Abstract
Sustained activation of the c-Jun NH2-terminal kinase (JNK) signaling pathway mediates the development and progression of experimental diet-induced nonalcoholic fatty liver disease (NAFLD). Delineating the mechanism of JNK overactivation in the setting of a fatty liver is therefore essential to understanding the pathophysiology of NAFLD. Both human and experimental NAFLD are associated with oxidative stress and resultant lipid peroxidation, which have been proposed to mediate the progression of this disease from simple steatosis to steatohepatitis. The ability of oxidants and the lipid peroxidation product 4-hydroxynonenal (HNE) to activate JNK signaling suggested that these two factors may act synergistically to trigger JNK overactivation. The effect of HNE on hepatocyte injury and JNK activation was therefore examined in cells under chronic oxidant stress from overexpression of the prooxidant enzyme cytochrome P450 2E1 (CYP2E1), which occurs in NAFLD. CYP2E1-generated oxidant stress sensitized a rat hepatocyte cell line to death from normally nontoxic concentrations of HNE. CYP2E1-overexpressing cells underwent a more profound depletion of glutathione (GSH) in response to HNE secondary to decreased γ-glutamylcysteine synthetase activity. GSH depletion led to overactivation of JNK/c-Jun signaling at the level of mitogen-activated protein kinase kinase 4 that induced cell death. Oxidant stress and the lipid peroxidation product HNE cause synergistic overactivation of the JNK/c-Jun signaling pathway in hepatocytes, demonstrating that HNE may not be just a passive biomarker of hepatic oxidant stress but rather an active mediator of hepatocellular injury through effects on JNK signaling.
Keywords: nonalcoholic fatty liver disease, glutathione, lipid peroxidation
the mitogen-activated protein kinase (MAPK) c-Jun-NH2-terminal kinase (JNK) regulates multiple cellular processes that are important in nonalcoholic fatty liver disease (NAFLD) including lipid metabolism, insulin resistance, and cell death. Recent studies have demonstrated that sustained JNK activation occurs in diet-induced models of murine steatohepatitis (33, 39). Both methionine- and choline-deficient and high-fat diet-induced steatohepatitis are associated with increased hepatic levels of phosphorylated JNK and its downstream target c-Jun (33, 39). In diet-treated jnk1 knockout mice, steatosis and liver injury were markedly decreased, demonstrating a critical function for JNK1 in the development of steatohepatitis (33, 39). An antisense oligonucleotide-induced knockdown of JNK1 in established steatohepatitis significantly decreased the degree of steatosis and liver injury, indicating that JNK1 function is also essential for the maintenance/progression of fat accumulation and hepatitis (39). Understanding the mechanism of JNK overactivation in fatty liver disease is critical to NAFLD prevention and treatment. However, the mechanism of hepatocyte JNK overactivation in the setting of steatosis is not known.
Oxidant stress has been implicated as a causal factor in NAFLD development. Both human and experimental NAFLD are associated with chronic oxidative stress and the accumulation of lipid peroxidation products including 4-hydroxynonenal (HNE) (6, 17, 21). The source of oxidative stress is controversial but may result in part from overexpression of the prooxidant enzyme cytochrome P450 2E1 (CYP2E1), which occurs in human and experimental NAFLD (7, 21, 43, 44). Reactive oxygen species (ROS) generated by CYP2E1 or other sources may mediate the progression from steatosis to hepatocyte injury by two mechanisms (11). The first is through impaired cellular function resulting from the direct oxidative modification of cellular macromolecules including lipids, proteins, and DNA. The second mechanism, which has been increasingly implicated in tissue injury, is by direct activation of cell death signaling pathways such as in the induction of hepatocyte apoptosis from menadione-generated superoxide by JNK/c-Jun activation (12). Activation of cell death signaling cascades may occur not only from ROS but also from oxidized byproducts of ROS generation. In particular, the lipid peroxidation product HNE has been shown to activate JNK (5, 31).
As a physiologically relevant model of the involvement of chronic hepatic oxidative stress in hepatocyte injury, the effects of oxidant stress generated by stable CYP2E1 overexpression have been investigated in vitro. Interestingly, CYP2E1 overexpression protected rather than sensitized hepatocytes, hepatoma cells, and NIH3T3 cells to death from acute menadione-generated oxidative stress (15, 19, 28). The explanation of this finding is that cells chronically stimulated by a potentially injurious oxidative stress develop mechanisms of resistance to ROS toxicity. However, it remains possible that injury is promoted by oxidized end products resulting from CYP2E1-dependent oxidant stress. In particular, the fact that CYP2E1 overexpression (22) and HNE (5, 31) are both known stimuli of JNK/c-Jun signaling suggested the hypothesis that ROS and HNE generated from CYP2E1 overexpression and/or other sources of oxidative stress may act in synergy to induce deleterious JNK overactivation.
To test this hypothesis, the ability of chronic oxidant stress from CYP2E1 expression to sensitize hepatocytes to death from HNE was examined. CYP2E1 overexpression sensitized cells to death from usually nontoxic concentrations of HNE. Death was mediated by sustained JNK/c-Jun activation resulting from cellular depletion of the antioxidant glutathione (GSH). Thus the increased levels of ROS and HNE that occur in the setting of a steatotic liver may synergistically activate JNK/c-Jun signaling that promotes liver injury.
MATERIALS AND METHODS
Cells and culture conditions.
Experiments were performed in the rat hepatocyte line RALA255-10G (RALA) cultured as previously described (20). This hepatocyte cell line is conditionally immortalized with a mutant SV40 virus that expresses a temperature-sensitive T antigen (9). Cells were cultured in DMEM (Mediatech, Herndon, VA) supplemented with 4% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and antibiotics (Invitrogen, Carlsbad, CA) at the permissive temperature of 33°C. For experiments, cells were cultured in DMEM, 2% fetal bovine serum, antibiotics, and 1 μM dexamethasone at the restrictive temperature of 37°C for 3 days and then placed in serum-free medium containing dexamethasone for 18 h. Under these conditions, T antigen expression is suppressed and the cells are nontransformed and display a differentiated hepatocyte phenotype (9, 10). From wild-type RALA cells, clonal cell lines with increased CYP2E1 expression were established by stable transfection with a pCI-Neo expression vector that contained the human CYP2E1 cDNA, as described previously (19). Clones with increased CYP2E1 expression (S-CYP cells) were identified by both Western blotting for CYP2E1 protein levels and chlorzoxazone assay for enzyme activity (19, 34). A polyclonal cell line transfected with the pCI-Neo vector lacking any insert (VEC cells) was also established and served as a control (34).
Cells were treated with HNE (EMD Chemicals, San Diego, CA) at the concentrations indicated, diethyl maleate (DEM; 0.3 mM), or varied concentrations of malondialdehyde (MDA) (Sigma, St. Louis, MO). Some HNE-treated cells were pretreated for 1 h with GSH ethyl ester (2 mM) or PD980159 (25 μM) (EMD Chemicals). Cells were also treated with catalase-polyethylene glycol (1,000 U/ml) (Sigma) at 48, 24, and 0.5 h before HNE treatment.
MTT assay.
Cell death was quantified by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (29). At 24 h after treatment, the cell culture medium was aspirated, and an equal volume of a 1 mg/ml MTT solution, pH 7.4, in DMEM was added to the cells. After incubation at 37°C for 1 h, the MTT solution was discarded, and 1.5 ml of N-propyl alcohol was added to solubilize the formazan product. The absorbance of this compound was measured at 560 nm in a spectrophotometer. The percentage of cell death was calculated by dividing the optical density of a treatment group by the optical density for untreated, control cells, multiplying by 100, and subtracting that number from 100.
Fluorescence microscopy.
The numbers of apoptotic and necrotic cells were quantified by fluorescence microscopy after costaining with acridine orange and ethidium bromide, as previously described (24). Cells with shrunken cytoplasm and condensed or fragmented nuclei as determined by acridine orange staining were considered apoptotic. Necrotic cells were detected by positive staining with ethidium bromide. A minimum of 400 cells per dish were examined, and the numbers of apoptotic and necrotic cells expressed as a percentage of the total number of cells that were counted.
ROS assay.
ROS levels were measured by means of the 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA) assay. Cells were treated with DCFH-DA (6.25 μg/ml) alone, or in combination with 85 μM HNE or 3.5 mM H2O2 (Sigma). After 2 h, the cells were washed and lysed in 0.1% Triton X-100, and the fluorescent intensity of the lysate was determined at excitation and emission wavelengths of 492 and 520 nm, respectively. Background fluorescence was determined from vehicle-treated cells and subtracted from the sample values. Values were expressed as relative fluorescent intensity compared with untreated VEC cells.
HNE-adduct ELISA.
Levels of HNE-histidine protein adducts were measured by ELISA using a commercial kit (Cell Biolaboratories, San Diego, CA).
GST activity.
GSH S-transferase (GST) activity was determined as previously described (30). Cell protein was isolated in 1% Triton X-100, and protein concentration was determined using the Bio-Rad (Hercules, CA) protein assay according to the manufacturer's instructions. The reaction mixture contained 50 μg of protein, 1 mM GSH, and 1 mM 1-chloro-2,4-dinitrobenzene (CDNB). The change in absorbance at 340 nm, indicating formation of the glutathione-CDNB (GS-CDNB) conjugate, was monitored spectrophotometrically. GST activity was expressed as nanomoles of GS-CDNB formed per minute per milligram of protein (ε =9,600 M−1 cm−1).
GSH assay.
Cellular total GSH content was determined by the 5,5′-dithiobis(2-nitrobenzoic acid)-GSH disulfide recycling assay (1), as previously described (46). Protein concentrations were determined on the same lysates, and GSH levels were calculated as nanomoles per milligram of protein.
GCS activity.
γ-Glutamylcysteine synthetase (GCS) activity was determined by a spectrophotometric assay, as previously described (37). The assay employed a reaction mixture of Tris·HCl (100 mM, pH 8.0), potassium chloride (150 mM), magnesium chloride (20 mM), disodium EDTA (2 mM), disodium ATP (5 mM), sodium phosphoenolpyruvate (2 mM), sodium l-glutamate (10 mM), l-α-aminobutyrate (10 mM), NADH (0.2 mM), pyruvate kinase (5 U), and lactate dehydrogenase (10 U) (Sigma). The reaction was initiated by the addition of sample, and the rate of NADH oxidation was measured by the decrease in absorbance at 340 nm over 3 min at 37°C. Sample protein concentration was determined as described above and GCS activity expressed as nanomoles per milligram of protein per minute.
Protein isolation and Western blotting.
For the isolation of total protein, cells were harvested in phosphate-buffered saline, centrifuged, and resuspended in cell lysis buffer containing 20 mM Tris, pH 7.5, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, and protease and phosphatase inhibitors, as described previously (42). The cells were then sonicated, and the lysate was used for Western blotting after determination of the protein concentration.
Western blotting was performed by denaturing 50 μg of protein at 100°C for 5 min in Laemmli sample buffer containing 62.5 mM Tris, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue, and 5% β-mercaptoethanol. Samples were applied to 12% SDS-polyacrylamide gels and resolved at 100 V over 3 h. Proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell Bioscience, Keene, NH) in transfer buffer containing 25 mM Tris, pH 8.3, 192 mM glycine, 0.01% SDS, and 15% methanol using a Bio-Rad Trans-blot SD semidry transfer cell to which 150 mA were applied for 90 min. Membranes were blocked in 5% nonfat dry milk, 20 mM Tris, pH 7.5, 500 mM sodium chloride, and 0.5% Tween 20 (TBS-T) for 1 h. Membranes were exposed to antibodies that recognized phosphorylated and total JNK1 and JNK2, phosphorylated and total c-Jun (Santa Cruz Biotechnology, Santa Cruz, CA), phosphorylated and total extracellular signal-regulated kinase (ERK) 1/2, phosphorylated MAPK kinase 4 (MKK4) (Cell Signaling, Beverly, MA), and protein disulfide isomerase (kind gift of Richard Stockert, Albert Einstein College of Medicine, Bronx, NY). These primary antibodies were used at 1:1,000 to 1:2,000 dilutions in 5% bovine serum albumin or nonfat milk for 18 h at 4°C. Membranes were exposed to anti-rabbit and anti-mouse secondary antibodies conjugated with horseradish peroxidase (KPL, Gaithersburg, MD) at a dilution of 1:10,000 in 5% nonfat milk TBS-T for 1 h at room temperature. Signals were detected with a chemiluminescence detection system (Western Lightning Chemiluminescence Plus; PerkinElmer Life Sciences, Boston, MA) and exposure to X-ray film.
JNK assay.
JNK activity was measured in cell lysates using a stress-activated protein kinase/JNK assay kit (Cell Signaling), according to the manufacturer's instructions. An NH2-terminal c-Jun-(1-89) fusion protein bound to GSH-Sepharose beads was used to immobilize JNK from cell lysates that contained 250 μg of total protein. After washing, the kinase reaction was performed in the presence of cold ATP using the c-Jun fusion protein as a substrate. Samples were resolved on 10% SDS-polyacrylamide gels, and the amount of phosphorylated c-Jun was detected with an antibody specific for c-Jun phosphorylated at serine 63. As a control for the loading of equivalent amounts of protein among samples, total c-Jun levels were analyzed by immunoblotting with a phosphorylation-independent c-Jun antibody (Santa Cruz Biotechnology). Proteins were visualized using a secondary antibody and chemiluminescence substrate as described above.
Luciferase assay.
RALA hepatocytes were cultured as previously described and transiently transfected with reporter genes using Lipofectamine Plus (Invitrogen) 18 h before HNE treatments. Cells were transfected with the activator protein-1 (AP-1)-driven firefly Luciferase reporter gene 2XTRELuc (14) and the constitutive Renilla luciferase vector pRL-TK (Promega, Madison, WI). Luciferase activities were assayed as previously described (24), and firefly luciferase activity was normalized to Renilla luciferase activity.
Adenovirus preparation and infection.
To inhibit c-Jun function, cells were infected with the adenovirus Ad5TAM that expresses TAM-67, a dominant negative c-Jun (4). Ad5LacZ, which expresses the Escherichia coli β-galactosidase gene (16), served as a control for the nonspecific effects of adenoviral infection. Viruses were amplified in 293 cells, purified by banding twice on CsCl gradients as previously described (45), and titered by plaque assay. Infections were performed as previously described (45), at an multiplicity of infection of 20.
Dephosphorylation assay.
The rate of JNK and c-Jun dephosphorylation following exposure of hepatocytes to heat shock was examined by a method adapted from Yaglom et al. (47). To activate JNK/c-Jun, heat shock was performed by putting the cells in 42°C medium and a 42°C incubator for 15 min. After heat shock, cells were treated with 10 mM 2-deoxyglucose and 5 μM rotenone to inhibit further protein phosphorylation. At different times, the cells were harvested on ice, washed twice with cold phosphate-buffered saline, supplemented with 1 mM sodium orthovanadate, and lysed in buffer containing 1% Triton X-100, 20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2 mM β-glycerophosphate, 5 mM sodium pyrophosphate, 2 mM sodium vanadate, 1 μg/ml leupeptin, 2 μg/ml pepstatin, and 1 mM PMSF. The lysates were subjected to Western blotting as described above and immunoblotted with JNK and c-Jun antibodies.
Statistical analysis.
All numerical results are reported as mean ± SE and represent data from a minimum of three independent experiments. Groups were compared by the Student's t-test. Statistical significance was defined as P < 0.05. Calculations were made with Sigma Plot (Jandel Scientific, San Rafael, CA).
RESULTS
CYP2E1 overexpression sensitizes to death from HNE.
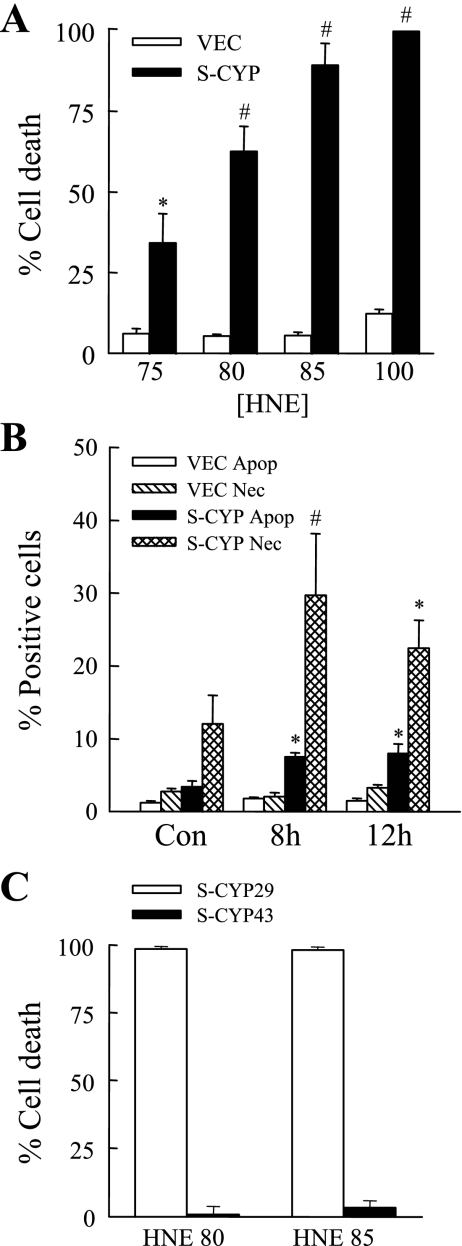
To determine whether chronic oxidant stress may synergize with lipid peroxides to induce hepatocyte injury, sensitivity to HNE-induced cell death was examined in a hepatocellular model of chronic oxidative stress induced by overexpression of the enzyme CYP2E1. Studies were performed in a polyclonal RALA hepatocyte cell line stably transfected with empty vector (VEC cells) that lacks CYP2E1 activity and in a clone with physiological overexpression of CYP2E1 following stable transfection with a CYP2E1 expression vector (S-CYP15 cells) (19, 34). VEC cells were resistant to HNE toxicity as reflected in their low levels of cell death (<10%) at HNE concentrations up to 100 μM as determined by 24-h MTT assay (Fig. 1A). In contrast, CYP2E1-overexpressing S-CYP15 cells were sensitized to death from HNE with levels of concentration-dependent cell death ranging from 34–100% with treatment with 75–100 μM HNE (Fig. 1A).
Fig. 1.
Cytochrome P450 2E1 (CYP2E1) overexpression sensitizes to death from 4-hydroxynonenal (HNE). A: pCI-NEO vector cells (VEC) and S-CYP15 cells (S-CYP) were treated with the indicated micromolar concentrations of HNE, and the percentage of cell death was determined at 24 h by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (*P < 0.02; #P < 0.00001 compared with VEC cells treated with the same HNE concentration). B: VEC and S-CYP15 cells were left untreated (Con) or treated with 80 μM HNE for 8 or 12 h and costained with acridine orange and ethidium bromide, and the numbers of apoptotic (Apop) and necrotic (Nec) cells were determined by fluorescence microscopy as described in materials and methods (*P < 0.01; #P < 0.03 compared with the same type of cell death in VEC cells). C: percentage of cell death by MTT assay in S-CYP29 and S-CYP43 cells after 24 h of treatment with 80 or 85 μM HNE. Results are means + SE from 3 independent experiments each with duplicate data points.
S-CYP15 cell sensitization to HNE toxicity was confirmed by fluorescence microscopic examination of acridine orange/ethidium bromide costained cells for the steady-state levels of apoptosis and necrosis. Untreated S-CYP15 cells had slight increases in the numbers of apoptotic and necrotic cells compared with VEC cells, as previously reported (19). At 8 and 12 h after treatment with HNE, steady-state levels of apoptotic and necrotic cells were increased significantly in S-CYP15 cells compared with VEC cells (Fig. 1B). The amount of necrosis was greater than the degree of apoptosis, but the numbers of necrotic cells may have been inflated by the inclusion of apoptotic cells undergoing secondary necrosis.
To insure that the sensitivity of S-CYP15 cells to HNE-mediated cell death was secondary to CYP2E1 overexpression and not nonspecific clonal variation, the extent of death following HNE treatment was examined in two additional S-CYP clones. The clone S-CYP29 has increased CYP2E1 protein levels and enzymatic activity similar to S-CYP15 cells, whereas S-CYP43 cells do not overexpress CYP2E1 despite transfection with the CYP2E1 expression vector and antibiotic selection (35). Similar to the findings in S-CYP15 cells, S-CYP29 cells were sensitized to death from HNE as reflected in their nearly 100% cell death from 80 and 85 μM HNE concentrations (Fig. 1C). In contrast, the S-CYP43 clone, which fails to overexpress CYP2E1, was resistant to HNE toxicity similar to the findings in VEC cells (Fig. 1C). Thus sensitization to HNE toxicity was the result of cellular effects of CYP2E1 overexpression.
S-CYP cells have constitutively increased ROS production but normal HNE levels.
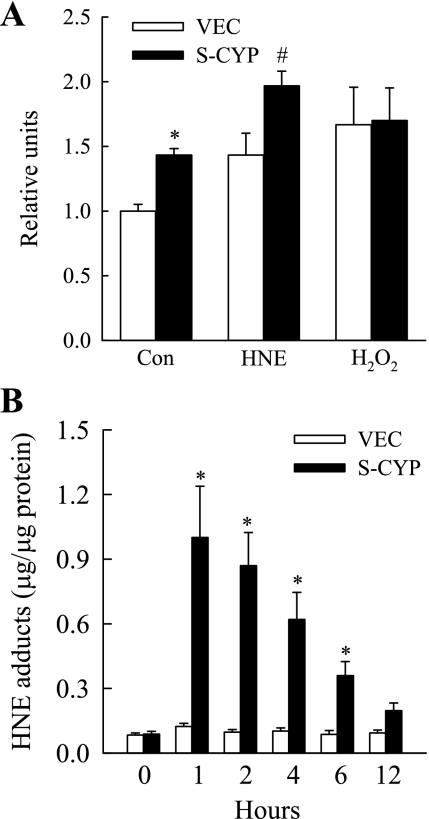
CYP2E1 overexpression generates ROS, which could result in the formation of lipid peroxidation products such as HNE if ROS levels exceed the antioxidant capacity of the hepatocyte. The increased HNE toxicity in S-CYP cells may result therefore from a greater concentration of HNE in these cells that is the sum of both endogenous and exogenous sources. As expected, untreated S-CYP15 cells had increased ROS levels by the DCFH-DA assay compared with VEC cells (Fig. 2A). HNE treatment increased ROS production in both cell types, but levels remained significantly greater in S-CYP cells (Fig. 2A). In contrast, the amount of ROS generated by treatment with the oxidant H2O2 was equivalent in the two cell types. Despite the constitutive increase in ROS in S-CYP cells, VEC and S-CYP15 cells had equivalent low levels of HNE as determined by the quantity of HNE-protein adducts (Fig. 2B). In response to HNE treatment, no increase in adducts occurred in VEC cells, but levels were markedly increased in S-CYP15 cells (Fig. 2B), suggesting a defect in HNE metabolism in these cells. Thus sensitization of S-CYP cells to death from HNE did not result simply from higher concentrations of HNE occurring from the combination of CYP2E1-generated endogenous production and exogenous supplementation.
Fig. 2.
S-CYP cells have constitutively increased reactive oxygen species production but normal levels of HNE. A: relative fluorescent intensity in untreated VEC and S-CYP15 (S-CYP) control cells (Con) and cells treated for 2 h with 85 μM HNE or 3.5 mM H2O2 (*P < 0.02; #P < 0.0001 compared with VEC cells with the same treatment). B: levels of HNE-histidine adducts in VEC and S-CYP cells untreated or treated for the indicated number of hours with 85 μM HNE (*P < 0.02 compared with VEC cells with the same treatment). Results are means + SE from 3 independent experiments each with duplicate data points.
S-CYP cell sensitivity to HNE toxicity is not secondary to decreased GST activity.
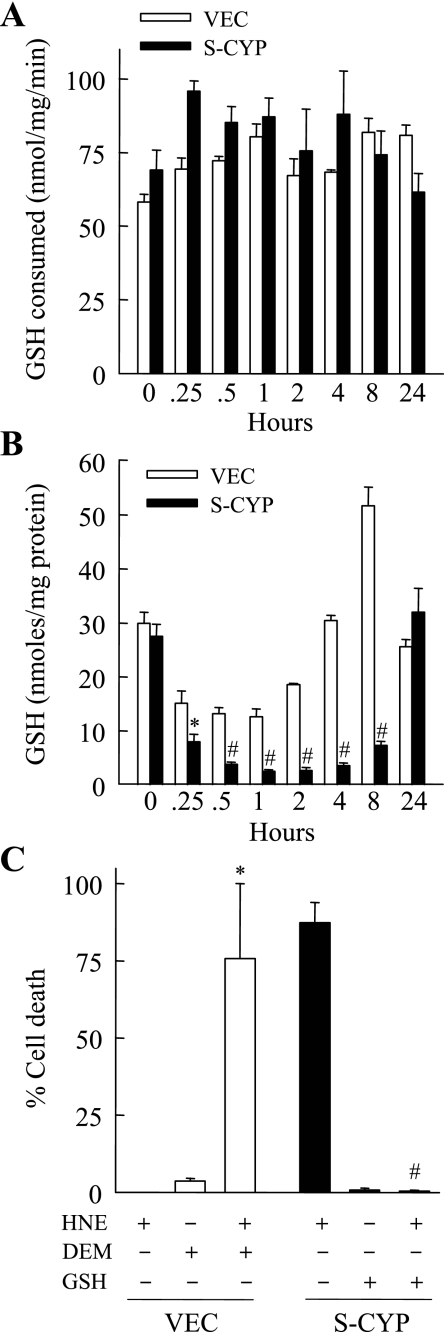
A possible mechanism of S-CYP cell sensitization to HNE toxicity could be the failure of S-CYP cells to metabolize and thereby eliminate this toxic hydroxyaldehyde. The relative increase in levels of HNE-protein adducts in S-CYP cells with HNE treatment was also suggestive of this possibility. In hepatocytes, the major pathway of HNE metabolism is through GST-mediated conjugation to GSH to form an HNE-GSH conjugate that then effluxes from the cell (13). To determine whether S-CYP cells had impaired GST function, GST activity was measured in untreated and HNE-treated VEC and S-CYP15 cells. Levels of GST activity were equivalent in the two cell types untreated and at various times after HNE treatment (Fig. 3A), demonstrating that S-CYP cell sensitization to HNE toxicity was not the result of decreased GST activity.
Fig. 3.
S-CYP cells undergo marked glutathione (GSH) depletion with HNE treatment. GSH S-transferase activity (A) and cellular GSH content (B) were measured as described in materials and methods in VEC and S-CYP15 (S-CYP) cells untreated and treated with 80 μM HNE for the indicated times shown. Data are from 3 independent experiments performed in duplicate (*P < 0.04; #P < 0.0001 compared with VEC cells with the same treatment). C: percentage cell death in VEC cells treated with 80 μM HNE, diethyl maleate (DEM), or a combination of the two and in S-CYP15 cells treated with 80 μM HNE, 2 mM GSH ethyl ester, or both agents together. Results are means + SE from 3 independent experiments (*P < 0.03; #P < 0.0002 compared with cells treated with HNE alone).
HNE toxicity results from GSH depletion in CYP2E1-overexpressing RALA hepatocytes.
Previous studies have shown that, in contrast to the ability of wild-type or VEC hepatocytes to survive a profound decrease in levels of the cellular antioxidant GSH, S-CYP cells undergo cell death in response to chemical GSH depletion by DEM (19). Upon exposure to the death receptor ligand TNF, only hepatocytes with CYP2E1 overexpression undergo GSH depletion and resultant cell death (22). Thus CYP2E1 overexpression makes hepatocytes susceptible to GSH depletion and resultant cell death, suggesting that the mechanism of HNE toxicity in S-CYP cells may be from GSH depletion resulting from GSH consumption for HNE conjugation. To examine for this possibility, GSH content was measured in VEC and S-CYP15 cells at different times after 80 μM HNE treatment. As previously reported (19), basal GSH levels were equivalent in the two cells types (Fig. 3B). GSH content decreased significantly within 15 min of HNE treatment in both VEC and S-CYP15 cells, consistent with the known rapid conjugation of HNE with GSH (13). However, at 0.5 h after HNE treatment, GSH levels were decreased 86% in S-CYP15 cells compared with only 57% in VEC cells (Fig. 3B). GSH levels reached their nadir in both cell types at 1 h after HNE treatment at which point levels in S-CYP15 cells were fivefold lower than in VEC cells (Fig. 3B). In addition, S-CYP cells experienced a lag in the recovery of their GSH content back to normal levels. GSH levels normalized in VEC cells within 4 h, whereas levels in S-CYP15 cells were still markedly depressed at 8 h (Fig. 3B). GSH levels did return to normal in S-CYP15 cells surviving at 24 h (Fig. 3B).
These data suggested that CYP2E1 overexpression may sensitize hepatocytes to death from HNE by promoting a fatal depletion of GSH. To determine the functional significance of GSH depletion in S-CYP cell death from HNE, the effect of GSH levels on cell death was examined. Chemical GSH depletion of VEC cells by DEM, which was nontoxic by itself, sensitized VEC cells to HNE toxicity (Fig. 3C). Conversely, pretreatment with 2 mM GSH ethyl ester, a membrane-permeable form of GSH (1), completely inhibited S-CYP15 cell death from 80 μM HNE (Fig. 3C). Thus the mechanism of HNE-induced death in CYP2E1-overexpressing RALA hepatocytes was dependent on prolonged depletion of the antioxidant GSH. This deficit in GSH led to decreased HNE conjugation and increased levels of HNE-protein adducts.
S-CYP cells fail to replete their GSH secondary to decreased GCS activity.
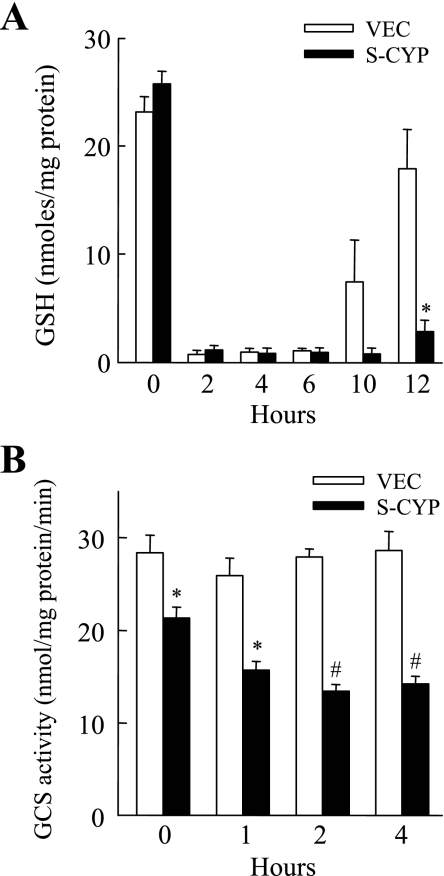
GSH conjugation to HNE irreversibly consumes intracellular GSH (26). Two possible mechanisms for the more profound HNE-induced GSH depletion in CYP2E1-overexpressing cells were that consumption of GSH was greater in S-CYP cells or that S-CYP cells had a decreased capacity for GSH synthesis that delayed normalization of their GSH content. To distinguish between these two possibilities, the recovery of GSH levels after chemically induced depletion from DEM was compared in VEC and S-CYP15 cells. DEM induced an equally profound depletion of GSH in VEC and S-CYP15 cells within 2 h (Fig. 4A). However, GSH content normalized at different rates in the two cell types. Within 12 h of DEM administration VEC cell GSH content had almost returned to baseline, whereas levels were still markedly depressed in S-CYP15 cells (Fig. 4A). These data suggested that S-CYP cells have an inherent problem in their GSH synthetic capacity that limits their ability to normalize GSH content after chemical- or oxidant-induced depletion.
Fig. 4.
S-CYP cells undergo increased GSH depletion from DEM and have decreased γ-glutamylcysteine synthetase (GCS) activity. A: GSH levels were determined in VEC and S-CYP15 (S-CYP) cells untreated and treated with DEM for the number of hours shown. Results are means + SE from 3 independent experiments (*P < 0.01 compared with VEC cells at the same time point). B: GCS activity in VEC and S-CYP15 cells untreated and treated for the indicated number of hours with 80 μM HNE. The data are means + SE from 4 independent experiments (*P < 0.003; #P < 0.001 compared with VEC cells at the same time point).
To examine whether S-CYP cells have an insufficiency in GSH synthesis, activity levels of GCS, the rate-limiting enzyme in GSH synthesis (26), were assayed in the two cell types. HNE has been reported to upregulate GSH synthesis in nonhepatic cells by inducing GCS expression (25). GCS activity was unchanged in VEC cells following HNE treatment (Fig. 4B). Activity was modestly but significantly reduced in untreated S-CYP15 cells compared with VEC cells (Fig. 4B). S-CYP15 cell GCS activity further decreased with HNE treatment to levels that were only 50% of those in VEC cells at 2 and 4 h (Fig. 4B).
HNE-induced death in S-CYP-overexpressing cells is associated with JNK/c-Jun overactivation.
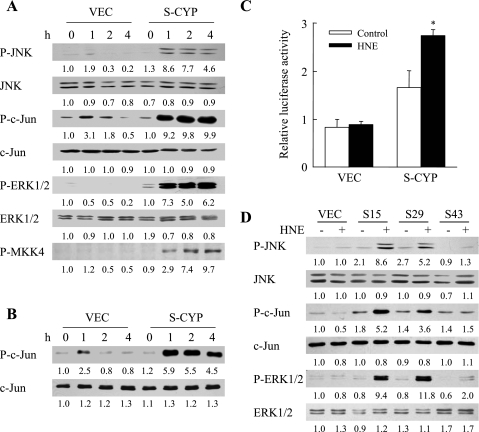
A possible mechanism for S-CYP cell death from HNE-induced GSH depletion could be that, in the absence of sufficient levels of the antioxidant GSH, CYP2E1-generated ROS have direct, injurious biochemical effects on cellular macromolecules. Alternatively, ROS or HNE itself may affect cell signaling pathways that regulate hepatocyte death and survival. Previous studies have demonstrated that CYP2E1 overexpression promotes a proapoptotic overactivation of the MAPK JNK in response to TNF (22). Activation of the JNK/c-Jun pathway in VEC and S-CYP15 cells by HNE treatment was therefore assessed by immunoblotting for levels of phosphorylated JNK and its downstream substrate c-Jun. In VEC cells treated with 80 μM HNE, JNK activation was barely detectable, and a transient and low-level activation of c-Jun occurred at 1–2 h as reflected in increased levels of the phosphorylated forms of JNK and c-Jun (Fig. 5A). In contrast, marked increases in phospho-JNK and phospho-c-Jun occurred in HNE-treated S-CYP15 cells at 1 h and persisted for greater than 12 h (Fig. 5A and data not shown). The effect of HNE on a second MAPK, ERK1/2, was also examined. HNE treatment induced a sustained overactivation of ERK1/2 in S-CYP15 but not VEC cells (Fig. 5A). No changes were observed in the levels of total JNK, c-Jun, or ERK1/2 in the two cell types with HNE treatment (Fig. 5A). HNE treatment in the setting of CYP2E1 overexpression therefore led to sustained activation of the JNK and ERK1/2 MAPK signaling pathways.
Fig. 5.
HNE treatment in the setting of CYP2E1 overexpression induces sustained c-Jun NH2-terminal kinase (JNK)/c-Jun activation. A: protein was isolated from VEC and S-CYP15 (S-CYP) cells untreated or treated with 80 μM HNE for the indicated hours. Aliquots of protein were immunoblotted with antibodies for phospho-JNK (P-JNK) and total JNK (JNK), phospho-c-Jun (P-c-Jun) and total c-Jun (c-Jun), phospho-extracellular signal-regulated kinase (P-ERK1/2) and total ERK1/2 (ERK1/2), and phospho-MAPK kinase 4 (P-MKK4). B: identically treated cells were assayed for JNK activity by in vitro kinase assay. JNK activity is indicated by levels of P-c-Jun on immunoblots, and levels of total c-Jun (c-Jun) serve as a control for protein loading. C: relative levels of activator protein-1 (AP-1)-dependent luciferase activity in VEC and S-CYP15 cells untreated (Con) and treated with 80 μM HNE for 6 h (*P < 0.000001 compared with HNE-treated VEC cells). D: immunoblots with proteins from VEC, S-CYP15 (S15), S-CYP29 (S29), and S-CYP43 (S43) cells untreated or treated with 80 μM HNE for 4 h. The results in A, B, and D are representative of 3 independent experiments, and the data in C are means + SE from 3 independent experiments performed in duplicate. Numerical results under the immunoblots represent the relative mean signal intensity among samples from densitometry scanning of 3 experiments.
To further confirm the presence of JNK/c-Jun overactivation in S-CYP15 cells, the effects of HNE treatment on JNK kinase activity and levels of c-Jun-dependent, AP-1-driven transcription were examined. JNK activity was measured by an in vitro kinase assay with c-Jun as substrate. HNE treatment induced a twofold increase in JNK activity in VEC cells at 1 h, but activity returned to baseline within 2 h (Fig. 5B). In CYP2E1-overexpressing S-CYP15 cells, HNE treatment led to a sixfold increase in JNK activity at 1 h that persisted for greater than 4 h (Fig. 5B). JNK phosphorylation of c-Jun increases its ability to transcriptionally activate the AP-1 promoter. Effects of HNE on AP-1 activity were determined in VEC and S-CYP15 cells transiently transfected with the AP-1-driven reporter 2XTRELuc. S-CYP15 cells had levels of AP-1 transcriptional activity that were increased significantly threefold over VEC cells after HNE treatment (Fig. 5C). In contrast, consistent with the minimal JNK/c-Jun activation that occurred in VEC cells, AP-1 activity was unaffected by HNE treatment in these cells (Fig. 5C). Therefore, CYP2E1 overexpression led to elevated AP-1 transcriptional activation that was further amplified by HNE treatment.
To confirm that JNK overactivation resulted from CYP2E1 overexpression and not nonspecific clonal variation, immunoblot analysis for phosphorylated JNK, c-Jun, and ERK1/2 was performed in two additional HNE-treated S-CYP clones. HNE induced JNK, c-Jun, and ERK1/2 overactivation in the CYP2E1-overexpressing clone S-CYP29 similar to S-CYP15 cells (Fig. 5D). As expected, JNK, c-Jun, and ERK1/2 overactivation did not occur in the S-CYP43 clone, which, despite transfection, fails to overexpress CYP2E1 (Fig. 5D). No changes were observed among cell types in the levels of total JNK, c-Jun, or ERK1/2.
Inhibition of JNK/c-Jun signaling blocks HNE-mediated death in CYP2E1-overexpressing cells.
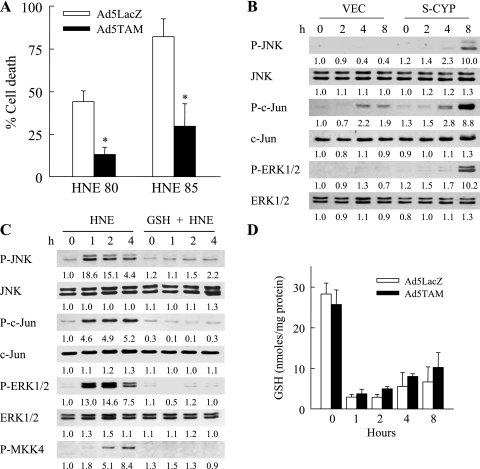
Prolonged activation of the JNK/c-Jun signaling pathway triggers cell death in hepatocytes (12, 23, 27, 36). To determine whether JNK/c-Jun function sensitizes S-CYP cells to death from HNE, the ability of JNK/c-Jun inhibition to block HNE-induced cell death was examined. JNK/c-Jun function was blocked by infecting cells with the adenovirus Ad5TAM that expresses TAM67, a dominant negative form of c-Jun lacking the transactivation domain (4). Ad5TAM effectively blocks c-Jun function in RALA hepatocytes as assessed previously by the effects of Ad5TAM on AP-1 transcriptional activity (23). S-CYP cells infected with the control adenovirus Ad5LacZ that expresses the β-galactosidase gene or Ad5TAM were treated with 80 or 85 μM HNE, and cell death was determined by MTT assay. Inhibition of c-Jun function decreased S-CYP15 cell death from 80 and 85 μM HNE by 75% and 64%, respectively (Fig. 6A). In contrast, cell death was unaffected by pharmacological inhibition of ERK1/2 function with PD98059 (data not shown).
Fig. 6.
HNE toxicity is mediated by JNK/c-Jun overactivation resulting from GSH depletion. A: percentage cell death by MTT assay in S-CYP15 cells preinfected with Ad5LacZ or Ad5TAM and treated with the indicated micromolar concentrations of HNE for 24 h. Data are means + SE from 3 independent experiments performed in duplicate (*P < 0.001 compared with similarly treated Ad5LacZ-infected cells). B: protein was isolated from VEC and S-CYP15 cells that were untreated or treated with DEM for the number of hours indicated. Protein aliquots were immunoblotted with antibodies for P-JNK, total JNK, P-c-Jun, total c-Jun, P-ERK1/2, and total ERK1/2. C: protein was isolated from S-CYP15 cells that were untreated or treated with 80 μM HNE alone or together with GSH ethyl ester (GSH) for the indicated hours. Aliquots of protein were immunoblotted with the previous antibodies along with an antibody for P-MKK4. Results in B and C are each representative of 3 independent experiments with the numerical results representing the relative mean signal intensity from densitometry scanning of these experiments. D: total GSH levels in S-CYP15 cells preinfected with Ad5LacZ or Ad5TAM and left untreated or treated with 80 μM HNE for the indicated hours. Data are means + SE from 3 independent experiments performed in duplicate.
JNK/c-Jun overactivation results from GSH depletion.
The mechanistic involvement of both GSH depletion and prolonged JNK/c-Jun signaling in S-CYP cell death from HNE suggested that GSH depletion might be the upstream event mediating JNK/c-Jun overactivation. Consistent with this possibility was the fact that the greater depletion of GSH in S-CYP cells after DEM treatment (Fig. 4A) was associated with increased MAPK activation as reflected in increased levels of phosphorylated JNK, c-Jun, and ERK1/2 (Fig. 6B). To specifically examine the role of GSH depletion in HNE-induced JNK/c-Jun activation, the effects of GSH ethyl ester supplementation on levels of HNE-induced JNK and c-Jun phosphorylation were determined. HNE-induced phosphorylation of JNK/c-Jun was completely blocked by GSH supplementation (Fig. 6C), demonstrating that JNK/c-Jun overactivation resulted from GSH depletion. ERK1/2 MAPK activation was similarly inhibited by GSH (Fig. 6C). To confirm that JNK/c-Jun activation was downstream of GSH depletion, the effect of c-Jun inhibition on HNE-induced GSH depletion was determined. Inhibition of c-Jun signaling by Ad5TAM infection failed to block HNE-induced GSH depletion (Fig. 6D). These data demonstrate that JNK/c-Jun overactivation is the downstream effect of GSH depletion, as GSH supplementation blocked JNK/c-Jun activation and death, whereas Ad5TAM inhibited cell death without affecting GSH levels.
Oxidative stress mediates S-CYP cell death from HNE.
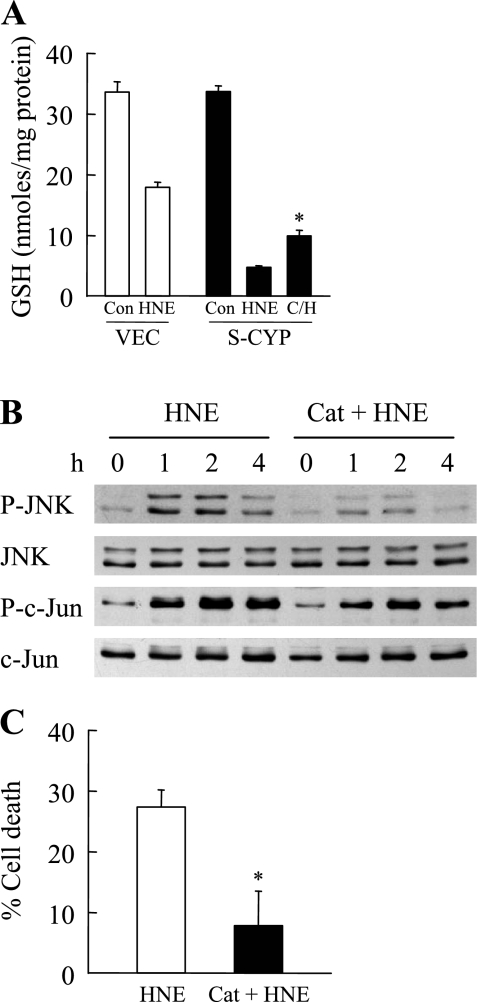
To demonstrate that oxidant stress resulting from CYP2E1 overexpression was the mechanism of cell sensitivity to death from HNE, the effect of the antioxidant catalase on death from HNE was examined. Catalase treatment significantly decreased the degree of GSH depletion in HNE-treated S-CYP cells (Fig. 7A). This inhibition of GSH depletion resulted in decreased HNE-induced JNK activation as determined by levels of phosphorylated JNK and c-Jun (Fig. 7B). As a result, catalase treatment blocked cell death from HNE (Fig. 7C). The increased production of ROS by CYP2E1 overexpression therefore mediated cell death from HNE.
Fig. 7.
Inhibition of oxidant stress inhibits GSH depletion, JNK activation, and cell death. A: GSH levels in VEC and S-CYP15 cells in untreated control (Con) cells and cells treated for 1 h with HNE alone (HNE) or catalase and HNE (C/H). Data are means + SE from 3 independent experiments (*P < 0.01 compared with HNE-treated S-CYP cells). B: Western blots of proteins isolated from S-CYP15 cells treated with HNE alone or catalase and HNE (Cat + HNE) for the indicated number of hours and probed for the antibodies shown. C: percentage cell death in S-CYP15 cells treated with HNE alone or catalase and HNE for 24 h. Data are means + SE from 3 independent experiments (*P < 0.004 compared with HNE-treated cells).
Increased phospho-JNK/c-Jun levels in HNE-treated S-CYP cells are secondary to increased MKK4 activation.
Steady-state levels of phosphorylated JNK and c-Jun induced by any stimulus including HNE reflect the balance between the rates of phosphorylation by upstream kinases and dephosphorylation by phosphatases. In addition, HNE has been demonstrated to directly bind and activate JNK in hepatic stellate cells (31). Immunoprecipitation of HNE-treated S-CYP15 cells with an anti-JNK or anti-HNE antibody followed by immunoblotting with the other antibody failed to detect JNK-bound HNE (data not shown). This finding suggested that JNK/c-Jun activation was the result of altered kinase and/or phosphatase activity and not a direct effect of HNE on JNK.
HNE-treated S-CYP15 cells were then examined for a differential upregulation of upstream kinase MKK4 of JNK. HNE treatment of VEC cells failed to cause any significant increase in levels of active, phosphorylated MKK4 as detected by immunoblotting (Fig. 5A). In contrast, a sustained increase in MKK4 activation occurred in HNE-treated S-CYP15 cells (Fig. 5A). This increase in MKK4 activation was the result of GSH depletion, as MKK4 phosphorylation was blocked by GSH supplementation (Fig. 6C).
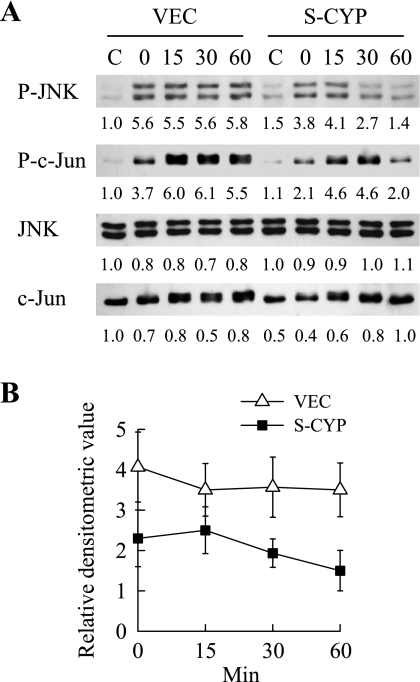
To assess whether an inherent decrease in JNK phosphatase activity also contributed to JNK overactivation in S-CYP cells, rates of JNK dephosphorylation were compared in the two cell types. The marked difference in levels of JNK phosphorylation in VEC and S-CYP cells in response to HNE made this stimulus unsuitable for comparative studies of dephosphorylation rates; therefore, heat shock was used to activate JNK. The amount of JNK phosphorylation induced by heat shock was equivalent in the two cell types (Fig. 8A). The rate of JNK dephosphorylation after heat shock was slightly greater in S-CYP15 cells than VEC cells, but the differences were not significant (Fig. 8, A and B). Overactivation of JNK in response to HNE therefore did not result from HNE binding to JNK or a deficiency in phosphatase activity but rather from increased activation of the upstream kinase MKK4.
Fig. 8.
S-CYP cells do not have a decreased rate of JNK dephosphorylation. A: VEC and S-CYP15 cells were heat shocked and treated with phosphorylation inhibitors as detailed in materials and methods. Total protein was isolated from untreated control cells (C) or cells at the indicated min after heat shock. Isolated protein was immunoblotted with antibodies to P-JNK, total JNK, P-c-Jun, and total c-Jun. Relative signal intensities from densitometric scanning of 3 independent experiments are indicated. B: rate of decline in JNK phosphorylation in VEC and S-CYP15 cells over time.
S-CYP cells are not sensitized to death from a lipid peroxidation product that fails to activate JNK/c-Jun.
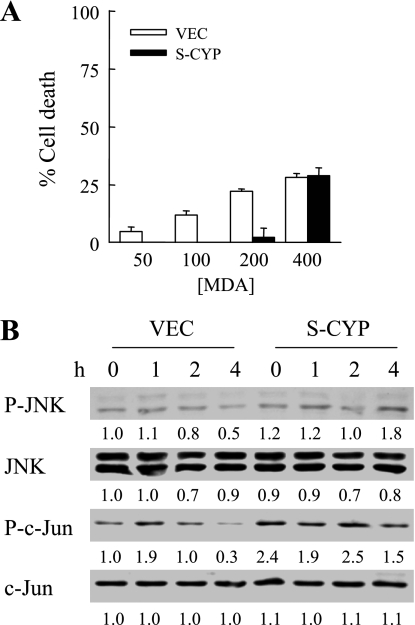
Finally, to determine the specificity of HNE toxicity in CYP2E1-overexpressing cells, the resistance of these cells to a second lipid peroxidation product, MDA, was investigated. In contrast to the findings with HNE, VEC and S-CYP15 cells were both resistant to high concentrations of MDA. At low levels of MDA, S-CYP cells were in fact more resistant to toxicity, and, at higher concentrations, low levels of cell death were equivalent in VEC and S-CYP cells (Fig. 9A). Consistent with its relative lack of toxicity, MDA treatment failed to induce JNK or c-Jun activation in either VEC or S-CYP15 cells (Fig. 9B). Thus CYP2E1-overexpressing cells were specifically sensitized to death from the lipid peroxide HNE.
Fig. 9.
S-CYP cells are not sensitized to death from malondialdehyde (MDA). A: VEC and S-CYP15 cells were treated with the indicated micromolar concentrations of MDA, and the percentage cell death was determined at 24 h by MTT assay. Results are means + SE from 3 independent experiments performed in duplicate. B: protein was isolated from VEC and S-CYP15 cells at the indicated hours after treatment with 200 μM MDA and immunoblotted with antibodies for P-JNK, total JNK, P-c-Jun, and total c-Jun. The mean densitometric signal intensities from 3 independent experiments are shown.
DISCUSSION
Overactivation of the MAPK JNK has been identified as a central mechanism in the development and progression of murine steatohepatitis (33, 39). However, the factors that trigger sustained JNK activation in these models are unknown. Both experimental and human NAFLD are associated with oxidant stress and increased levels of HNE generated by ROS-induced lipid peroxidation (6, 17, 21). The lipid peroxidation products produced in this disease are considered passive markers of the effects of oxidative stress. However, similar to ROS, lipid peroxidation products may affect cell signaling pathways (5, 31) and thereby actively alter cellular responses to injury. This study specifically examined whether HNE promoted hepatocyte death pathway signaling in the setting of chronic oxidative stress. Overexpression of CYP2E1 was employed as a model of chronic oxidative stress because expression of this enzyme is increased in both nonalcoholic and alcoholic fatty liver disease (43, 44). Findings of increased CYP2E1 expression in concert with oxidant stress have suggested a prooxidant etiological role for this enzyme in the development of steatohepatitis; however, the function of CYP2E1 in these diseases remains controversial. Nonetheless, CYP2E1 expression serves as a physiological model for the effects of chronic oxidant stress on hepatocytes. Although CYP2E1 overexpression is protective against an acute exogenous oxidant stress (15, 19, 28), this chronic oxidative stress sensitizes hepatocytes to death from other factors (22, 34). The known induction of the proapoptotic JNK/c-Jun pathway by oxidants and HNE suggested that the synergistic effects of these two factors may be a mechanism of JNK/c-Jun overactivation and hepatocyte death.
RALA hepatocytes transfected with vector alone were resistant to high concentrations of HNE, consistent with prior findings in primary hepatocytes (38). CYP2E1 overexpression sensitized these cells to toxicity from HNE concentrations of 75 μM or greater. Death did not occur from an increased baseline concentration of HNE from CYP2E1 overexpression, as untreated S-CYP15 cells had normal HNE levels despite elevated ROS production. Mean steady-state whole liver HNE levels in response to oxidative stress are ∼10 μM (32, 41), and localized cellular concentrations may reach as high as 5 mM (2, 18). Our in vitro model employed a single treatment with HNE, which was metabolized rapidly by the hepatocyte within a few minutes (13, 38). Therefore, RALA hepatocyte HNE exposure in our experiments was relatively limited compared with the sustained elevations that hepatocytes are exposed to in vivo in liver disease. These facts suggest that the sensitization of CYP2E1-overexpressing hepatocytes to death occurred at a physiologically relevant HNE concentration.
Death from HNE in CYP2E1-overexpressing cells was associated with overactivation of JNK/c-Jun signaling as demonstrated by increased levels of 1) phospho-JNK and phospho-c-Jun by Western blotting, 2) JNK activity by in vitro kinase assay, and 3) AP-1-driven transcriptional activity. JNK/c-Jun signaling mediated cell death from HNE as expression of the c-Jun-dominant negative TAM67 significantly increased cell survival. HNE has been identified as a JNK activator in other cell types (5, 31), but we believe this to be the first report to identify HNE as an inducer of JNK signaling in hepatocytes. A mechanism of JNK activation by HNE in some cells is through HNE binding to JNK (31). Direct HNE-JNK interaction was not the mechanism of JNK activation in HNE-treated S-CYP cells, as HNE and JNK did not coimmunoprecipitate and activation of the upstream JNK kinase MKK4 occurred. The mechanism of JNK/c-Jun overactivation resulting from CYP2E1 overexpression and HNE treatment was a prolonged reduction in levels of the principal nonenzymatic antioxidant GSH. GSH depletion, JNK overactivation, and cell death were mediated by CYP2E1-induced oxidant stress, as the antioxidant catalase inhibited all of these events. MAPK activity is known to be redox sensitive, and the fact that ROS can inactivate MAPK phosphatases (40) suggested that prolonged JNK activation may be secondary to a failure to dephosphorylate this protein. However, the rate of JNK dephosphorylation in S-CYP cells was equivalent to that in control VEC cells. Instead HNE-treated S-CYP cells had marked overactivation of MKK4, demonstrating that the effect of GSH depletion occurred upstream of JNK. The mechanism by which HNE activates MKK4 remains to be determined.
The susceptibility of S-CYP cells to GSH depletion was not specific for HNE, as these cells also underwent a more profound GSH reduction in response to the chemical DEM. The initial GSH depletion was marked with both treatments, equivalent in the two cell types after DEM treatment, but significantly greater in HNE-treated S-CYP cells than in VEC cells. Both treatments led to a more sustained period of GSH depletion in S-CYP cells. Rat liver GSH has a rapid 2–3-h turnover, and GCS is the rate-limiting enzyme in GSH synthesis (26). GCS activity was reduced in S-CYP cells especially after HNE treatment. Levels of GCS activity can regulate hepatocyte injury as evidenced by the ability of GCS overexpression to ameliorate acetaminophen-induced liver injury resulting from GSH depletion (3). The more extensive GSH depletion in S-CYP cells therefore had two mechanisms. One was the greater degree of GSH depletion that occurred from the combined effects of CYP2E1-generated oxidative stress and HNE conjugation. Second, GSH levels in these cells could not recover as quickly because of reduced GCS activity and GSH synthetic capacity. These findings suggest the presence of a novel mechanism of decreased GCS activity in S-CYP cells that remains to be defined. It is somewhat surprising that S-CYP cells would downregulate an antioxidant pathway that might be expected to be upregulated as a compensatory protective response to their chronic oxidant stress. However, the effect on GCS might be the result of the upregulation of a critical compensatory signaling response that had a secondary deleterious effect of decreasing GCS activity. Consistent with this speculation is that we previously demonstrated that S-CYP cells have increased ERK1/2 signaling that protects them from oxidant stress (19) but also acts to sensitize them to death from fatty acids (34).
The results of this study differ from those previously reported in HepG2 hepatoma cells in which stable CYP2E1 overexpression increased their resistance to HNE toxicity (28). This discrepancy is likely secondary to differences between the nontransformed RALA hepatocytes employed in the present studies and hepatoma cells. Transformed cell lines have marked differences in their antioxidant levels compared with normal cells. HepG2 cells have GSH levels that are threefold greater than those in primary hepatocytes or RALA cells (8). In contrast, HepG2 cells have a 10-fold decrease in their GST activity compared with normal liver (28). Therefore, the regulation of GST expression and resultant HNE toxicity obviously differs in these cells compared with normal liver and not surprisingly from RALA hepatocytes as well. Despite an upregulation of GSH synthesis in CYP2E1-overexpressing HepG2 cells, consistent with our findings in RALA hepatocytes is that CYP2E1-overexpressing HepG2 cells undergo cell death from chemical GSH depletion (8), suggesting that these cells also have an impaired ability to replete GSH.
The present findings are a novel demonstration of the ability of ROS and a lipid peroxidation product to have synergistic effects on cellular JNK MAPK signaling and provide a physiologically relevant mechanism for the overactivation of JNK/c-Jun signaling in the steatotic liver. Although the hepatocyte can adapt to the oxidant stress, the cell is still vulnerable to injury from HNE because of cumulative effects on JNK signaling. Thus the lipid peroxide HNE generated in NAFLD may not be merely a passive marker of oxidative stress in the liver but an actual mediator of liver injury. This finding supplies a new potential mechanism by which oxidant stress may promote the development of this disease.
GRANTS
This work was supported by National Institutes of Health grant DK61498 to M. J. Czaja.
ACKNOWLEDGMENTS
We thank David Brenner for supplying the adenoviruses, Richard Stockert for the protein disulfide isomerase antibody, and Laura Nagy for helpful discussions. Jörn M. Schattenberg's current affiliation is I. Medizinische Klinik und Poliklinik Johannes Gutenberg Universität, Mainz, Germany.
REFERENCES
- 1.Anderson ME, Powrie F, Puri RN, Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys 239: 538–548, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Benedetti A, Comporti M, Fulceri R, Esterbauer H. Cytotoxic aldehydes originating from the peroxidation of liver microsomal lipids. Identification of 4,5-dihydroxydecenal. Biochim Biophys Acta 792: 172–181, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Botta D, Shi S, White CC, Dabrowski MJ, Keener CL, Srinouanprachanh SL, Farin FM, Ware CB, Ladiges WC, Pierce RH, Fausto N, Kavanagh TJ. Acetaminophen-induced liver injury is attenuated in male glutamate-cysteine ligase transgenic mice. J Biol Chem 281: 28865–28875, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bradham CA, Hatano E, Brenner DA. Dominant-negative TAK1 induces c-Myc and G0 exit in liver. Am J Physiol Gastrointest Liver Physiol 281: G1279–G1289, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bruckner SR, Estus S. JNK3 contributes to c-jun induction and apoptosis in 4-hydroxynonenal-treated sympathetic neurons. J Neurosci Res 70: 665–670, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 99: 1497–1502, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 37: 544–550, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Cederbaum AI. Cytotoxicity and apoptosis produced by cytochrome P450 2E1 in Hep G2 cells. Mol Pharmacol 53: 638–648, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Chou JY. Temperature-sensitive adult liver cell line dependent on glucocorticoid for differentiation. Mol Cell Biol 3: 1013–1020, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou JY, Yeoh GC. Tyrosine aminotransferase gene expression in a temperature-sensitive adult rat liver cell line. Cancer Res 47: 5415–5420, 1987 [PubMed] [Google Scholar]
- 11.Czaja MJ. Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis 27: 378–389, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Czaja MJ, Liu H, Wang Y. Oxidant-induced hepatocyte injury from menadione is regulated by ERK and AP-1 signaling. Hepatology 37: 1405–1413, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Hartley DP, Ruth JA, Petersen DR. The hepatocellular metabolism of 4-hydroxynonenal by alcohol dehydrogenase, aldehyde dehydrogenase, and glutathione S-transferase. Arch Biochem Biophys 316: 197–205, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Hattori M, Tugores A, Westwick JK, Veloz L, Leffert HL, Karin M, Brenner DA. Activation of activating protein 1 during hepatic acute phase response. Am J Physiol Gastrointest Liver Physiol 264: G95–G103, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Huan JY, Koop DR. Tightly regulated and inducible expression of rabbit CYP2E1 using a tetracycline-controlled expression system. Drug Metab Dispos 27: 549–554, 1999 [PubMed] [Google Scholar]
- 16.Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhoven R, Grisham JW, Brenner DA. NFκB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest 101: 802–811, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S, Kawada N, Arakawa T, Ueda M. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology 43: 506–514, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Iles KE, Liu RM. Mechanisms of glutamate cysteine ligase (GCL) induction by 4-hydroxynonenal. Free Radic Biol Med 38: 547–556, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Jones BE, Liu H, Lo CR, Koop DR, Czaja MJ. Cytochrome P450 2E1 expression induces hepatocyte resistance to cell death from oxidative stress. Antioxid Redox Signal 4: 701–709, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Jones BE, Lo CR, Liu H, Srinivasan A, Streetz K, Valentino KL, Czaja MJ. Hepatocytes sensitized to tumor necrosis factor-α cytotoxicity undergo apoptosis through caspase-dependent and caspase-independent pathways. J Biol Chem 275: 705–712, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest 105: 1067–1075, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Jones BE, Bradham C, Czaja MJ. Increased cytochrome P-450 2E1 expression sensitizes hepatocytes to c-Jun-mediated cell death from TNF-α. Am J Physiol Gastrointest Liver Physiol 282: G257–G266, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Lo CR, Czaja MJ. NF-κB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology 35: 772–778, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Lo CR, Jones BE, Pradhan Z, Srinivasan A, Valentino KL, Stockert RJ, Czaja MJ. Inhibition of c-Myc expression sensitizes hepatocytes to tumor necrosis factor-induced apoptosis and necrosis. J Biol Chem 275: 40155–40162, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Liu RM, Borok Z, Forman HJ. 4-Hydroxy-2-nonenal increases γ-glutamylcysteine synthetase gene expression in alveolar epithelial cells. Am J Respir Cell Mol Biol 24: 499–505, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 13: 1169–1183, 1999 [PubMed] [Google Scholar]
- 27.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem 281: 12093–12101, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Mari M, Cederbaum AI. Induction of catalase, alpha, and microsomal glutathione S-transferase in CYP2E1 overexpressing HepG2 cells and protection against short-term oxidative stress. Hepatology 33: 652–661, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Pabst MJ, Habig WH, Jakoby WB. Glutathione S-transferase A. A novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J Biol Chem 249: 7140–7147, 1974 [PubMed] [Google Scholar]
- 31.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Waeg G, Gentilini P, Dianzani MU. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest 102: 1942–1950, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J 227: 629–638, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology 43: 163–172, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Schattenberg JM, Wang Y, Rigoli RM, Koop DR, Czaja MJ. CYP2E1 overexpression alters hepatocyte death from menadione and fatty acids by activation of ERK1/2 signaling. Hepatology 39: 444–455, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Schattenberg JM, Wang Y, Singh R, Rigoli RM, Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J Biol Chem 280: 9887–9894, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNFα- and Fas-mediated apoptosis in hepatocytes. FASEB J 18: 720–722, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Seelig GF, Meister A. Glutathione biosynthesis; γ-glutamylcysteine synthetase from rat kidney. Methods Enzymol 113: 379–390, 1985 [DOI] [PubMed] [Google Scholar]
- 38.Siems WG, Zollner H, Grune T, Esterbauer H. Metabolic fate of 4-hydroxynonenal in hepatocytes: 1,4-dihydroxynonene is not the main product. J Lipid Res 38: 612–622, 1997 [PubMed] [Google Scholar]
- 39.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology 49: 87–96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell 121: 667–670, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Tsukamoto H, Horne W, Kamimura S, Niemela O, Parkkila S, Yla-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest 96: 620–630, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Schattenberg JM, Rigoli RM, Storz P, Czaja MJ. Hepatocyte resistance to oxidative stress is dependent on protein kinase C-mediated down-regulation of c-Jun/AP-1. J Biol Chem 279: 31089–31097, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology 27: 128–133, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology 111: 1645–1653, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Bialik S, Jones BE, Iimuro Y, Kitsis RN, Srinivasan A, Brenner DA, Czaja MJ. NF-κB inactivation converts a hepatocyte cell line TNF-α response from proliferation to apoptosis. Am J Physiol Cell Physiol 275: C1058–C1066, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, Jones BE, Neufeld DS, Czaja MJ. Glutathione modulates rat and mouse hepatocyte sensitivity to tumor necrosis factor toxicity. Gastroenterology 115: 1229–1237, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Yaglom J, O'Callaghan-Sunol C, Gabai V, Sherman MY. Inactivation of dual-specificity phosphatases is involved in the regulation of extracellular signal-regulated kinases by heat shock and hsp72. Mol Cell Biol 23: 3813–3824, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]