Abstract
Background: Disruption of the circadian system may be causal for manifestations of the metabolic syndrome (MetS).
Objective: The objective was to study the associations of 5 CLOCK polymorphisms with MetS features by analyzing fatty acid (FA) composition from dietary and red blood cell (RBC) membrane sources.
Design: Participants (n = 1100) in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study were included. Dietary intake was estimated with a validated questionnaire. Anthropometric and biochemical measurements and genotypes were determined. Postprandial lipids and the FA composition of RBC membranes were analyzed.
Results: CLOCK single nucleotide polymorphisms were significantly associated with obesity and individual components of MetS. For single nucleotide polymorphism rs4580704, minor allele carriers had a 46% lower risk of hypertension than did noncarriers. The monounsaturated fatty acid (MUFA) content of RBC membranes, particularly oleic acid, changed according to CLOCK genetic variants (P < 0.05). We identified significant gene-diet interactions associated with MetS at the CLOCK locus. By dichotomizing MUFA intake, we found different effects across rs4580704 genotypes for glucose (P = 0.020) and insulin resistance (P = 0.026). The protective effect of the minor allele on insulin sensitivity was only present when MUFA intake was >13.2% of energy. We also found different effects across CLOCK 3111T→C genotypes for saturated fatty acid intake (% of energy) (P = 0.017). The deleterious effect of gene variants on waist circumference was only found with high saturated fatty acid intakes (>11.8%).
Conclusions: CLOCK polymorphisms interact with FAs to modulate MetS traits. The dietary source and membrane content of MUFAs are implicated in the relations between alterations in the circadian system and MetS.
INTRODUCTION
Recent clinical and epidemiologic studies have shown significant relations between chronobiology and certain components of the metabolic syndrome (MetS). Thus, shift work, sleep deprivation and bright-light exposure at night have been associated with an increased level of adiposity and prevalence of MetS (1).
Although our understanding of the biological clock model continues to evolve, it is already known that CLOCK (Circadian Locomotor Output Cycles Kaput), one of the transcription factors from the positive limb of the molecular clock, is involved in metabolic alterations (2). It has been shown in animal models that mice with Clock gene disruptions are prone to develop a phenotype resembling MetS (3). In 2004, Rudic et al (4) showed that mutations in the Clock gene were associated with impaired glucose tolerance, which indicates that the disruption of the circadian system may be causal for the expression of some of the MetS components.
Lipids, especially fatty acids (FAs), play a critical metabolic role (5). In particular, the composition of membrane FAs greatly influences membrane function, and the interplay between dietary FAs, membrane lipid composition, and several MetS components has been reviewed (6). Moreover, mounting evidence suggests that FAs might regulate different chronobiological functions. FAs affect pineal function, which is implicated in the sleep-wake rhythm and may play a role in the regulation of locomotor and exploratory activity (7). Studies performed in experimental models show that changes in the pineal membrane FA composition are associated with a lessening of the melatonin rhythm and a weakening of the endogenous functioning of the circadian clock and play a role in nocturnal sleep disturbances, as described in attention deficit/hyperactivity disorder (7). Conversely, the circadian system has been reported to influence lipid metabolism through the regulation of expression and/or activity of some metabolic enzymes and transport systems involved in FA metabolism (8, 9). As such, several nuclear receptors involved in lipid metabolism have been found to exhibit circadian rhythmicity of expression (10). Peroxisome proliferator–activated receptor α (PPARα), a nuclear receptor family member, provides an example of coordination between circadian and metabolic processes (11). It has been shown that CLOCK/BMAL1-mediated transcription of period (PER) and cryptochrome (CRY) is modulated by PPARα/RXRα, which suggests that there may be crosstalk between PPARα/RXRα- and CLOCK/BMAL1-regulated systems and lipid metabolism (12–14).
More recently, these research efforts have been extended to gain further understanding about the role of CLOCK variants in human obesity and MetS risk. Thus far, only 2 studies have specifically focused on MetS, and no study has yet related CLOCK gene polymorphisms to any particular MetS component (15, 16). Interestingly, despite the potential influence of membranes' FA composition in both, the regulation of the circadian system regulation and the risk of the MetS, there has been study of the associations between FA erythrocyte membrane composition and dietary intake and human CLOCK gene polymorphisms. Therefore, the objective of this investigation was to study the associations of CLOCK genetic polymorphisms with MetS features and describe environmental interactions such as those associations modified by dietary FAs.
SUBJECTS AND METHODS
Study participants and study design
The study sample consisted of 540 men and 560 women who participated in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study. GOLDN is part of the Program for Genetic Interactions Network and is funded by the National Institutes of Health through the University of Alabama at Birmingham and in collaboration with the University of Utah, Washington University, Tufts University, University of Texas, University of Michigan, University of Minnesota, and Fairview-University of Minnesota Medical Center. Most participants were re-recruited from 3-generational pedigrees from two National Heart, Lung, and Blood Institute Family Heart Study field centers (Minneapolis, MN, and Salt Lake City, UT) (17). All individuals were of European descent. The details of the study design were previously described (18). The protocol was approved by the Institutional Review Boards at the University of Alabama, the University of Minnesota, the University of Utah, and Tufts University.
Anthropometric measurements
We measured weight with a beam balance, hip circumference at maximal hip girth, and waist circumference at the umbilicus. Body mass index (BMI) was calculated as weight (kg)/height2 (m), and obesity was defined as BMI ≥ 30. We administered clinical and lifestyle questionnaires and created an interviewer-administered direct data entry system for the diet-history questionnaire (DHQ) developed by the National Cancer Institute.
Dietary intake
Dietary intake was estimated by use of the DHQ, a food-frequency questionnaire developed by staff at the Risk Factor Monitoring and Methods Branch. It consists of 124 food items and includes both portion size and dietary supplement questions. Two studies were conducted to assess its validity (19, 20). The food list and nutrient database used with the DHQ are based on national dietary data (21).
Biochemical analyses
We drew venous blood after the study participants had fasted overnight. Plasma samples were stored and analyzed together. Blood collection, plasma separation and processing, and biochemical analyses for plasma lipids (including triglycerides, total cholesterol, HDL cholesterol, and LDL cholesterol), glucose, insulin, adiponectin, and interleukin-6 (IL6) were previously described (22, 23).
Postprandial study fat challenge
The postprandial study fat challenge consisted of a meal formulated according to the protocol of Patsch et al (24).The meal, which participants were instructed to consume within 15 min, contained 700 kcal/m2 body surface area (2.93 MJ/m2 body surface area); 3% of energy was derived from protein, 14% from carbohydrate, and 83% from fat sources. The cholesterol content was 240 mg, and the ratio of polyunsaturated fatty acid (PUFA) to saturated fatty acid (SFA) was 0.06. The average person consumed 175 mL heavy whipping cream (39.5% fat) and 7.5 mL powdered, instant, nonfat dry milk blended with ice and 15 mL chocolate- or strawberry-flavored syrup to increase palatability. We drew blood samples immediately before (time 0) and 3.5 and 6 h after the high-fat meal.
Erythrocyte membrane fatty acid determination
Fasting blood samples were collected into EDTA-containing tubes. Erythrocyte membrane separation and FA extraction followed procedures previously described (25, 26). The final product was dissolved in heptane and injected into a capillary Varian (Palo Alto, CA) CP7420 100-m column with a Hewlett-Packard (Palo Alto, CA) 5890 gas chromatograph equipped with a HP6890A autosampler. FA methylesters from 12:0 through 24:1(n−9) were separated, identified, and expressed as a percentage of total FAs.
DNA isolation and CLOCK genotyping
We selected tag single nucleotide polymorphisms (tag SNPs) as effective proxies for untyped SNPs in strong linkage disequilibrium (LD) by using the Tagger (27) based on HapMap Caucasian European Utah data (28) with a minor allele frequency (MAF) ≥0.10 and a minimum r2 of 0.8. Tagger uses an algorithm that selects tag SNPs to construct single- and multi-marker tests to capture alleles of interest based on the computed correlation r2 between them.
For haplotype analysis, we estimated haplotype frequencies using the expectation-maximization algorithm implemented in HelixTree for a subset of SNPs selected on the basis of individual association with a given trait (29). To determine the association between haplotypes and phenotypes, we examined the association between haplotypes and given traits using linear regression models, while treating carriers or noncarriers of a haplotype as a predictor (30). Analyses were adjusted for potential confounders.
DNA was isolated from blood samples by using routine DNA isolation sets (Qiagen, Hilden, Germany). We performed genotyping of CLOCK gene polymorphisms using a TaqMan assay with allele-specific probes on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) according to standardized laboratory protocols (31).
Bioinformatics analysis
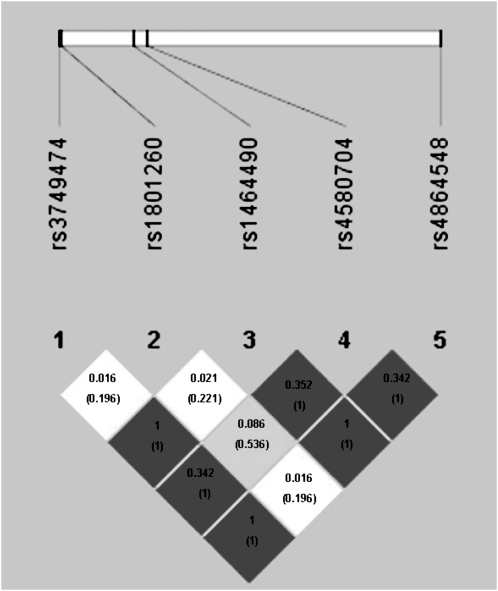
The LD plot between the studied SNPs is shown in Figure 1. SNPs were selected by using 3 criteria: literature reports of genetic associations or biological function of interest, bioinformatics functional assessment, and LD structure. In conjunction with the selection of tag SNPs, we also performed a bioinformatics analysis of the genomic DNA sequence encompassing the different SNPs in order to ascertain putative biological consequences of the different alleles. SNPs mapping to regions upstream of the transcription start site or within introns were studied with MAPPER (32) to identify potential allele-specific transcription factor binding sites. No intronic SNPs altered splice acceptor or donor sites or other signals recognized by the splicing machinery, such as the poly-pyrimidine tract. Polymorphisms within the 3′-UTR of the mRNA can exert an effect on the folding of the mRNA with concomitant changes in mRNA stability. Such was tested with RNAfold (33) within the Vienna RNA package.
FIGURE 1.
Linkage disequilibrium (LD) plot between the single nucleotide polymorphisms (SNPs) selected and the LD plot across the CLOCK gene. The horizontal white bar depicts the 113-kb DNA segment of chromosome 4q12 analyzed in the sample. The 5 tag SNP locations are indicated by hatch marks. In the LD plot depicted in the bottom part of the figure, each diamond represents the magnitude of LD for a single pair of markers. The dark tones indicate strong LD (r2 = 1.0), the white tones indicate no LD (r2 = 0), and the gray tones indicate intermediate LD. The numbers inside the diamonds indicate the r2 (D′) value.
Statistical analysis
Those variables, which were not normally distributed, were log transformed. We applied analysis of variance and Student's t test to compare crude means across genotype groups. We tested different genetic inherent models, and a dominant model was applied in the final analyses for all the SNPs selected, except for rs1801260 (3111T→C), which followed a recessive model. We carried out multivariate adjustments of the associations by analysis of covariance and estimated adjusted means. We adjusted analyses for sex, age, and family relationships. We also tested the statistical homogeneity of the effects by sex in the corresponding regression model with interaction terms. The familial relationships within the population were adjusted by using a generalized linear model implemented in the GENMOD procedure in SAS assuming an exchangeable correlation structure within pedigree (34, 35) We fitted logistic regression models to estimate the odds ratios (ORs) and 95% CIs of obesity and particular MetS components, such as high triglycerides, high glucose, high blood pressure and abdominal obesity associated with the CLOCK polymorphism. We used routine regression diagnostic procedures to ensure the appropriateness of the models. Statistical analyses were done by using SPSS 15.0 software (SPSS Inc, Chicago, IL). A 2-tailed P value <0.05 was considered statistically significant.
RESULTS
Characteristics of the population studied, including MetS features and FA intake and composition, are shown in Table 1. Briefly, 37.3% of our population had MetS. Significant inverse correlations were found between oleic acid and the total MUFA content of erythrocyte membranes and MetS traits (oleic acid and total MUFAs, respectively): BMI (r = −0.092, P = 0.003; r = −0.18, P = 0.0001), glucose (r = −0.08, P = 0.006; r = −0.80, P = 0.001), and insulin (r = −0.10, P = 0.001; r = −0.13, P = 0.001); conversely, we found significant correlations for adiponectin (r = 0.10, P = 0.001; r = −0.14, P = 0.0001).
TABLE 1.
General characteristics of the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) participants1
| Total (n = 1100) | Men (n = 540) | Women (n = 560) | |
| Age (y) | 48.6 ± 16.42 | 49.0 ± 16.4 | 48.2 ± 16.4 |
| Weight (kg) | 82.9 ± 18.2 | 90.4 ± 16.3 | 75.9 ± 17.23 |
| Height (m) | 1.71 ± 0.1 | 1.78 ± 0.1 | 1.65 ± 0.13 |
| BMI (kg/m2) | 28.3 ± 5.6 | 28.5 ± 4.9 | 28.0 ± 6.3 |
| Waist circumference (cm) | 96.5 ± 16.2 | 100.5 ± 13.9 | 92.7 ± 17.23 |
| Hip circumference (cm) | 107.4 ± 11.6 | 105.8 ± 8.9 | 108.8 ± 13.53 |
| Triglycerides (mg/dL) | 139 ± 115.9 | 153 ± 142.3 | 125 ± 82.23 |
| Total cholesterol (mg/dL) | 191 ± 38.9 | 190 ± 37.5 | 191 ± 40.2 |
| HDL cholesterol (mg/dL) | 47.1 ± 13.1 | 41.5 ± 9.8 | 52.3 ± 13.73 |
| LDL cholesterol (mg/dL) | 122 ± 31 | 123 ± 30 | 120 ± 32 |
| Fasting glucose (mg/dL) | 101 ± 18.7 | 106 ± 20.8 | 98 ± 15.73 |
| Fasting insulin (mU/L) | 13.7 ± 8.1 | 14.1 ± 8.4 | 13.3 ± 7.9 |
| Systolic BP (mm Hg) | 115 ± 16 | 118 ± 15 | 112 ± 173 |
| Diastolic BP (mm Hg) | 68 ± 9.3 | 71 ± 9.1 | 66 ± 8.93 |
| Adiponectin (ng/mL) | 8341 ± 4798 | 6329 ± 3519 | 10,204 ± 50673 |
| IL-6 (pg/mL) | 1.97 ± 3.1 | 2.07 ± 4.15 | 1.89 ± 1.64 |
| Energy intake (kcal/d) | 2116 ± 1182 | 2492 ± 1403 | 1768 ± 7873 |
| Dietary fat composition | |||
| Total fat (% of energy) | 35.4 ± 6.9 | 35.9 ± 6.9 | 34.9 ± 6.93 |
| SFA (% of energy) | 11.8 ± 2.8 | 12.2 ± 2.8 | 11.6 ± 2.73 |
| MUFA (% of energy) | 13.3 ± 2.9 | 13.7 ± 2.8 | 12.9 ± 2.83 |
| PUFA (% of energy) | 7.6 ± 2.2 | 7.4 ± 2.0 | 7.9 ± 2.33 |
| Total fat (g/d) | 85.4 ± 50.7 | 101.2 ± 58.3 | 70.6 ± 36.53 |
| SFA (g/d) | 28. ± 18.4 | 34.5 ± 21.4 | 23.4 ± 12.83 |
| MUFA (g/d) | 32.1 ±19.4 | 38.5 ± 22.3 | 26.2 ± 13.83 |
| PUFA (g/d) | 18.1 ±10.7 | 20.6 ± 11.9 | 15.8 ± 8.83 |
| RBC membrane (%) | |||
| cis Oleic acid | 16.1 ± 1.1 | 16.25 ± 1.11 | 16.03 ± 1.03 |
| trans Oleic acid | 1.40 ± 0.42 | 1.38 ± 0.42 | 1.42 ± 1.03 |
| Total n−9 FAs | 16.2 ± 1.04 | 16.33 ± 1.03 | 16.18 ± 0.433 |
| MUFA | 17.97 ± 1.08 | 18.03 ± 1.09 | 17.91 ± 1.03 |
| PUFA | 34.13 ± 1.52 | 34.15 ± 1.56 | 34.11 ± 1.07 |
| SFA | 35.01 ± 1.31 | 34.93 ± 1.24 | 35.08 ± 1.48 |
| Total trans FAs | 1.69 ± 0.48 | 1.67 ± 0.48 | 1.71 ± 1.37 |
| Metabolic syndrome [n (%)] | 418 (37.3) | 223 (41.4) | 195 (33.6) |
| Obesity [n (%)] | 373 (33.2) | 176 (32.5) | 197 (33.8) |
| Diabetes or high blood sugar [n (%)] | 90 (8.0) | 53 (9.8) | 37 (6.4) |
| CLOCK polymorphism [n (%)] | |||
| rs4580704 | |||
| GG | 141 (12.8) | — | — |
| CG | 478 (43.4) | — | — |
| CC | 483 (43.8) | — | — |
| rs1801260 (3111T→C) | |||
| GG | 88 (5.8) | — | — |
| AG | 652 (43.3) | — | — |
| AA | 746 (49.5) | — | — |
| rs3749474 | |||
| TT | 146 (13.4) | — | — |
| TC | 520 (46.8) | — | — |
| CC | 424 (38.9) | — | — |
BP, blood pressure; FA, fatty acid; IL-6, interleukin-6; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; RBC, red blood cell; SFA, saturated fatty acid.
Mean ± SD (all such values).
Significantly different from men, P < 0.05 (Student's t test).
Genotype frequency of CLOCK variants in the GOLDN population
SNP rs1464490 was selected as the tag SNP for a large LD block (LD1); rs3749474, also from LD1, was selected from bioinformatics functional assessment because of its potential effect on mRNA structure, and rs4864584 was selected because of its previous association with overweight/obesity (15, 16). From the different SNPs in LD2, inclusion of rs4580704 was based on its previous relation with BMI (16). From LD3, we selected rs18012602 (3111T→C) based on previous reports showing associations with sleep alterations (36) and binge-eating disorders (37). Their locations in the CLOCK gene, Hardy-Weinberg equilibrium, and MAF of the SNPs studied are listed in Table 2. CLOCK genotype frequencies did not deviate from Hardy-Weinberg equilibrium expectations. Because rs3749474, rs1464490, and rs4864548 were almost in complete LD and displayed a similar pattern of phenotypic associations, only the results for the rs3749474 SNP are presented.
TABLE 2.
Description of CLOCK single nucleotide polymorphisms1
| Name | Location | P value2 | MAF | Alleles | Minor allele |
| rs3749474 | 3′-UTR | 0.9618 | 0.406 | C/T | T |
| rs1801260 (3111T→C) | 3′-UTR | 0.1816 | 0.372 | A/G | G |
| rs1464490 | Intron 11 | 1 | 0.415 | C/T | T |
| rs4580704 | Intron 9 | 0.8464 | 0.332 | C/G | G |
| rs4864548 | Promoter | 0.9618 | 0.406 | G/A | A |
MAF, minor allele frequency; UTR, untranslated region.
Hardy-Weinberg equilibrium expectations.
We first examined the association between the CLOCK SNPs and MetS components (Table 3). We did not detect sex heterogeneity for any of the SNPs examined. Therefore, we present the results for men and women combined. We found significant associations with weight and BMI for SNPs rs4580704 and rs1801260 (3111T→C) (Table 3). These SNPs were also associated with glucose and insulin-resistance related variables such as plasma insulin, homeostasis model assessment of insulin resistance (HOMA), and adiponectin concentrations. Moreover, for rs4580704, minor allele carriers had a 31% lower risk of diabetes than did noncarriers (Table 4). We found no associations between CLOCK SNPs and fasting lipids such as triglycerides, HDL cholesterol, LDL cholesterol, VLDL cholesterol, and total cholesterol. Conversely, postprandial triglyceridemia after a fat-loading test was lower in carriers than in noncarriers for the rs4580704 allele (P = 0.045). However, associations found for rs4580704 were lost when data were adjusted for BMI (Tables 3 and 4).
TABLE 3.
Association of CLOCK with metabolic syndrome (MetS) components, dietary fatty acids (FAs), and erythrocyte membrane FAs (n = 1100)1
| rs45807042 |
rs18012603 (3111T→C) |
rs37494742 |
||||||||||
| Genotype (minor allele = G) | Mean ± SEM | P4 | P5 | Genotype (minor allele = G) | Mean ± SEM | P4 | P5 | Genotype (minor allele = T) | Mean ± SEM | P4 | P5 | |
| Weight (kg) | GG+CG | 83.52 ± 0.85 | 0.017 | 0.072 | GG | 87.21 ± 1.33 | 0.020 | 0.330 | TT+TC | 85.58 ± 0.79 | 0.168 | 0.043 |
| CC | 86.24 ± 0.91 | AG+AA | 84.21 ± 0.69 | CC | 83.65 ± 0.98 | |||||||
| BMI (kg/m2) | GG+CG | 28.09 ± 0.26 | 0.014 | GG | 29.33 ± 0.42 | 0.017 | TT+TC | 28.63 ± 0.24 | 0.554 | |||
| CC | 29.04 ± 0.28 | AG+AA | 28.36 ± 0.21 | CC | 28.40 ± 0.30 | |||||||
| MetS components | ||||||||||||
| Waist circumference (cm) | GG+CG | 96.64 ± 0.74 | 0.193 | 0.234 | GG | 100.19 ± 1.20 | 0.223 | 0.312 | TT+TC | 97.70 ± 0.69 | 0.390 | 0.653 |
| CC | 98.07 ± 0.81 | AG+AA | 96.62 ± 0.60 | CC | 96.75 ± 0.87 | |||||||
| Hip circumference (cm) | GG+CG | 107.19 ± 0.56 | 0.033 | 0.993 | GG | 109.4 ± 0.91 | 0.075 | 0.077 | TT+TC | 108.36 ± 0.51 | 0.203 | 0.090 |
| CC | 108.97 ± 0.61 | AG+AA | 107.64 ± 0.46 | CC | 107.32 ± 0.64 | |||||||
| Fasting glucose (mg/dL) | GG+CG | 101.79 ± 0.88 | 0.715 | 0.812 | GG | 102.21 ± 1.30 | 0.914 | 0.496 | TT+TC | 102.18 ± 0.75 | 0.780 | 0.590 |
| CC | 102.26 ± 0.97 | AG+AA | 102.05 ± 0.65 | CC | 101.84 ± 0.94 | |||||||
| log Fasting insulin (mU/L) | GG+CG | 1.08 ± 0.01 | 0.016 | 0.469 | GG | 1.13 ± 0.02 | 0.043 | 0.010 | TT+TC | 1.09 ± 0.01 | 0.347 | 0.096 |
| CC | 1.12 ± 0.01 | AG+AA | 1.09 ± 0.01 | CC | 1.11 ± 0.01 | |||||||
| HOMA-IR | GG+CG | 3.63 ± 0.14 | 0.055 | 0.719 | GG | 3.96 ± 0.21 | 0.009 | 0.019 | TT+TC | 3.57 ± 0.11 | 0.168 | 0.056 |
| CC | 3.91 ± 0.15 | AG+AA | 3.72 ± 0.11 | CC | 3.91 ± 0.14 | |||||||
| Adiponectin (ng/mL) | GG+CG | 8194 ± 208 | 0.387 | 0.889 | GG | 7356 ± 339 | 0.001 | 0.090 | TT+TC | 8142 ± 196 | 0.072 | 0.084 |
| CC | 7925 ± 229 | AG+AA | 8485 ± 169 | CC | 8600 ± 245 | |||||||
| Systolic BP (mm Hg) | GG+CG | 113 ± 0.70 | 0.001 | 0.036 | GG | 116 ± 1.16 | 0.001 | 0.002 | TT+TC | 115 ± 0.66 | 0.666 | 0.742 |
| CC | 117 ± 0.77 | AG+AA | 115 ± 0.58 | CC | 115 ± 0.83 | |||||||
| Diastolic BP (mm Hg) | GG+CG | 67 ± 0.43 | 0.005 | 0.033 | GG | 69 ± 0.71 | 0.227 | 0.116 | TT+TC | 68 ± 0.41 | 0.453 | 0.963 |
| CC | 69 ± 0.47 | AG+AA | 68 ± 0.35 | CC | 68 ± 0.51 | |||||||
| IL-6 (pg/mL) | GG+CG | 2.03 ± 0.12 | 0.100 | 0.024 | GG | 2.058 ± 1.615 | 0.168 | 0.346 | TT+TC | 1.88 ± 1.65 | 0.006 | 0.002 |
| CC | 1.91 ± 0.14 | AG+AA | 1.974 ± 3.199 | CC | 2.14 ± 1.84 | |||||||
| Dietary FAs | ||||||||||||
| Total fat (g/d) | GG+CG | 82.64 ± 1.921 | 0.038 | 0.200 | GG | 89.62 ± 3.95 | 0.288 | 0.224 | TT+TC | 88.20 ± 1.82 | 0.021 | 0.005 |
| CC | 88.32 ± 2.16 | AG+AA | 84.91 ± 1.98 | CC | 80.66 ± 2.83 | |||||||
| SFA (g/d) | GG+CG | 27.92 ± 0.63 | 0.031 | 0.158 | GG | 30.08 ± 1.41 | 0.348 | 0.140 | TT+TC | 30.19 ± 0.80 | 0.009 | 0.002 |
| CC | 30.20 ± 0.77 | AG+AA | 28.60 ± 0.70 | CC | 26.80 ± 1.01 | |||||||
| MUFA (g/d) | GG+CG | 31.36 ± 0.72 | 0.053 | 0.216 | GG | 33.67 ± 1.52 | 0.337 | 0.228 | TT+TC | 33.53 ± 0.87 | 0.029 | 0.008 |
| CC | 33.61 ± 0.82 | AG+AA | 32.03 ± 0.76 | CC | 30.48 ± 1.09 | |||||||
| PUFA (g/d) | GG+CG | 17.71 ± 0.41 | 0.043 | 0.253 | GG | 19.21 ± 0.85 | 0.203 | 0.458 | TT+TC | 18.79 ± 0.49 | 0.066 | 0.020 |
| CC | 18.91 ± 0.47 | AG+AA | 17.99 ± 0.43 | CC | 17.34 ± 0.61 | |||||||
| RBC membrane FAs | ||||||||||||
| Total oleic acids | GG+CG | 16. 25 ± 0. 43 | 0.0001 | 0.001 | GG | 15.97 ± 0.08 | 0.012 | 0.121 | TT+TC | 16.06 ± 0.05 | 0.005 | 0.005 |
| CC | 15. 9 ± 0. 49 | AG+AA | 16.91 ± 0.04 | CC | 16.28 ± 0.06 | |||||||
| Total MUFA | GG+CG | 18.09 ± 0.43 | 0.00001 | 0.00003 | GG | 17.92 ± 0.08 | 0.560 | 0.267 | TT+TC | 17.89 ± 0.04 | 0.003 | 0.003 |
| CC | 17.81 ± 0.49 | AG+AA | 17.97 ± 0.03 | CC | 18.09 ± 0.05 | |||||||
| AUC | ||||||||||||
| Triglycerides | GG+CG | 2624 ± 98 | 0.048 | 0.467 | GG | 2738 ± 157 | 0.870 | 0.643 | TT+TC | 2809 ± 93 | 0.381 | 0.942 |
| GG | 2887 ± 105 | AG+AA | 2765 ± 81 | CC | 2678 ± 116 | |||||||
AUC, area under the curve; BP, blood pressure; HOMA-IR, homeostasis model assessment of insulin resistance; IL-6, interleukin-6; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; RBC, red blood cell; SFA, saturated fatty acid. Multivariate adjustments of the associations were obtained by ANCOVA and estimated adjusted means.
Dominant model.
Recessive model.
After adjustment for age, sex, and family relationships.
After adjustment for BMI.
TABLE 4.
Odds ratios (ORs) for the prevalence of metabolic syndrome components across CLOCK (rs4580704) genotype groups1
| Elevated blood pressure |
High fasting glucose |
|||
| rs4580704 | OR | 95% CI | OR | 95% CI |
| CC (n = 483) | 1.46 | 1.080, 1.997 | 1.310 | 1.00, 1.70 |
| GG+CG (n = 619) | 1 | — | 1 | — |
| P | 0.014 | — | 0.047 | — |
| P2 | 0.036 | — | 0.591 | — |
Cutoffs were based on National Cholesterol Education Program criteria (1). Estimated means and P values were adjusted for age, sex, and family relationships.
Adjusted for age, sex, family relationships, and BMI (logistic regression model).
Systolic and diastolic blood pressure values were also strongly associated with SNP rs4580704. Minor allele carriers had lower blood pressure values than did carriers of the common allele. These data were consistent with further logistic regression analysis, which showed that minor allele carriers had a 45% lower risk of having hypertension. These results were statistically significant after adjustment for BMI (Table 4).
We found significant associations between each of the SNPs examined and oleic acid and total MUFA FA membrane composition, with rs4580704 and rs3749474 following a dominant pattern, whereas 3111T→C followed a recessive pattern of inheritance (Table 3). Total fat intake was also associated with CLOCK gene polymorphisms. Minor allele carriers of SNP rs3749474 showed a higher intake of fat, whereas the opposite was true for rs4580704. After the adjustment for total fat intake, associations with dietary FAs disappeared, whereas those found for membrane FAs remained statistically significant, even after adjustment for n−3 and n−6 PUFAs. Moreover, addition of BMI to the statistical model did not affect the significance of most of the main effect associations (Table 3). To assess sleep quality, serum IL-6 was measured. After adjusting for BMI, we found significant associations between serum IL-6 values and rs4580704 and rs3749474 SNPs.
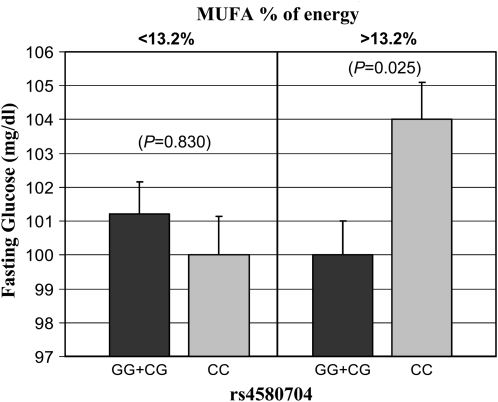
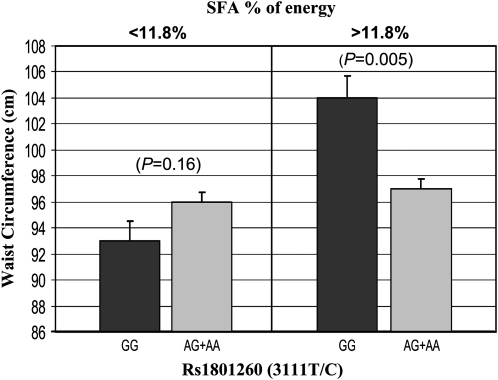
We identified significant gene-diet interactions associated with MetS at the CLOCK locus. By dichotomizing MUFA intake, we found significantly different effects across rs4580704 genotypes for glucose (P = 0.020) and insulin resistance (HOMA) (P = 0.026). When the MUFA intake (% of energy) was below the median (<13.2%), no significant differences were found for plasma glucose concentrations and HOMA between carriers and noncarriers (P > 0.05). However, when MUFA intake was ≥13.2%, minor allele carriers had significantly lower plasma glucose concentrations (P = 0.025) (Figure 2) and HOMA (P = 0.004) values than did noncarriers. We also found different effects across CLOCK 3111T→C genotypes for SFA intake (% of energy) (P = 0.017) (Figure 3). Similarly, when the SFA intake was <11.8%, no significant differences were found for waist circumference between carriers and noncarriers (P = 0.153). Conversely, when SFA intake was ≥11.8%, minor allele carriers had larger waist circumferences than noncarriers (P = 0.028).
FIGURE 2.
Mean (±SE) fasting plasma glucose concentrations by rs4580704 polymorphism at the CLOCK gene according to monounsaturated fatty acid (MUFA) intakes below and above the population median (13.2% of energy). Estimated means were adjusted for sex, age, and familial relationships. P values for the interaction (P = 0.020) terms between fat intake and the corresponding polymorphism were obtained in the hierarchical multivariate interaction model containing MUFA intake as a categorical variable with additional control for the other covariates.
FIGURE 3.
Mean (±SE) waist circumference by rs1801260 (3111T→C) polymorphism at the CLOCK gene according to saturated fatty acid (SFA) intakes below and above the population median (11.8% of energy). Estimated means were adjusted for sex, age, and familial relationships. P values for the interaction (P = 0.017) terms between fat intake and the corresponding polymorphism were obtained in the hierarchical multivariate interaction model containing SFA intake as a categorical variable with additional control for the other covariates.
Haplotype analysis
To examine the combined effects of multiple variants of CLOCK, we conducted a haplotype analysis using a subset of CLOCK SNPs. Although the best method among proposed strategies to select SNPs for haplotype analysis is debatable, we selected 3 SNPs that exhibited significant or marginally significant association with any given trait: rs3749474, rs45807041, and rs1801260 (3111T→C).
There were 7 haplotypes with frequencies ranging from 1% to 31%, which accounted for 100% of all haplotypes in this population. For further analysis we selected those haplotypes with frequencies >4%, such as TCA (31%), CGA (29%), CCG (29%), and TCG (4.3%). The 3 SNPs in haplotypes were arranged in the order rs3749474, rs4580704, 3111T→C. After adjustment for covariates, haplotype analysis showed that carriers of the haplotype CGA had a lower BMI, weight, waist circumference, adiponectin concentration, and blood pressure than did noncarriers (Table 5). In addition, CLOCK haplotype CGA was significantly associated with oleic acid RBC membrane composition. Haplotype was composed of the major allele of rs3749474, minor allele of 4580704, and major allele of rs1801260. Data were consistent with previous associations between the different variables studied and the particular CLOCK SNPs. The frequency of CLOCK haplotype CGA was 60.6% in noncarriers and 39.4% in carriers.
TABLE 5.
Significant associations with haplotype CGA (rs3749474/rs4580704/rs1801260)1
| Mean ± SEM | P value | |
| BMI (kg/m2) | ||
| CGA noncarrier | 29.04 ± 0.26 | 0.016 |
| CGA carrier | 28.06 ± 0.31 | |
| Weight (kg) | ||
| CGA noncarrier | 86.34 ± 0.81 | 0.003 |
| CGA carrier | 82.65 ± 0.95 | |
| Waist circumference (cm) | ||
| CGA noncarrier | 98.80 ± 0.75 | 0.020 |
| CGA carrier | 96.08 ± 0.88 | |
| Adiponectin (ng/mL) | ||
| CGA noncarrier | 7962 ± 177 | 0.006 |
| CGA carrier | 8671 ± 186 | |
| Systolic BP (mm Hg) | ||
| CGA noncarrier | 116 ± 0.63 | 0.006 |
| CGA carrier | 113 ± 0.66 | |
| Diastolic BP (mm Hg) | ||
| CGA noncarrier | 69 ± 0.37 | 0.0001 |
| CGA carrier | 67 ± 0.38 | |
| cis Oleic acid (RBC membrane) (%) | ||
| CGA noncarrier | 16.01 ± 0.05 | 0.0001 |
| CGA carrier | 16.33 ± 0.06 | |
| Total n−9 FAs (RBC membrane) (%) | ||
| CGA noncarrier | 29.19 ± 0.08 | 0.001 |
| CGA carrier | 29.51 ± 0.10 | |
| MUFA (RBC membrane) (%) | ||
| CGA noncarrier | 17. 85 ± 0. 04 | 0.0003 |
| CGA carrier | 18. 08 ± 0. 04 | |
BP, blood pressure; FA, fatty acid; MUFA, monounsaturated fatty acid; RBC, red blood cell.
DISCUSSION
In this study we replicated, in a large US white population, the previously shown associations between CLOCK gene polymorphisms and BMI (15, 16, 37). Moreover, we showed novel significant associations with individual MetS components such as waist, glucose metabolism-related variables and blood pressure. Carriers of the CGA (rs3749474/rs4580704/rs1801260 (3111T→C) haplotype had lower BMI, waist circumference, blood pressure, and insulin resistance. In addition, erythrocyte membrane MUFA composition was modulated by variability at the CLOCK gene. Furthermore, the genetic effect was also modulated by dietary MUFA content. This is the first report to show such diet-CLOCK gene interactions.
We recently provided evidence of CLOCK gene expression in human adipose tissue and showed its association with different components of MetS (38). In the current study, BMI was associated with SNPs rs4580704 and 3111T→C. Moreover, subjects with those genetic variants associated with higher BMI were also associated with a high consumption of fat. It has been reported in experimental models that high-fat feeding, particularly a high intake of saturated fat, modifies circadian synchronization to light and leads to metabolic abnormalities that mimic human MetS, including obesity and insulin resistance (39). Carriers of the minor allele of rs4580704 (CC+CG) and noncarriers of 3111T→C had lower plasma insulin concentrations. Circadian control of glucose metabolism has been recognized from studies showing variation in glucose tolerance and insulin action across the day (40). In humans, it has been repeatedly shown that oral glucose tolerance is impaired in the afternoon and evening compared with the morning hours. This situation has been ascribed to the impaired insulin sensitivity of the peripheral tissues and to a relative decrease in insulin secretion during evening hours (40). Adiponectin, highly related to insulin sensitivity and defined as a protector cytokine against MetS disturbances (41), is a circadian protein that exhibits both ultradian pulsatility and diurnal variation (42). In the present population, homozygotes for the minor G allele for 3111T→C had higher BMI values than noncarriers, which could account for the lower adiponectin plasma values observed in these subjects (41). In fact, when data were adjusted for BMI, most of the variables related to insulin resistance did not show statistical significance for this particular SNP, which suggests that, for 3111T→C, associations with glucose metabolism are mediated by obesity.
Of particular interest to MetS is the effect that circadian system has on blood pressure. In our study, minor allele carriers of rs4850704 showed lower blood pressure values than major allele carriers. These data appear independent to obesity because after adjustment for BMI, associations with CLOCK gene SNPs remained significant. Consistent with this finding, the risk of hypertension was 46% lower in minor allele carriers than in noncarriers, even after adjustment for BMI. Blood pressure displays a circadian rhythmicity, rising during the day and dipping at night (43). The loss of this pattern has been correlated with insulin resistance and with increased end-organ damage (44, 45). SNP rs4580704 is predicted to produce an allele-specific CREM (cAMP responsive element modulator) binding site (C allele on forward strand binds CREM, G allele does not). CREM has been shown most recently to be responsible for the circadian expression in the mouse liver of many genes that could be implicated in cardiovascular disease risk (46).
We found no associations between CLOCK gene polymorphisms and fasting state lipids, such as triglycerides, HDL cholesterol, LDL cholesterol, VLDL cholesterol, and total cholesterol. These results agree with previous studies performed by Scott et al (15), and Sookoian et al (16). However, after the fat-load test, we found that the response of plasma triglycerides during the entire 6-h period was associated with the CLOCK rs4580704 SNP, which suggests either decreased triglyceride-rich lipoprotein production or faster clearance of minor allele carriers. This association disappeared after BMI was adjusted for, which suggests that obesity influences these results. Related to our finding was the previous report by Romon et al (47), who showed that postprandial lipid, lipoprotein, and apolipoprotein concentrations were affected by circadian factors. In general, associations between erythrocyte membrane FA composition and MetS risk were previously reviewed (6). Moreover, we reported in this population significant differences in the erythrocyte FA profile between MetS and non-MetS subjects (48). Our results indicate that the oleic acid contents of erythrocyte membranes were inversely associated with obesity and insulin-resistant traits, which supports the protective role of MUFAs against MetS risk. However, our study goes further by demonstrating that this protective effect may differ according to the genetic background, in this particular case exemplified by the CLOCK gene. Thus, minor allele carriers of any of the SNPs examined had lower oleic acid and total MUFA contents in RBC membranes than did homozygotes for the major allele, except for rs4580704 SNP, for which the higher MUFA content was associated with the presence of the minor allele. These effects remained significant even after adjustment for obesity.
Dietary fat intake correlated with membrane FA lipid composition, with PUFA showing a tighter correlation than MUFA (49). Oleic acid can be synthesized de novo through elongation and desaturation processes involving the stearoyl-CoA-desaturase-1 (50). The circadian system regulates lipid metabolism throughout the expression and/or activity of some metabolic enzymes involved in FA metabolism (8, 9). CLOCK also functions as a transcriptional regulator of different nuclear receptors, which are known to respond to lipids, particularly PPARα and REV-ERBα (NR1D1) (10, 11).
We hypothesized that differences in the MUFA content of RBCs between carriers and noncarriers of CLOCK gene variants could be due to CLOCK-related changes in the circadian regulation of lipid metabolism. Indeed, our analysis with RNAfold (33) rs3749474 suggests that the T→C base change in the CLOCK 3′-UTR transforms the structure of this particular region, potentially affecting CLOCK mRNA stability or cellular localization. Altered CLOCK mRNA stability could account for a diminished activity of stearoyl-CoA-desaturase-1 in RBC membranes, resulting in the lower MUFA content characteristic of carriers of the CLOCK variant.
Furthermore, a reduced membrane MUFA content could also account for the progression of metabolic disorders in minor allele carriers. It has been postulated that the FA composition of RBCs is a reliable biomarker of dysregulation of lipid and glucose metabolism in the liver. Furthermore, insulin resistance correlates with changes in the FA composition of RBC membranes (51). In our population, subjects with those genetic variants associated with a lower oleic acid content in RBCs also displayed MetS alterations. We could speculate as well that changes in RBC membrane FA composition correlates with those in other cell membranes such as the pineal gland affecting circadian system regulation (7).
The dietary lipid profile has a direct and substantial effect on diseases linked to MetS. It has been shown that dietary MUFAs exert a protective role, whereas SFA intake is directly correlated with obesity and insulin resistance (5, 52, 53). In the present study, analyses of gene-diet interactions showed that dietary MUFA intake could influence the association between insulin resistance and the circadian system. Indeed, for the rs4580704 SNP, the improved insulin sensitivity associated with the minor allele was present only at high MUFA intakes. Furthermore, for SNP 3111T→C, we found a significant gene-diet interaction, by which the deleterious effect of this gene variant on visceral fat was present only in association with high intakes of SFAs.
Our data show strong associations between CLOCK gene polymorphisms and plasma IL-6 concentrations. For SNP rs4580704, the data showed that carriers of the major allele (CC) who reported high BMI also had decreased plasma cytokine concentrations, which could result in decreased sleep (54). For rs3749474, carriers of the major allele who had higher membrane-MUFA concentrations and reported a lower intake of fat, an increase in serum IL-6 concentrations associated with a better sleep quality was observed. It is well known that different cytokines exert effects on sleep time (55–57). In particular, IL-6 is considered to be a sleep factor because concentrations are a good indicator of sleep pattern and fatigue (57–61). These data as a whole assume the greatest importance of sleep quality and duration in obesity and metabolic alterations and confirm experimental and epidemiological studies linking sleep deprivation with obesity. One limitation of the present study was the problem of multiple comparisons. False-positive results make genetic association studies particularly susceptible to publication bias. However, this study is a replication of previous work performed by Sookoian et al (16) and Scott et al (15), which suggest the consistency of associations.
In summary, our results in a US white population support the notion that genetic variation at the CLOCK gene is associated with MetS features, including BMI, waist circumference, glucose-related variables, and blood pressure. Moreover, RBC MUFA composition changes among genotypes. Most interesting is the finding showing that these genetic effects on insulin resistance and obesity phenotypes could be modulated by the dietary intake of MUFAs and SFAs. All of these results reinforce the importance of CLOCK genes in MetS risk and the relevant role of membrane FA composition. This new information offers new insights to the mechanisms linking the circadian system and metabolic alterations.
Acknowledgments
The authors' responsibilities were as follows—MG: collection and analysis of data and writing of the manuscript; Y-CL: collection and analysis of data; JS: analysis of data; LDP: provision of significant advice; DKA and MYT: design of the experiment; C-QL: provision of significant advice; JMO: design of the experiment, critical reading of the manuscript, and provision of significant advice. There were no potential conflicts of interest.
REFERENCES
- 1.Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol 2009;20:127–34 [DOI] [PubMed] [Google Scholar]
- 2.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science 2008;230:1074–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005;308:1043–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudic RD, McNamara P, Curtis AM, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2004;2:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garaulet M, Pérez-Llamas F, Pérez-Ayala M, et al. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr 2001;74:585–91 [DOI] [PubMed] [Google Scholar]
- 6.Hulbert AJ, Turner N, Storlien LH, Else PL. Dietary fats and membrane function: implications for metabolism and disease. Biol Rev Camb Philos Soc 2005;80:155–69 [DOI] [PubMed] [Google Scholar]
- 7.Lavialle M, Champeil-Potokar G, Alessandri JM, et al. An (n−3) polyunsaturated fatty acid-deficient diet disturbs daily locomotor activity, melatonin rhythm, and striatal dopamine in Syrian hamsters. J Nutr 2008;138:1719–24 [DOI] [PubMed] [Google Scholar]
- 8.Froy O. The relationship between nutrition and circadian rhythms in mammals. Front Neuroendocrinol 2007;28:61–71 [DOI] [PubMed] [Google Scholar]
- 9.Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab 2007;18:4–11 [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Downes M, Yu RT, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell 2006;126:801–10 [DOI] [PubMed] [Google Scholar]
- 11.Lemberger T, Saladin R, Vázquez M, et al. Expression of the peroxisome proliferator-activated receptor alpha gene is stimulated by stress and follows a diurnal rhythm. J Biol Chem 1996;271:1764–9 [DOI] [PubMed] [Google Scholar]
- 12.Canaple L, Rambaud J, Dkhissi-Benyahya O, et al. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol 2006;20:1715–27Epub 2006 Mar 23 [DOI] [PubMed] [Google Scholar]
- 13.Goh BC, Wu X, Evans AE, Johnson ML, Hill MR, Gimble JM. Food entrainment of circadian gene expression altered in PPARalpha-/- brown fat and heart. Biochem Biophys Res Commun 2007;360:828–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura K, Inoue I, Takahashi S, Komoda T, Katayama S. Cryptochrome and period proteins are regulated by the CLOCK/BMAL1 gene: crosstalk between the PPARs/RXRalpha-regulated and CLOCK/BMAL1-regulated systems. PPAR Res 2008;348610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–62 [DOI] [PubMed] [Google Scholar]
- 16.Sookoian S, Gemma C, Gianotti TF, Burgueño A, Castaño G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr 2008;87:1606–15 [DOI] [PubMed] [Google Scholar]
- 17.Higgins M, Province M, Heiss G, et al. NHLBI Family Heart Study: objectives and design. Am J Epidemiol 1996;143:1219–28 [DOI] [PubMed] [Google Scholar]
- 18.Corella D, Arnett DK, Tsai MY, et al. The -256T>C polymorphism in the apolipoprotein A-II gene promoter is associated with body mass index and food intake in the genetics of lipid lowering drugs and diet network study. Clin Chem 2007;53:1144–52Epub 2007 [DOI] [PubMed] [Google Scholar]
- 19.Thompson FE, Subar AF, Brown CC, et al. Cognitive research enhances accuracy of food frequency questionnaire reports: results of an experimental validation study. J Am Diet Assoc 2002;102:212–25 [DOI] [PubMed] [Google Scholar]
- 20.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block. Willett and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol 2001;154:1089–99 [DOI] [PubMed] [Google Scholar]
- 21.US Department of Agriculture, Agriculture Research Service 1994 Continuing Survey of Food Intakes by Individuals and the 1994 Diet and Health Knowledge Survey. Springfield, VA: National Technical Information Service, 1996. Accession No. PB97-502058 (CD-ROM) [Google Scholar]
- 22.Shen J, Arnett DK, Peacock JM, et al. Interleukin1beta genetic polymorphisms interact with polyunsaturated fatty acids to modulate risk of the metabolic syndrome. J Nutr 2007;137:1846–51 [DOI] [PubMed] [Google Scholar]
- 23.Tsai MY, Hanson NQ, Straka RJ, et al. Effect of influenza vaccine on markers of inflammation and lipid profile. J Lab Clin Med 2005;145:323–7 [DOI] [PubMed] [Google Scholar]
- 24.Patsch JR, Miesenbock G, Hopferwieser T, et al. Relation of triglyceride metabolism and coronary artery disease: studies in the postprandial state. Arterioscler Thromb 1992;12:1336–45 [DOI] [PubMed] [Google Scholar]
- 25.Reed CF, Swisher SN, Marinetti GV, Enen EG. Studies of the lipids of the erythrocyte. I. Quantitative analysis of the lipids of normal human red blood cells. J Lab Clin Med 1960;56:281–9 [PubMed] [Google Scholar]
- 26.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res 1964;5:600–8 [PubMed] [Google Scholar]
- 27.de Bakker PI. Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet 2005;37:1217–23 [DOI] [PubMed] [Google Scholar]
- 28.Caucasian European Utah data. Available from: www.hapmap.org (cited 28 July 2009)
- 29.HelixTree manual. Version 5.3.0. Available from: http://goldenhelix.com/HelixTreeManual/compositehaplotypemethodchm.html#x133-75400023.7 (cited 28 July 2009)
- 30.Lai CQ, Demissie S, Cupples LA. Influence of the APOA5 locus on plasma triglyceride, lipoprotein subclasses, and CVD risk in the Framingham Heart Study. J Lipid Res 2004;45:2096–105 [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ. Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet Anal 1999;14:143–9 [DOI] [PubMed] [Google Scholar]
- 32.Marinescu VD. Kohane IS, Riva A. MAPPER: a search engine for the computational identification of putative transcription factor binding sites in multiple genomes. BMC Bioinformatics 2005;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruber AR, Lorenz R, Bernhart SH, Neuböck R, Hofacker IL.2008. The Vienna RNA website. Nucleic Acids Res 36 (Web Server issue):W70-4. Available from: http://rna.tbi.univie.ac.at/cgibin/RNAfold.cgi (cited 28 July 2009) [DOI] [PMC free article] [PubMed]
- 34.Pérez-Martínez P, Ordovás JM, López-Miranda J, et al. Polymorphism exon 1 variant at the locus of the scavenger receptor class B type I gene: influence on plasma LDL cholesterol in healthy subjects during the consumption of diets with different fat contents. Am J Clin Nutr 2003;77:809–13 [DOI] [PubMed] [Google Scholar]
- 35.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute Inc, 1996 [Google Scholar]
- 36.Iwase T, Kajimura N, Uchiyama M, et al. Mutation screening of the human Clock gene in circadian rhythm sleep disorders. Psychiatry Res 2002;109:121–8 [DOI] [PubMed] [Google Scholar]
- 37.Monteleone P, Tortorella A, Docimo L, et al. Investigation of 3111T/C polymorphism of the CLOCK gene in obese individuals with or without binge eating disorder: association with higher body mass index. Neurosci Lett 2008;435:30–3 [DOI] [PubMed] [Google Scholar]
- 38.Gómez-Abellán P, Hernandez-Morante JJ, Lujan JA, et al. Clock genes are implicated in the human metabolic syndrome. Int J Obes 2008;32:121–8 [DOI] [PubMed] [Google Scholar]
- 39.Mendoza J, Pevet P, Challet E. High-fat feeding alters the clock synchronization to light. J Physiol 2008;586:5901–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.la Fleur SE, Kalsbeek A, Wortel J, et al. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes 2001;50:1237–43 [DOI] [PubMed] [Google Scholar]
- 41.Garaulet M, Hernández-Morante JJ, de Heredia FP, Tébar FJ. Adiponectin, the controversial hormone. Public Health Nutr 2007;10:1145–50 [DOI] [PubMed] [Google Scholar]
- 42.Barnea M, Madar Z, Froy O. High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology 2009;150:161–8 [DOI] [PubMed] [Google Scholar]
- 43.Young ME, Bray MS. Potential role for peripheral circadian clock dyssynchrony in the pathogenesis of cardiovascular dysfunction. Sleep Med 2007;8:656–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamada T, Murata T, Narita K, et al. The clinical significance of abnormal diurnal blood pressure variation in healthy late middle-aged and older adults. Blood Press 2008;17:134–40 [DOI] [PubMed] [Google Scholar]
- 45.Gupta AK, Greenway FL, Cornelissen G, Pan W, Halberg F. Prediabetes is associated with abnormal circadian blood pressure variability. J Hum Hypertens 2008;22:627–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes ME, DiTacchio L, Hayes KR, et al. Harmonics of circadian gene transcription in mammals. PLoS Genet 2009;5:e1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romon M, Le Fur C, Lebel P, Edmé JL, Fruchart JC, Dallongeville J. Circadian variation of postprandial lipemia. Am J Clin Nutr 1997;65:934–40 [DOI] [PubMed] [Google Scholar]
- 48.Kabagambe EK, Tsai MY, Hopkins PN, et al. Erythrocyte fatty acid composition and the metabolic syndrome: a National Heart, Lung, and Blood Institute GOLDN study. Clin Chem 2008;54:154–62 [DOI] [PubMed] [Google Scholar]
- 49.Poppitt SD, Kilmartin P, Butler P, Keogh GF. Assessment of erythrocyte phospholipid fatty acid composition as a biomarker for dietary MUFA, PUFA or saturated fatty acid intake in a controlled cross-over intervention trial. Lipids Health Dis 2005;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warensjo E, Ohrvall M, Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr Metab Cardiovasc Dis 2006;16:128–36 [DOI] [PubMed] [Google Scholar]
- 51.Bakan E, Yildirim A, Kurtul N, Polat MF, Dursun H, Cayir K. Effects of type 2 diabetes mellitus on plasma fatty acid composition and cholesterol content of erythrocyte and leukocyte membranes. Acta Diabetol 2006;43:109–13 [DOI] [PubMed] [Google Scholar]
- 52.Hernández-Morante JJ, Larqué E, Luján JA, Zamora S, Garaulet M. N-6 from different sources protect from metabolic alterations to obese patients: a factor analysis. Obesity (Silver Spring) 2009;17:452–9 [DOI] [PubMed] [Google Scholar]
- 53.Garaulet M, Marín C, Pérez-Llamas F, Canteras M, Tebar FJ, Zamora S. Adiposity and dietary intake in cardiovascular risk in an obese population from a Mediterranean area. J Physiol Biochem 2004;60:39–49 [DOI] [PubMed] [Google Scholar]
- 54.Tizard I. Sickness behavior, its mechanisms and significance. Anim Health Res Rev 2008;9:87–99 [DOI] [PubMed] [Google Scholar]
- 55.Obal F, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci 2003;8:d520–50 [DOI] [PubMed] [Google Scholar]
- 56.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin and increased body mass index. PLoS Med 2004;1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation 2005;12:131–40 [DOI] [PubMed] [Google Scholar]
- 58.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S. Sleep duration and biomarkers of inflammation. Sleep 2009;32(2):200–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol 2009;82:12–7Epub 2009 May 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanitallie TB. Sleep and energy balance: interactive homeostatic systems. Metabolism 2006;55(suppl 2):S30–5(Review) [DOI] [PubMed] [Google Scholar]
- 61.Morrow JD, Opp MR. Sleep-wake behavior and responses of interleukin−6-deficient mice to sleep deprivation. Brain Behav Immun 2005;19:28–39 [DOI] [PubMed] [Google Scholar]