Abstract
Background: Elevated serum triglyceride and low HDL-cholesterol concentrations have been reported in persons with HIV.
Objective: The effect of a dietary intervention plus n−3 (ω−3) fatty acid supplementation on serum triglycerides and markers of insulin sensitivity was investigated.
Design: Fifty-four persons with HIV and elevated serum triglycerides (>150 mg/dL) and/or abnormal Quantitative Insulin Sensitivity Check Index values (<0.35 but >0.30) were recruited for a dietary intervention in which total fat, type of fat, fiber, and glycemic load were controlled along with supplementation with n−3 fatty acids to achieve an intake of 6 g/d. The subjects were randomly assigned to an intervention or control group, and serum lipids, markers of insulin sensitivity, and serum phospholipid fatty acids were measured in both groups at baseline, 3 wk, and 13 wk.
Results: Triglycerides in the intervention group decreased from a median of 180 mg/dL (interquartile range: 141, 396) to 114 mg/dL (interquartile range: 84, 169) from baseline to 3 wk, whereas they remained stable in the control group (P = 0.003). Serum phospholipid fatty acids indicated a decrease in de novo lipogenesis and a decrease in arachidonic acid (% nmol; P ≤ 0.001) in the intervention group. At 3 wk, the insulin area under the curve decreased but not significantly.
Conclusions: Diet and n−3 fatty acid supplementation dramatically reduced serum triglycerides, decreased arachidonic acid in the phospholipids fraction, and appeared to decrease the de novo lipogenesis associated with the metabolic syndrome in the intervention group.
INTRODUCTION
Alterations in lipid metabolism with elevations in serum triglycerides and low concentrations of HDL cholesterol have been reported in persons with HIV infection before (1) and after (2–4) the advent of highly active antiretroviral therapy (HAART). The contribution of the long-term effects of HIV infection and the effect of HAART on these changes in lipid metabolism are still unclear.
n−3 Fatty acids have been shown to increase fatty acid oxidation in the liver and to decrease the hepatic output of triglycerides (5, 6) in animal studies. In a review by Harris (7) of 36 well-controlled crossover studies in humans with elevated serum triglycerides, but without HIV infection, supplementation with n−3 fatty acids at 1.5–7.0 g/d resulted in an average decrease in serum triglycerides of 25–30%. Four studies have reported that supplementation with n−3 fatty acids can also be effective in lowering serum triglycerides in subjects with HIV infection (8–11).
The possible role of the n−3 fatty acids in insulin resistance was recently reviewed (12, 13). Elevated serum triglycerides and free fatty acids have been shown to be involved in the development of insulin resistance (14–16) and of abnormalities in insulin secretion (17). Diets high in saturated fat are known to increase the development of insulin resistance and the metabolic syndrome (18–20). Saturated fatty acids have been implicated in the destruction of β-cells in the pancreas, which results in loss of an adequate insulin response to the usual stimuli of insulin secretion (17). In contrast, increased dietary polyunsaturated fat intakes have been shown to be associated with greater insulin sensitivity (21–23), with n−3 fatty acids having the greatest effect (24–26). Foods high in fiber (27, 28) or with a lower glycemic index or glycemic load (28–30) can also improve metabolic variables associated with diabetes.
Drug treatment of elevated triglycerides may lead to further risk of drug-drug interactions, so there is a need for nonpharmacologic interventions to treat these abnormalities in the HIV-infected population. Therefore, we performed a randomized trial of a dietary intervention designed to reduce serum lipids and increase insulin sensitivity. The dietary intervention consisted of reduced total fat, reduced saturated fat, increased fiber, lowered glycemic load, and an increased intake of foods containing high amounts of n−3 fatty acid and n−3 fatty acid supplementation from fish oil. Serum phospholipid fatty acids were determined to verify changes in the intervention group and to evaluate the role of change in specific fatty acids with biochemical measures of serum lipids and insulin status.
SUBJECTS AND METHODS
Subjects
Fifty-four subjects with HIV infection were recruited between July 2003 and March 2007. Eligibility criteria included HIV infection, age >18 y, a body mass index (kg/m2) between 19 and 30, and a fasting plasma triglyceride concentration >150 mg/dL or a Quantitative Insulin Sensitivity Check Index (QUICKI) score of <0.35 but >0.30 at any time over the past year (31). QUICKI is a mathematical model used to estimate insulin sensitivity by using the following equation:
A lower QUICKI score indicates more insulin resistance (31). The eligibility criteria for the study included elevated serum triglycerides and/or an abnormal QUICKI score because of their association with insulin resistance by using the classic oral glucose tolerance test (31, 32). Subjects also had to be free of any opportunistic infections, have no active injection drug use, and not have taken any n−3 fatty acid supplements for ≥3 mo before the study started. Potential subjects were excluded if their triglyceride concentration was >550 mg/dL, which could indicate liver damage and interfere with the efficacy of the intervention. Persons taking HMG-CoA reductase inhibitors (statins) were also excluded. A medical history was taken, which also included a list of current antiretroviral medications as well as other medications and vitamin supplements. Potential subjects were questioned regarding their weight stability and their willingness to remain weight stable (±3% change) over the 6 mo of the study. Written informed consent was obtained from all study participants, and the study protocol was approved by the Tufts–Medical Center Institutional Review Board.
Recruitment
Subjects were recruited via the network of groups and sites that we used and maintained during our large observational study in the HIV population in the Boston area (33, 34). Fifty-four subjects were recruited into the study.
Experimental design
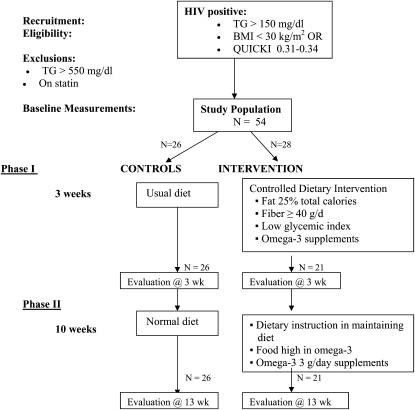
The study was a randomized nutrition intervention trial, and the study design is shown in Figure 1. Randomization was done by block design to ensure equal numbers in each group for every 10 subjects recruited. Those randomly assigned to the intervention came in each day to eat one meal at the Clinical Research Center (CRC) and to have their 2 other meals and snacks packed to take home for the first 3 wk of the study. These meals were formulated for the daily intake to be lower in fat (≈25% of energy from total fat), be lower in saturated fat (≈7% of energy), have a saturated:monounsaturated:polyunsaturated fatty acid ratio of 1:1:1, provide a dietary intake of n−3 fatty acids of 3 g/d, provide a dietary fiber level of ≥40 g/d, and to have a reduced glycemic load, achieved by reducing the use of simple sugars and carbohydrate foods low in fiber. The Harris-Benedict Equation with an activity factor was used to estimate the caloric requirements of each subject in determining their individual caloric need to maintain their current body weight. This calculation proved to be very reliable in accomplishing the goal of weight stability. In addition, the intervention subjects took a supplement of n−3 fatty acids that totaled 3.0 g 20:5n−3 [eicosapentaenoic acid (EPA)] + 22:6n−3 [docosahexaenoic acid (DHA)] daily (Omega Rx; Sear Laboratories, Marblehead, MA). Each capsule contained 0.600 g n−3 fatty acids as EPA (0.400 g) and DHA (0.200 g). Five capsules were taken per day. The total intake of EPA + DHA during phase 1 was ≈6.0 g/d, coming equally from diet and supplements. Subjects in the intervention were also given a supplement of vitamin E (100 mg/d; Perfect Source, Fullerton, CA) because of their higher intake of polyunsaturated fatty acids (35, 36), which are more prone to oxidation.
FIGURE 1.
Study design of randomized dietary intervention trial. TG, triglycerides; QUICKI, Quantitative Insulin Sensitivity Check Index.
Food typically consumed by the intervention group included a 6-oz (170.1-g) salmon fillet on 2 of the 3-d repeating menu. Lean chicken or turkey was given on the third day. Other sources of dietary n−3 fatty acids came from intakes of walnut oil (1–2 Tbsp/d, or 14.79–29.58 mL) and, 2–4 Tbsp (15–30 g) walnuts, depending on caloric needs. In addition, each day, subjects might have consumed 2–4 slices of high-fiber bread (5 g fiber/slice), 1 cup brown rice (195 g), 1 cup barley (157 g), 1 cup all-bean salad (185 g), and 2–4 Tbsp bean dip (28–56 g). The serving sizes of vegetables (all high in fiber) were increased.
During the first 3 wk of the intervention, the subjects were individually counseled on how to select foods to maintain the low total and saturated fat, high-fiber, and low glycemic load profile of the intervention diet given in phase 1. During the next 10 wk (phase 2), those in the intervention group came into the CRC once per week to receive a package of food for the week that contained frozen salmon, canned sardines, high-fiber bread, high-fiber cereal, walnuts, and a salad dressing made with walnut oil plus a week’s supply of the n−3 fatty acid supplement (3.0 g EPA + DHA/d). At weekly visits, goals of the dietary intervention were discussed, and all of the subjects' questions were addressed. A 3-d food record was collected every week of this 10-wk period of phase 2. The subjects randomly assigned to the control group were seen at baseline, 3 wk, and 13 wk, and dietary data were collected at that time.
Dietary analysis
Dietary intake was obtained from all subjects at baseline, 3 wk, and 13 wk by using a 3-d food record or a 24-h dietary recall, if a 3-d food record was not available. To reflect the current food marketplace throughout the study, the 3-d food record and 24-h dietary recalls were analyzed by using the Nutrition Data System for Research software versions 4.06 and 5.0, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. Final calculations were completed by using Nutrition Data System for Research version 2006. The Nutrition Data System for Research time-related database updates analytic data while maintaining nutrient profiles true to the version used for data collection. The analysis includes food separately and total intake of food and supplements.
Blood lipids, glucose, and insulin
All blood was drawn between 0700 and 1000 after a fast of ≥8 h for serum lipids, glucose, insulin, and highly sensitive C-reactive protein (CRP). Insulin was assessed by using a radioimmunoassay technique (ADVIA Centaur Immunoassay; Bayer Diagnostics, Tarrytown, NY). QUICKI was determined by using measurements from the fasting blood (31). The following determinations were analyzed by using the Beckman LX-20 in the Tufts–Medical Center Clinical Laboratory: glucose, total cholesterol, HDL cholesterol, and triglycerides. LDL was calculated by using the Friedewald equation (37). Highly-sensitive CRP was determined by using a turbidimetric in vitro immunoassay (Equal Diagnostics, Exton, PA).
A frequently sampled oral glucose tolerance test was carried out in all subjects at baseline and at 3 and 13 wk of the study. The test was carried out between 0730 and 1000 after an 8-h overnight fast. Blood samples were collected at −15, 0, 15, 30, 45, 60, 90, 120, 150, and 180 min after an oral glucose load of 75 g glucose, and the blood samples were analyzed for glucose and insulin. The area under the curve (AUC) for glucose and insulin was determined by using the method of Matthews et al (38).
Blood samples for fatty acid measurements
Blood from the study subjects was collected after an 8-h fast in tubes containing EDTA, and the serum was separated within 2 h of collection. Butylated hydroxytoluene (BHT; 40 μL/mL) was added to all samples as an antioxidant. An aliquot was covered by nitrogen gas to protect against oxidation of fatty acids and then stored at –80°C until analysis within 12 mo.
Fatty acid measurements
Briefly, an aliquot of 50 μL of a 1 mg/mL solution of diheptadecanoyl phosphatidylcholine (Avanti Polar Lipids Inc, Alabaster, AL) and triheptadecanoyl glycerol (Sigma, St Louis, MO) was added as an internal standard to 500 μL serum before lipid extraction. A liquid-liquid extraction was carried out on the serum with 6 volumes of chloroform-methanol (2:1, vol:vol) centrifuged at 800 × g for 10 min, and the resulting lower phase was aspirated. Lipid extracts from the serum samples were fractionated into triglycerides and phospholipids by aminopropyl column chromatography (39). The eluate was evaporated to dryness under nitrogen gas and methylated by adding 0.5 mL methanolic base (Acros Organics, Morris Plains, NJ), and the sample was placed in a heating block at 100°C for 3 min. After the sample was cooled on ice, 0.5 mL BF3 reagent (Sigma-Aldrich, St Louis, MO) was added, and the sample was placed in a heating block at 100°C for 1 min, removed, and allowed to cool on ice. N-Hexane was added (0.5 mL) and incubated at 100°C for 1 min (40). After being cooled, 6.5 mL of a sodium chloride–saturated salt solution was added, and the reaction mixture was centrifuged at 800 × g for 2 min. The upper hexane level was aspirated. Fatty acid methyl esters were analyzed by gas chromatography with a Hewlett-Packard 5890A gas chromatograph with the use of Supelcowax-10 (0.25 mm internal diameter column; Supelco, Bellefonte, PA). Fatty acid methyl ester peaks were identified by comparison of retention times of standard mixtures against internal standards. The fatty acids are reported as concentrations (nmol/dL) to show the absolute change, whereas the major results of the levels of phospholipid fatty acids are standardized by expressing them as the mole percent of the total fatty acid content in the sample (% nmol).
Statistical analysis
All analyses were conducted by using SAS version 9.1 (SAS Institute, Cary, NC). Nonparametric statistics were generally used, except when looking at the change in triglycerides, change in insulin AUC, and change in the glucose AUC, which were normally distributed. For comparisons of characteristics between groups, the Wilcoxon’s rank-sum test was used for continuous variables, and the chi-square test (or Fisher’s exact test where there were small cells) was used for categorical variables. To determine the significance of within-group changes between visits, we used Wilcoxon’s signed-rank test. To compare changes over time between the intervention and control groups, we used the 2-sample t test to test the significance of the difference in mean changes between groups. For triglycerides and insulin AUC, the changes over time between the 2 study groups were adjusted for baseline values by using PROC-REG in SAS. Spearman’s correlations were used to examine the correlation between changes in fatty acids over the 3- and 13-wk periods and changes in the 3 major outcomes (triglycerides, insulin AUC, and glucose AUC).
Because this was an intention-to-treat analysis, we analyzed our results using a multiple imputations method for missing data with SAS MI and MIANALYZE. Because the results with and without the imputed data did not differ, we report final results using data from the 47 subjects who completed the study.
RESULTS
Retention
Of the 54 subjects who were eligible for the study, who signed consent forms and were randomly assigned in the study, 7 dropped out before the week 13 visit—all from the intervention group. Five of these subjects dropped out before starting the intervention diet. Of the remaining 2 drop-outs in the intervention arm, one dropped out because of the difficulty of coming in each day for meals, and 1 dropped out because of gas and discomfort associated with the meals. The final group of 47 provided data at baseline and at 3 and 13 wk of follow-up.
Demographics
The median age of the 54 subjects was 46.8 y [interquartile range (IQR): 41.5, 51.3] and the body mass index was 25.4 (IQR: 23.5, 28.3), as shown in Table 1. Eighty percent of the subjects were male, and 20% were female. The study group was 43% African American, 50% white, and 7% other. The randomization into the intervention and control groups was successful in maintaining balance in age, body mass index, and sex. Eighty-seven percent of the subjects were receiving HAART, and the 2 groups were well balanced concerning the medical status of their HIV infection. More subjects in the control group were taking ritonavir or kaletra, HIV medications known to increase serum triglyceride concentrations, but this difference was not significant (P = 0.10). Forty-seven percent of the study subjects had the metabolic syndrome, defined as ≥3 of the 5 criteria (32); 66% had triglyceride concentrations >150 mg/dL at baseline, 37% had a fasting glucose concentration >100 mg/dL, and 33% had glucose intolerance (glucose ≥120 mg/dL at hour 2 in the frequently sampled oral-glucose-tolerance test). Fifty-four percent had insulin resistance, defined as a QUICKI score <0.35 (31). The randomization was successful in maintaining a balance between these indicators of the metabolic syndrome between the intervention and control groups. The control group had a greater number of persons with elevated blood pressure, but the difference between the 2 groups was not statistically significant (P = 0.057). A greater number (P = 0.010, Fisher’s exact test) of subjects dropped out of the intervention group (n = 7) than out of the control group (n = 0). Comparison of the 7 subjects who dropped out with the 47 who completed the 13-wk study showed no statistical difference between those who remained in the study and those who did not (data not shown).
TABLE 1.
Demographics of the study subjects1
| Variable | All (n = 54) | Intervention group (n = 28) | Control group (n = 26) | P value2 |
| Age (y) | 46.8 (41.5, 51.3)3 | 45.3 (41.1, 52.2) | 47.3 (42.4, 49.1) | 0.94 |
| BMI (kg/m2) | 25.4 (23.5, 28.3) | 25.0 (23.3, 28.6) | 25.8 (24.4, 27.1) | 0.80 |
| Male [n (%)] | 43 (80) | 24 (86) | 19 (73) | 0.25 |
| HIV status | ||||
| CD4 count (cells/mm3)4 | 468 (357, 656) | 512 (383, 687) | 465 (348, 656) | 0.35 |
| Log10 viral load (copies/mL)5 | 2.3 (2.3, 2.8) | 2.3 (2.3, 2.3) | 2.3 (2.3, 3.4) | 0.39 |
| No HIV medication use [n (%)] | 7 (13) | 4 (14) | 3 (12) | 1.006 |
| HAART without PI [n (%)] | 13 (24) | 6 (21) | 7 (27) | 0.64 |
| HAART with PI [n (%)] | 34 (63) | 18 (64) | 16 (62) | 0.83 |
| Ritonavir or kaletra [n (%)] | 27 (50) | 11 (39) | 16 (62) | 0.10 |
| Metabolic syndrome [n (%)] | ||||
| Has metabolic syndrome4 | 25 (47) | 11 (41) | 14 (54) | 0.34 |
| Fasting triglycerides >150 mg/dL [n (%)]2 | 35 (66) | 17 (63) | 18 (69) | 0.63 |
| Fasting glucose ≥100 mg/dL | 20 (37) | 12 (43) | 8 (31) | 0.36 |
| Waist >102 cm (M), >88 cm (F) | 8 (15) | 2 (7) | 6 (23) | 0.146 |
| HDL <40 mg/dL (M), <50 mg/dL (F)4 | 43 (81) | 24 (89) | 19 (73) | 0.14 |
| Systolic >130 mm Hg, diastolic >85 mm Hg | 20 (37) | 7 (25) | 13 (50) | 0.057 |
| Has peripheral atrophy [n (%)] | 12 (22) | 5 (18) | 7 (27) | 0.42 |
| Has central adiposity [n (%)] | 4 (7) | 1 (4) | 3 (12) | 0.346 |
| QUICKI score | 0.35 (0.32, 0.37) | 0.34 (0.32, 0.37) | 0.36 (0.32, 0.37) | 0.63 |
| Glucose >120 mg/dL at 2 h in OGTT [n (%)] | 18 (33) | 9 (32) | 9 (35) | 0.85 |
The study group was 43% African American, 50% white, and 7% other. Thirty-three percent of the study group was glucose intolerant, and 54% was insulin resistant; these characteristics were not significantly different between the intervention and control groups. HAART, highly active antiretroviral therapy; QUICKI, Quantitative Insulin Sensitivity Check Index; OGTT, oral-glucose-tolerance test; PI, protease inhibitor.
Determined with a chi-square test for discrete values, except where noted, and with Wilcoxon’s rank-sum test for continuous values.
Median; interquartile range in parentheses (all such values).
Data missing for 1 participant.
Data missing for 10 participants.
Fisher’s exact test.
Dietary data
Dietary intakes, assessed at baseline, 3 wk, and 13 wk, are shown in Table 2. At baseline, there were no significant differences in the nutrition variables between the groups. The dietary intake in the intervention group at 3 wk represents the prepared meals that were served to the individual subjects for days 1–21 or 3 wk. The comparison between the intervention and control dietary intakes indicated that the goals of the intervention diet were achieved during the 3 wk of phase 1. There was a statistically significantly lower intake of dietary cholesterol (P = 0.002), total percentage fat (P = 0.003), and percentage saturated fat (P ≤ 0.001) in the intervention group than in the control group at 3 wk; the percentage of saturated:monounsaturated:polyunsaturated fatty acid was significantly different between groups (data not shown). The intervention group had a statistically higher intake of n−3 fatty acids (P ≤ 0.001) and fiber (P ≤ 0.001) and a significantly lower n−6/n−3 ratio of 2.2 at week 3 (P < 0.001) compared with the control group. The intervention group had higher calorie and protein intakes at 3 wk than did the control group (P = 0.032 and 0.001, respectively). At 13 wk (phase 2), the dietary goals were partially maintained in the intervention group. During phase 2, the subjects were given selected foods and counseled on maintaining the intervention diet, but were no longer taking their meals at the CRC. Calorie and protein intakes were now not statistically different between the 2 groups, but dietary cholesterol (P = 0.034) and saturated fat (P ≤ 0.001) intakes were still statistically lower in the intervention group. Total n−3 fatty acids, EPA, and DHA intakes were still statistically higher in the intervention group (all P < 0.001), and the n−6/n−3 ratio was still statistically lower (P ≤ 0.001). Whereas fiber intake dropped in the intervention group from 60 g at week 3 to 37 g at week 13, it was still statistically higher in the intervention group than in the control group (37 g compared with 22 g; P = 0.002). However, the total percentage of fat in the diet went from 28% to 39% in the intervention group between week 3 and week 13 and was no longer significantly different from that of the control group at week 13 (P = 0.75).
TABLE 2.
Diet comparison between the intervention and control groups at baseline, week 3, and week 131
| Baseline |
Week 3 |
Week 13 |
|||||||
| n | Median (IQR) | P value2 | n | Median (IQR) | P value2 | n | Median (IQR) | P value2 | |
| Energy intake (kcal/d) | |||||||||
| Intervention | 28 | 2527 (2000, 3250) | 21 | 2724 (2400, 3138) | 21 | 2306 (1803, 2631) | |||
| No intervention | 26 | 2579 (1862, 3162) | 0.88 | 26 | 2188 (1708, 2804) | 0.032 | 26 | 2201 (1610, 3672) | 0.61 |
| Protein (g) | |||||||||
| Intervention | 28 | 112 (92, 133) | 21 | 142 (126, 153) | 21 | 113 (92, 133) | |||
| No intervention | 26 | 109 (73, 141) | 0.37 | 26 | 92 (79, 107) | 0.001 | 26 | 106 (63, 138) | 0.52 |
| Cholesterol (g) | |||||||||
| Intervention | 28 | 387 (282, 626) | 21 | 203 (176, 236) | 21 | 230 (203, 406) | |||
| No intervention | 26 | 296 (210, 551) | 0.17 | 26 | 312 (234, 423) | 0.002 | 26 | 441 (287, 611) | 0.034 |
| Fat (% of energy) | |||||||||
| Intervention | 28 | 35 (30, 42) | 21 | 28 (27, 30) | 21 | 39 (32, 41) | |||
| No intervention | 26 | 33 (27, 40) | 0.58 | 26 | 34 (29, 38) | 0.003 | 26 | 37 (33, 39) | 0.75 |
| Saturated fat (% of energy) | |||||||||
| Intervention | 28 | 12 (9, 15) | 21 | 8 (7, 8) | 21 | 9 (6, 11) | |||
| No intervention | 26 | 11 (9, 13) | 0.25 | 26 | 12 (11, 14) | <0.001 | 26 | 13 (10, 14) | <0.001 |
| PUFA (% of energy) | |||||||||
| Intervention | 28 | 7 (5, 9) | 21 | 8 (8, 9) | 21 | 11 (7, 15) | |||
| No intervention | 26 | 6 (5, 9) | 0.97 | 26 | 6 (5, 8) | <0.001 | 26 | 6 (6, 7) | 0.002 |
| MUFA (% of energy) | |||||||||
| Intervention | 28 | 13 (11, 14) | 21 | 8 (8, 9) | 21 | 11 (8, 13) | |||
| No intervention | 26 | 12 (10, 16) | 0.86 | 26 | 12 (10, 14) | <0.001 | 26 | 14 (12, 15) | <0.001 |
| α-Linolenic acid (% of energy) | |||||||||
| Intervention | 28 | 1 (0, 1) | 21 | 1 (1, 1) | 21 | 1 (1, 2) | |||
| No intervention | 26 | 1 (0, 1) | 0.59 | 26 | 1 (0, 1) | <0.001 | 26 | 1 (0, 1) | <0.001 |
| n−6 FA (% of energy) | |||||||||
| Intervention | 28 | 6 (5, 7) | 21 | 6 (6, 7) | 21 | 8 (5, 10) | |||
| No intervention | 26 | 6 (4, 8) | 0.92 | 26 | 5 (4, 7) | 0.038 | 26 | 5 (5, 6) | 0.028 |
| n−3 FA (% of energy) | |||||||||
| Intervention | 28 | 1 (1, 1) | 21 | 3 (2, 3) | 21 | 4 (3, 5) | |||
| No intervention | 26 | 1 (0, 1) | 0.31 | 26 | 1 (0, 1) | <0.001 | 26 | 1 (1, 1) | <0.001 |
| EPA intake (mg) | |||||||||
| Intervention | 28 | 0.028 (0.011, 0.144) | 21 | 2.2368 (2.367, 2.370) | 21 | 2.415 (2.370, 2.581) | |||
| No intervention | 26 | 0.019 (0.009, 0.039) | 0.21 | 26 | 0.031 (0.011,0.101) | <0.001 | 26 | 0.055 (0.006, 0.254) | <0.001 |
| DHA intake (mg) | |||||||||
| Intervention | 28 | 0.133 (0.040, 0.288) | 21 | 2.277 (2.275, 2.283) | 21 | 2.308 (2.262, 2.454) | |||
| No intervention | 26 | 0.077 (0.031, 0.156) | 0.29 | 26 | 0.086 (0.036, 0.182) | <0.001 | 26 | 0.118 (0.038, 0.278) | <0.001 |
| n−6 FA/n−3 FA ratio | |||||||||
| Intervention | 28 | 8.3 (5.9, 9.4) | 21 | 2.2 (2.0, 2.7) | 21 | 2.2 (1.7, 3.0) | |||
| No intervention | 26 | 8.2 (7.1, 10.7) | 0.36 | 26 | 7.4 (6.5, 8.9) | <0.001 | 26 | 8.0 (5.8, 9.5) | <0.001 |
| Fiber (g) | |||||||||
| Intervention | 28 | 21 (16, 28) | 21 | 60 (52, 71) | 21 | 37 (29, 47) | |||
| No intervention | 26 | 25 (18, 35) | 0.069 | 26 | 22 (12, 28) | <0.001 | 26 | 22 (14, 32) | 0.002 |
At baseline, 61% of the intervention group and 38% of the control group completed the 3-d food record (P = 0.1); at 3 wk, 100% of the intervention group and 73% of the control group completed the 3-d food record (P = 0.012); and at 13 wk, 86% of the intervention group and 77% of the control group completed the 3-d food record (P = 0.87). The remaining participants completed a 24-h dietary recall. FA, fatty acid; IQR, interquartile range; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
The difference between the 2 groups was tested with Wilcoxon’s signed-rank test.
Outcome measures
The outcomes measures are presented in Table 3. The data are presented as both changes from baseline within each group and the comparison of changes over time between study groups. The within-group differences clearly show a significant decrease in serum triglycerides from a median of 180 mg/dL (IQR: 141, 396) at baseline to 114 (IQR: 84, 169) and 110 (IQR: 76, 220) mg/dL, in the intervention group at 3 and 13 wks, respectively (P ≤ 0.001 and P = 0.005, respectively). The control group showed no significant change in triglycerides from 175 mg/dL (IQR: 135, 222) at baseline to 183 (IQR: 125, 300) and 205 (IQR: 146, 283) mg/dL at 3 and 13 wk (P = 0.74 and P = 0.50, respectively). Comparison of the change in triglycerides concentrations between the 2 groups at 3 and 13 wk showed a significant difference in the change from baseline between groups at weeks 3 and 13 (P = 0.003 and 0.02, respectively).
TABLE 3.
Change from baseline in outcome variables by group and differences in changes between groups1
| Change from baseline within groups |
|||||||
| Intervention group |
Control group |
P for difference in change between groups3 | |||||
| Variable and visit | n | Median (IQR) | P value2 | n | Median (IQR) | P value2 | |
| Fasting triglycerides (mg/dL) | |||||||
| Baseline | 21 | 180 (141, 396) | 26 | 175 (135, 222) | |||
| Week 3 | 21 | 114 (84, 169) | <0.001 | 26 | 183 (125, 300) | 0.74 | 0.003 |
| Week 13 | 21 | 110 (76, 220) | 0.005 | 26 | 205 (146, 283) | 0.5 | 0.02 |
| Fasting glucose (mg/dL) | |||||||
| Baseline | 22 | 94 (84, 105) | 26 | 95 (86, 102) | |||
| Week 3 | 21 | 94 (86, 104) | 0.43 | 26 | 93 (81, 102) | 0.38 | 0.8 |
| Week 13 | 21 | 95 (88, 108) | 0.24 | 26 | 93 (89, 102) | 0.76 | 0.99 |
| Fasting insulin (μIU/mL) | |||||||
| Baseline | 22 | 10 (6, 14) | 26 | 8 (5, 13) | |||
| Week 3 | 21 | 7 (5, 11) | 0.34 | 26 | 8 (6, 14) | 0.34 | 0.49 |
| Week 13 | 21 | 8 (7, 11) | 0.73 | 26 | 9 (6, 13) | 0.077 | 0.61 |
| Insulin AUC (μIU · min/mL)4 | |||||||
| Baseline | 22 | 8261 (5273, 10,868) | 26 | 5299 (4418, 8153) | |||
| Week 3 | 21 | 5955 (4575, 10,543) | 0.066 | 24 | 4868 (3284, 9724) | 0.91 | 0.78 |
| Week 13 | 20 | 9028 (5914, 15,359) | 0.076 | 26 | 5719 (3630, 8993) | 0.85 | 0.069 |
| Glucose AUC (mg · min/dL) | |||||||
| Baseline | 22 | 22,110 (19,388, 26,295) | 25 | 21,660 (18,668, 27,218) | |||
| Week 3 | 21 | 23,520 (20,963, 25,598) | 0.65 | 25 | 20,985 (18,630, 23,400) | 0.41 | 0.41 |
| Week 13 | 21 | 23,760 (20,873, 26,955) | 0.20 | 26 | 21,308 (17,850, 26,520) | 0.84 | 0.64 |
| QUICKI | |||||||
| Baseline | 22 | 0.34 (0.32, 0.37) | 26 | 0.36 (0.32, 0.37) | |||
| Week 3 | 21 | 0.35 (0.32, 0.38) | 0.58 | 26 | 0.34 (0.32, 0.37) | 0.27 | 0.54 |
| Week 13 | 21 | 0.34 (0.32, 0.36) | 0.85 | 26 | 0.34 (0.32, 0.36) | 0.11 | 0.38 |
| Total cholesterol (mg/dL) | |||||||
| Baseline | 21 | 208 (140, 221) | 26 | 184 (153, 210) | |||
| Week 3 | 21 | 186 (138, 207) | 0.011 | 26 | 189 (171, 203) | 0.14 | 0.005 |
| Week 13 | 21 | 172 (141, 225) | 0.78 | 26 | 204 (169, 223) | <0.001 | 0.14 |
| LDL cholesterol (mg/dL) | |||||||
| Baseline | 20 | 102 (80, 139) | 25 | 106 (79, 133) | |||
| Week 3 | 20 | 114 (85, 153) | 0.32 | 26 | 108 (82, 135) | 0.82 | 0.34 |
| Week 13 | 19 | 105 (82, 167) | 0.14 | 24 | 110 (92, 144) | 0.045 | 0.52 |
| HDL cholesterol (mg/dL)5 | |||||||
| Baseline | 21 | 28 (19, 32) | 26 | 33 (24, 45) | |||
| Week 3 | 21 | 26 (21, 37) | 0.074 | 26 | 37 (27, 45) | <0.001 | 0.19 |
| Week 13 | 21 | 33 (23, 38) | 0.006 | 26 | 33 (27, 49) | 0.02676 | 0.74 |
| Ultrasensitive CRP (mg/L) | |||||||
| Baseline | 21 | 1.97 (1.02, 2.98) | 22 | 1.42 (0.79, 2.71) | |||
| Week 13 | 21 | 1.81 (1.00, 3.01) | 0.93 | 21 | 1.86 (0.80, 2.62) | 0.75 | 0.51 |
| CD4 (count/mm3) | |||||||
| Baseline | 22 | 531 (409, 635) | 25 | 465 (348, 656) | |||
| Week 13 | 20 | 456 (329, 639) | 0.43 | 26 | 487 (309, 687) | 0.45 | 0.14 |
| EPA (nmol/mL) | |||||||
| Baseline | 21 | 24 (19, 53) | 26 | 30 (21, 51) | |||
| Week 3 | 21 | 239 (193, 291) | <0.001 | 26 | 47 (32, 66) | 0.033 | <0.001 |
| Week 13 | 21 | 244 (153, 342) | <0.001 | 26 | 32 (26, 57) | 0.41 | <0.001 |
| DHA (nmol/mL) | |||||||
| Baseline | 21 | 129 (109, 201) | 26 | 128 (91, 165) | |||
| Week 3 | 21 | 235 (203, 346) | <0.001 | 26 | 164 (124, 191) | 0.016 | 0.071 |
| Week 13 | 21 | 252 (208, 308) | <0.001 | 26 | 151 (101, 192) | 0.16 | 0.012 |
| AA (nmol/mL) | |||||||
| Baseline | 21 | 575 (471, 793) | 26 | 600 (521, 736) | |||
| Week 3 | 21 | 444 (338, 511) | <0.001 | 26 | 615 (565, 702) | 0.78 | 0.001 |
| Week 13 | 21 | 387 (324, 532) | <0.001 | 26 | 590 (521, 676) | 0.53 | <0.001 |
Total n = 47. At baseline, the intervention and control groups were similar except where noted. QUICKI, Quantitative Insulin Sensitivity Check Index; IQR, interquartile range; CRP, C-reactive protein; AA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; AUC, area under the curve.
Wilcoxon’s signed-rank test of difference from baseline.
Two-sample t test of the difference in change between the 2 groups.
Borderline significantly different at baseline (P = 0.13); the distribution was uneven.
Borderline significantly different at baseline (P = 0.12).
Change is significant because those below the median at baseline had significant increases at week 13, whereas those at or above the median had no change.
There was a large difference in the insulin AUC between the 2 groups at baseline, although this difference was not statistically significant (P = 0.13; data not shown). A decrease in insulin AUC was seen in the intervention group from baseline to week 3 (during controlled feeding), but it was not statistically significant (P = 0.066). At 13 wk the insulin AUC in the intervention group appeared to rebound to exceed baseline levels, but was not statistically significant from baseline (P = 0.076). With this rebound, the comparison between the intervention group and the control group at week 13 was almost statistically significant (P = 0.069), which reflected both the higher initial value of insulin AUC in the intervention group and the large rebound that took place in week 13.
The intervention group also showed a significant decrease in total cholesterol at 3 wk (P = 0.011) from 208 (IQR: 140, 221)mg/dL to 186 (IQR:138, 207) mg/dL and an increase in HDL-cholesterol from 28(IQR:19, 32)mg/dL at baseline to 33 (IQR:23, 38) mg/dL at 13 wk (P = 0.006), but no difference at 3 wk. A significant increase in total cholesterol was seen in the control group from184 (IQR:153, 210) mg/dL at baseline to 204 (IQR:169, 223) mg/dL at 13 wk (P ≤ 0.001). The comparison of change in total cholesterol between the 2 groups shows a significance of P = 0.005 at week 3, reflecting the significant decrease in total cholesterol over 3 wk in the intervention group but no change in the control group.
HDL cholesterol in the control group was erratic, with a significant increase between baseline and 3 wk from 33 to 37 mg/dL (P < 0.001) that decreased again back to a median of 33 mg/dL at week 13, but was still statistically higher (P = 0.026) than at baseline because of a change in the distribution. There was not a significant difference in the change in the HDL-cholesterol values between the 2 groups at either 3 or 13 wk.
Table 3 also contains data on the concentrations of EPA, DHA, and arachidonic acid (AA) (nmol/dL) in the phospholipid fraction. EPA and DHA in the phospholipid fraction increased significantly in absolute amounts in the intervention group (P ≤ 0.001), and AA decreased significantly in the intervention group at weeks 3 and 13 (P ≤ 0.001 for both). The differences in the change between groups for EPA and AA at each time point were all statistically significant. The change in DHA was statistically different between groups only at week 13 (P = 0.012). It should be noted that, in the control group, a small but significant increase was seen in the serum concentrations of EPA and DHA at week 3, expressed as nmol/mL (P = 0.033 and 0.016, respectively).
Phospholipid fatty acids
Serum phospholipid fatty acids, expressed as % nmol, are presented in Table 4. Data for baseline, 3 wk, and 13 wk are included, and statistical comparisons are made within and between the intervention and control groups. Statistically significant decreases in 16:1 (P = 0.015) were observed at week 13, whereas decreases in 18:1 were observed at both 3 wk (P ≤ 0.001) and 13 wk (P ≤ 0.001) in the intervention group. These changes are significantly different between the 2 groups at both time periods only for the 18:1 fatty acid (P ≤ 0.001 for both).
TABLE 4.
Differences in serum phospholipids (PLs) within groups and between groups1
| Change from baseline within groups |
|||||||
| Intervention group |
Control group |
P for difference in change between groups3 | |||||
| Variable and visit | n | Median (IQR) | P value2 | n | Median (IQR) | P value2 | |
| % nmol | % nmol | ||||||
| PL 16:0 (PA) | |||||||
| Baseline | 21 | 29 (28, 31) | 26 | 30 (28, 31) | |||
| Week 3 | 21 | 30 (29, 31) | 0.24 | 26 | 30 (28, 32) | 0.28 | 0.72 |
| Week 13 | 21 | 30 (29, 32) | 0.28 | 26 | 30 (29, 32) | 0.3 | 0.89 |
| PL 16:1n−7 (PO) | |||||||
| Baseline | 21 | 0.65 (0.59, 0.77) | 26 | 0.80 (0.58, 0.88) | |||
| Week 3 | 21 | 0.51 (0.38, 0.72) | 0.12 | 26 | 0.76 (0.57, 0.87) | 0.61 | 0.22 |
| Week 13 | 21 | 0.44 (0.35, 0.60) | 0.015 | 26 | 0.71 (0.57, 0.92) | 0.5 | 0.074 |
| PL 18:0 (SA) | |||||||
| Baseline | 21 | 15 (15, 16) | 26 | 14 (14, 16) | |||
| Week 3 | 21 | 16 (15, 17) | 0.11 | 26 | 15 (14, 16) | 0.95 | 0.22 |
| Week 13 | 21 | 16 (15, 16) | 0.1 | 26 | 15 (14, 16) | 0.7 | 0.58 |
| PL 18:1n−9 (OL) | |||||||
| Baseline | 21 | 12 (11, 14) | 26 | 13 (11, 14) | |||
| Week 3 | 21 | 11 (8.9, 11) | <0.001 | 26 | 13 (12, 14) | 0.87 | 0.001 |
| Week 13 | 21 | 9.9 (9.3, 11) | <0.001 | 26 | 12 (11, 14) | 0.7 | <0.001 |
| PL 18:2n−6 (LA) | |||||||
| Baseline | 21 | 21 (19, 23) | 26 | 22 (21, 24) | |||
| Week 3 | 21 | 18 (17, 21) | 0.002 | 26 | 20 (19, 23) | 0.72 | 0.075 |
| Week 13 | 21 | 20 (19, 22) | 0.1 | 26 | 21 (19, 23) | 0.78 | 0.9 |
| PL 18:3n−3 (ALA) | |||||||
| Baseline | 21 | 0.25 (0.14, 0.33) | 26 | 0.28 (0.17, 0.36) | |||
| Week 3 | 21 | 0.28 (0.17, 0.33) | 0.092 | 26 | 0.29 (0.19, 0.35) | 0.93 | 0.28 |
| Week 13 | 21 | 0.27 (0.17, 0.33) | 0.24 | 26 | 0.27 (0.21, 0.32) | 0.45 | 0.18 |
| PL 20:3n−9 (mead) | |||||||
| Baseline | 21 | 0.21 (0.15, 0.35) | 26 | 0.28 (0.18, 0.42) | |||
| Week 3 | 21 | 0.14 (0.07, 0.26) | 0.04 | 26 | 0.30 (0.16, 0.36) | 0.99 | 0.069 |
| Week 13 | 21 | 0.12 (0.00, 0.29) | 0.048 | 26 | 0.26 (0.15, 0.40) | 0.81 | 0.35 |
| PL 20:3n−6 (DGLA) | |||||||
| Baseline | 21 | 3.7 (2.9, 4.9) | 26 | 4.3 (3.4, 4.8) | |||
| Week 3 | 21 | 2.0 (1.6, 2.7) | <0.001 | 26 | 3.5 (2.7, 4.4) | 0.01 | 0.003 |
| Week 13 | 21 | 2.3 (1.7, 2.8) | <0.001 | 26 | 4.2 (3.1, 4.7) | 0.35 | <0.001 |
| PL 20:4n−6 (AA) | |||||||
| Baseline | 21 | 11 (8.9, 13) | 26 | 12 (9.5, 13) | |||
| Week 3 | 21 | 9.3 (7.9, 11) | <0.001 | 26 | 12 (8.5, 13) | 0.16 | 0.025 |
| Week 13 | 21 | 8.6 (7.2, 9.9) | <0.001 | 26 | 12 (9.2, 13) | 0.46 | <0.001 |
| PL 20:5n−3 (EPA) | |||||||
| Baseline | 21 | 0.57 (0.43, 0.83) | 26 | 0.57 (0.45, 0.88) | |||
| Week 3 | 21 | 5.7 (4.2, 6.6) | <0.001 | 26 | 0.86 (0.52, 1.2) | 0.082 | <0.001 |
| Week 13 | 21 | 5.4 (3.8, 7.0) | <0.001 | 26 | 0.63 (0.45, 0.94) | 0.72 | <0.001 |
| PL 22:4n−6 (adrenic) | |||||||
| Baseline | 21 | 0.35 (0.15, 0.51) | 26 | 0.48 (0.37, 0.56) | |||
| Week 3 | 21 | 0.16 (0.09, 0.24) | <0.001 | 26 | 0.37 (0.26, 0.49) | 0.011 | 0.097 |
| Week 13 | 21 | 0.17 (0.10, 0.21) | <0.001 | 26 | 0.47 (0.29, 0.57) | 0.5 | <0.001 |
| PL-22:5n−6 (osbond) | |||||||
| Baseline | 21 | 0.32 (0.27, 0.43) | 26 | 0.38 (0.24, 0.52) | |||
| Week 3 | 21 | 0.12 (0.08, 0.16) | <0.001 | 26 | 0.33 (0.25, 0.46) | 0.35 | 0.001 |
| Week 13 | 21 | 0.11 (0.07, 0.14) | <0.001 | 26 | 0.38 (0.26, 0.53) | 0.89 | <0.001 |
| PL 22:5n−3 (DPA) | |||||||
| Baseline | 21 | 0.67 (0.40, 0.78) | 26 | 0.74 (0.48, 0.89) | |||
| Week 3 | 21 | 1.1 (0.73, 1.2) | <0.001 | 26 | 0.67 (0.54, 0.90) | 0.76 | 0.011 |
| Week 13 | 21 | 1.0 (0.84, 1.3) | <0.001 | 26 | 0.72 (0.55, 0.88) | 0.99 | 0.02 |
| PL-22:6n-3 (DHA) | |||||||
| Baseline | 21 | 2.8 (2.0, 3.2) | 26 | 2.4 (1.7, 2.8) | |||
| Week 3 | 21 | 5.9 (4.9, 6.5) | <0.001 | 26 | 3.0 (2.1, 3.4) | 0.02 | <0.001 |
| Week 13 | 21 | 5.6 (5.0, 6.2) | <0.001 | 26 | 2.7 (2.1, 3.2) | 0.16 | <0.001 |
| PL SFA | |||||||
| Baseline | 21 | 45 (43, 45) | 26 | 44 (43, 45) | |||
| Week 3 | 21 | 45 (45, 46) | 0.003 | 26 | 44 (44, 45) | 0.068 | 0.17 |
| Week 13 | 21 | 46 (45, 46) | 0.002 | 26 | 45 (44, 46) | 0.044 | 0.6 |
| PL MUFA | |||||||
| Baseline | 21 | 13 (12, 14) | 26 | 14 (12, 15) | |||
| Week 3 | 21 | 11 (9.3, 12) | <0.001 | 26 | 14 (12, 15) | 0.95 | 0.001 |
| Week 13 | 21 | 11 (9.8, 11) | <0.001 | 26 | 13 (12, 15) | 0.76 | <0.001 |
| PL PUFA (includes DUFA) | |||||||
| Baseline | 21 | 43 (41, 43) | 26 | 43 (42, 44) | |||
| Week 3 | 21 | 44 (43, 45) | <0.001 | 26 | 43 (40, 44) | 0.39 | 0.015 |
| Week 13 | 21 | 44 (43, 45) | 0.023 | 26 | 42 (40, 43) | 0.068 | 0.01 |
| PL n−6 | |||||||
| Baseline | 21 | 38 (36, 39) | 26 | 39 (36, 39) | |||
| Week 3 | 21 | 31 (29, 32) | <0.001 | 26 | 37 (34, 39) | 0.018 | <0.001 |
| Week 13 | 21 | 30 (28, 33) | <0.001 | 26 | 38 (36, 39) | 0.057 | <0.001 |
| PL n−3 | |||||||
| Baseline | 21 | 4.4 (3.0, 5.0) | 26 | 3.9 (3.5, 4.7) | |||
| Week 3 | 21 | 13 (11, 14) | <0.001 | 26 | 4.6 (3.7, 5.8) | 0.033 | <0.001 |
| Week 13 | 21 | 13 (10, 15) | <0.001 | 26 | 4.1 (3.4, 5.3) | 0.42 | <0.001 |
| PL n−6/n−3 ratio | |||||||
| Baseline | 21 | 8.28 (7.08, 11.42) | 26 | 10.01 (8.02, 11.39) | |||
| Week 3 | 21 | 2.35 (2.07, 2.91) | <0.001 | 26 | 7.70 (6.26, 9.68) | 0.021 | <0.001 |
| Week 13 | 21 | 2.35 (1.91, 3.08) | <0.001 | 26 | 9.06 (7.28, 11.07) | 0.29 | <0.001 |
| PL EPA and DHA | |||||||
| Baseline | 21 | 3.5 (2.5, 4.0) | 26 | 3.0 (2.6, 3.9) | |||
| Week 3 | 21 | 11 (9.6, 13) | <0.001 | 26 | 3.9 (2.8, 4.8) | 0.033 | <0.001 |
| Week 13 | 21 | 11 (9.1, 13) | <0.001 | 26 | 3.2 (2.6, 4.2) | 0.28 | <0.001 |
Total n = 47. PA, palmitic acid; PO, palmitoleic acid; SA, stearic acid; OL, oleic acid; LA, linoleic acid; ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid; AA, arachidonic acid; EPA, eicosopentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; DUFA, diunsaturated fatty acid; IQR, interquartile range.
Wilcoxon’s signed-rank test of the change from baseline.
Two-sample t test of the difference in change between the 2 groups.
A statistically significant decrease in AA was seen within the intervention group at 3 wk (P ≤ 0.001) and 13 wk (P ≤ 0.001). The comparison of the difference in change between the intervention and control groups in AA is significant at 3 wk (P = 0.025) and 13 wk (P ≤ 0.001). AA values in the control group were very stable over this time period. Decreases in the long-chain n−6 fatty acids 20:3n−6 [dihomo-γ-linolenic acid (DGLA)], 22:4n−6, and 22:5n−6 were observed at both 3 and 13 wk within the intervention group (P < 0.001 for all). The differences in change between the 2 groups are statistically significant for the 2 fatty acids 20:3n−6 and 22:5n−6 at both time periods, whereas for 22:4n−6, the comparison between the intervention and control groups was only significant at week 13.
It is apparent that the supplementation of the fatty acids EPA and DHA in the intervention group resulted in significant and large increases in these fatty acids in the phospholipid fraction. At baseline, EPA accounted for 0.57% of the total % nmol of fatty acids, whereas at 3 and 13 wk in the intervention group this value was 5.7% and 5.4%, respectively (P < 0.001 for both). This is an ≈10-fold increase in EPA values over time. The values of these fatty acids in the control group remained relatively stable. The change in DHA in the intervention group was from 2.8 % nmol at baseline to 5.9% and 5.6% nmol at weeks 3 and 13, respectively—a 2-fold increase. There was also a statistically significant increase in DHA in the control group, from 2.4% to 3.0% from baseline to 3 wk (P = 0.02). Investigating the dietary intake of n−3 fatty acids in the control group over this period, we discovered that 3 control subjects had fish once a week (as allowed in those randomly assigned into the control group), but the large quantities consumed (14 oz, or 392 g/serving) may have accounted for the increases in mean DHA values observed in the control group. The ratio of the n−6/n−3 fatty acids in the serum phospholipid fatty acids was significantly and dramatically lowered in the intervention group, decreasing from 8.28 to 2.35 at both week 3 and at week 13 (P < 0.001 for both). This was due to both a decrease in total n−6 fatty acids and an increase in n−3 fatty acids in the intervention group, which is closely reflected in their proportions in the phospholipid fraction, expressed as % nmol and as nmol/dL in Table 3.
Evaluation of the effects of the 3 categories of antiretroviral treatment [HAART with protease inhibitor (PI), HAART without PI, or no treatment] indicated that there was no independent effect of the medications on the outcomes of triglycerides, insulin AUC, glucose AUC, or highly sensitive CRP (data not shown).
Correlation of phospholipid fatty acids and outcome measures
Evaluation of the correlations between changes in fatty acids and changes in the 3 major outcome variables (Table 5) over the first 3 wk of the study indicated that changes in 20:3n−6 (DGLA) were directly correlated to changes in serum triglyceride concentrations (r = 0.37, P = 0.01). Changes in DGLA were negatively associated with changes in glucose AUC with an r = −0.31 (P = 0.037). Total n−3 fatty acids and EPA+DHA showed a negative correlation with serum triglycerides: r = −0.31 (P = 0.035) and r = −0.34 (P = 0.021), respectively. Therefore, the greater the increase in total n−3 fatty acids or EPA+DHA, the greater the decrease in serum triglyceride concentrations. The fatty acids 16:1, 18:1, 20:3n−6 (DGLA), and 22:5n−6 and the percentage of monounsaturated fatty acids were all positively and significantly associated with an increase in serum triglyceride concentrations, with correlations ranging from 0.32 to 0.44. EPA+DHA, EPA (data not shown), and the percentage of polyunsaturated fatty acids (data not shown) were negatively associated with an increase in serum triglycerides, but the associations were weaker.
TABLE 5.
Correlations between the change in phospholipid fatty acids (PL FAs; % nmol) and the change in outcome measures from baseline to 3 wk and from baseline to 13 wk1
| 3 wk |
13 wk |
|||
| r | P | r | P | |
| PL FAs and serum triglycerides | — | — | — | — |
| 16:1 (palmitoleic) | — | NS | 0.32 | 0.030 |
| 18:1 (oleic) | — | NS | 0.44 | 0.002 |
| 20:3n−6 (DGLA) | 0.37 | 0.010 | 0.35 | 0.015 |
| 22:5n−6 | — | NS | 0.39 | 0.006 |
| 20:5n−3+22:6n−3 (EPA+DHA) | −0.34 | 0.021 | −0.29 | 0.046 |
| Total n−3 fatty acids | −0.31 | 0.035 | — | NS |
| MUFA | — | NS | 0.44 | 0.002 |
| PL FAs and glucose AUC | ||||
| 20:3n−9 | 0.30 | 0.043 | — | NS |
| 20:3n−6 (DGLA) | −0.31 | 0.037 | — | NS |
| 22:5n−3 (DPA) | — | NS | 0.31 | 0.033 |
| 22:5n−6 | — | NS | −0.30 | 0.043 |
| 20:5n−3 (EPA) | — | NS | 0.39 | 0.007 |
| 22:6n−3 (DHA) | — | NS | 0.34 | 0.020 |
| EPA+DHA | — | NS | 0.39 | 0.007 |
| Total n−3 fatty acids | — | NS | 0.40 | 0.006 |
| Total n−6 fatty acids | — | NS | −0.32 | 0.033 |
| n−6/n−3 ratio | — | NS | −0.32 | 0.030 |
| PL FAs and insulin AUC | ||||
| 20:5n−3 (EPA) | — | NS | 0.36 | 0.013 |
= 47. This table includes only covariates for which r ≥ 0.30. AUC, area under the curve; DGLA, dihomo-γ-linolenic acid; EPA, eicosopentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid; MUFA, monounsaturated fatty acid.
Changes in the levels of specific fatty acids, expressed as % nmol, were correlated with changes in the glucose AUC at week 13. Increases in EPA, DHA, total n−3 fatty acids, and EPA+DHA were positively associated with increases in the glucose AUC. Changes in total n−6 fatty acids and the n−6/n−3 ratio were negatively associated with the change in the glucose AUC, so increases in n−6 fatty acids and increases in the n−6/n−3 ratio were associated with decreases in the glucose AUC. In addition, an increase in the specific n−6 fatty acid 22:5n−6 was associated with a decrease in the glucose AUC (r = −0.30, P = 0.043). These associations were not strong. EPA (20:5n−3) was the only fatty acid associated with insulin AUC. At 13 wk, it was positively associated with insulin AUC (r = 0.36, P = 0.013).
DISCUSSION
Published reports in the HIV-infected population have shown that supplementation with n−3 fatty acids at levels of 2–6 g/d were effective at lowering serum triglycerides by 15–25%, even in individuals treated with HAART (8–11). This nutritional intervention study showed that adherence to a diet high in n−3 fatty acids (4–6 g/d) was effective at producing a significant and sustained median drop in serum triglycerides in the intervention group of 46% (IQR: −65%, 10%) (data not shown). In addition, we observed a significant decrease in the proportion of AA in phospholipid fatty acids in the intervention group (P ≤ 0.001) at 13 wk and a significant difference between the groups (P ≤ 0.001). This decrease would be expected to decrease the production of inflammatory cytokines for which AA is the primary substrate (41, 42). Oleic acid (18:1n−9) was significantly reduced in the intervention group at week 13 (P ≤ 0.001) and was significantly lower than in the control group (P < 0.001). This suggests a decrease in de novo lipogenesis. Increased lipogenesis is a common observation in the metabolic syndrome.
The intervention group maintained their dietary goals into week 13, except for total fat intake. Dietary cholesterol and saturated fat intakes continued to be low into week 13, which suggested that many of the changes could be maintained long term. The daily intake of a large portion of fish would present the largest barrier to long-term dietary change, whereas the use of n−3 supplements presented no problem. The total serum cholesterol concentration increased significantly from baseline to week 13 in the control group with no change in body weight or calorie intake, but a significant increase in saturated fat (P = 0.011) during this period could explain this increase (data not shown).
The literature supports an important role for n−3 fatty acids in increasing insulin sensitivity via interaction with transcription factors known to be critical modulators of insulin sensitivity, such as peroxisome proliferators–activated receptors, steroid regulating element binding protein, signaling compounds such as diacylglycerol, and critical enzymes such as phosphatidyl inositol–3 kinase or acute phase proteins such as highly sensitive CRP underlying processes of inflammation, lipogenesis, and fatty acid oxidation (12, 13, 19, 43–47). n−3 Fatty acids can reverse insulin resistance in a rat model of dietary-induced insulin resistance, initiated by increased intake of saturated fat, and improve glucose metabolism and insulin sensitivity in healthy subjects, but are not able to reverse insulin resistance in persons with established insulin resistance (48). In 2 studies, the expected effects of n−3 fatty acids were not seen in the presence of a high-fat diet (49, 50).
We approached this lack of consistency of an effect of n−3 fatty acids on insulin sensitivity as being indicative of the need to control both macronutrient intake, especially fat intake, and to supply n−3 fatty acid supplementation with an n−6/n−3 ratio of ≈2 or less. This would provide higher availability of the n−3 fatty acids, which are in competition with the n−6 fatty acids (51). The potential validation of this proposition is supported by the improvement in insulin sensitivity at week 3 when we provided the intervention diet. However, this decrease in insulin AUC was not statistically significant (P = 0.066). This may be due to a small sample size or because only 62% of the subjects in the intervention group had insulin resistance at baseline (data not shown).
Serum phospholipid fatty acids have been used as a good estimate of membrane phospholipid composition, which influence many metabolic functions. At 3 wk, the change in phospholipid fatty acids, EPA+DHA, and total n−3 fatty acids were both negatively correlated with the change in total serum triglycerides, consistent with previous reports. Of interest was the observation of a direct relation between change in the fatty acid DGLA (20:3n−6) with serum triglyceride changes (r = 0.37, P = 0.01). This fatty acid might accumulate in the presence of a lower activity of Δ-5 desaturase. The decrease in this fatty acid and serum triglycerides in the intervention group at week 3 is consistent with an interpretation of a lower Δ-5 desaturase activity.
Our earlier publication (52) showed evidence of increased activity of Δ-6 and Δ-5 desaturase in only the HIV-infected patients, which was ascribed to the disease and the treatment medications. In this nutrition intervention study, HIV subjects in the intervention showed indications of a reduction in the activity of these 2 desaturases, as evidenced by statistically lower values of 20:3n−6 (DGLA), 20:4n−6 (AA), 22:4n−6, and 22:5n−6, which are products of these desaturases in the n−6 fatty acid series. n−3 Fatty acids have been reported to down-regulate Δ-9 desaturase in rats (53), where it increased fatty acid oxidation and suppressed hepatic fatty acid synthesis to reverse lipid build-up in the liver and decrease the secretion of triglycerides. The reduction in the activity of these desaturases could be ascribed to their down-regulation by the highly elevated levels of n−3 fatty acids supplied as a supplement.
If the ratios of the product to precursor are used as an indication of desaturase activity, increases in Δ-9 and Δ-6 desaturase and decreases in Δ-5 desaturases have been reported in persons with the metabolic syndrome and insulin resistance (54–56). Protease inhibitors have been reported to increase Δ-9 and Δ-6 desaturase enzymes and decrease Δ-5 desaturase in children with HIV infection (57).
The role of Δ-5 desaturase is based on product-precursor ratios and has only been analyzed directly in type 1 diabetes, where it was suppressed compared with controls (58, 59). This reported suppression of Δ-5 desaturase in diabetes, with the reduced ability to synthesize longer-chain unsaturated fatty acids, could be expected to be detrimental in persons with diabetes who have high intakes of saturated fat and low intakes of polyunsaturated fat (60). In the presence of high intakes of highly unsaturated fatty acids (either n−6 or n−3), elevated activity of Δ-5 desaturases would be assumed to be less critical.
Subjects in the intervention group, treated with diet and n−3 supplementation, showed lower levels of products of Δ-5 desaturase, but this was in the setting of increased activity related to HIV infection and its treatment and presumably does not have the same implications for persons with diabetes, independent of HIV infection.
A weakness of the study was its small sample size, which decreased our chances to determine the effect of the intervention on markers of insulin sensitivity. The control group was not seen as often as the intervention group, which was reflected in the lower percentage of subjects who provided a 3DFR.
In summary, our HIV study subjects who received HAART and had elevated serum triglycerides and/or insulin resistance, had high levels of fatty acids formed by the Δ-9, -6, and -5 desaturase enzymes, increased de novo lipogenesis, and increased levels of AA (20:4n−6), which is characteristic of increased inflammation. All of these effects were ameliorated through the consumption of a healthier diet that was low in total and saturated fats, that was higher in fiber and n−3 fatty acids, and that had a lower n−6/n−3 ratio.
Acknowledgments
We thank the nursing staff and the laboratory staff at the CRC for their assistance with the clinical aspects of the study. We particularly acknowledge the support and input of Haewook Han (chief dietitian and study coordinator at the CRC) and Jeanette Queenan, who provided expertise in the dietary analysis of the food records. We also acknowledge the expert statistical advice of Norma Terrin. Lisa Gualtieri managed the storage of all specimens and the extraction of all samples for fatty acid analysis.
The authors’ responsibilities were as follows—MNW: design of analysis, interpretation of study data, and writing of the manuscript; CAW: study design, interpretation of study data, and review of medications, clinical data of the subjects, and the manuscript; P-RL: supervision and interpretation of the phospholipids analysis, review of the manuscript; KMH: study design, interpretation of the dietary data, and review of the methods and the manuscript; AMT: responsible for statistical analysis methodology and interpretation of data and review of the manuscript; TAK (liason to the CRC and study physician): review of the manuscript; CEA: phospholipid analysis, interpretation of the data, and review of the manuscript, KRD: responsible for the dietary intervention methods, data review, interpretation of the data, and review of the manuscript; SS: study analysis and methodology and review of the manuscript; and BRB: supervision of the phospholipid analysis, interpretation and discussion, and review of the manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Hellerstein MK, Grunfeld C, Wu K, et al. Increased de novo hepatic lipogenesis in human immunodeficiency virus infection. J Clin End Metab 1993;76:559–65 [DOI] [PubMed] [Google Scholar]
- 2.d’Arminio A, Sabin CA, Phillips AN, et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS 2004;18:1811–7 [DOI] [PubMed] [Google Scholar]
- 3.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005;352:48–62 [DOI] [PubMed] [Google Scholar]
- 4.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007;356:1723–35 [DOI] [PubMed] [Google Scholar]
- 5.Rustan AC, Nossen JO, Christiansen EN, Drevon CA. Eicosapentaenoic acid reduces hepatic synthesis and secretion of triacylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglyerol acyltransferase. J Lipid Res 1988;29:1417–26 [PubMed] [Google Scholar]
- 6.Madsen L, Rustan AC, Vaagenes H, et al. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids 1999;34:951–63 [DOI] [PubMed] [Google Scholar]
- 7.Harris WS. n–3 Fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 1997;65(suppl 5):1645S–54S [DOI] [PubMed] [Google Scholar]
- 8.Wohl DA, Tien HC, Busby M, et al. Randomized study of the safety and efficacy of fish oil (omega-3 fatty acid) supplementation with dietary and exercise counseling for the treatment of antiretroviral therapy-associated hypertriglyceridemia. Clin Infect Dis 2005;41:1498–504 [DOI] [PubMed] [Google Scholar]
- 9.Carter VM, Woolley I, Jelley D, et al. A randomized controlled trial of omega-3 fatty acid supplementation for treatment of hypertriglycridemia in HIV-infected males on highly active antiretroviral therapy. Sex Health 2006;3:287–90 [DOI] [PubMed] [Google Scholar]
- 10.De Truchis P, Kirstetter M, Perier A, et al. Reduction in triglyceride level with N-3 polyunsaturated fatty acids in HIV-infected patients taking potent antiretroviral therapy: a randomized prospective study. J Acquir Immune Defic Syndr 2007;44:278–85 [DOI] [PubMed] [Google Scholar]
- 11.Manfredi R, Calza L, Chiodo F. Polyunsaturated ethyl esters of n−3 fatty acids in HIV-infected patients with moderate hypertiglyceridemia: comparison with dietary and lifestyle changes, and fibrate therapy. J Acquir Immune Defic Syndr 2004;36:878–80 (letter) [DOI] [PubMed] [Google Scholar]
- 12.Lombardo YB, Chicco AG. Effects of dietary polyunsaturated n−3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J Nutr Biochem 2006;17:1–13 [DOI] [PubMed] [Google Scholar]
- 13.Carpentier YA, Portois L, Malaisse WJ. n−3 Fatty acids and the metabolic syndrome. Am J Clin Nutr 2006;83(suppl):1499S–504S [DOI] [PubMed] [Google Scholar]
- 14.Silveira LR, Fiamoncini J, Hirabara SM, et al. Updating the effect of fatty acids on skeletal muscle. J Cell Physiol 2008;217:1–12 [DOI] [PubMed] [Google Scholar]
- 15.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr 2005;25:391–406 [DOI] [PubMed] [Google Scholar]
- 16.Boden G, Chen X, Rosner J, Barton M. Effects of a 48 hour fat infusion on insulin secretion. Secretion and glucose utilization. Diabetes 1995;44:1239–42 [DOI] [PubMed] [Google Scholar]
- 17.El-Assaad W, Buteau J, Peyot ML, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic B-cell death. Endocrinology 2003;144:4154–63 [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, van Dam RM, Liu S. Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia 2001;44:805–17 [DOI] [PubMed] [Google Scholar]
- 19.Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr 2009;139:1–4 [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Pinnamaneni S, Eo S, et al. Saturated, but not n−6 polyunsaturated fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol 2006;100:1467–74 [DOI] [PubMed] [Google Scholar]
- 21.Stein DT, Steverson BE, Chester MW, et al. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J Clin Invest 1997;100:398–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borkman MB, Storlien LH, Pan DA, et al. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 1993;328:238–44 [DOI] [PubMed] [Google Scholar]
- 23.Browning LM. N-3 Polyunsaturated fatty acids, inflammation and obesity-related disease. Proc Nutr Soc 2003;62:447–53 [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Real JM, Vendrell J, Broch M, Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care 2003;26:1362–8 [DOI] [PubMed] [Google Scholar]
- 25.Taouis M, Dagou C, Ster C, et al. N-3 Polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab 2001;282:E664–71 [DOI] [PubMed] [Google Scholar]
- 26.Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley L. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000;342:1392–8 [DOI] [PubMed] [Google Scholar]
- 27.Venn BJ, Mann JI. Cereal grains, legumes and diabetes. Eur J Clin Nutr 2004;58:1443–61 [DOI] [PubMed] [Google Scholar]
- 28.Jenkins DJA, Kendall CW, McKeown-Eyssen G, et al. Effect of a low glycemic index or a high cereal fiber diet on type 2 diabetes: a randomized trial. JAMA 2008;300:2742–53 [DOI] [PubMed] [Google Scholar]
- 29.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr 2008;87(suppl):258S–68S [DOI] [PubMed] [Google Scholar]
- 30.Brand-Miller J, Hayne S, Petocz P, Colagoiro S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–7 [DOI] [PubMed] [Google Scholar]
- 31.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–10 [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Brewer H, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109:433–8 [DOI] [PubMed] [Google Scholar]
- 33.Forrester JE, Spiegelman D, Woods MN, Knox TA, Fauntleroy JM, Gorbach SL. Weight loss and body composition in cohort of HIV positive men and women. Public Health Nutr 2001;4:743–7 [DOI] [PubMed] [Google Scholar]
- 34.Woods MN, Spiegelman D, Knox TA, et al. Nutrient intake and body weight in a large HIV cohort that includes women and minorities. J Am Diet Assoc 2002;102:203–11 [DOI] [PubMed] [Google Scholar]
- 35.Meydani M, Natiello F, Goldin B, et al. Effect of long-term fish oil supplementation on vitamin E status and lipid peroxidation in women. J Nutr 1991;121:484–91 [DOI] [PubMed] [Google Scholar]
- 36.Palozza P, Sgarlata E, Luberto C, et al. n−3 Fatty acids induce oxidative modifications in human erythrocytes depending on dose and duration of dietary supplementation. Am J Clin Nutr 1996;64:297–304 [DOI] [PubMed] [Google Scholar]
- 37.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 38.Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez JG, Touchstone JC. Separation of acidic and neutral lipids by aminopropyl-bonded silica gel column chromatography. J Chromatogr 2005;557:142–5 [DOI] [PubMed] [Google Scholar]
- 40.Alvarez JG, Storey B. Differential incorporation of fatty acids into and peroxidative loss of fatty acid from phospholipids of human spermatozoa. Mol Reprod Dev 1995;42:334–6 [DOI] [PubMed] [Google Scholar]
- 41.Cook JA, Wise WC, Knapp DR, Halushka PV. Essential fatty acid deficient rats: a new model for evaluating arachidonate metabolism in shock. Adv Shock Res 1981;6:93–105 [PubMed] [Google Scholar]
- 42.Ling PR, Boyce P, Bistrian BR. Role of arachidonic acid in the regulation of the inflammatory response in TNF-alpha-treated rats. JPEN J Parenter Enteral Nutr 1998;22:268–75 [DOI] [PubMed] [Google Scholar]
- 43.Timmers S, Schrauiven P, deVogt J. Muscular diacylglycerol metabolism and insulin resistance. Physiol Behav 2008;94:242–51 [DOI] [PubMed] [Google Scholar]
- 44.Svegliati-Baroni G, Candelaresi C, Saccomanno S, et al. A model of insulin resistance and nonalcoholic steatohepatitis in rats. Am J Pathol 2006;169:846–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calder PC. n−3 Polyunsaturated fatty acids, inflammation and inflammatory diseases. Am J Clin Nutr 2006;83(suppl):1505S–19S [DOI] [PubMed] [Google Scholar]
- 46.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr 2004;23:447–56 [DOI] [PubMed] [Google Scholar]
- 47.Rytter E, Vessby B, Asgard R, et al. Glycemic status in relation to oxidative stress and inflammation in well-controlled type 2 diabetes subjects. Br J Nutr 2009;101:1423–6 [DOI] [PubMed] [Google Scholar]
- 48.Delarue J, LeFoll C, Corporeau C, Lucas D. n−3 Long chain polyunsaturated acids: a nutritional tool to prevent insulin resistance associated to type 2 diabetes and obesity? Reprod Nutr Dev 2004;44:289–99 [DOI] [PubMed] [Google Scholar]
- 49.Vessby B, Uusitupa M, Hermansen K, et al. Substituting dietary saturated fat for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU study. Diabetologia 2001;44:312–9 [DOI] [PubMed] [Google Scholar]
- 50.Kraegen EW, Cooney GJ, Turner N. Muscle insulin resistance: a case of fat overconsumption, not mitochondrial dysfunction. Proc Natl Acad Sci USA 2008;105:7627–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care 2004;7:137–44 [DOI] [PubMed] [Google Scholar]
- 52.Woods MN, Wanke CA, Ling PR, et al. Metabolic syndrome and serum fatty acids patterns in serum phospholipids in hypertriglyceridemic persons with human immunodeficiency virus (HIV). Am J Clin Nutr 2009;89:1180–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HJ, Takahashi M, Ezaki O. Fish oil feeding decreases mature SREBP-1 by down-regulation of SREBP-1c mRNA in mouse liver. J Biol Chem 1999;274:25892–8 [DOI] [PubMed] [Google Scholar]
- 54.Vessby B, Gustafsson IB, Tengblad S, Boberg M, Dandersson A. Desaturation and elongation of fatty acids and insulin action. Ann N Y Acad Sci 2002;967:183–95 [DOI] [PubMed] [Google Scholar]
- 55.Vessby B. Dietary fat, fatty acid composition and the metabolic syndrome. Curr Opin Lipidol 2003;14:15–9 [DOI] [PubMed] [Google Scholar]
- 56.Pelikánová T, Kazdova L, Chvojkova S, Base J. Serum phospholipid fatty acid composition and insulin action in type 2 diabetic patients. Metabolism 2001;50:1472–8 [DOI] [PubMed] [Google Scholar]
- 57.Aldamiz-Echevarria L, Pocheville I, Sanjurjo P, et al. Abnormalities in plasma fatty acid composition in human immunodeficiency virus-infected children treated with protease inhibitors. Acta Paediatr 2005;94:672–7 [DOI] [PubMed] [Google Scholar]
- 58.Rimoldi JO, Finarelli GS, Brenner RR. Effects of diabetes and insulin on hepatic delta 6-desaturase gene expression. Biochem Biophys Res Commun 2001;283:323–6 [DOI] [PubMed] [Google Scholar]
- 59.El Boustani S, Causse JE, Descomps B, Monnier L, Mendy F, Crastes de Paulet A. Direct in vivo characterization of delta5-desaturase activity in humans by deuterium labeling: effect of insulin. Metabolism 1989;38:315–21 [DOI] [PubMed] [Google Scholar]
- 60.Hodge AM, English DR, O’Dea K, et al. Plasma phospholipids and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–97 [DOI] [PubMed] [Google Scholar]