Abstract
Background: An analysis of data from the National Health and Nutrition Examination Survey indicated that in older adults exposed to folic acid fortification, the combination of low serum vitamin B-12 and elevated folate is associated with higher concentrations of homocysteine and methylmalonic acid and higher odds ratios for cognitive impairment and anemia than the combination of low vitamin B-12 and nonelevated folate. These findings await confirmation in other populations.
Objective: The purpose was to compare metabolic indicators of vitamin B-12 status, cognitive function, and depressive symptoms among elderly Latinos with elevated and nonelevated plasma folate.
Design: Cross-sectional data were analyzed for 1535 subjects (age: ≥60 y) from the Sacramento Area Latino Study on Aging. Subjects were divided into 4 groups on the basis of plasma vitamin B-12 (< or ≥148 pmol/L) and folate (≤ or >45.3 nmol/L). Homocysteine, methylmalonic acid, holotranscobalamin, ratio of holotranscobalamin to vitamin B-12, Modified Mini-Mental State Examination, delayed recall, and depressive symptom scores were compared between the groups.
Results: Individuals with low vitamin B-12 and elevated folate (n = 22) had the highest concentrations of homocysteine and methylmalonic acid and the lowest concentration of holotranscobalamin and ratio of holotranscobalamin to vitamin B-12 when compared with all other groups (P ≤ 0.003). No differences in Modified Mini-Mental State Examination, delayed recall, and depressive symptom scores were observed between the low vitamin B-12 and elevated-folate group compared with other groups.
Conclusions: Low vitamin B-12 is associated with more pronounced metabolic evidence of vitamin B-12 deficiency when folate is elevated than when folate is not elevated. These data should be considered when assessing the potential costs, risks, and benefits of folic acid and vitamin B-12 fortification programs.
See corresponding editorial on page 1449.
INTRODUCTION
Folic acid fortification of the food supply in the United States, Canada, and other countries has been in effect since the mid- to late-1990s. The primary goal of this fortification is to prevent neural tube defects (spina bifida, anencephaly, and related disorders). In this regard, the program has been efficacious, having reduced the incidence of neural tube defects by 20–50% (1, 2). In addition, there has been a large reduction in the prevalence of both folate deficiency and elevated plasma homocysteine concentrations (hyperhomocysteinemia) in the general population (3, 4), and there is some epidemiologic evidence that an observed reduction in fatal stroke incidence in the United States and Canada coincided with and may have resulted from folic acid fortification (5). Thus, folic acid fortification is a highly successful public health intervention for its intended purpose, and it may have had additional benefits.
However, some concern has been expressed about folic acid fortification. Geometric mean serum folate concentrations have increased from ≈12 to ≈30 nmol/L in the United States (6), with some of the serum folate now detectable as unmetabolized folic acid (7). Moreover, the percentage of vitamin supplement users in the United States who are consuming an amount of folic acid above the upper tolerable limit of 1 mg/d has increased from ≈1% to ≈11% (8). Folic acid is an unsubstituted and oxidized form of folate that is not generally found in natural food sources. It is therefore possible that high intake of this unnatural compound could have unintended consequences, affecting at least segments of the population. Areas of concern, based on biochemical and physiologic considerations and epidemiologic association studies, include potential promotion of tumor progression (9–12), inhibition of natural killer cell activity (7), and alterations in epigenetic programming in utero (13, 14).
An area of particular concern is the potential for folic acid to mask or exacerbate vitamin B-12 deficiency. Severe vitamin B-12 deficiency, such as that caused by pernicious anemia, an autoimmune disorder in which the physiologic mechanism of vitamin B-12 absorption is impaired, is characterized by macrocytic anemia and neurologic disturbances (15). On the basis of early case reports of patients with pernicious anemia who were treated with folic acid rather than with vitamin B-12, it has been observed that folic acid will partially or temporarily alleviate the macrocytic anemia of vitamin B-12 deficiency but will allow the neurologic disturbances to progress unabated. Some have suggested that folic acid may even precipitate or exacerbate the neurologic symptoms (16, 17), although experimental data from animal and human studies in support of this notion are limited.
Recent cross-sectional association studies have reinvigorated interest in this issue. With the use of data collected on older adults from 1999 to 2002 in the National Health and Nutrition Examination Survey (NHANES), Morris et al (18) found that the odds ratios for both cognitive impairment and anemia were higher for subjects with the combination of low vitamin B-12 status and high serum folate compared with the combination of low vitamin B-12 and normal but not elevated folate. In a follow-up study, again using NHANES data, Selhub et al (19) found that the combination of low vitamin B-12 and high folate was also associated with higher homocysteine and methylmalonic acid concentrations than the combination of low vitamin B-12 and nonelevated folate. From these data, it was concluded that excess folate may exacerbate vitamin B-12 deficiency both clinically and metabolically. These controversial findings remain to be confirmed in other populations.
In the present study, we investigated associations between the combination of low vitamin B-12 and high folate and the metabolic indicators of vitamin B-12 status, homocysteine, and methylmalonic acid, in a representative population of community-dwelling older Latinos. We also assessed associations with holotranscobalamin and the ratio of holotranscobalamin to total plasma vitamin B-12 (holotranscobalamin:vitamin B-12), as well as associations with tests of cognitive function and depressive symptoms. For folate status, we report on red cell folate as well as serum folate. Measures of hematologic status in the study sample were not available.
SUBJECTS AND METHODS
Subjects
Data analyzed for this study came from the Sacramento Area Latino Study on Aging (SALSA), a cohort study of physical and cognitive functioning in community-dwelling elderly Latinos (n = 1789; age ≥ 60 y) (20–25). Baseline recruitment occurred between February 1998 and August 1999—ie, after the start of mandatory folic acid fortification of cereals and grains in the United States. For this study, subjects were considered “Latino” if they, their parents, or their grandparents were born in Mexico, Central America, or South America. The details of sampling and recruitment are described elsewhere (20, 21). Of the original 1789 subjects, 1535 had valid measurements of both plasma vitamin B-12 and plasma folate and are included in the present study. The University of California Davis Institutional Review Board approved the study, and all subjects provided written informed consent.
Sample collection and analyses
Fasting blood samples were collected from all subjects during home visits. The blood was transported on ice to the University of California Davis Medical Center Clinical Laboratory for processing within 4 h of collection. Plasma and serum were isolated and stored at −80°C until analysis. Total plasma vitamin B-12 and plasma folate were measured by radioligand binding assay (Bio-Rad Diagnostics, Hercules, CA); red blood cell (RBC) folate was measured by using automated chemiluminescence [ACS 180; Chiron Diagnostics (now Siemens Healthcare Diagnostics), Tarrytown, NY]; plasma holotranscobalamin was measured by monoclonal antibody capture of the holotranscobalamin followed by radioligand binding (Axis-Shield, Oslo, Norway) (26); total plasma homocysteine was measured by HPLC with fluorescence detection (27); plasma methylmalonic acid was measured by liquid chromatography-tandem mass spectrometry at ARUP Laboratories, Salt Lake City, UT (28); and serum creatinine was measured by the Jaffe rate reaction method using a SYNCHRON LX20 instrument (Beckman Coulter, Fullerton, CA). The holotranscobalamin:vitamin B-12 was calculated and expressed as a percentage. The cutoff value for low plasma vitamin B-12 was defined as 148 pmol/L (standard clinical reference value), and the cutoff for elevated plasma folate was defined as 45.3 nmol/L (the upper limit of the standard curve for the assay). Subjects with plasma folate above the standard curve of the assay were nominally defined as >45.3 nmol/L.
Neuropsychological and depressive symptom assessments
The Modified Mini-Mental State Examination (3MSE) (29) was used to assess overall or global cognitive function. The 3MSE evaluates memory, orientation, attention, and language on a scale of 0–100 points. A 3MSE score ≤78 is indicative of cognitive impairment (21). The ability to learn and recall verbal information was assessed on a 0–15-point scale with the use of a delayed recall test (30). A score ≤6 on the delayed recall test is indicative of cognitive impairment (21). Subjects were given the choice of taking the neuropsychological tests in English or Spanish. Depressive symptoms were assessed on a scale of 0–60 points with the use of the Center for Epidemiologic Studies Depression scale (31). A score on the Center for Epidemiologic Studies Depression scale ≥16 is indicative of elevated depressive symptoms (31).
Vitamin supplement use
Vitamin use was assessed through an inventory carried out during home visits. All nutritional supplements were coded according to the Centers for Disease Control and Prevention National Center for Health Statistics Ambulatory Care Drug Database System (http://www.cdc.gov/nchs/about/major/ahcd/ambulatory.htm) in which nutritional supplements are coded as “Group 0913—Vitamins/Minerals.” The list of ingredients was then reviewed to classify each supplement for the presence of B vitamins. Vitamins were coded as “multivitamin with B vitamins,” “only B vitamins,” or “other vitamins.”
Statistical analysis
Subjects were divided into 4 groups on the basis of low or nonlow total plasma vitamin B-12 (< or ≥148 pmol/L) and nonelevated or elevated plasma folate (≤ or >45.3 nmol/L) concentrations. A Scheffe test was used to compare mean values among the groups for age, all blood analytes, both cognitive function scores, and depressive symptom score. Chi-square analysis was used to compare sex distributions, percentage of supplement users, percentage with low cognitive function scores, and percentage with elevated depressive symptoms among the groups. Interactions between total plasma vitamin B-12 (low and nonlow) and plasma folate (nonelevated and elevated) on homocysteine, methylmalonic acid, holotranscobalamin, holotranscobalamin:vitamin B-12, and RBC folate were assessed by 2-factor analysis of variance. Because the values for homocysteine, methylmalonic acid, holotranscobalamin, holotranscobalamin:vitamin B-12, and RBC folate were not normally distributed (ie, there was tailing toward higher values), these variables were natural log-transformed before analysis, and geometric means with 95% CIs are presented. For the blood analytes, cognitive function scores, and depressive symptom scores, the statistical analyses included control for potential confounding by age, sex, education, supplement use, and creatinine. The statistical analyses were carried out with the use of STATVIEW for Macintosh and Windows (version 5.0.1; Abacus Concept, Berkeley, CA). Statistical significance was defined as P < 0.05.
RESULTS
Characteristics of the study sample, divided by low and nonlow plasma vitamin B-12 and nonelevated and elevated plasma folate, are presented in Table 1. For subjects with low vitamin B-12, no significant differences were observed between those with nonelevated folate and those with elevated folate in sex distribution, age, vitamin B-12, creatinine, cognitive function scores, percentage with low cognitive function scores, depressive symptom scores, and percentage with elevated depressive symptom scores. A higher percentage of subjects in the low vitamin B-12/elevated-folate group was taking a supplement containing folate or vitamin B-12 or both (alone or in a multivitamin) compared with the low vitamin B-12/non-elevated-folate group (28.6% vs 7.7%, respectively). The non–low vitamin B-12/elevated-folate group had the highest percentage of B vitamin supplement use (53.7%). In addition, non–low vitamin B-12/elevated-folate subjects were more likely to be women, were older, had higher plasma vitamin B-12, and had a higher global cognitive function score than did those with nonelevated folate. Controlling for confounding by age, sex, education, supplement use, and creatinine did not significantly affect any of these findings.
TABLE 1.
Sacramento Area Latino Study on Aging (SALSA) sample grouped by plasma vitamin B-12 and folate concentrations1
| Vitamin B-12 < 148 pmol/L |
Vitamin B-12 ≥ 148 pmol/L |
|||
| Folate ≤ 45.3 nmol/L (n = 78) | Folate > 45.3 nmol/L (n = 22) | Folate ≤ 45.3 nmol/L (n = 1055) | Folate > 45.3 nmol/L (n = 380) | |
| Sex (% female) | 53 | 45 | 56 | 6423 |
| Age (y) | 74 ± 8.04 | 72 ± 6.2 | 70 ± 6.82 | 72 ± 7.023 |
| Vitamin B-12 (pmol/L) | 106 ± 30 | 93 ± 34 | 344 ± 17025 | 386 ± 157235 |
| Folate (nmol/L) | 25.4 ± 9.3 | >45.323 | 26.7 ± 9.35 | >45.323 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.5 | 0.9 ± 0.6 |
| 3MSE6 | 83 ± 15 | 88 ± 9 | 84 ± 15 | 87 ± 133 |
| 3MSE score ≤78 (%) | 23.4 | 14.3 | 21.3 | 13.33 |
| Delayed recall test7 | 7.7 ± 3.5 | 8.6 ± 2.8 | 8.4 ± 3.2 | 8.6 ± 3.1 |
| Delayed recall score ≤6 (%) | 23.7 | 15.0 | 17.2 | 13.8 |
| CES-D8 | 8.0 ± 8.7 | 7.4 ± 7.9 | 10.1 ± 10.7 | 9.6 ± 10.6 |
| CES-D score ≥16 (%) | 16.4 | 9.5 | 26.9 | 23.4 |
| Supplement users (%)9 | 7.7 | 28.62 | 20.92 | 53.7235 |
3MSE, Modified Mini-Mental State Examination; CES-D, Center for Epidemiologic Studies Depression Scale. Interactive effects of vitamin B-12 (low or nonlow) and folate (nonelevated or elevated) were assessed by 2-factor ANOVA. No significant interactions were observed. Group values were compared by using either a Scheffe test (means) or chi-square test (percentages).
Significantly different from low vitamin B-12/non-elevated-folate group, P ≤ 0.05.
Significantly different from non–low vitamin B-12/non-elevated-folate group, P ≤ 0.02.
Mean ± SD (all such values).
Significantly different from low vitamin B-12/elevated-folate group, P ≤ 0.02.
Assessed on a scale of 0–100 points, with a score ≤78 being indicative of cognitive impairment.
Assessed on 0–15-point scale, with a score ≤6 being indicative of cognitive impairment.
Assessed on a scale of 0–60 points, with a score ≥16 being indicative of elevated depressive symptoms.
Participants taking any supplements containing folic acid or vitamin B-12, either alone or included in multivitamins.
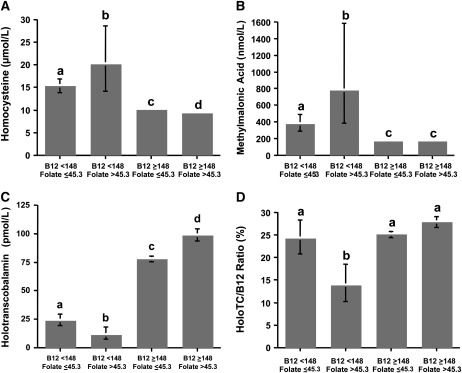
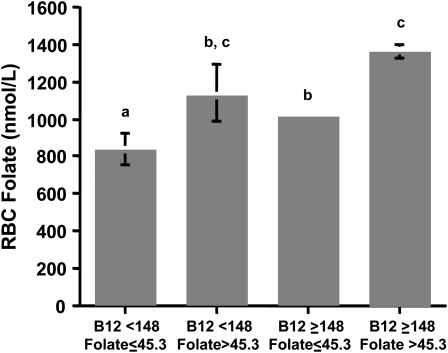
With respect to metabolic indicators of vitamin B-12 status, significant interactions between vitamin B-12 and folate, grouped as low or nonlow and as nonelevated or elevated, respectively, were observed for homocysteine, methylmalonic acid, holotranscobalamin, and holotranscobalamin:vitamin B-12 (P < 0.001). Geometric mean values for the 4 groups are compared in Figure 1. Regardless of folate concentration, homocysteine and methylmalonic acid concentrations were higher, and holotranscobalamin concentration was lower in those subjects with low vitamin B-12 than in those with nonlow vitamin B-12 (P < 0.001). However, the highest values for both homocysteine (P ≤ 0.003) and methylmalonic acid (P < 0.001) and the lowest value for holotranscobalamin (P < 0.001) were observed in the group with low vitamin B-12 and elevated folate. The holotranscobalamin:vitamin B-12 also was lowest in the group with low vitamin B-12 and elevated folate, whereas the ratio was not different among the other 3 groups. The geometric mean values for RBC folate concentrations among the 4 groups are shown in Figure 2. RBC folate was higher in those subjects with elevated plasma folate regardless of vitamin B-12 concentration. Controlling for confounding by age, sex, education, supplement use, and creatinine did not significantly affect any of these findings.
FIGURE 1.
Indicators of vitamin B-12 status in elderly Latinos. Subjects were grouped by low and nonlow plasma vitamin B-12 (< or ≥148 pmol/L) and nonelevated and elevated plasma folate (≤ or >45.3 nmol/L). Bars represent geometric means with 95% CIs for homocysteine (A), methylmalonic acid (B), holotranscobalamin (C), and the ratio of holotranscobalamin to total vitamin B-12 (holoTC/B12 ratio) (D). Sample sizes for each group are as follows: low vitamin B-12/nonelevated folate, n = 78; low vitamin B-12/elevated folate, n = 22; nonlow vitamin B-12/nonelevated folate, n = 1055; nonlow vitamin B-12/elevated folate, n = 380. Interactive effects of vitamin B-12 (low or nonlow) and folate (nonelevated or elevated) for all 4 dependent variables were detected, P < 0.001 (2-factor ANOVA). Differences between the groups were assessed by Scheffe test, controlled for age, sex, education, supplement use, and creatinine. Different letters represent significantly different values, P ≤ 0.001.
FIGURE 2.
Geometric mean (with 95% CI) red blood cell (RBC) folate concentrations in elderly Latinos. Subjects were grouped by low and nonlow plasma vitamin B-12 (< or ≥148 pmol/L) and nonelevated and elevated plasma folate (≤ or >45.3 nmol/L). Sample sizes for each group are as follows: low vitamin B-12/nonelevated folate, n = 78; low vitamin B-12/elevated folate, n = 22; nonlow vitamin B-12/nonelevated folate, n = 1055; nonlow vitamin B-12/elevated folate, n = 380. No interactive effect of vitamin B-12 (low or nonlow) and folate (nonelevated or elevated) for RBC folate was detected (2-factor ANOVA). Differences among the groups were assessed by Scheffe test, controlled for age, sex, education, supplement use, and creatinine. Different letters represent significantly different values, P ≤ 0.001.
DISCUSSION
In the SALSA study population, which has been exposed to folic acid fortification, subjects with the combination of low vitamin B-12 and elevated folate had higher homocysteine and methylmalonic acid concentrations than did those with low vitamin B-12 and nonelevated folate. This is consistent with NHANES data previously reported (19). In addition, both holotranscobalamin and holotranscobalamin:total vitamin B-12 were lower in the low vitamin B-12/elevated-folate group than in the low vitamin B-12/non-elevated-folate group, an observation that has not been reported previously. In contrast to NHANES analyses (18), we did not observe significant differences in cognitive function scores between these groups, nor did we see differences in depressive symptom scores (not assessed in the NHANES study). Thus, we confirm that metabolic evidence of vitamin B-12 deficiency is more pronounced in older individuals when plasma folate is elevated, but we do not confirm an association with cognitive function.
The primary limitation of this study, as well as the NHANES study (18, 19), is that it is cross-sectional in design, and as such it is not possible to discern a cause-and-effect relation between elevated folate and the metabolic indicators of vitamin B-12 status. Nonetheless, Selhub et al (19) have put forth the hypothesis that excess folic acid and some of its metabolites serve as electron acceptors (ie, oxidizers) during their metabolism, which may exacerbate vitamin B-12 deficiency by causing the irreversible oxidation of intracellular vitamin B-12 in a manner analogous to that caused by exposure to nitrous oxide (32). Such oxidation of vitamin B-12 by nitrous oxide causes a primary impairment of methionine synthase, the vitamin B-12–dependent enzyme that catalyzes the conversion of homocysteine to methionine with the use of methyltetrahydrofolate as the methyl donor. The irreversible oxidation of the vitamin B-12 cofactor associated with methionine synthase ultimately leads to reduced availability of vitamin B-12 as a cofactor for the mitochondrial reaction in which methylmalonyl-coenzyme A (CoA) mutase catalyzes the rearrangement of methylmalonyl-CoA to succinyl-CoA. As a consequence, both vitamin B-12–dependent reactions are compromised, vitamin B-12 deficiency is precipitated or exacerbated, homocysteine and methylmalonic acid accumulate, and hematologic and neurologic consequences ensue (15). Whether folic acid has the same oxidative effect on vitamin B-12 as does nitrous oxide awaits rigorous experimental testing.
The novel findings in the present study are the observed differences in holotranscobalamin and holotranscobalamin:total vitamin B-12 among the groups. Holotranscobalamin is the fraction (typically 20–30%) of total plasma vitamin B-12 that is available for delivery of vitamin B-12 to all cells (15). The remainder and majority of plasma vitamin B-12 is bound to a second vitamin B-12 transport protein, haptocorrin, which is taken up nonspecifically by the liver. Holotranscobalamin has therefore received significant attention in recent years as a potential alternative measure of vitamin B-12 status (15, 24–26). The finding that the lowest holotranscobalamin and the lowest holotranscobalamin:total vitamin B-12 are observed in the low vitamin B-12/elevated-folate group indicates that the metabolic associations observed by Selhub et al (19) may extend beyond intracellular metabolism of vitamin B-12 (as reflected by homocysteine and methylmalonic acid concentrations) to relative availability and delivery of vitamin B-12 to the tissues.
It must be noted that 2 additional explanations for the associations observed in the NHANES and SALSA studies have been put forward that do not invoke direct effects of folic acid on vitamin B-12 concentrations or delivery to tissues. First, Berry et al (33) suggested that the criteria by which subjects are classified as low vitamin B-12 with nonelevated folate, or low vitamin B-12 with elevated folate, artificially places individuals with more severe vitamin B-12 deficiency in the low vitamin B-12/elevated-folate group. The reasoning is that these are individuals, by virtue of their high plasma folate concentrations, who presumably consume multivitamins and ready-to-eat cereals that contain both folic acid and vitamin B-12. That they have low vitamin B-12 status may indicate that they are the individuals within the study samples who have unrecognized vitamin B-12 malabsorption (eg, pernicious anemia) and thus more severe vitamin B-12 deficiency than their counterparts in the low vitamin B-12/non-elevated-folate group. Consistent with this hypothesis is our observation that the low vitamin B-12–elevated-folate group had a higher percentage of subjects taking B vitamin–containing supplements than the low vitamin B-12/non-elevated-folate group. Also potentially consistent with this hypothesis are the lower holotranscobalamin values observed in the low vitamin B-12–elevated-folate group. It has been suggested that low holotranscobalamin concentrations reflect malabsorption of vitamin B-12 (34, 35). However, we found no difference in total plasma vitamin B-12 between the low vitamin B-12/non-elevated-folate and low vitamin B-12/elevated-folate groups. One would expect that among individuals with low plasma vitamin B-12, mean vitamin B-12 concentrations would be significantly lower in individuals with pernicious anemia than in individuals who have other causes of low vitamin B-12 status. However, we do not have data on ready-to-eat cereals or overall dietary folate and vitamin B-12 intakes, concentrations of folic acid and vitamin B-12 in the reported supplements, or absorption or other indicators of pernicious anemia in the SALSA population. We therefore cannot rule out this alternative hypothesis.
The second alternative explanation for the NHANES and SALSA data has been put forward by Quinlivan (36) who invokes the so-called methyl-trap hypothesis (37). In folate metabolism, the conversion of methylenetetrahydrofolate to methyltetrahydrofolate, catalyzed by methylenetetrahydrofolate reductase, is an irreversible reaction under physiologic conditions. Because methyltetrahydrofolate is the methyl donor for the vitamin B-12–dependent conversion of homocysteine to methionine, it will accumulate within the cell under conditions of vitamin B-12 deficiency because it cannot be converted to tetrahydrofolate or revert back to methylenetetrahydrofolate. Tetrahydrofolate is an obligate precursor for intracellular polyglutamation of folate, a modification to the folate molecule that results in its retention within the cell. In vitamin B-12 deficiency, folate not only accumulates as methyltetrahydrofolate, but it also remains in the monoglutamate form, because it is not converted to the tetrahydrofolate form necessary for polyglutamate synthesis. The monoglutamate can more easily pass out of the cell back into the circulation. Thus, in vitamin B-12 deficiency, an elevation in plasma folate with a concomitant fall in RBC folate often occurs because of a lack of intracellular retention of the vitamin (38, 39). Arguing against the proposition that our low vitamin B-12/elevated-folate group simply selected for individuals with more severe vitamin B-12 deficiency is our finding that RBC folate concentrations were actually significantly higher in the low vitamin B-12–elevated plasma folate group than in the low vitamin B-12–nonelevated plasma folate group. This is consistent with a higher folate intake in the low vitamin B-12/elevated-folate group and is not consistent with the explanation that implicates the methyl-folate trap.
No significant differences were observed in cognitive function scores between the low vitamin B-12/non-elevated-folate and low vitamin B-12/elevated-folate groups in the SALSA study sample. Many factors affect cognitive function in older adults, including Alzheimer's disease, vascular disease, diabetes, depression, and genetics (eg, apolipoprotein E genotype). These conditions are highly prevalent in the SALSA population (20–24). Coupled with the fact that the low vitamin B-12/elevated-folate group consisted of only 22 subjects, our power to see associations with cognitive function scores may have been inadequate. Moreover, cognitive function consists of various domains that could be differentially affected by different factors. In the NHANES study, cognitive function was evaluated with the use of the Digit Symbol-Coding subtest of the Wechsler Adult Intelligence Scale III (18, 40). This test assesses response speed, sustained attention, visual spatial skills, associative learning, and memory, and it is purported to be a more sensitive measure of dementia than is the 3MSE (18). In the present study, we used the Modified Mini-Mental State Examination (29) and delayed recall (30). These tests may have limited sensitivity for the effects of vitamin B-12 deficiency on cognitive function.
In conclusion, cross-sectional observations of more pronounced metabolic evidence of vitamin B-12 deficiency in individuals with elevated plasma folate compared with nonelevated plasma folate have now been observed in 2 distinct study samples of older adults exposed to folic acid fortification. The mechanistic nature of this association remains to be determined, as do the ramifications, if any, for the safety of current and future folic acid fortification programs, including proposals to increase the current fortification and the widespread practice of adding folic acid supplements to breakfast cereals and other foods. We note that in the SALSA study sample, only 28.6% and 53.7% of the subjects with high plasma folate in the low vitamin B-12 and nonlow vitamin B-12 groups, respectively, were taking folic acid-containing supplements. This indicates that high concentrations of folate can be achieved from natural and fortified food sources without additional supplementation in pill form. These observations should be considered by nutrition and health professionals when making recommendations at the individual and population levels about the optimum and maximum quantities of folate used as supplements and for fortification. These data also should be evaluated in relation to current proposals to fortify staple foods with vitamin B-12.
Acknowledgments
We thank Teresa Ortiz and the staff of the SALSA study for subject recruitment, phlebotomy, data collection, and data management. We thank Rebecca Cotterman and the UC Davis Clinical Laboratory for blood sample processing and biochemical assessments.
The authors' responsibilities were as follows—JWM: participated in the concept and design of the study, supervised blood processing and biochemical analyses, performed the statistical analysis, participated in the interpretation of the data, and was responsible for drafting the article; MGG: participated in the statistical analysis and interpretation of the data and provided input into the final draft of the article; LHA (principal investigator of USDA grant 00-35200-9073): participated in the concept and design of the study and provided input into the final draft of the article; MNH (principal investigator of the SALSA, NIH grant AG12975): participated in the concept and design of the study; was responsible for recruitment of study subjects, acquisition of blood samples, data collection, and data management; participated in the statistical analysis and interpretation of the data; and provided input into the final draft of the article; and RG: participated in the concept and design of the study and interpretation of data and provided input into the final draft of the article. None of the authors had a conflict of interest.
REFERENCES
- 1.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LYC. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001;285:2981–6 [DOI] [PubMed] [Google Scholar]
- 2.De Wals P, Tairou F, Van Allen MI, et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med 2007;357:135–42 [DOI] [PubMed] [Google Scholar]
- 3.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med 1999;340:1449–54 [DOI] [PubMed] [Google Scholar]
- 4.Choumenkovitch SF, Jacques PF, Nadeau MR, Wilson PW, Rosenberg IH, Selhub J. Folic acid fortification increases red blood cell folate concentrations in the Framingham study. J Nutr 2001;131:3277–80 [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Botto LD, Erickson JD, et al. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation 2006;113:1335–43 [DOI] [PubMed] [Google Scholar]
- 6.Ganji V, Kafai MR. Trends in serum folate, RBC folate, and circulating total homocysteine concentrations in the United States: analysis of data from National Health and Nutrition Examination surveys, 1988-1994, 1999-2000, and 2001-2002. J Nutr 2006;136:153–8 [DOI] [PubMed] [Google Scholar]
- 7.Troen AM, Mitchell B, Sorensen B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr 2006;136:189–94 [DOI] [PubMed] [Google Scholar]
- 8.Choumenkovitch SF, Selhub J, Wilson PWF, Rader JI, Rosenberg IH, Jacques PF. Folic acid intake from fortification in United States exceeds predictions. J Nutr 2002;132:2792–8 [DOI] [PubMed] [Google Scholar]
- 9.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr 2004;80:1123–8 [DOI] [PubMed] [Google Scholar]
- 10.Mason JB, Dickstein A, Jacques PF, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev 2007;16:1325–9 [DOI] [PubMed] [Google Scholar]
- 11.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007;297:2351–9 [DOI] [PubMed] [Google Scholar]
- 12.Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, et al. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr 2006;83:895–904 [DOI] [PubMed] [Google Scholar]
- 13.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol 2003;23:5293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yajnik CS, Deshpande SS, Jackson AA, et al. Vitamin B(12) and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia 2008;51:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green R, Miller JW. Vitamin B12. : Zempleni J, Rucker RB, McCormick DB, Suttie JW, Handbook of vitamins. 4th ed Boca Raton, FL: Taylor and Francis, 2007 [Google Scholar]
- 16.Chodos RB, Ross JF. The effects of combined folic acid and liver extract therapy. Blood 1951;6:1213–33 [PubMed] [Google Scholar]
- 17.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol 2006;5:949–60 [DOI] [PubMed] [Google Scholar]
- 18.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr 2007;85:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selhub J, Morris MS, Jacques PF. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci U S A 2007;104:19995–20000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CC, Mungas D, Petkov CI, et al. Brain structure and cognition in a community sample of elderly Latinos. Neurology 2002;59:383–91 [DOI] [PubMed] [Google Scholar]
- 21.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc 2003;51:169–77 [DOI] [PubMed] [Google Scholar]
- 22.Miller JW, Green R, Ramos MI, et al. Homocysteine and cognitive function in the Sacramento Area Latino Study on Aging. Am J Clin Nutr 2003;78:441–7 [DOI] [PubMed] [Google Scholar]
- 23.Ramos MI, Allen LH, Mungas DM, et al. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr 2005;82:1346–52 [DOI] [PubMed] [Google Scholar]
- 24.Garrod MG, Green R, Allen LH, et al. Fraction of total plasma vitamin B12 bound to transcobalamin correlates with cognitive function in elderly Latinos with depressive symptoms. Clin Chem 2008;54:1210–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JW, Garrod MG, Rockwood AL, et al. Measurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiency. Clin Chem 2006;52:278–85 [DOI] [PubMed] [Google Scholar]
- 26.Ulleland M, Eilertsen I, Quadros EV, et al. Direct assay for cobalamin bound to transcobalamin (holo-transcobalamin) in serum. Clin Chem 2002;48:526–32 [PubMed] [Google Scholar]
- 27.Gilfix BM, Blank DW, Rosenblatt DS. Novel reductant for determination of total plasma homocysteine. Clin Chem 1997;43:687–8 [PubMed] [Google Scholar]
- 28.Kushnir MM, Komaromy-Hiller G, Shushan B, Urry FM, Roberts WL. Analysis of dicarboxylic acids by tandem mass spectrometry: high-throughput quantitative measurement of methylmalonic acid in serum, plasma, and urine. Clin Chem 2001;47:1993–2002 [PubMed] [Google Scholar]
- 29.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–8 [PubMed] [Google Scholar]
- 30.Mungas D, Reed BR, Marshall SC, Gonzalez HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology 2000;14:209–23 [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 32.Drummond JT, Matthews RG. Nitrous oxide inactivation of cobalamin-dependent methionine synthase from Escherichia coli: characterization of the damage to the enzyme and prosthetic group. Biochemistry 1994;33:3742–50 [DOI] [PubMed] [Google Scholar]
- 33.Berry RJ, Carter HK, Yang Q. Cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr 2007;86:265–7 [DOI] [PubMed] [Google Scholar]
- 34.Lindgren A, Kilander A, Bagge E, Nexo E. Holotranscobalamin - a sensitive marker of cobalamin malabsorption. Eur J Clin Invest 1999;29:321–9 [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Remacha AF, Sarda MP, Carmel R. Influence of cobalamin deficiency compared with that of cobalamin absorption on serum holo-transcobalamin II. Am J Clin Nutr 2005;81:110–4 [DOI] [PubMed] [Google Scholar]
- 36.Quinlivan EP. In vitamin B12 deficiency, higher serum folate is associated with increased homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci U S A 2008;105:E7.(letter) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbert V, Zalusky R. Interrelations of vitamin B12 and folic acid metabolism: folic acid clearance studies. J Clin Invest 1962;41:1263–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavoie A, Tripp E, Hoffbrand AV. The effect of vitamin B12 deficiency on methylfolate metabolism and pterylpolyglutamate synthesis in human cells. Clin Sci Mol Med 1974;47:617–30 [DOI] [PubMed] [Google Scholar]
- 39.Stokstad ELR, Reisenauer A, Kusano G, Keating JN. Effect of high levels of dietary folate on folate metabolism in vitamin B12 deficiency. Arch Biochem Biophys 1988;265:407–14 [DOI] [PubMed] [Google Scholar]
- 40.Wechsler D. Wechsler Adult Intelligence Scale–III. San Antonio, TX: The Psychological Corporation, 1997 [Google Scholar]