Abstract
The premature activation of digestive proenzymes, specifically proteases, within the pancreatic acinar cell is an early and critical event during acute pancreatitis. Our previous studies demonstrate that this activation requires a distinct pathological rise in cytosolic Ca2+. Furthermore, we have shown that a target of aberrant Ca2+ in acinar cells is the Ca2+/calmodulin-dependent phosphatase calcineurin (PP2B). In this study, we hypothesized that PP2B mediates in vivo protease activation and pancreatitis severity. To test this, pancreatitis was induced in mice over 8 h by administering hourly intraperitoneal injections of the cholecystokinin analog caerulein (50 μg/kg). Treatment with the PP2B inhibitor FK506 at 1 and 8 h after pancreatitis induction reduced trypsin activities by greater than 50% (P < 0.005). Serum amylase and IL-6 was reduced by 86 and 84% relative to baseline (P < 0.0005) at 8 h, respectively. Histological severity of pancreatitis, graded on the basis of pancreatic edema, acinar cell vacuolization, inflammation, and apoptosis, was reduced early in the course of pancreatitis. Myeloperoxidase activity from both pancreas and lung was reduced by 93 and 83% relative to baseline, respectively (P < 0.05). These data suggest that PP2B is an important target of the aberrant acinar cell Ca2+ rise associated with pathological protease activation and pancreatitis.
Keywords: acinar cell, tacrolimus, zymogen activation, calcium signaling, protein phosphatase 2B
acute pancreatitis is a life-threatening inflammatory disease that affects 1 to 2 in 1,000 patients each year (51). One quarter with severe disease die, and the overall mortality rate approaches 5–10%. Activation of secreted pancreatic proenzymes, or zymogens, normally occurs in the intestinal lumen. In contrast, the premature activation of zymogens, particularly proteases, within the pancreatic acinar cell plays an early and critical role in the pathogenesis of acute pancreatitis. This conclusion is based on four fundamental observations. First, severe pancreatitis presents with morphological changes that strongly resemble those that are typical of digestive necrosis (49). Second, prior to evidence of acinar cell injury, pancreatic and serum levels of activated proteases increase early in the course of disease (11). Third, trypsinogen activation and pancreatitis are blocked following pretreatment with serine protease inhibitors (40, 45). Fourth, mutations in the cationic trypsinogen gene that may lead to enhanced activation are precursors to hereditary pancreatitis (56).
An aberrant rise in cytosolic Ca2+ is required for this pathological protease activation (42, 45). Ca2+ concentrations are governed by a number of membrane pumps, buffers, second messengers, and Ca2+ channels (3). Following secretagogue-induced stimulation of acinar cells, the initial rise in Ca2+ is predominantly controlled by release from intracellular Ca2+ pools. We have recently shown that Ca2+ released by the intracellular Ca2+ channel, the ryanodine receptor (RyR), mediates premature protease activation (24). The RyR was selectively distributed in the basolateral region of the acinar cell, where protease activation is first observed. Relatively high basolateral Ca2+ elevations were dependent on RyR opening. Finally, RyR inhibition reduced premature protease activation in isolated acinar cells and in vivo. Thus, although aberrant Ca2+ release is central to the pathogenesis of protease activation and acute pancreatitis, targets of the pathological Ca2+ signal have not been identified.
An important target of Ca2+ in eukaryotic cells is the Ca2+/calmodulin-dependent serine/threonine phosphatase calcineurin (PP2B) (43). It is a heterodimeric protein with a catalytic subunit A (CN-A) and a regulatory Ca2+-binding subunit B (CN-B). The primary sequence of both subunits is highly conserved. PP2B is widely distributed in mammalian tissues and cells, including the pancreas and the pancreatic acinar cell. Although PP2B is regulated by several factors, including oxidative stress (53) and unsaturated long-chain fatty acids (26), its major activators are Ca2+ and calmodulin.
We hypothesized that PP2B is a key target of the aberrant acinar cell Ca2+ signals generated after a pancreatitis insult. We have previously shown in isolated acinar cells that PP2B inhibition reduces intra-acinar protease activation (23). In the present study, we examined whether PP2B mediates in vivo protease activation and subsequent long-term effects of pancreatitis. Acute treatment of mice with the specific PP2B inhibitor FK506 reduced intrapancreatic protease activation after 1 h of caerulein hyperstimulation and mitigated the inflammatory response over an 8-h time course. These data suggest that Ca2+-dependent events target PP2B activation early in pancreatitis and may modulate disease severity.
METHODS
Reagents and animals.
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Male Swiss-Webster mice (25–30 g) were purchased from Charles River Laboratories (Wilmington, MA). Mice were fed standard laboratory chow, given free access to water, and randomly assigned to control or experimental groups. All animal treatments and euthanasia protocols were approved by the Animal Care and Use Committee.
Induction of pancreatitis.
After a 12-h fast, pancreatitis was induced in mice by administering hourly intraperitoneal injections of caerulein (50 μg/kg body wt) (36). Saline-injected animals served as controls.
Preparation of serum and tissue samples.
Mice were euthanized at intervals between 1 and 8 h after the first intraperitoneal injection of caerulein. Whole blood samples were centrifuged at 5,000 g for 10 min at 4°C. Serum amylase and IL-6 was measured by use of Phadebas (Amersham Pharmacia, Rochester, NY) and Bio-plex cytokine assay kits (Bio-Rad, Hercules, CA), respectively. Tissue from pancreas and lung was fixed at room temperature for 2 h in 10% formalin solution with 125 mM phosphate buffer (pH 7.4) and then transferred to 70% ethanol. Paraffin-embedded sections were stained with hematoxylin and eosin and graded at ×20 magnification over three separate fields in a blinded manner by a gastrointestinal pathologist (D. Jain) for edema, acinar cell vacuole formation, inflammation, and apoptosis (modified from Ref. 57). Another portion of pancreas and lung was immediately frozen in liquid nitrogen and stored at −80°C. For measurement of trypsin activity, thawed pancreas samples were homogenized in iced medium containing 5 mM MOPS, 250 mM sucrose, and 1 mM MgSO4 (pH 7.0). Samples were then centrifuged at 5,000 g for 5 min at 4°C. Trypsin was measured in supernatant with use of a fluorogenic substrate as previously described (29). Myeloperoxidase (MPO) activity was performed as described (7). Briefly, thawed pancreas and lung samples were homogenized in 10 times the volume per tissue weight of 50 mM phosphate buffer (pH 7.4) containing protease inhibitors and centrifuged at 16,000 g for 15 min at 4°C. The pellet was resuspended in fresh buffer and again centrifuged. The resulting pellet was then resuspended in 100 mM phosphate buffer (pH 6.0) containing 0.5% hexadecylmethylammonium bromide and 10 mM EDTA. The suspension was disrupted three times by sonication and freeze-thaw and then centrifuged at 16,000 g for 15 min at 4°C. MPO was measured in supernatant by use of the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine. Protein was measured by a pyrogallol red-molybdate protein dye-binding assay (Bio-Rad, Hercules, CA). Phosphatase activity was measured from tissue lysates by using the Calbiochem Calcineurin Cellular Activity Assay Kit (EMD Biosciences, La Jolla, CA).
Western blot for HSP70 expression.
Blots were performed as previously described (4). Briefly, 25 mg of freshly isolated pancreas was snap frozen and stored at −80°C. Samples were then thawed and homogenized in 400 μl of buffer containing 1 mM EDTA, 10 mM Tris, 100 mM NaCl, and protease inhibitor cocktail (Roche, Indianapolis, IN). Protein content was assayed following the method of Bradford and 10 μg were loaded per lane on a 10% SDS gel. Proteins were transferred to a nitrocellulose membrane and heat shock protein 70 (HSP70) was probed for using a monoclonal antibody (Stressgen, SPA-810, Ann Arbor, MI). Bands were detected using horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:2,500) and ECL chemiluminescent reagents (Buckinghamshire, UK). Results were normalized to actin expression and quantified using densitometry.
Statistical analysis.
Data are expressed as means ± SD unless otherwise stated. Statistical significance was determined by a Student's t-test.
RESULTS
FK506 reduces pancreatic PP2B activity.
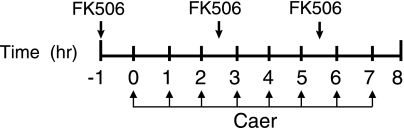
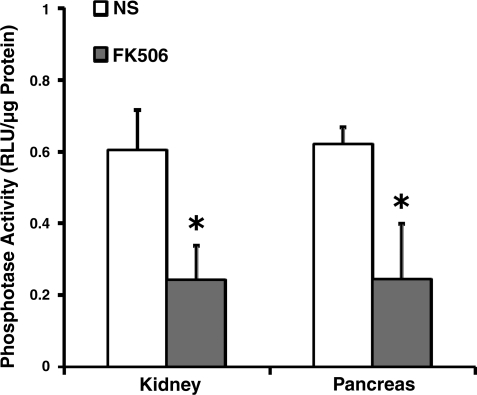
FK506 (1 mg/kg) was injected intraperitoneally in mice three times over an 8-h period to inhibit PP2B activity (Fig. 1) (22). We hypothesized that pancreatic PP2B activation modulates pancreatitis by inducing pathological intra-acinar protease activation, an event that occurs within minutes of a pancreatitis insult. For this reason, FK506 was administered prophylactically 1 h before caerulein hyperstimulation as well as therapeutically during the course of pancreatitis. The aim of the study was to inhibit pancreatic PP2B. FK506 administration reduced pancreatic PP2B phosphatase activity by 61% (Fig. 2). As a positive control of the FK506 dosing regimen in another tissue, PP2B activity was reduced by 60% in kidney.
Fig. 1.
Schema for in vivo Ca2+/calmodulin-dependent calcineurin (PP2B) inhibition using FK506 (1 mg/kg) and pancreatitis induction using caerulein (Caer; 50 μg/kg).
Fig. 2.
Pancreatic PP2B activity can be reduced with FK506 administration. Mice received intraperitoneal injections of FK506 (1 mg/kg) as described in methods and PP2B activity was assayed. Activity from kidney was used as a positive control (n = 3). NS, normal saline. *P < 0.05 compared with the saline-treated group.
FK506 reduces intrapancreatic protease activation.
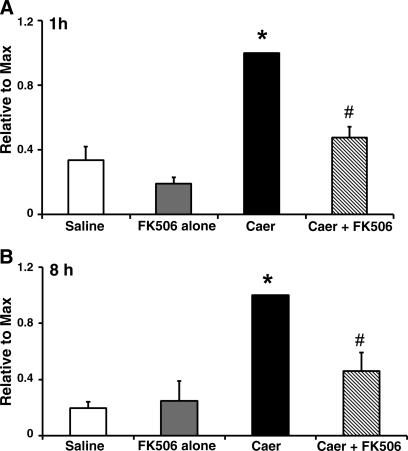
In C57BL/6 mice, intrapancreatic activation of trypsin with caerulein hyperstimulation peaks at 1 and 8 h (19). The trend appeared similar in our study with Swiss-Webster mice (Fig. 3). Intrapancreatic factors primarily mediate early protease activation, whereas at later time points immune cell infiltrates also play a role (16). Notably, FK506 treatment reduced trypsin activity by 82% relative to basal levels at 1 h vs. 52% at 8 h postcaerulein hyperstimulation. There was no change in trypsin activity with FK506 administration alone at 1 and 8 h. At the early time point, pancreatic PP2B might be more influential than nonpancreatic sources. The results are consistent with our previous study, which demonstrated that FK506 pretreatment of isolated acinar cells reduced intra-acinar protease activation 1 h after caerulein hyperstimulation (23). To confirm that the in vivo results were not strain specific, a reduction in protease activation was also shown in limited experiments with C57BL/6 mice (data not shown).
Fig. 3.
FK506 treatment reduces pancreatic trypsin activity in vivo. Trypsin activity was assayed from pancreatic homogenates 1 (A) and 8 h (B) after the first caerulein dose (n = 5 animals per group). *, #P < 0.005 compared with saline-treated and caerulein-alone groups, respectively. Values are expressed as means ± SE and are normalized to a maximum value.
FK506 reduces inflammation in pancreatitis.
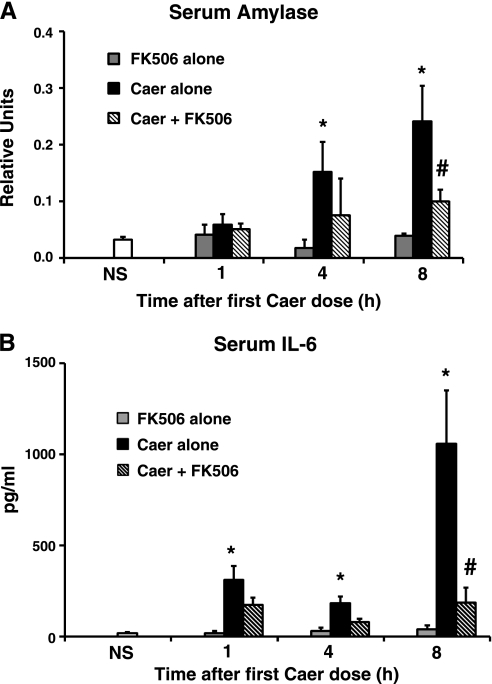
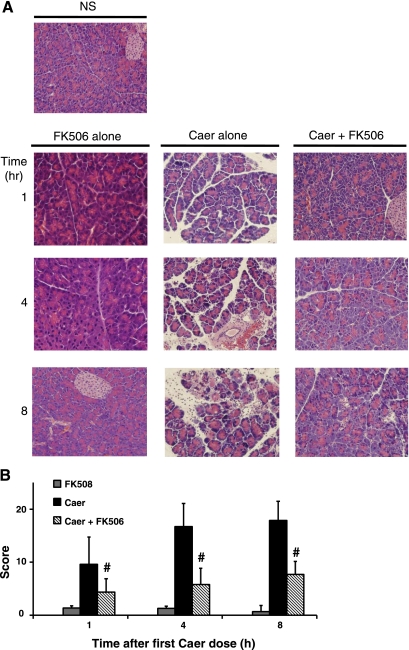
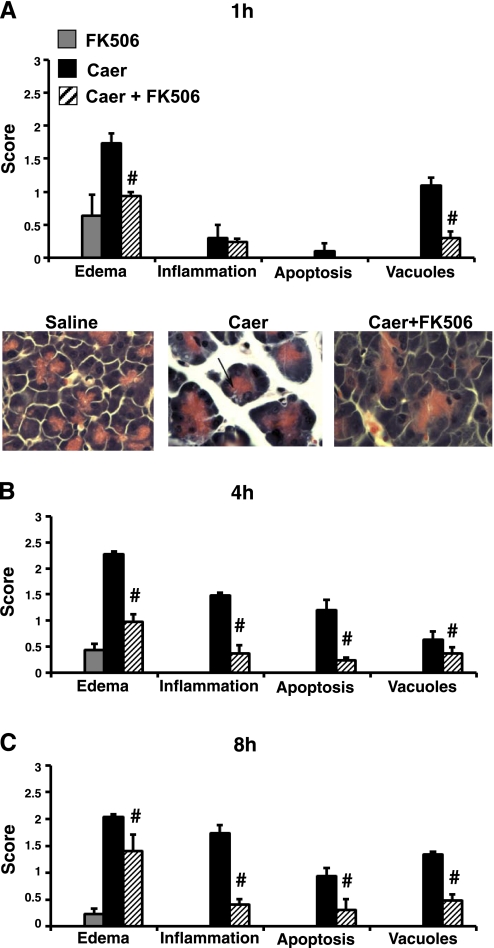
A time-dependent linear rise in serum amylase was observed with caerulein hyperstimulation over 8 h (Fig. 4). FK506 treatment reduced serum amylase levels by 86% at 8 h, compared with basal levels. Serum IL-6 followed a similar trend; FK506 reduced levels at 1, 4, and 8 h by 47, 63, and 84%, respectively. It has been shown in previous studies that caerulein hyperstimulation caused a mild to moderate interstitial pancreatitis with features of pancreatic edema, basolateral vacuole formation in acinar cells, inflammatory infiltration, and apoptosis (28). Consistent with these findings, we report that FK506 treatment reduced the cumulative score at 1, 4, and 8 h by 56, 67, and 58%, respectively (Fig. 5). Saline-only and FK506-alone-treated animals had no increase in any of these parameters. Edema and vacuolization appeared within the first 1 h, consistent with previous reports (21, 40, 42, 48, 55). FK506 treatment reduced those parameters at the early time point (Fig. 6). At later time points, all parameters were significantly reduced, including inflammation and apoptosis. Mice receiving FK506 alone at 1, 4, and 8 h were indistinguishable in serum levels or histological analysis from control saline-only treated animals (Figs. 4 and 5A).
Fig. 4.
FK506 treatment reduces serum amylase and IL-6 elevations observed during pancreatitis. Caerulein hyperstimulation caused a time-dependent increase in serum amylase and IL-6. FK506 administration reduced levels as early as 4 h (n = 5 animals per group). *P < 0.005; #P < 0.05 compared with saline-treated and caerulein-alone groups, respectively. SE shown for IL-6. Serum values from FK506-only treated animals did not rise above baseline at 1, 4, or 8 h after caerulein stimulation (shaded bar; n = 3). Representative images at 1 h demonstrate edema and vacuolization (arrow).
Fig. 5.
FK506 treatment reduces histological severity of pancreatitis. A: representative hematoxylin and eosin sections of pancreas from saline- (top left) or caerulein-treated animals over an 8-h time course. B: histological scoring of pancreatitis severity with or without FK506 administration (n = 5 animals per group). #P < 0.05, P < 0.001, P < 0.005 compared with caerulein-alone groups at 1, 4, and 8 h, respectively.
Fig. 6.
Analysis of histological parameters shows that FK506 treatment causes a reduction in severity early in the course of pancreatitis. A score of 0–3 was given on the basis of the degree of edema, inflammation, apoptosis, and vacuole formation at 1 (A), 4 (B), and 8 h (C) after the first caerulein dose (n = 5 animals per group). #P < 0.005 compared with caerulein-alone groups. All parameters were minimally increased for saline controls or FK506-alone animals. Representative images demonstrate edema and vacuolization (arrow).
Tissue MPO activity measures neutrophil sequestration and is a reliable marker of the acute inflammatory response during pancreatitis. In pancreas, caerulein treatment by 8 h caused an increase in MPO of 15-fold above the saline-only treated group, FK506 caused a 93% reduction relative to basal levels (Table 1). In lung, within 4 h, a 4.4-fold increase was observed with caerulein treatment, but FK506 reduced MPO levels by 83%. These data demonstrate that FK506 treatment mitigates the effects of pancreatitis early on, as noted by a reduction in edema and acinar vacuole formation. This attenuation is further exhibited at later time points with findings of reduced inflammation.
Table 1.
FK506 treatment reduces pancreatic and lung MPO activity
| Saline | Caer | Caer+FK506 | |
|---|---|---|---|
| Pancreas | 0.02+0.01 | 0.30+0.19* | 0.04+0.01† |
| Lung | 0.16+0.09 | 0.70+0.07* | 0.25+0.12† |
Results are expressed as RLU/μg protein.
P < 0.05 compared with saline- and caerulein (caer)-only treated groups, respectively.
FK506 does not increase HSP70 expression.
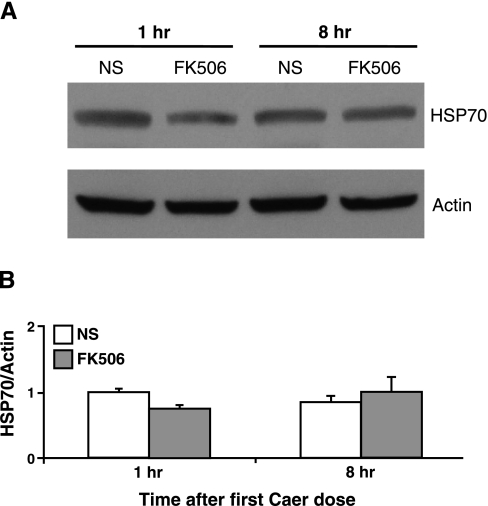
Although FK506 is a highly specific PP2B inhibitor, isolated reports in kidney suggest that it can increase the expression of HSP70 independent of PP2B (4). HSP70 has recently been shown to be protective against the development of pancreatitis (33, 44). However, Western blotting for HSP70 in pancreas from mice treated with FK506 demonstrated no effect at 1 or 8 h postadministration (Fig. 7).
Fig. 7.
The FK506 dosing regimen does not affect pancreatic heat shock protein 70 (HSP70) expression. A: pancreatic tissue was isolated for Western blot analysis from mice receiving hourly injections of normal saline or FK506 (1 mg/kg) after 1 and 8 h of treatment. Representative blot demonstrates no increase in HSP70 expression with FK506 treatment. B: quantification of HSP70 expression, normalized to actin (n = 3).
DISCUSSION
In this study, we have shown that acute treatment in vivo with the PP2B inhibitor FK506 reduced early protease activation as well as later indexes of inflammation associated with pancreatitis. Physiologically, PP2B is necessary for CCK-induced acinar cell growth (17, 46, 50) and may have a modest effect on enzyme secretion (8, 13, 23, 54). Pathological roles have also been described in other tissues such as in heart, where activation of PP2B produces cardiac hypertrophy (20). The findings of our studies are an extension of our previous work in isolated acinar cells, which demonstrated that inhibition of acinar cell PP2B reduced intra-acinar protease activation. The present study indirectly supports the postulate that a Ca2+-dependent acinar cell cascade is primarily responsible for the pathological activation of proteases. Previous evidence for this dependence comes from studies that examine chelation of cytosolic Ca2+ by BAPTA. When BAPTA is administered either to CCK-stimulated acinar cells in isolation (42, 45) or during in vivo bile duct ligation (32), trypsinogen activation is blocked. Furthermore, we and others have shown that an aberrant pattern of acinar cell Ca2+ signaling is necessary for protease activation. This is either due to a shift of intracellular Ca2+ release from an apical to a basolateral region of the cell (24) and/or due to sustained cytosolic Ca2+ elevations throughout the cell (42). Although the cellular targets of this pathological Ca2+ signal remain unclear, here we report a role, in vivo, for the Ca2+/calmodulin-dependent phosphatase PP2B acting as a Ca2+ sensor in the cascade, leading to protease activation. The regulatory subunit CN-B contains four high-affinity Ca2+ binding EF-hand domains, which in conjunction with calmodulin mediate the phosphatase activity of the catalytic subunit CN-A (1). Among several known PP2B phosphatase substrates, Ca2+-regulated heat-stable protein of 24 kDa (CRHSP-24) may contribute to premature protease activation (5, 14). CRHSP-24 is confined to the basolateral region of the acinar cell, and this localization overlaps with intracellular protease activation.
We used FK506 in our study because it is a highly specific PP2B inhibitor. It forms a complex with FK506-binding protein (FKBP) that hinders protein substrates from approaching the PP2B catalytic site (9, 12). We decided to use FK506 over another PP2B inhibitor cyclosporine (CsA) because, unlike FK506, CsA is known to affect mitochondrial pathways by inhibiting the mitochondrial permeability transition pore in the pancreatic acinar cell (2, 38). Although FK506 is a potent PP2B inhibitor, there may be less common PP2B-independent effects contributing to the improvement in pancreatitis. For example, in the thymus, FK506 can associate with HSP90 (27, 41) and, in the kidney, it can induce expression of HSP70 (59), proteins which have recently been shown to limit acinar cell injury during pancreatitis (2, 34). It was therefore possible that the ameliorative effects of FK506 were due to induction of HSP70. We, however, saw no elevation of HSP70 expression with FK506 treatment, consistent with our hypothesis that FK506 is acting primarily through PP2B inhibition.
The results of the study further substantiate a role for FK506 in long-term effects of pancreatitis such as inflammation. They suggest that treatment with FK506, a widely used immunosuppressant, might be clinically useful in reducing the severity of pancreatitis. Previous reports of FK506 show conflicting results. A few clinical case reports suggest an association of FK506 use with pancreatitis (31, 37, 39, 47, 52). However, in the experimental literature, some reports demonstrate improvement of disease with FK506 (15, 30, 58), whereas others show worsening (10, 25) or no effect (18). An examination of both the clinical and experimental papers suggests that chronic FK506 exposure may actually predispose individuals to pancreatitis whereas acute doses improve outcome. Although we have not critically examined this posit, our data would support a beneficial role of acute, prophylactic, and intercurrent FK506 administration. The results may imply that FK506 treatment might reduce the risk of pancreatitis in high-risk situations, such as during endoscopic retrograde cholangiopancreatography.
Although we hypothesize that inhibition of pancreatic acinar PP2B leads to a reduction in protease activation and pancreatitis, a limitation of the present study is its inability to determine the primary PP2B tissue source involved. Although the observed reduction in protease activation, edema, and vacuole formation with FK506 within the first 1 h of pancreatitis induction supports our hypothesis, two major sources of PP2B expression, lymphocytes and neurons, might also contribute to the inflammatory response (35, 58). Future genetic studies designed to selectively knock down PP2B in acinar cells, for example using an elastase-specific promoter, are necessary. Another approach that examines the contribution of immune cells would be to transplant wild-type bone marrow into PP2B-deficient transgenics and thereby establish a mouse chimera that produces immune cells without a PP2B defect. Yet despite the potential sources of PP2B that might contribute to the pancreatitis response, we report here that FK506 treatment, given acutely in vivo, reduces protease activation and long-term indexes of inflammation associated with pancreatitis. The findings support our hypothesis that PP2B is an important target of the aberrant Ca2+ signal generated early in the course of pancreatitis.
GRANTS
This work was supported by National Institutes of Health Grants R01 DK083327, R03 DK078707, K08 DK68116, K12 HD001401, and DK34989 (Yale Liver Center), and a Children's Digestive Health and Nutrition Young Investigator Award (to S. Z. Husain).
ACKNOWLEDGMENTS
We thank Drs. F. Gorelick and M. Nathanson for helpful discussion throughout the study.
REFERENCES
- 1.Aitken A, Klee CB, Cohen P. The structure of the B subunit of calcineurin. Eur J Biochem 139: 663–671, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Baggaley EM, Elliott AC, Bruce JIE. Oxidant-induced inhibition of the plasma membrane Ca2+-ATPase in pancreatic acinar cells: role of the mitochondria. Am J Physiol Cell Physiol 295: C1247–C1260, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bhagat L, Singh VP, Hietaranta AJ, Agrawal S, Steer ML, Saluja AK. Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation. J Clin Invest 106: 81–89, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhuri A, Kolodecik TR, Gorelick FS. Effects of increased intracellular cAMP on carbachol-stimulated zymogen activation, secretion, and injury in the pancreatic acinar cell. Am J Physiol Gastrointest Liver Physiol 288: G235–G243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawra R, Ku YS, Sharif R, Dhaulakhandi D, Phillips P, Dudeja V, Saluja AK. An improved method for extracting myeloperoxidase and determining its activity in the pancreas and lungs during pancreatitis. Pancreas 37: 62–68, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Doi R, Inoue K, Chowdhury P, Kaji H, Rayford PL. Structural and functional changes of exocrine pancreas induced by FK506 in rats. Gastroenterology 104: 1153–1164, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Dumont FFJ. FK506, an immunosuppressant targeting calcineurin function. Curr Med Chem 7: 731–748, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Echigo Y, Inoue K, Kogire M, Doi R, Higashide S, Sumi S, Kaji H, Imamura M. Effects of cyclosporine and tacrolimus (FK 506) on acute pancreatitis in mice. Arch Surg 130: 64–68, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Grady T, Saluja A, Kaiser A, Steer M. Edema and intrapancreatic trypsinogen activation precede glutathione depletion during caerulein pancreatitis. Am J Physiol Gastrointest Liver Physiol 271: G20–G26, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Griffith JP, Kim JL, Kim EE, Sintchak MD, Thomson JA, Fitzgibbon MJ, Fleming MA, Caron PR, Hsiao K, Navia MA. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell 82: 507–522, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Groblewski GE, Wagner AC, Williams JA. Cyclosporin A inhibits Ca2+/calmodulin-dependent protein phosphatase and secretion in pancreatic acinar cells. J Biol Chem 269: 15111–15117, 1994 [PubMed] [Google Scholar]
- 14.Groblewski GE, Yoshida M, Bragado MJ, Ernst SA, Leykam J, Williams JA. Purification and characterization of a novel physiological substrate for calcineurin in mammalian cells. J Biol Chem 273: 22738–22744, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Gukovskaya AS, Hosseini S, Satoh A, Cheng JH, Nam KJ, Gukovsky I, Pandol SJ. Ethanol differentially regulates NF-kappaB activation in pancreatic acinar cells through calcium and protein kinase C pathways. Am J Physiol Gastrointest Liver Physiol 286: G204–G213, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, Holland S, Pandol SJ. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology 122: 974–984, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Gurda GT, Guo L, Lee SH, Molkentin JD, Williams JA. Cholecystokinin activates pancreatic calcineurin-NFAT signaling in vitro and in vivo. Mol Biol Cell 19: 198–206, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackert T, Pfeil D, Hartwig W, Fritz S, Gebhard MM, Klar E, Werner J. Ciclosporin aggravates tissue damage in ischemia reperfusion-induced acute pancreatitis. Pancreas 32: 145–151, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest 106: 773–781, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hofbauer B, Saluja AK, Lerch MM, Bhagat L, Bhatia M, Lee HS, Frossard JL, Adler G, Steer ML. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol 275: G352–G362, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Karl IE. Dantrolene ameliorates the metabolic hallmarks of sepsis in rats and improves survival in a mouse model of endotoxemia. Proc Natl Acad Sci USA 91: 3039–3043, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husain SZ, Grant WM, Gorelick FS, Nathanson MH, Shah AU. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol 292: G1594–G1599, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proc Natl Acad Sci USA 102: 14386–14391, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito T, Kimura T, Furukawa M, Yamaguchi H, Goto M, Nakano I, Nawata H. Protective effects of gabexate mesilate on acute pancreatitis induced by tacrolimus (FK-506) in rats in which the pancreas was stimulated by caerulein. J Gastroenterol 29: 305–313, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Kessen U, Schaloske R, Aichem A, Mutzel R. Ca2+/calmodulin-independent activation of calcineurin from Dictyostelium by unsaturated long chain fatty acids. J Biol Chem 274: 37821–37826, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Klettner A, Herdegen T. FK506 and its analogs — therapeutic potential for neurological disorders. Curr Drug Targets 2: 153–162, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol 373: 97–117, 1977 [DOI] [PubMed] [Google Scholar]
- 29.Lu Z, Karne S, Kolodecik T, Gorelick FS. Alcohols enhance caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 282: G501–G507, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer JM, Laine VJ, Gezgin A, Kolodziej S, Nevalainen TJ, Storck M, Beger HG. Single doses of FK506 and OKT3 reduce severity in early experimental acute pancreatitis. Eur J Surg 166: 734–741, 2000 [DOI] [PubMed] [Google Scholar]
- 31.McDiarmid SV, Klintmalm G, Busuttil RW. FK 506 rescue therapy in liver transplantation: outcome and complications. Transplant Proc 23: 2996–2999, 1991 [PubMed] [Google Scholar]
- 32.Mooren F, Hlouschek V, Finkes T, Turi S, Weber IA, Singh J, Domschke W, Schnekenburger J, Kruger B, Lerch MM. Early changes in pancreatic acinar cell calcium signaling after pancreatic duct obstruction. J Biol Chem 278: 9361–9369, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Moretti AI, Rios EC, Soriano FG, de Souza HP, Abatepaulo F, Barbeiro DF, Velasco IT. Acute pancreatitis: hypertonic saline increases heat shock proteins 70 and 90 and reduces neutrophil infiltration in lung injury. Pancreas 38: 507–514, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Moretti AI, Rios EC, Soriano FG, de Souza HP, Abatepaulo F, Barbeiro DF, Velasco IT. Acute pancreatitis: hypertonic saline increases heat shock proteins 70 and 90 and reduces neutrophil infiltration in lung injury. Pancreas 38: 507–514, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Nathan JD, Peng RY, Wang Y, McVey DC, Vigna SR, Liddle RA. Primary sensory neurons: a common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol 283: G938–G946, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Niederau C, Ferrell LD, Grendell JH. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology 88: 1192–1204, 1985 [DOI] [PubMed] [Google Scholar]
- 37.Nieto Y, Russ P, Everson G, Bearman SI, Cagnoni PJ, Jones RB, Shpall EJ. Acute pancreatitis during immunosuppression with tacrolimus following an allogeneic umbilical cord blood transplantation. Bone Marrow Transplant 26: 109–111, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Odinokova IV, Sung KF, Mareninova OA, Hermann K, Evtodienko Y, Andreyev A, Gukovsky I, Gukovskaya AS. Mechanisms regulating cytochrome c release in pancreatic mitochondria. Gut 58: 431–442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogunseinde BA, Wimmers E, Washington B, Iyob M, Cropper T, Callender CO. A case of tacrolimus (FK506)-induced pancreatitis and fatality 2 years postcadaveric renal transplant. Transplantation 76: 448–448, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Otani T, Chepilko SM, Grendell JH, Gorelick FS. Codistribution of TAP and the granule membrane protein GRAMP-92 in rat caerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 275: G999–G1009, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Peattie DA, Harding MW, Fleming MA, DeCenzo MT, Lippke JA, Livingston DJ, Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci USA 89: 10974–10978, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, Petersen OH. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA 97: 13126–13131, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev 80: 1483–1521, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Saluja A, Dudeja V. Heat shock proteins in pancreatic diseases. J Gastroenterol Hepatol 23, Suppl 1: S42–S45, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer ML. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 276: G835–G842, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Sans MD, Williams JA. Calcineurin is required for translational control of protein synthesis in rat pancreatic acini. Am J Physiol Cell Physiol 287: C310–C319, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Sastry J, Young S, Shaw PJ. Acute pancreatitis due to tacrolimus in a case of allogeneic bone marrow transplantation. Bone Marrow Transplant 33: 867–868, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Sherwood MW, Prior IA, Voronina SG, Barrow SL, Woodsmith JD, Gerasimenko OV, Petersen OH, Tepikin AV. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA 104: 5674–5679, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steer ML, Saluja AK. Experimental acute pancreatitis: studies of the early events that lead to cell injury. In: The Pancreas: Biology, Pathobiology, and Disease, edited by Go VL, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA. New York: Raven, 1993, p. 489–500 [Google Scholar]
- 50.Tashiro M, Samuelson LC, Liddle RA, Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol 286: G784–G790, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Topazian M, Gorelick FS. Acute pancreatitis. In: Textbook of Gastroenterology, edited by Yamada T, Alpers DH, Kaplowitz N, Laine L, Owyang C, Powell DW. Philadelphia, PA: Lippincott Williams & Wilkins, 2003, p. 2026–2060 [Google Scholar]
- 52.Uemoto S, Tanaka K, Honda K, Tokunaga Y, Sano K, Katoh H, Yamamoto E, Takada Y, Ozawa K. Experience with FK506 in living-related liver transplantation. Transplantation 55: 288–292, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Culotta VC, Klee CB. Superoxide dismutase protects calcineurin from inactivation. Nature 383: 434–437, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Waschulewski IH, Hall DV, Kern HF, Edwardson JM. Effects of the immunosuppressants cyclosporin A and FK 506 on exocytosis in the rat exocrine pancreas in vitro. Br J Pharmacol 108: 892–900, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe O, Baccino FM, Steer ML, Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol 246: G457–G467, 1984 [DOI] [PubMed] [Google Scholar]
- 56.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 14: 141–145, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Wildi S, Kleeff J, Mayerle J, Zimmermann A, Bottinger EP, Wakefield L, Buchler MW, Friess H, Korc M. Suppression of transforming growth factor β signalling aborts caerulein induced pancreatitis and eliminates restricted stimulation at high caerulein concentrations. Gut 56: 685–692, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada T, Hashimoto T, Sogawa M, Kobayashi S, Kaneda K, Nakamura S, Kuno A, Sano H, Ando T, Kobayashi S, Aoki S, Nakazawa T, Ohara H, Nomura T, Joh T, Itoh M. Role of T cells in development of chronic pancreatitis in male Wistar Bonn/Kobori rats: effects of tacrolimus. Am J Physiol Gastrointest Liver Physiol 281: G1397–G1404, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Yang CW, Ahn HJ, Han HJ, Kim WY, Li C, Shin MJ, Kim SK, Park JH, Kim YS, Moon IS, Bang BK. Pharmacological preconditioning with low-dose cyclosporine or FK506 reduces subsequent ischemia/reperfusion injury in rat kidney. Transplantation 72: 1753–1759, 2001 [DOI] [PubMed] [Google Scholar]