq/11 protein, whereas its receptor appears to couple to Gαi3 proteins. Gs proteins appear to increase whereas Gq/11 proteins inhibit the levels of PGE2.
Fig. 10.
A: effect of G protein Abs on contraction induced by PGF2α (10−6 M) in normal permeable colon muscle cells. Abs against Gαi3 blocked the contraction induced by PGF2α (10−6 M) (*P < 0.01 by ANOVA), whereas Abs against Gαq/11, Gαi1/2, and Gαs had no effect. Values are means ± SE of n = 4. B: Gαs protein Ab treatment in normal permeable cells almost completely abolished the inhibition of contraction induced by PGE2 (10−6 M) compared with that induced in control muscle cells (#P < 0.005 by ANOVA). Values are means ± SE of n = 3.
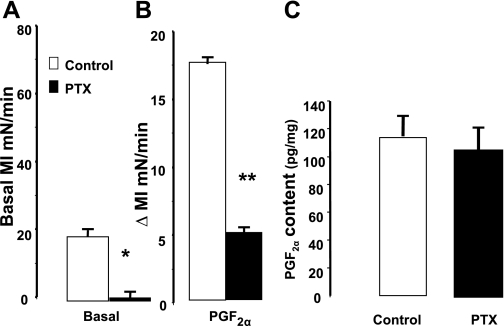
The role of these G proteins in PGF2α generation and in mediating induced contraction was supported by additional studies with PTX in muscle strips. PTX (100 μg/ml) treatment for 1 h had no effect on the genesis of PGF2α (Fig. 11C) but significantly reduced the basal MI (Fig. 11A) and blocked the contraction induced by PGF2α (Fig. 11B). PTX inhibits the functions of Gαi proteins.
Fig. 11.
Effect of pertussis toxin (PTX) (100 μg/ml) on muscle contraction induced by PGF2α (10−6 M) and basal levels of PGF2α in intact colon muscle cells. A: PTX reduced the basal MI (*P < 0.01 by ANOVA). B: it also decreased the contraction induced by PGF2α (10−6 M) (**P < 0.01 by ANOVA). C: PTX had no effect on the PGF2α levels. Values are means ± SE of n = 4.
DISCUSSION
The present studies show that P4 reduces the basal MI in guinea pigs in vivo by altering the normal levels of prostaglandin that induced contraction (PGF2α) and relaxation (PGE2). P4 decreases PGF2α levels because of downregulation of Gαq/11 proteins and increases PGE2 levels by upregulating Gαs proteins. P4 also reduces the basal MI by impairing the contraction induced by PGF2α through downregulation of Gαi3 proteins, and, by upregulating Gαs proteins, it may increase the relaxation induced by PGE2. Furthermore, consistent with previous observations, treatment with P4 in vivo had no effect on receptor- and G protein-independent agonists. P4 treatment had no effect on KCl that contracts muscle cells by increasing calcium influx through calcium channels and had no effect on muscle relaxation induced by 8B-cAMP, a second messenger that relaxes colon muscle cells through receptor- and G protein-independent mechanisms by stimulating protein kinase A (7). The specificity of the actions of P4 was confirmed by the finding that pretreatment with the P4 receptor antagonist RU-486 blocked the prostaglandin changes induced by P4. These findings suggest that the rising P4 levels during gestation are responsible for the changes in the pattern of G proteins (19, 32) and slower gastrointestinal transit (2, 23, 24, 26).
Selective and nonselective inhibitors of COX enzymes reproduced the effects of P4 on the basal MI of the colon muscle strips and on prostaglandin levels. COX-1 inhibitor reduced the basal MI and lowered PGF2α levels. In contrast, a COX-2 inhibitor increased the basal MI without altering the levels of PGF2α. It is possible that the increase in the basal MI induced by the COX-2 inhibitor was attributable to reductions in PGE2 levels. We have previously shown that constitutive levels of COX-2 enzyme generate PGE2 in human muscle cells (11).
PGF2α appears to contribute to the genesis of the colon basal MI because the specific PGF2α receptor antagonist Al-1810 blocked the contraction induced by this prostaglandin and abolished the basal MI of muscle strips treated with TTX. Al-1810, however, had no effect on the normal response of the colon muscle to ACh. It is likely that PGE2 also participates in the genesis of the basal MI by inhibiting the muscle contraction through EP2 and/or EP4 prostaglandin receptors known to mediate the inhibitory actions of this prostaglandin. However, further studies are needed using specific antagonists of the four PGE2 receptors in colon muscle strips to confirm this hypothesis.
These prostaglandins appear to be generated by the circular muscle cells acting as autacoids or paracrine factors. First, they are present in the colon muscle cells. Second, NEM, a nonspecific receptor blocker (13), did not block the PGF2α and PGE2 receptors because NEM-treated muscle strips and cells responded normally to exogenous PGF2α and PGE2. Third, NEM had no effect on the basal MI of TTX-treated muscle strips, suggesting that these prostaglandins generated by the muscle cells are occupying and protecting their receptors. In contrast, NEM blocked the effects induced by neurotransmitters ACh and VIP. These results are in agreement with previous reports showing that NEM blocks the contraction induced by exogenous ligands or neurotransmitters because it inactivates unoccupied membrane receptors.
Our findings also suggest that P4 modulates the levels of prostaglandins through its effects on G proteins. Treatment with GTPγS, the G protein activator, increased the levels of PGF2α levels, whereas GDPβS, the nonspecific G protein blocker, reduced its levels. Furthermore, specific antibodies against Gαq/11 proteins reduced PGF2α levels, whereas Gαs protein antibodies lowered the levels of PGE2. G proteins also mediate the prostaglandin effects on muscle contraction. PGF2α contracts muscle cells by acting on receptors that couple to Gαi3 proteins because its antibody and PTX, which inhibits all Gαi proteins, blocked the PGF2α-induced contraction. P4 is known to downregulate Gi3 proteins in muscle cells (5).
In summary, treatment with P4 in vivo decreases the basal MI by 1) reducing the levels of excitatory prostaglandins (PGF2α) and increasing the levels of inhibitory prostaglandins (PGE2) and 2) by blocking the actions of PGF2α and probably by increasing the inhibitory actions of PGE2. The effects of P4 on prostaglandins levels appear to be mediated through the P4 actions on G proteins and COX enzymes. Thus P4 may also affect the colon transit time by reducing the basal MI and neurotransmitter-induced motility.
GRANTS
This work was supported by the grant from National Institutes of Health R01-DK27389.
REFERENCES
- 1.Arkinstall SJ, Jones CT.Pregnancy suppresses G protein coupling to phosphoinositide hydrolysis in guinea pig myometrium. Am J Physiol Endocrinol Metab 259: E57–E65, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Baron Ramirez TH, Richter J. Gastrointestinal motility disorders during pregnancy. Ann Intern Med 118: 366–375, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Bitar KN, Makhlouf GM. Relaxation of isolated gastric smooth muscle cells by vasoactive intestinal polypeptide. Science 216: 531–533, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Cao W, Harnett KM, Behar J, Biancani P. PGF (2α)-induced contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Am J Physiol Gastrointest Liver Physiol 283: G282–G289, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Chitinavis V, Xiao ZL, Yu PR, Oh S, Biancani P, Behar J. Impaired G protein function in gallbladder muscle from progesterone-treated guinea pigs. Am J Physiol Gastrointest Liver Physiol 274: G283–G289, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Lee K, Xiao ZL, Biancani P, Behar J. Mechanism of gallbladder relaxation in the cat: role of norepinephrine. J Pharmacol Exp Ther 285: 475–479, 1998 [PubMed] [Google Scholar]
- 7.Chen Q, Amaral J, Oh S, Biancani P, Behar J. Gallbladder relaxation in patients with pigment and cholesterol stones. Gastroenterology 113: 930–937, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Xiao ZL, Biancani P, Behar J. Down regulation of Gαq-11 protein expression in guinea pig antral and colonic circular muscle during pregnancy. Am J Physiol Gastrointest Liver Physiol 276: G895–G900, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Cong P, Pricolo V, Biancani P, Behar J. High levels of caveolar cholesterol inhibit progesterone-induced genomic actions in human and guinea pig gallbladder muscle. Am J Physiol Gastrointest Liver Physiol 296: G948–G954, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cong P, Pricolo V, Biancani P, Behar J. Abnormalities of prostaglandins and cyclooxygenase enzymes in female patients with slow-transit constipation. Gastroenterology 133: 445–453, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Cong P, Xiao ZL, Biancani P, Behar J. Prostaglandins mediate tonic contraction of the guinea pig and human gallbladder. Am J Physiol Gastrointest Liver Physiol 292: G409–G418, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Datta S, Hey VM, Pleuvry BJ. Effects of pregnancy and associated hormones in mouse intestinal in vivo and in vitro. Pflügers Arch 346: 87–95, 1974 [DOI] [PubMed] [Google Scholar]
- 13.DeLegge M, Murthy KS, Grider JR, Makhlouf GM. Characterization of distinct receptors for the peptidyl leukotrie LTC4 and LTD4/LTE4 coupled to the same signaling pathway in isolated gastric muscle cells. J Pharmacol Exp Ther 266: 857–863, 1993 [PubMed] [Google Scholar]
- 14.Elwardy-Merezak J, Maltier JP, Cohen-Tannoudji J, Lecrivain JL, Vivat V, Legrand C. Pregnancy-related modifications of rat myometrial Gs proteins: ADP-ribosylation, immunoreactivity and gene expression studies. J Mol Endocrinol 13: 23–37, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Europe-Finner GN, Phaneuf S, Watson SP, Lopez BA. Identification and expression of G-proteins in human myometrium: up-regulation of G alpha s in pregnancy. Endocrinology 132: 2484–2490, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Griffen BW, Klimko P, Crider JY, Sharif NA. A novel prostaglandin F2α analog with selective antagonism effect at the prostaglandin F2α (FP) receptor. J Pharmacol Exp Ther 290: 1278–1284, 1999 [PubMed] [Google Scholar]
- 17.Guarino MPL, Xiao ZL, Biancani P, Behar J. PAF-induced gallbladder muscle contraction is mediated by different pathways in guinea pigs. Am J Physiol Gastrointest Liver Physiol 285: G1189–G1197, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Jin JG, Murthy KS, Grider JR, Makhlouf GM. Activation of distinct C'AMP- and cGMP-dependent pathways by relaxant agents in isolated gastric muscle cells. Am J Physiol Gastrointest Liver Physiol 264: G470–G477, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Joelle CT, Sakina M, Jamila EM, Lecrivain JL, Robin MT, Legrand C, Maltier JP. Regulation of myometrial Gi2, Gi3, and Gq expression during pregnancy: effect of progesterone and estradiol. Biol Reprod 53: 55–64, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Kubota Y, Nomura Kamm KN M, Mumby MC, Stull JT. GTPγS-dependent regulation of smooth muscle contractile elements. Am J Physiol Cell Physiol 262: C405–C410, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Misra S, Murthy KS, Zhou H, Grider JR. Coexpression of Y1, Y2 and Y4 receptors in smooth muscle coupled to distinct signaling pathways. J Pharmacol Exp Ther 311: 1154–1162, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Okamoto T, Ikezu T, Murayama Y, Ogata E, Nishimato I. Measurement of GTP gamma S binding to specific G proteins in membranes using G-protein antibodies. FEBS Lett 305: 125–128, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Ryan JP, Pellechia D. Effect of progesterone pretreatment on guinea pig gallbladder motility in vitro. Gastroenterology 83: 81–83, 1982 [PubMed] [Google Scholar]
- 24.Ryan JP. Effect of pregnancy on intestinal transit: comparison of results using radioactive and non-radioactive test meals. Life Sci 31: 2635–2640, 1982 [DOI] [PubMed] [Google Scholar]
- 25.Ryan JP, Bhojwani A, Wang MB. Effect of pregnancy on gastric motility in vivo and vitro in the guinea pig. Gastroenterology 93: 29–34, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Ryan JP, Bhojwani A. Colonic transit in rats: effect of ovariectomy, sex steroid hormones, and pregnancy. Am J Physiol Gastrointest Liver Physiol 251: G46–G50, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Scott LD, Lester R, Van Thiel DH, Wald A. Pregnancy related changes in small intestinal myoelectric activity in the rat. Gastroenterology 84: 301–305, 1983 [PubMed] [Google Scholar]
- 28.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology 129: 1518–1532, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Tanfin Z, Goureau O, Milligan G, Harbon S. Characterization of G proteins in rat myometrium: a differential modulation of Gi2a and Gi3a during gestation. FEBS Lett 278: 4–8, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Xiao ZL, Chen Q, Biancani P, Behar J. Mechanisms of pregnancy-induced gallbladder hypomotility in guinea pigs. Gastroenterology 116: 411–419, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Xiao ZL, Pricolo V, Biancani P, Behar J. Role of progesterone signaling in the regulation of G-protein levels in female chronic constipation. Gastroenterology 128: 667–75, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Xiao ZL, Chen Q, Biancani P, Behar J. Mechanisms of gallbladder hypomotility in pregnant guinea pigs. Gastroenterology 116: 411–9, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Yu PR, DePetris G, Biancani P, Amaral J, Behar J. Cholecystokinin-coupled intracellular signaling in human gallbladder muscle. Gastroenterology 106: 763–770, 1994 [DOI] [PubMed] [Google Scholar]