Abstract
Background: Children with rs9939609 FTO variant alleles (homozygous = AA and heterozygous = AT) are predisposed to greater adiposity than are those with 2 wild-type alleles (TT).
Objective: Because FTO is highly expressed in hypothalamic regions that are important for appetite, FTO genotype may affect energy balance by influencing eating behavior. Loss of control (LOC) eating, a behavior commonly reported by overweight youth, predicts excessive weight gain in children. However, the relation between FTO genotype and LOC eating has not been previously examined.
Design: Two-hundred eighty-nine youth aged 6–19 y were genotyped for rs9939609, underwent body-composition measurements, and were interviewed to determine the presence or absence of LOC eating. A subset (n = 190) participated in a lunch buffet test meal designed to model an LOC eating episode. Subjects with AA and AT genotypes were grouped together for comparison with wild-type TT subjects.
Results: Subjects with at least one A allele (67.7%) had significantly greater body mass indexes, body mass index z scores (P < 0.01), and fat mass (P < 0.05). Of the AA/AT subjects, 34.7% reported LOC compared with 18.2% of the TT subjects (P = 0.002). Although total energy intake at the test meal did not differ significantly by genotype (P = 0.61), AA/AT subjects consumed a greater percentage of energy from fat than did the TT subjects (P < 0.01).
Conclusions: Children and adolescents with 1 or 2 FTO rs9939609 obesity-risk alleles report more frequent LOC eating episodes and select foods higher in fat at a buffet meal. Both LOC eating and more frequent selection of energy-dense, palatable foods may be mechanisms through which variant FTO alleles lead to excess body weight.
INTRODUCTION
Association studies have shown that common single nucleotide polymorphisms (SNPs) in the first intron of the FTO (fat mass and obesity–associated) gene (16q12.2) are consistently associated with higher body mass index (BMI; in kg/m2) and adiposity in both children and adults (1–5). However, the mechanisms by which these FTO locus risk alleles affect the development of obesity are not well understood. Rodent studies indicate that FTO mRNA is highly expressed in brain areas important for regulation of energy- and reward-driven consumption (6, 7). Hypothalamic expression of FTO and another nearby coregulated gene, Ftm, has been found to be affected by energy intake (7). Food deprivation increases FTO expression in the mouse hypothalamus (6, 7), consistent with the hypothesis that genes at the FTO locus play a role in governing eating behavior. Preliminary data suggest a link between FTO and eating behavior in humans. Children and adults with at least one rs9939609 FTO obesity-risk allele (homozygous = AA and heterozygous = AT) were found to report greater energy intake, on average, than youth with 2 wild-type alleles (TT) (8, 9). Two small studies, one of 4–5-y-old children and another of 4–10-y-old children, have suggested that rs9939609 AA/AT children may eat more at laboratory meals than do TT children (10, 11). Taken together, these studies further bolster the possibility that energy intake plays a significant role in the relation between FTO and body weight. However, specific eating behavior phenotypes associated with this genetic polymorphism have not yet been identified. One compelling possibility is that high-risk variants in FTO exert their influence on obesity via their effect on eating behaviors that promote excessive weight gain.
Binge-eating episodes, defined as discrete time periods during which the consumption of a large amount of food is accompanied by a sense of loss of control (LOC) over eating (12), are common among overweight adults. LOC eating—defined as the experience of LOC while eating, regardless of whether the reported amount of food consumed is unambiguously large—is common in youth (13). The term LOC eating therefore encompasses both binge episodes as well as eating episodes during which the amount of food consumed may not be unambiguously large. Most of the literature on pediatric LOC eating describes children who report at least one episode in the month before assessment (13). LOC eating in children is associated with overweight and high body fat mass (13). Both reported binge (14–16) and LOC (17) eating in youth predict excessive body weight gain in longitudinal studies of children and adolescents. One study has suggested that young, overweight children who self-report binge-eating episodes consume more energy than do those without such behaviors at laboratory test meals (18). A second laboratory meal study, performed in a sample of nonoverweight and overweight children and adolescents, found that those who reported LOC eating consumed more energy from carbohydrate and less energy from protein than did those without LOC eating (19).
To our knowledge, no study has examined the relation between the FTO rs9939609 obesity risk allele and LOC eating. We therefore genotyped children and adolescents for FTO rs9939609 and conducted interviews to determine the presence or absence of LOC eating episodes. We hypothesized that LOC eating would be reported more frequently among children with rs9939609 AA and AT alleles than among those with TT. In addition, a subset of these children participated in a standardized buffet meal in the laboratory. On the basis of extant literature (8–11), we hypothesized that those with at least one obesity risk allele would consume more energy than would those without FTO variant alleles.
SUBJECTS AND METHODS
Participants
Children and adolescents aged 6–19 y who were participating in nonintervention metabolic protocols (clinicaltrials.gov: NCT00320177, NCT00001195, and NCT00001522) were included to assess associations between genotype, body composition, and the presence or absence of LOC eating. Those participating in one protocol (NCT00320177) had also been tested with a laboratory buffet meal paradigm intended to assess energy intake at a “binge” meal. By design, youth were enriched for overweight (BMI ≥95th percentile for age and sex) (20). Participants were recruited through posted flyers, mailings to local family physicians and pediatricians, and mailings to parents in Montgomery County and Prince George's County, MD, school districts requesting children willing to participate in studies investigating hormones and growth in youth. Individuals were excluded if they had a significant medical condition, were taking medication known to affect body weight, had a psychiatric disorder that might impede protocol compliance, or had abnormal hepatic, renal, or thyroid function. Pregnant girls were not eligible for the study, nor were children who had lost >5 lb (2.3 kg) in the past 3 mo or who were undergoing weight-loss treatment. The institutional review board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development approved the clinical protocols. After a complete description of the study was given to children and their parents, written informed assent and consent for their participation and genetic analyses were obtained. Data collection took place between July 1996 and February 2009.
Procedures
All children were screened for eligibility after fasting overnight. Each participant's weight and height were measured by using calibrated electronic instruments as described previously (21). BMI was calculated as weight (in kg) divided by the square of height (in m). BMI SD scores (BMI z scores) were calculated according to the Centers for Disease Control and Prevention 2000 growth charts (22). Body composition was measured as previously described (21, 23) by using air-displacement plethysmography (Life Measurement Inc, Concord, CA) to determine fat-free mass and fat mass. All participants underwent a medical history and a physical examination performed by an endocrinologist or a trained nurse practitioner.
FTO genotyping
Each child supplied a whole blood sample from which genomic DNA was isolated by using the QIAamp DNA Blood Maxi Kit (QIAGEN Inc, Valencia, CA) or by a commercial company (Lofstrand Laboratories Ltd, Gaithersburg, MD). Genotyping of the FTO SNP rs9939609 was performed by using a TaqMan SNP Genotyping Assay (Applied Biosystems Inc, Foster City, CA). All assays were performed in duplicate, and an automatic allele calling quality value of 0.95 was used to determine genotype assignment. Subjects with indeterminate results with the TaqMan assay were genotyped by direct sequencing of the polymerase chain reaction amplicon containing the rs9939609 locus, which was amplified using forward primer 5′-CTATGGTTCTACAGTTCCAGTCATTT-3′ and reverse primer 5′-AGGATAGTTTCGATCTATTGACCTC-3′.
Loss of control eating
The Eating Disorder Examination version 12OD/C.2 (EDE) (24) or the EDE adapted for children (25) was administered to each participant to determine the presence or absence of LOC eating as described previously (26, 27). The EDE has good interrater reliability for all episode types (Spearman correlation coefficients: ≥0.70) (28). Tests of the EDE adapted for children have also shown good interrater reliability (Spearman rank correlations from 0.91 to 1.00) and discriminant validity in eating disordered samples and matched control subjects aged 8–14 y (29). In the nonoverweight and overweight 6–13-y-olds studied in our laboratory, the child version of the EDE showed excellent interrater reliability with a Cohen's κ for presence of the different eating episode categories of 1.00 (P < 0.001) (26).
Buffet test meal
During a screening visit, participants were acclimated to the laboratory food intake setting by consuming a high-calorie shake (787 kcal, 52% carbohydrate, 11% protein, and 37% fat). Those unable to consume at least one-half of the shake under these conditions were considered unable to acclimate and were deemed ineligible. To ensure that participants found the foods offered at the buffet test meal to be acceptable, participants completed a food-preference questionnaire on which they rated how much they liked 57 foods that children commonly consume (30) (including the 28 foods offered on our buffet) using 10-point Likert scales (31). If children disliked >50% of the foods offered on the buffet, they were excluded from the study.
For the test meal, the experimental design was based on the adult literature that has examined binge-eating behaviors in a laboratory setting, where most studies used a prolonged deprivation period before meal consumption (32). Children arrived in the morning after an overnight fast and were provided with a standard 280-kcal (74% carbohydrate, 7% protein, and 19% fat) breakfast. Participants remained at the National Institutes of Health Clinical Center for the next 6 h, during which they were observed to ensure that they consumed no calorie-containing items. Children were allowed to participate only in sedentary activities. In the afternoon, children reported on their level of hunger by completing a visual analog scale ranging from “not at all” to “extremely” hungry. Each participant was then presented with a multiple-item, 9835-kcal test meal buffet designed to contain an array of palatable foods, as previously described (18). To model a binge-eating episode, participants received prerecorded instructions to “Let yourself go, and eat as much as you want,” and were given unrestricted time to consume their meal while viewing preselected episodes of a television show devoid of any references to food and from which the commercials had been removed. Subjects ate alone in a room free of food-containing stimuli. All food items presented were weighed to the nearest 0.1 g before and after the test session. Nutrient intakes were calculated by using data from the US Department of Agriculture (USDA) National Nutrient Database for Standard Reference (release 16; USDA, Agricultural Research Service) and manufacturer information, when available. After completion of the test meal, the families left the National Institutes of Health, and no further data were collected regarding children's intake during the rest of the day.
Data analytic plan
All analyses were performed with SPSS 16.0 (33). Logarithmic transformations were made for total energy intake, and arcsine transformations were conducted for the percentage of macronutrient content (fat, protein, and carbohydrate) intake. Departure of genotype distribution from Hardy-Weinberg equilibrium was assessed by using chi-square analysis; t tests and chi-square analyses were used to analyze participant demographics based on genotype. Analyses of covariance (ANCOVA), accounting for age, sex, and race, were used to examine the association of FTO rs9939609 (AA/AT and TT) with BMI. The same covariates in addition to height were considered in the model examining body fat mass. Pearson's chi-square tests and binary logistic regression accounting for BMI z score were used to examine associations between genotype and LOC eating presence. ANCOVAs were used to examine total energy intake (kcal) and percentages of energy intake from protein, carbohydrate, and fat based on genotype as well as the interaction of genotype by LOC status. The interaction term was not significant in any model; therefore, the data are not shown. Each model included sex, age, race (coded non-Hispanic white and all other racial-ethnic minorities), socioeconomic status (34) (coded into 2 categories based on the median split), fat-free mass, percentage fat mass as covariates, and the number of buffet foods reported as acceptable. Reported means and SEs were adjusted for all variables included in each model. Differences were considered significant when P values were <0.05. All tests were 2-tailed.
RESULTS
Two-hundred eighty-nine children and adolescents were genotyped. Fifty-three (18.3%) were homozygous for the obesity risk allele (AA) for the FTO SNP rs9939609, 137 (47.4%) were heterozygous (AT), and 99 (34.3%) were wild type (TT). The overall frequency of the A allele was 0.43. Genotype frequencies did not differ significantly (χ2 = 0.22, P = 0.90) from Hardy-Weinberg equilibrium expectations (AA = 17.7%, AT = 48.7%, and TT = 33.6%).
Participant demographics by genotype are shown in Table 1. As has been done in prior studies, homozygous (AA) and heterozygous (AT) risk allele youth were grouped together (8, 9). The presence of at least one copy of the A allele was associated with significantly higher BMI and BMI z scores (P < 0.01) and adiposity (P < 0.05; Table 1).
TABLE 1.
Demographic characteristics of the participants by genotype
| TT (n = 99) | AT or AA (n = 190) | P value | |
| Age (y) | 13.60 ± 0.261 | 13.34 ± 0.20 | 0.45 |
| Female sex (%) | 44.9 | 51.6 | 0.28 |
| Race (%) | |||
| Non-Hispanic white | 54.1 | 59.5 | 0.38 |
| Other | 45.9 | 40.5 | |
| Median socioeconomic status score | 2 | 2 | 0.47 |
| BMI (kg/m2) | 22.85 ± 0.8 | 25.87 ± 0.6 | 0.002 |
| BMI z score | 0.71 ± 0.1 | 1.2 ± 0.1 | 0.005 |
| Fat mass (kg) | 16.86 ± 1.7 | 21.40 ± 1.2 | 0.028 |
| Fat-free mass (kg) | 46.83 ± 1.2 | 45.70 ± 0.8 | 0.43 |
| Hunger before meal2 | 73.6 ± 2.4 | 72.5 ± 1.7 | 0.70 |
| Total energy intake (kcal)3 | 1448.8 ± 104.5 | 1428.3 ± 103.0 | 0.79 |
Mean ± SE (all such values).
Hunger was rated on a visual analog scale ranging from 0 (“not at all”) to 100 (“extremely”).
Total energy intake was adjusted for covariates as described in Subjects and Methods.
FTO genotype in relation to reported LOC eating and energy intake
On the basis of their responses to the EDE interview, 84 children reported at least one episode of LOC eating in the month before assessment. Of the TT group, only 18 (18.2%) children reported LOC eating, compared with 66 (34.7%) children with the FTO gene variant (χ2 = 8.65, Fisher's exact P = 0.002). Furthermore, even after the contribution of BMI z score was accounted for, having at least one A allele was associated with a nearly 2 times greater odds of describing LOC eating episodes [B = 0.68, Wald statistic = 4.7, (Exp)B = 1.98, P = 0.029].
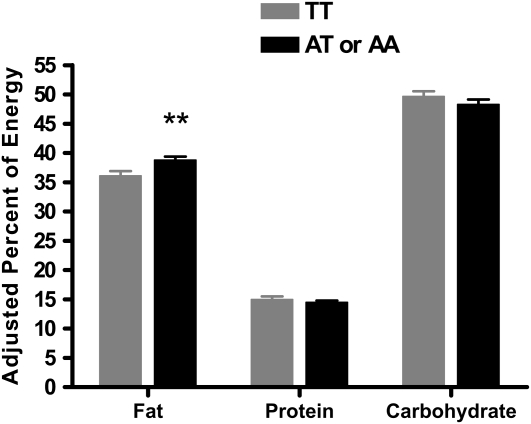
A subset of youth (n = 190) participated in the laboratory buffet meal test. These children did not differ from the entire sample with regard to any variable except age; children who participated were somewhat younger than those who did not participate (13.2 ± 0.2 compared with 13.9 ± 0.3; P = 0.03). Once covariates such as fat-free mass (as described in Subjects and Methods) were taken into account, children with (n = 128) and without (n = 62) the FTO variant did not differ with regard to the amount of energy consumed at the buffet binge meal (Table 1). This finding persisted when total energy intake expressed as percentage of the participants' daily energy requirements was examined (P = 0.40) (35). However, youth with at least one A allele consumed a significantly greater percentage of energy from fat than did the TT subjects (P = 0.008; Figure 1). No difference was found for the percentage of energy consumed from either protein or carbohydrate (Figure 1). No significant group differences were noted in palatability ratings for foods found in the buffet meal. After completion of the meal, perceived fullness did not differ significantly between youth with and without the A allele (P = 0.72).
FIGURE 1.
Macronutrient content of the test meal by genotype. Participants with the AA or AT genotype consumed a significantly greater percentage of energy from fat than did the participants with the TT genotype. n = 190. **P < 0.008. Adjusted back-transformed arcsine percentage data are presented.
DISCUSSION
In a sample of nontreatment children and adolescents, we replicated prior findings that youth carrying at least one rs9939609 FTO risk allele are heavier and have more adiposity than children with no A alleles (1, 2). In addition, youth with variant alleles were more likely to report episodes of LOC eating than were children identified as wild type, even after the control of BMI adjusted for age and sex. We also found that the presence of the FTO variant was associated with the consumption of a greater percentage of energy from fat at a test meal buffet designed to capture the experience of a binge or LOC eating episode. No differences were identified between children with and without the A allele with regard to total energy intake, percentage of energy consumed from protein or carbohydrate, or fullness after the meal.
The finding that children with at least one A allele are more likely to report LOC eating is novel and may provide important information regarding the mechanism of weight gain for some children. This relation was significant even after the contribution of BMI z score was accounted for; indeed, youth with the FTO A allele were almost twice as likely as were those with the wild-type allele to report LOC eating. To date, no study of the FTO gene has identified a distinct, potentially modifiable behavioral eating phenotype that is associated with the allele, although contradictory data exist for the effect of the interaction between FTO genotype and exercise on body weight (36–38).
The effect of FTO genetic variation on human energy expenditure is somewhat unclear. Fischer et al (39) found that inactivation of FTO in mice resulted in leanness due to increased energy expenditure. In human samples, the data are mixed. No human studies have found changes in resting energy expenditure associated with FTO genotype (11, 40); however, 2 studies, one making use of the doubly labeled water method in children (11) and the second based on self-report data in adults (41), found evidence of greater activity energy expenditure among those with 1 or 2 A alleles. In contrast, a prospective study of youth based on self-reported data found no association with energy expenditure (42).
Our findings associating the presence of the A allele with LOC eating may have important implications. Preliminary data suggest that reducing binge and LOC eating may be effective for both weight loss (43) and obesity prevention (44). Therefore, modifying a reported eating behavior phenotype associated with the FTO variant may offer promise for youth with this genetic predisposition for excess body weight gain. An important next research step should examine whether response to interventions for binge and LOC eating is affected by rs9939609 genotype.
Contrary to expectations, children with the presence of an FTO variant did not consume more energy at a meal designed to model an episode of binge or LOC eating. Although consistent with one study that made use of food records (42), this finding is in contrast with that of 2 relatively small studies that reported that youth with the variant consumed more energy at laboratory test meals (10, 11). However, the paradigm carried out in these studies differed from the present analysis in that our participants were given a standard breakfast and all were very hungry before the test meal. Studies examining the relation between food deprivation and disinhibited eating have yielded mixed results, with some data supporting a relation (45, 46) and other research finding no correlation (47, 48). As suggested by the data of Wardle et al (10), children with the FTO variant may be more susceptible to overeating in the absence of hunger rather than to overeating when hungry. Indeed, experiencing LOC eating would seem, at least in the latter parts of an eating episode, to potentially predispose toward eating in the absence of hunger. Interestingly, youth with A alleles consumed more energy from fat at the laboratory test meal. This finding is particularly notable because children with and without the A allele did not differ significantly in their reported palatability for the buffet foods. If the observed style of eating promotes consumption of more energy-dense foods outside of the laboratory setting, our findings may help explain why youth with the FTO A allele are heavier than TT children. Supporting these observations, greater energy density consumption has been associated with the FTO variant in children making use of a test meal paradigm (11) as well as food records (8). However, a third study based on 3-d food diaries found that dietary energy density was not modified by FTO variants (49).
The strengths of this study include the use of a semistructured clinical interview for the assessment of LOC eating and air-displacement plethysmography to determine body composition. In addition, the study involved a well-controlled laboratory meal, and the inclusion of boys and girls who were not seeking weight-loss treatment and who were of a broad age and weight range. A primary limitation was that LOC eating was positively correlated with increased body weight. Although the relation between LOC eating and the A allele was significant even after the contribution of BMI z score was accounted for, it is difficult to disentangle whether such associations are causes or consequences of the relation. Other limitations include that all participants were provided the same standardized breakfast before the test meal, regardless of body weight. It is possible that heavier youth required substantially more energy than what was provided for breakfast to have equal satiety, thereby potentially masking some differences between groups with regard to genotype. However, we accounted for body composition in all analyses. Furthermore, because all participants in our study were very hungry when they were served the test meal, our paradigm may not have captured the dysregulated eating potentially attributable to the A allele. Although laboratory feeding studies have been shown to illuminate behavioral differences in intake effectively (32), such paradigms may not fully capture eating patterns observable in a naturalistic setting. Indeed, the use of a large buffet and the instruction provided to the children may have stimulated even restrained youth to overeat. Finally, having children watch television while eating may have been a limitation. Television viewing has been shown to increase food intake even in those who are unrestrained, healthy eaters (50).
In conclusion, report of LOC eating is a behavioral phenotype more frequently reported by children and adolescents with 1 or 2 FTO rs9939609 obesity risk alleles. Furthermore, children with the A allele appear to have a preference for fat when offered a variety of palatable foods at a buffet meal. Both LOC eating and more frequent selection of energy-dense palatable foods may be mechanisms through which variant FTO alleles lead to excess body weight.
Acknowledgments
The authors' responsibilities were as follows—MT-K, JCH, SZY, and JAY: primarily responsible for developing the study design and conceived the hypothesis for this article; MT-K, JCH, and JAY: supervised the data collection; MT-K, JCH, LBS, and JAY: conducted the data analysis; and MK, KA, KMC, LEW, CE, LMR, and CAR: contributed to data collection and provided critical input on data analyses and on versions of the manuscript. All authors participated in the interpretation of the results and approved the final version of the manuscript. JCH, JAY, and MK, are commissioned officers in the US Public Health Service, Department of Health and Human Services. None of the authors reported any conflicts of interest.
REFERENCES
- 1.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 2007;39:724–6 [DOI] [PubMed] [Google Scholar]
- 3.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 2007;3:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt SC, Stone S, Xin Y, et al. Association of the FTO gene with BMI. Obesity (Silver Spring) 2008;16:902–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinney A, Nguyen TT, Scherag A, et al. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS One 2007;2:e1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerken T, Girard CA, Tung YC, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007;318:1469–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stratigopoulos G, Padilla SL, LeDuc CA, et al. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol 2008;294:R1185–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timpson NJ, Emmett PM, Frayling TM, et al. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr 2008;88:971–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity (Silver Spring) 2008;16:1961–5 [DOI] [PubMed] [Google Scholar]
- 10.Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes (Lond) 2009;33:42–5 [DOI] [PubMed] [Google Scholar]
- 11.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CNA. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med 2008;359:2558–66 [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric association Diagnostic and statistical manual of mental disorders DSM-IV-TR. Washington, DC:American Psychiatric association, 2000 [Google Scholar]
- 13.Tanofsky-Kraff M.Binge eating among children and adolescents Jelalian E, Steele R, Handbook of child and adolescent obesity. New York, NY: Springer, 2008:41–57 [Google Scholar]
- 14.Field AE, Austin SB, Taylor CB, et al. Relation between dieting and weight change among preadolescents and adolescents. Pediatrics 2003;112:900–6 [DOI] [PubMed] [Google Scholar]
- 15.Stice E, Cameron RP, Killen JD, Hayward C, Taylor CB. Naturalistic weight-reduction efforts prospectively predict growth in relative weight and onset of obesity among female adolescents. J Consult Clin Psychol 1999;67:967–74 [DOI] [PubMed] [Google Scholar]
- 16.Tanofsky-Kraff M, Cohen ML, Yanovski SZ, et al. A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics 2006;117:1203–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int J Eat Disord 2009;42:26–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirch MC, McDuffie JR, Yanovski SZ, et al. Effects of binge eating on satiation, satiety, and energy intake of overweight children. Am J Clin Nutr 2006;84:732–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, et al. Laboratory assessment of the food intake of children and adolescents with loss of control eating. Am J Clin Nutr 2009;89:738–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA 2002;288:1728–32 [DOI] [PubMed] [Google Scholar]
- 21.Russell DL, Keil MF, Bonat SH, et al. The relation between skeletal maturation and adiposity in African American and Caucasian children. J Pediatr 2001;139:844–8 [DOI] [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000;Jun 8:1–27 [PubMed] [Google Scholar]
- 23.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc 1995;27:1692–7 [PubMed] [Google Scholar]
- 24.Fairburn CG, Cooper Z. The Eating Disorder Examination: Fairburn CG, Wilson GT, Binge eating, nature, assessment and treatment. 12th ed.New York, NY: Guilford, 1993:317–60 [Google Scholar]
- 25.Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the eating disorder examination with children: a pilot study. Int J Eat Disord 1996;19:391–7 [DOI] [PubMed] [Google Scholar]
- 26.Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, Marmarosh C, Morgan CM, Yanovski JA. Eating disordered behaviors, body fat, and psychopathology in overweight and normal weight children. J Consult Clin Psychol 2004;72:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanofsky-Kraff M, Goossens L, Eddy KT, et al. A multisite investigation of binge eating behaviors in children and adolescents. J Consult Clin Psychol 2007;75:901–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizvi SL, Peterson CB, Crow SJ, Agras WS. Test-retest reliability of the eating disorder examination. Int J Eat Disord 2000;28:311–6 [DOI] [PubMed] [Google Scholar]
- 29.Christie D, Watkins B, Lask B. Assessment: Lask B, Bryant-Waugh RJ, Anorexia nervosa and related eating disorders in childhood and adolescence. 2 ed.East Essex, United Kingdom: Psychology Press, 2000:105–25 [Google Scholar]
- 30.Block G, Norris JC, Mandel RM, DiSogra C. Sources of energy and six nutrients in diets of low-income Hispanic-American women and their children: quantitative data from HHANES, 1982-1984. J Am Diet Assoc 1995;95:195–208 [DOI] [PubMed] [Google Scholar]
- 31.Hetherington MRB. Methods of investigating human behavior: Toates FRN, Feeding and drinking. Amsterdam, Netherlands: Elsevier Science Publishers BV, 1987:77–109 [Google Scholar]
- 32.Walsh BT, Boudreau G. Laboratory studies of binge eating disorder. Int J Eat Disord 2003;34(suppl):S30–8 [DOI] [PubMed] [Google Scholar]
- 33.SPSS SPSS 14.0 Chicago, IL: SPSS Inc, 2006 [Google Scholar]
- 34.Hollingshead A. Four factor index of social status. New Haven, CT: Yale University, 1975 [Google Scholar]
- 35.Otten JJ, Pitzi JH, Meyers LD. The dietary reference intakes: the essential guide to nutrient requirements. Washington, DC: The National Academies Press, 2006 [Google Scholar]
- 36.Andreasen CH, Stender-Petersen KL, Mogensen MS, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes 2008;57:95–101 [DOI] [PubMed] [Google Scholar]
- 37.Rampersaud E, Mitchell BD, Pollin TI, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch Intern Med 2008;168:1791–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vimaleswaran KS, Li S, Zhao JH, et al. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. Am J Clin Nutr 2009;90:425–8 [DOI] [PubMed] [Google Scholar]
- 39.Fischer J, Koch L, Emmerling C, et al. Inactivation of the Fto gene protects from obesity. Nature 2009;458:894–8 [DOI] [PubMed] [Google Scholar]
- 40.Haupt A, Thamer C, Staiger H, et al. Variation in the FTO gene influences food intake but not energy expenditure. Exp Clin Endocrinol Diabetes 2009;117:194–7 [DOI] [PubMed] [Google Scholar]
- 41.Jonsson A, Franks PW. Obesity, FTO gene variant, and energy intake in children. N Engl J Med 2009;360:1571–2, author reply 1572 [DOI] [PubMed] [Google Scholar]
- 42.Hakanen M, Raitakari OT, Lehtimaki T, et al. FTO genotype is associated with body mass index after the age of seven years but not with energy intake or leisure-time physical activity. J Clin Endocrinol Metab 2009;94:1281–7 [DOI] [PubMed] [Google Scholar]
- 43.Jones M, Luce KH, Osborne MI, et al. Randomized, controlled trial of an internet-facilitated intervention for reducing binge eating and overweight in adolescents. Pediatrics 2008;121:453–62 [DOI] [PubMed] [Google Scholar]
- 44.Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, et al. Interpersonal psychotherapy for the prevention of excess weight gain in adolescent girls with and without loss of control eating: a pilot study. Montreal, Canada: Eating Disorders Research Society, 2008 [Google Scholar]
- 45.Hetherington MM, Stoner SA, Andersen AE, Rolls BJ. Effects of acute food deprivation on eating behavior in eating disorders. Int J Eat Disord 2000;28:272–83 [DOI] [PubMed] [Google Scholar]
- 46.Wardle J. Compulsive eating and dietary restraint. Br J Clin Psychol 1987;26:47–55 [DOI] [PubMed] [Google Scholar]
- 47.Engelberg MJ, Gauvin L, Steiger H. A naturalistic evaluation of the relation between dietary restraint, the urge to binge, and actual binge eating: a clarification. Int J Eat Disord 2005;38:355–60 [DOI] [PubMed] [Google Scholar]
- 48.Timmerman GM. Caloric intake patterns of nonpurge binge-eating women. West J Nurs Res 1998;20:103–18 [DOI] [PubMed] [Google Scholar]
- 49.Johnson L, van Jaarsveld CH, Emmett PM, et al. Dietary energy density affects fat mass in early adolescence and is not modified by FTO variants. PLoS One 2009;4:e4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hetherington MM, Anderson AS, Norton GN, Newson L. Situational effects on meal intake: a comparison of eating alone and eating with others. Physiol Behav 2006;88:498–505 [DOI] [PubMed] [Google Scholar]