Abstract
Background: The nitrogen isotope ratio (expressed as δ15N) of red blood cells (RBCs) is highly correlated with the RBC long-chain ω−3 (n−3) fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in Yup'ik Eskimos. Because δ15N can also be measured in hair samples, it could provide a noninvasive, retrospective biomarker for EPA and DHA intakes.
Objectives: We investigated the agreement between δ15N in hair and RBCs and then evaluated the relations between hair δ15N and RBC EPA and DHA. We also assessed the agreement in carbon isotope ratios (δ13C) between hair and RBCs, because δ13C has been proposed as a dietary biomarker in other populations.
Design: We assessed relations between hair and RBC δ15N and δ13C in a community-based sample of 144 Yup'ik Eskimos and examined the correlations between δ15N and RBC EPA and DHA in a subset of these participants (n = 44).
Results: We showed a 1:1 relation with good agreement between hair and RBC δ15N (r = 0.91) and δ13C (r = 0.87). Hair isotope ratios were greater than RBC ratios by 1.5‰ for δ15N and by 2.3‰ for δ13C. There were strong correlations between hair δ15N and RBC EPA and DHA (r = 0.83 and 0.84, respectively).
Conclusions: These results support the use of hair δ15N values as a biomarker of EPA and DHA intakes. Because hair collection is noninvasive and the samples require no special processing, studies of EPA and DHA intakes in large populations could use biomarkers rather than self-reports to assess these fatty acids.
INTRODUCTION
Naturally occurring variations in stable-isotope ratios are gaining attention for their potential to serve as unbiased biomarkers of diet (1–4). Recently, the nitrogen isotope ratio (expressed as δ15N) of red blood cells (RBCs) was shown to be highly correlated with RBC omega-3 (n−3) fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and validated as a biomarker of EPA and DHA intake in Yup'ik Eskimos (4). δ15N acts as a biomarker for EPA and DHA in this population because all 3 markers are elevated in marine subsistence foods (3), which are an important component of the traditional diet (5). Because measurement of RBC δ15N is inexpensive and highly accurate, it is ideal for large-scale epidemiological studies of EPA and DHA intakes and disease in Alaska Natives. However, sampling blood is invasive and processing and storing samples is expensive. Here we investigate whether δ15N in hair is equally valid as a biomarker of RBC EPA and DHA, thus allowing measurement to be entirely noninvasive. Isotopic markers in hair have an additional advantage over blood; because hair is grown continuously, it provides a continuous record of the biomarker back through time. This could allow analysis of seasonal or annual dietary change.
Stable isotope ratios of nitrogen (δ15N) and carbon (δ13C) have also been used as markers of animal protein intake (1, 6, 7) and traditional food intake in Greenland Eskimos (3), Amazonian Indians (8), and Gidra-speaking Papuans (9). In these studies, isotope ratios were measured in either hair or fingernails, and the isotopic relation between these tissues is well understood (7, 10). However, several recent studies report stable isotope ratios in blood only (4, 11, 12), and the relation between isotope ratios in human blood and hair has not been characterized. An understanding of how isotopic markers are related in blood and hair will facilitate comparison among these and future studies, especially where investigations take advantage of blood collected from clinical or epidemiological studies (11). Although we investigate both δ15N and δ13C here, we note that δ13C is not related to either animal protein intake or intake of marine foods in our population (4).
Here we examine the relation and agreement between stable isotope ratios (δ15N and δ13C) in hair and RBCs from 144 participants in the Center for Alaska Native Health Research I (CANHR I) study (13). We also investigate the relation between hair δ15N and RBC EPA and DHA in a subset of 44 participants. RBC EPA and DHA vary with EPA and DHA intakes and are validated biomarkers for these important ω−3 fatty acids (14–16). The CANHR I study population is ideal for testing the relation between hair δ15N and RBC EPA and DHA, because participants have widely varying levels of EPA and DHA intakes, depending on the degree to which individuals adhere to a traditional, marine-based diet (5, 17).
SUBJECTS AND METHODS
The CANHR population
Data are from the Center for Alaska Native Health Research I (CANHR I) study, a cross-sectional, community-based participatory research study of biological, genetic, nutritional, and psychosocial risk factors for obesity and related disease in Yup'ik Eskimos. Between 2003 and 2005, 1003 men and women aged ≥14 y were recruited from 10 communities in Southwest Alaska, as described in detail elsewhere (13, 18). At entry into the study, participants completed an extensive interviewer-administered questionnaire covering demographic characteristics, economic status, ethnicity, and medical history. Diet interviews, body measurements, blood pressure, and biological samples were also collected. The CANHR study was approved by the University of Alaska Institutional Review Board, the National and Area Indian Health Service Institutional Review Board, and the Yukon-Kuskokwim Health Corporation Human Studies Committee.
Study sample
Our study sample was drawn from the last 3 communities to participate in the CANHR I study, because these were the only CANHR I participants from whom both hair and blood samples were available. Of 210 participants from these 3 communities, 144 were included in this study based on having hair >3 cm in length. Blood samples were available for all 144 of these participants. Analyses of RBC fatty acids were conducted for study participants from the first of these 3 communities (n = 44). Comparisons between hair δ15N and RBC EPA and DHA were based on that sample.
Specimen collection
Blood samples were collected into 10-mL K3 EDTA-treated evacuated whole-blood tubes (15% solution, 0.117 mL, 17.55 mg) and centrifuged for 15 minutes at 1000 rpm. The RBC portion was frozen at −12°C, transported to the University of Alaska Fairbanks, and placed in an ultralow freezer at −80°C. Aliquots were removed for both stable isotope and fatty acid analysis (see below).
Three hairs were collected by either pulling or cutting 3 hairs from the back of the head, and most participants elected to pull their own hair samples. Samples were taped with the follicle end labeled and stored in plastic bags. The follicle was removed with a razor blade, and the 2 cm of hair closest to the scalp was selected for analysis. The length of the hair sample was chosen to correspond with the average age of RBCs in the blood, and therefore the dietary inputs recorded by the RBC. Hair grows at a rate of ≈1 cm/mo (19–21); thus, our samples were expected to be reflective of the last 2 mo of intake. RBCs have a lifespan of ≈90–120 d (22–24) and a mean age of ≈50 d (23). Therefore, the hair sample bracketed the time when most RBCs were synthesized.
Stable isotope analyses
Aliquots (250 μL) of RBCs were autoclaved for 20 min at 121°C to destroy blood-borne pathogens. Samples were then apportioned into 3.5 × 3.75 mm tin capsules and dried to a final mass of 0.2 to 0.4 mg. Hair samples were cleaned with triplicate 30-min rinses in 2:1 methanol chloroform. Hairs were chopped into small pieces, placed into 11 × 8 mm tin capsules, and oven dried at 50°C for 24 h. The resulting sample masses ranged from 0.1 to 0.6 mg. Capsules were crushed into balls and loaded into an autosampler for isotope analysis.
Blood and hair samples were analyzed for carbon and nitrogen isotope ratios at the Alaska Stable Isotope Facility by using continuous-flow isotope ratio mass spectrometry. A Costech ECS4010 Elemental Analyzer (Costech Scientific Inc, Valencia, CA) combusted samples to carbon dioxide and nitrogen gas, which were carried in a constant flow of helium to a Finnigan Delta Plus XP isotope ratio mass spectrometer via the Conflo III interface (Thermo-Finnigan Inc, Bremen, Germany). Data are presented in the accepted delta notation as δX = (Rsample – Rstandard)/(Rstandard) × 1000‰, where R is the ratio of heavy to light isotope (for both nitrogen and carbon) and the internationally recognized standards are atmospheric nitrogen and Pee Dee Belemnite for carbon (25). We concurrently weighed and ran multiple peptone standards (δ15N = 7.0‰, δ13C = −15.8‰) to assess analytical accuracy and precision; these gave values of δ15N = 7.1 ± 0.3‰ (SD) and δ13C = −15.7 ± 0.2‰ (SD). The purity of the samples was assessed through the molar C:N ratios, which were 3.0 ± 0.1 for hair and 3.3 ± 0.1 for blood. The C:N ratios for hair were consistent with keratin values from other published studies (7, 10, 26). Expected C:N ratios for RBC have not been previously published; however, our values are consistent with those expected for a protein-rich tissue.
RBC fatty acid measurements
The RBC fatty acids were analyzed at the Fred Hutchinson Cancer Research Center in Seattle, WA, as described in detail elsewhere (4). Fatty acids were extracted from RBCs by using modified methods of Rose and Oklander (27). The lipid extract was transesterified and processed according to Lepage and Roy (28). Fatty acid methyl esters (FAMEs) were recovered in hexane, dried under nitrogen (40°C) and redissolved in 100 μL hexane for gas chromatography.
FAMEs were injected in a split mode (1:50) and were separated by using an SP-2560 capillary column (100 m × 0.25 mm × 0.2 μm) (Supelco, Bellefonte, PA) on a Hewlett-Packard, model 5890B gas chromatograph (GC-FID) (now Agilent, Santa Clara, CA). This method allowed the resolution of 46 different membrane fatty acids. The accuracy of the chromatographic system was monitored by using commercial standards (GLC-87, NIH-D, and NIH-F; NU-CHEK, Elysian, MN). The precision of the RBC fatty acids was monitored with repeat analysis of an in-house RBC quality-control pool that was included in each batch of 23 study samples. The CV for EPA (20:5n−3) was 2.7% and for DHA (22:6n−3) was 2.0%. Fatty acid composition is reported as the percentage by weight of total RBC fatty acids.
Statistical analyses
All statistical analyses were performed by using JMP version 8 (SAS Institute, Cary, NC). Differences between sex and age strata were assessed by using analysis of variance models, using the Tukey-Kramer honest significant difference test for individual comparisons. Relations between isotope signatures in hair and blood and between isotope signatures and fatty acid biomarkers were assessed by using correlation analysis (presented as Pearson's r). Agreement between hair and blood isotope ratios was evaluated as the mean and SD of their differences, according to Bland and Altman (29). Where parametric assumptions were met, we tested the effect of age and sex on the relation between δ15N and fatty acids using a factorial linear model and described the slope of the isotopic relations between blood and hair with linear regression. Normality of residuals was confirmed by using the Shapiro-Wilks test, and outliers were identified by using Mahalobnis Distance > 3. A significance level of 0.05 was used throughout analyses.
RESULTS
The demographic characteristics of our study population, including age, sex, and body mass index (BMI) distribution, are shown in Table 1. Because we only collected hair samples when hair was >3 cm in length, women are overrepresented in our study sample (“hair isotope data set”; n = 144) when compared with all CANHR participants from the communities presented here (76% compared with 53%; χ2 = 30.3, P < 0.0001; Table 1). We also present age, sex, and BMI distributions for samples from the single community for which we also measured RBC fatty acids (“fatty acid subsample”; n = 44; Table 1). The sex, age, and BMI distributions did not differ between the hair isotope data set and the fatty acid subsample (P > 0.05 in all cases).
TABLE 1.
Age, sex, and BMI distribution of all participants recruited by the Center for Alaska Native Health Research (CANHR) in the 3 study communities (n = 210), in the set of participants with hair isotopic measurements (n = 144), and in the subsample of participants with fatty acid data (n = 44)1
| All CANHR participants (n = 210)2 | Hair isotope data set (n = 144) | Fatty acid subsample (n = 44) | |
| Age (y) | 37.3 ± 17.73 | 39.4 ± 18.0 | 37.3 ± 17.2 |
| 14–24 y (%) | 17 | 29 | 36 |
| 25–39 y (%) | 43 | 26 | 21 |
| 40–54 y (%) | 29 | 27 | 27 |
| ≥55 y (%) | 11 | 18 | 16 |
| Men (%) | 47 | 24 | 32 |
| Women (%) | 53 | 76 | 68 |
| BMI (%) | |||
| <18.5 kg/m2 | 1 | 1 | — |
| 20–25 kg/m2 | 38 | 35 | 43 |
| 25–30 kg/m2 | 33 | 34 | 25 |
| >30 kg/m2 | 28 | 30 | 32 |
The distribution of BMI, age, and sex is not significantly different between the 2 study subsamples by chi-square test (in all cases, P > 0.05), although both include significantly more women than the recruited study population (P < 0.0001).
From the 3 communities sampled for this study.
Mean ± SD (all such values).
Descriptive statistics for the δ15N and δ13C values of RBC and hair and EPA and DHA in RBCs are provided in Table 2. Mean δ15N and δ13C values did not differ between males and females for either RBC or hair in the hair isotope data set. For the fatty acid subsample, RBC δ13C values were slightly but significantly higher in females than in males (0.4‰; P = 0.006). The mean EPA and DHA values did not differ by sex (EPA, P = 0.77; DHA, P = 0.17 in the fatty acid subsample).
TABLE 2.
Means and distributions of biomarker variables for participants in the hair isotope data set (n = 144) and the fatty acid subsample (n = 44)1
| Hair | Blood | |
| Hair isotope data set | ||
| δ15N (‰) | 10.8 ± 1.9 (8.5) | 9.3 ± 1.7 (7.0) |
| δ13C (‰) | −17.5 ± 0.7 (3.5) | −19.8 ± 0.6 (3.2) |
| Fatty acid subsample | ||
| δ15N (‰) | 10.4 ± 1.8 (7.2) | 9.1 ± 1.7 (6.4) |
| δ13C (‰) | −17.7 ± 0.6 (2.7) | −19.9 ± 0.6 (2.6) |
| EPA (%) | — | 2.7 ± 2.0 (6.3) |
| DHA (%) | — | 6.1 ± 1.6 (5.8) |
All values are means ± SDs; ranges in parentheses. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Relation between hair and blood isotope values
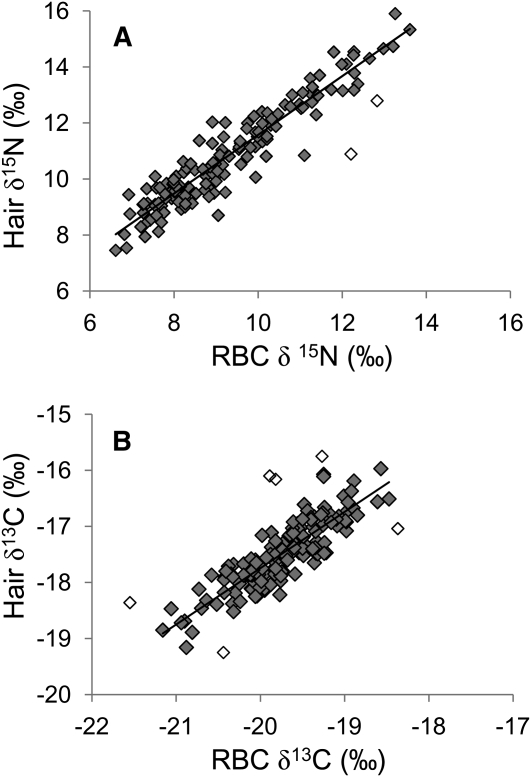
RBC isotope ratios were very strongly correlated with hair isotope ratios (n = 144) for the 2 cm of hair closest to the scalp (r = 0.93 for δ15N and r = 0.81 for δ13C; Table 3; Figure 1). Hair values were consistently elevated over RBCs; however, agreement between the measures was very good once this bias was accounted for (mean difference = 1.5 ± 0.6‰ for δ15N and 2.3 ± 0.4‰ for δ13C). Linear regression of isotope values in hair against blood gave slopes that were not statistically different from 1.0 (slopes = 1.01 for δ15N and 0.96 for δ13C); however, residuals were nonnormal because of the influence of several outliers (2 outliers from the relation between blood and hair δ15N and 6 outliers from the relation between blood and hair δ13C). Removal of these outliers normalized the residuals and altered slopes to 1.05 for δ15N and 1.00 for δ13C. Neither age nor sex had any effect on the relation between isotope ratios in hair and blood, nor were there any significant interactions.
TABLE 3.
Pearson product-moment correlations between isotope values in blood and hair (hair isotope data set; n = 144) and between fatty acids and δ15N values in red blood cells (RBCs) and hair (fatty acid subsample; n = 44)1
| Pearson correlation | Lower 95% CI | Upper 95% CI | |
| Hair isotope data set | |||
| RBC δ15N vs hair δ15N | 0.93 | 0.91 | 0.95 |
| RBC δ13C vs hair δ13C | 0.81 | 0.75 | 0.86 |
| Fatty acid subsample | |||
| RBC δ15N vs hair δ15N | 0.91 | 0.85 | 0.95 |
| EPA vs hair δ15N | 0.83 | 0.71 | 0.91 |
| EPA vs RBC δ15N | 0.92 | 0.87 | 0.96 |
| DHA vs hair δ15N | 0.84 | 0.73 | 0.91 |
| DHA vs RBC δ15N | 0.90 | 0.82 | 0.94 |
EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. P < 0.0001 for all coefficients.
FIGURE 1.
Relations between isotope ratios in red blood cells (RBCs) and hair for δ15N (A) and δ13C (B); n = 144. Open symbols denote samples identified as outliers by using Mahalobnis Distance > 3.0, which were removed for linear regression and calculation of the slope. Nitrogen and carbon isotope ratios are presented in δ notation as δX = (Rsample – Rstandard)/(Rstandard) × 1000‰, where R is the ratio of heavy to light isotope and standards are atmospheric nitrogen and Pee Dee Belemnite for carbon. Carbon isotope ratios are negative, because samples contained less 13C than the standard.
Relation between isotope values and fatty acids
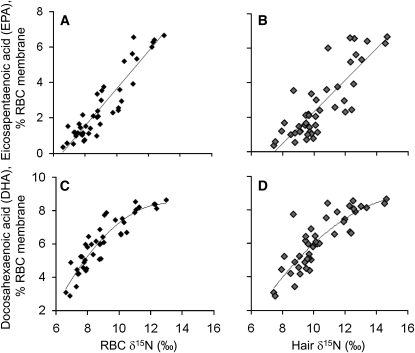
Both hair and RBC δ15N values correlated (n = 44) strongly with the percentage of EPA and DHA in RBCs (all r > 0.8 and P < 0.0001; Table 3). For both fatty acids, the correlation with RBC δ15N was stronger than with hair δ15N; however, the 95% CIs indicate that this difference was only significant for EPA (Table 3). The relation between RBC δ15N and EPA was linear (slope = 1.03, P < 0.0001; Figure 2A) and did not differ when controlled for sex and age. The relation between hair δ15N and EPA was also linear (slope = 0.87, P < 0.0001; Figure 2B) and was significantly stronger in older participants (slope = 0.42 for those aged <40 y and 0.96 for those aged >40 y; Page = 0.04, Page×hair δ15N = 0.001). No other interactions were significant. As previously observed (4), the relation between RBC DHA and hair and RBC δ15N was nonlinear (Figure 2, C and D).
FIGURE 2.
A–D: Red blood cell (RBC) and hair δ15N were significantly correlated with RBC fatty acids eicosapentaenoic acid and docosahexaenoic acid; n = 44. P < 0.0001 for all. Correlation coefficients are given in Table 3.
DISCUSSION
Hair and RBC isotope ratios were tightly correlated in our study population (r = 0.93 for δ15N and 0.81 for δ13C; n = 144), and showed good agreement. Hair δ15N was strongly correlated with RBC polyunsaturated fatty acids EPA and DHA in a subset of these participants (r = 0.83 and 0.84 respectively; n = 44). The relations of EPA and DHA with RBC δ15N were stronger than with hair δ15N, although the difference was only significant for EPA. Because RBC δ15N EPA and DHA are established biomarkers for EPA and DHA intakes (4, 30–33), this demonstrates the validity of hair δ15N to also serve as an intake biomarker for EPA and DHA in this population.
The finding that hair δ15N is significantly correlated with RBC EPA and DHA is an important development for investigators interested in the effect of EPA and DHA intakes on health outcomes, especially in Alaska Natives. Hair can be collected easily and noninvasively, which makes it an ideal tissue to sample for the large-scale epidemiologic studies needed to investigate the health consequences of dietary change in this population. An additional advantage of hair is that it grows continuously and does not remodel after growth; therefore, it provides a dietary record over the length of the participant's hair (≈1 mo/cm of hair). High-resolution sampling along the length of hair provides the opportunity to assess seasonal variation in EPA and DHA intakes, which has not been addressed in this population and which is likely to show important variation. ω-3 Fatty acids, particularly EPA and DHA, have been long suspected to protect against diabetes and other chronic disease in northern indigenous populations (30–33). However, until recently, there have been no longitudinal studies to clearly link diet to disease incidence in Alaska Natives. This biomarker will greatly enhance our ability to detect these associations.
Stable-isotope analysis of hair has been a widely used tool for detecting dietary patterns in anthropologic and archeologic studies (26, 34, 35) and has been increasingly applied to modern populations (1, 3, 6–8, 10, 36, 37). However, specimens collected for medical or epidemiologic studies are likely to include blood but not hair (4, 11). This study was the first to measure isotope ratios in both blood and hair and describes the relation between δ15N and δ13C values in the 2 tissues. For both isotopes, the relation between hair and blood was 1:1, with hair enriched relative to blood by 1.5 ± 0.6‰ for δ15N and 2.3 ± 0.4‰ for δ13C. Animal studies have also shown isotopic enrichment of hair over blood (38–40); however, the magnitude of these enrichments can vary taxonomically. Having this relation determined for a human population provides a basis for comparison between human studies based on one or the other tissue. However, further work is necessary to evaluate how universal these relations are among other human populations.
Although correlations between blood and hair stable-isotope ratios were strong and followed the expected 1:1 relation, we found outliers in both the relations between blood and hair δ15N and δ13C (particularly for carbon, for which ≈4% of observations were classified as outliers). It is possible that these outliers represent analytic errors, although we consider this unlikely given the accuracy and precision of the isotope analyses. Alternatively, the comparatively poor isotopic match between hair and blood for a small number of samples may result from the fact that ≈10–15% of head hairs are in the telogen phase and not growing (41) and we only sampled 2–3 hairs in our analysis. The diet reflected by nongrowing hairs would not be synchronized with that of the blood if a participant's diet varied over time, as might be expected for participants relying on seasonal subsistence foods. We recommend that future studies homogenize ≥10 head hairs to minimize potential error caused by nongrowing hairs.
This study had several limitations. The study sample overrepresented women relative to the eligible population because hair length >3 cm was required. The sample size for comparisons with fatty acids was small; however, there was still sufficient power to detect a modest difference in correlation between hair and blood δ15N and EPA. Finally, the range of DHA and EPA intakes in Yup'ik Eskimos is far broader than most populations. Whereas this variability is ideal for detecting relations among EPA and DHA biomarkers, relations may be less strong in populations with a more restricted intake of marine foods.
In summary, we found that hair δ15N correlated strongly with the ω−3 fatty acids EPA and DHA measured in RBCs. We propose that hair δ15N can be used as a noninvasive, inexpensive, high-throughput biomarker to estimate EPA and DHA intakes. Noninvasive sampling methods decrease participant burden and facilitate inclusion of biomarker data in a broad range of health studies. We also showed very tight correlation and good agreement between hair and RBC δ15N and δ13C. These findings will enable meaningful comparisons to be made between studies sampling these different tissues. There have been recent calls for the development of biomarker-based methods of diet investigation (42–44). This study helps advance the application of stable isotope measurements to dietary assessment.
Acknowledgments
We gratefully acknowledge our participants in the YK Delta and the CANHR field team, especially Field Research Coordinator Scarlett Hopkins. We thank Rebecca Church, Renee Pasker, and M Alyssa Jeannet for their assistance with sample preparation and Tim Howe and Norma Haubenstock at the Alaska Stable Isotope Facility. This manuscript was improved by comments from Trixie Lee, Steffen Oppel, and Caroline van Hemert.
The authors' responsibilities were as follows—SHN: data analysis and interpretation and manuscript preparation; ARK: data interpretation; BBB: sample collection and data interpretation; IBK: fatty acid analyses; JSM: stable isotope analyses; and DMO: study design, data interpretation, and manuscript preparation. All authors contributed to the final draft of the manuscript. None of the authors had any conflicts of interest.
REFERENCES
- 1.Petzke KJ, Boeing H, Klaus S, Metges CC. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr 2005;135:1515–20 [DOI] [PubMed] [Google Scholar]
- 2.Jahren AH, Saudek C, Yeung EH, Kao WHL, Kraft RA, Caballero B. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr 2006;84:1380–4 [DOI] [PubMed] [Google Scholar]
- 3.Buchardt B, Bunch V, Helin P. Fingernails and diet: stable isotope signatures of a marine hunting community from modem Uummannaq, North Greenland. Chem Geol 2007;244:316–29 [Google Scholar]
- 4.O'Brien DM, Kristal AR, Jeannet MA, Wilkinson MJ, Bersamin A, Luick B. Red blood cell δ15N: a novel biomarker of dietary eicosapentaenoic acid and docosahexaenoic acid intake. Am J Clin Nutr 2009;89:913–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bersamin A, Zidenberg-Cherr S, Stern JS, Luick BR. Nutrient intakes are associated with adherence to a traditional diet among Yup'ik Eskimos living in remote Alaska Native communities: the CANHR Study. Int J Circumpolar Health 2007;66:62–70 [DOI] [PubMed] [Google Scholar]
- 6.Bol R, Pflieger C. Stable isotope (C-13, N-15 and S-34) analysis of the hair of modern humans and their domestic animals. Rapid Commun Mass Spectrom 2002;16:2195–200 [DOI] [PubMed] [Google Scholar]
- 7.O'Connell TC, Hedges REM. Investigations into the effect of diet on modern human hair isotopic values. Am J Phys Anthropol 1999;108:409–25 [DOI] [PubMed] [Google Scholar]
- 8.Nardoto GB, Silva S, Kendall C, et al. Geographical patterns of human diet derived from stable-isotope analysis of fingernails. Am J Phys Anthropol 2006;131:137–46 [DOI] [PubMed] [Google Scholar]
- 9.Yoshinaga J, Minagawa M, Suzuki T, et al. Stable carbon and nitrogen isotopic composition of diet and hair of Gidra-speaking Papuans. Am J Phys Anthropol 1996;100:23–34 [DOI] [PubMed] [Google Scholar]
- 10.O'Connell TC, Hedges REM, Healey MA, Simpson AHRW. Isotopic comparison of hair, nail and bone: modern analyses. J Archaeol Sci 2001;28:1247–55 [Google Scholar]
- 11.Kraft RA, Jahren AH, Saudek CD. Clinical-scale investigation of stable isotopes in human blood: delta13C and delta15N from 406 patients at the Johns Hopkins Medical Institutions. Rapid Commun Mass Spectrom 2008;22:3683–92 [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson MJ, Yai Y, O'Brien DM. Age-related variation in red blood cell stable isotope ratios (delta C-13 and delta N-15) from two Yupik villages in Southwest Alaska: a pilot study. Int J Circumpolar Health 2007;66:31–41 [DOI] [PubMed] [Google Scholar]
- 13.Mohatt GV, Plaetke R, Klejka J, et al. The center for Alaska Native Health Research study: a community-based participatory research study of obesity and chronic disease-related protective and risk factors. Int J Circumpolar Health 2007;66:8–18 [DOI] [PubMed] [Google Scholar]
- 14.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n−3 fatty acids in humans. Am J Clin Nutr 2006;83:1467S–76S [DOI] [PubMed] [Google Scholar]
- 15.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 1997;38:2012–22 [PubMed] [Google Scholar]
- 16.Vidgren HM, Agren JJ, Schwab U, Rissanen T, Hanninen O, Uusitupa MI. Incorporation of n−3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids 1997;32:697–705 [DOI] [PubMed] [Google Scholar]
- 17.Bersamin A, Luick BR, King IB, Stern JS, Zidenberg-Cherr S. Westernizing diets influence fat intake, red blood cell fatty acid composition, and health in remote Alaskan Native communities in the center for Alaska Native health study. J Am Diet Assoc 2008;108:266–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer BB, Mohatt GV, Lardon C, et al. Building a community-based participatory research center to investigate obesity and diabetes in Alaska Natives. Int J Circumpolar Health 2005;64:281–90 [DOI] [PubMed] [Google Scholar]
- 19.Cernichiari E, Toribara TY, Liang L, et al. The biological monitoring of mercury in the Seychelles study. Neurotoxicology 1995;16:613–28 [PubMed] [Google Scholar]
- 20.Miyazawa N, Uematsu T. Analysis of ofloxacin in hair as a measure of hair growth and as a time marker for hair analysis. Ther Drug Monit 1992;14:525–8 [DOI] [PubMed] [Google Scholar]
- 21.Saitoh M, Uzuka M, Sakamoto M, Kobori T. Rate of hair growth. : Montagna W, Dobson RL, Hair growth. Oxford, United Kingdom: Pergamon Press, 1969 [Google Scholar]
- 22.Berlin NI, Waldmann TA, Weissman SM. Life span of red blood cell. Physiol Rev 1959;39:577–616 [DOI] [PubMed] [Google Scholar]
- 23.Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 2008;112:4284–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eadie GS, Brown IW., Jr The potential life span and ultimate survival of fresh red blood cells in normal healthy recipients as studied by simultaneous Cr51 tagging and differential hemolysis. J Clin Invest 1955;34:629–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoefs J. Stable isotope geochemistry. Berlin, Germany: Springer, 1997 [Google Scholar]
- 26.O'Connell TC, Hedges REM. Isotopic comparison of hair and bone: archaeological analyses. J Archaeol Sci 1999;26:661–5 [Google Scholar]
- 27.Rose HG, Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res 1965;6:428–31 [PubMed] [Google Scholar]
- 28.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986;27:114–20 [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10 [PubMed] [Google Scholar]
- 30.Dewailly E, Blanchet C, Gingras S, Lemieux S, Holub BJ. Cardiovascular disease risk factors and n−3 fatty acid status in the adult population of James Bay Cree. Am J Clin Nutr 2002;76:85–92 [DOI] [PubMed] [Google Scholar]
- 31.Dewailly E, Blanchet C, Lemieux S, et al. n−3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr 2001;74:464–73 [DOI] [PubMed] [Google Scholar]
- 32.Ebbesson SO, Tejero ME, Nobmann ED, et al. Fatty acid consumption and metabolic syndrome components: the GOCADAN study. J Cardiometab Syndr 2007;2:244–9 [DOI] [PubMed] [Google Scholar]
- 33.Nobmann ED, Ponce R, Mattil C, et al. Dietary intakes vary with age among Eskimo adults of northwest Alaska in the GOCADAN study, 2000-2003. J Nutr 2005;135:856–62 [DOI] [PubMed] [Google Scholar]
- 34.Macko SA, Engel MH, Andrusevich V, Lubec G, O'Connell TC, Hedges REM. Documenting the diet in ancient human populations through stable isotope analysis of hair. Philos Trans R Soc Lond B Biol Sci 1999;354:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White CD. Isotopic determination of seasonality in diet and death from Nubian mummy hair. J Archaeol Sci 1993;20:657–66 [Google Scholar]
- 36.Petzke KJ, Boeing H, Metges CC. Choice of dietary protein of vegetarians and omnivores is reflected in their hair protein 13C and 15N abundance. Rapid Commun Mass Spectrom 2005;19:1392–400 [DOI] [PubMed] [Google Scholar]
- 37.Minagawa M. Reconstruction of human diet from δ13C and δ15N in contemporary Japanese hair—a stochastic method for estimating multisource contribution by double isotopic tracers. Appl Geochem 1992;7:145–58 [Google Scholar]
- 38.Hobson KA, Schell DM, Renouf D, Noseworthy E. Stable carbon and nitrogen isotopic fractionation between diet and tissues of captive seals: implications for dietary reconstructions involving marine mammals. Can J Fish Aquat Sci 1996;53:528–33 [Google Scholar]
- 39.Miller JF, Millar JS, Longstaffe FJ. Carbon- and nitrogen-isotope tissue—diet discrimination and turnover rates in deer mice, Peromyscus maniculatus. Can J Zool 2008;86:685–91 [Google Scholar]
- 40.Tieszen LL, Boutton TW, Tesdahl KG, Slade NA. Fractionation and turnover of stable carbon isotopes in animal-tissues: implications for δ13C analysis of diet. Oecologia 1983;57:32–7 [DOI] [PubMed] [Google Scholar]
- 41.Schwertl M, Auerswald K, Schnyder H. Reconstruction of the isotopic history of animal diets by hair segmental analysis. Rapid Commun Mass Spectrom 2003;17:1312–8 [DOI] [PubMed] [Google Scholar]
- 42.Bingham SA. Biomarkers in nutritional epidemiology. Public Health Nutr 2002;5:821–7 [DOI] [PubMed] [Google Scholar]
- 43.Taren D, Dwyer J, Freedman L, Solomons NW. Dietary assessment methods: where do we go from here? Public Health Nutr 2002;5:1001–3 [DOI] [PubMed] [Google Scholar]
- 44.Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet 2009;125:507–25 [DOI] [PubMed] [Google Scholar]