Abstract
Obesity is often associated with dyslipidemia, insulin resistance, and hypertension. Together, these metabolic perturbations greatly increase the risk of developing cardiovascular disease and diabetes. Although fish oil is a well-established hypolipidemic agent, the mechanisms by which it mediates its lipid-lowering effects are not clear. In addition, it has not been established whether dietary fish oil has different effects in lean and obese mice. LDL receptor deficient (LDLR−/−) and leptin deficient mice on a LDLR−/− background (ob/ob;LDLR−/−) were fed a high fat diet (39% total fat) supplemented with 6% olive oil or fish oil for 6 wk. Fish oil supplementation resulted in lower concentrations of plasma total cholesterol (P < 0.01), triglycerides (P < 0.01), and free fatty acids (P < 0.001) in lean LDLR−/− mice, but not in ob/ob;LDLR−/− mice. In contrast, a fish oil diet did not modulate insulin sensitivity in lean LDLR−/− mice, but it improved insulin sensitivity in ob/ob;LDLR−/− mice (P < 0.05) compared with olive oil fed ob/ob;LDLR−/− mice. Interestingly, plasma adiponectin concentrations were significantly higher and hepatic steatosis was reduced in both mouse models upon fish oil feeding. Finally, fish oil fed LDLR−/− mice exhibited higher hepatic AMP activated protein kinase (AMPK) phosphorylation (P < 0.05), whereas AMPK phosphorylation was not elevated by fish oil feeding in ob/ob;LDLR−/− mice. Taken together, our data suggest that fish oil reduces hepatic steatosis in both lean and obese mice, has potent plasma lipid lowering effects in lean mice, and exerts insulin sensitizing effects in obese mice.
Introduction
Obesity is a global epidemic, affecting both adults and children, and is associated with significant morbidity and mortality. It is often associated with impaired hepatic fatty acid oxidation and increased de novo lipogenesis that may contribute to the development of hypertriglyceridemia, an important risk factor for the development of cardiovascular disease. Strategies to improve hepatic fatty acid metabolism, including dietary interventions, are therefore important for the prevention of obesity-associated comorbidities.
It has been reported that a dose-response relationship exists between the intake of (n-3) fatty acids, such as eicosapentaenoic acid (EPA)6 and docosahexaenoic acid (DHA), and plasma triglyceride (TG) lowering (1). Even a very low intake of fish oil (<2 g/d) has been shown to produce significant reductions in plasma TG (2). In fact, the hypotriglyceridemic effects of these (n-3) fatty acids found in fish oil are well established and are believed to account for their cardioprotective effects (3,4). Several mechanisms have been proposed for how the fish oil-derived (n-3) fatty acids may reduce plasma TG concentrations [reviewed in (4)]. For example, fish oil has been reported to induce hepatic β-oxidation and to reduce de novo fatty acid synthesis in the liver (5). Furthermore, fish oil has been shown to inhibit hepatic VLDL secretion (6) and to promote TG clearance from plasma (7).
It is possible that fish oil has different effects in obese mice as there are many pathophysiological consequences of obesity that could be modulated by dietary fish oils. In addition, fish oil could alter the storage and secretory properties of adipose tissue and thus mediate its beneficial effects. For example, fish oil has shown to increase plasma concentrations of adiponectin, an adipose tissue-derived adipokine, which is implicated in regulating hepatic lipid metabolism. In fact, our previous study demonstrated that supplementation with 6% fish oil improved lipid metabolism by reducing dyslipidemia and hepatic steatosis, and was associated with an increase in plasma adiponectin in lean LDL receptor deficient (LDLR−/−) mice (8). Likewise, studies by other investigators have demonstrated that fish oil increases plasma adiponectin concentrations in animal models (9,10). The effects of fish oil on adiponectin in humans are somewhat different from studies in animal models. For example, whereas fish oil has been reported to increase plasma adiponectin concentrations in obese humans (11,12) this effect was not seen in postmenopausal women with diabetes (13). However, it is widely accepted that fish oil may mediate some of its beneficial effects on metabolic syndrome via adiponectin. As opposed to adiponectin, reports on the effect of fish oil on plasma leptin concentrations have been inconsistent. Whereas some studies have shown that plasma leptin concentrations decrease upon fish oil feeding (14,15), several other studies have demonstrated that fish oil feeding is associated with an increase in plasma leptin concentrations (9,16,17). Moreover, EPA has been shown to induce leptin mRNA and protein concentrations in 3T3-L1 cells in vitro (18). Thus, it is possible that fish oil may have different effects in the presence of increased adipose tissue mass, possibly by modulating adiponectin and leptin concentrations.
Leptin deficient (ob/ob) mice are one of the most commonly used models of obesity. However, these mice, and other obese mouse models, have lipoprotein profiles characterized by elevations in HDL cholesterol rather than the atherogenic VLDL and LDL cholesterol (19). We have developed a novel mouse model to study the effect of fish oil on dyslipidemia in obesity by crossing ob/ob mice onto an LDLR−/− background (ob/ob;LDLR−/−) (20,21). Lean LDLR−/− mice have normal leptin signaling and insulin sensitivity, but demonstrate mild elevations in plasma cholesterol concentrations when fed an unpurified diet. When fed a high fat diet, these mice become mildly insulin resistant and develop more extreme elevations in plasma cholesterol and TG concentrations (22). Surprisingly, the ob/ob;LDLR−/− mice are not only obese and insulin resistant, they display extreme hypercholesterolemia and hypertriglyceridemia even when they are fed a low fat unpurified diet. Thus, both LDLR−/− mice and ob/ob;LDLR−/− mice become dyslipidemic when fed a high fat diet. Therefore, by comparing the effects of dietary fish oil in lean LDLR−/− mice with ob/ob;LDLR−/− mice, we can begin to determine potential differences in how fish oil mediates its beneficial effects on plasma lipids and insulin sensitivity in the presence and absence of obesity and its associated metabolic abnormalities.
Materials and Methods
Animals and diets.
LDLR−/− and ob/+ mice on the C57BL/6 background were originally purchased from Jackson Laboratory. We generated ob/ob;LDLR−/− mice by crossing ob/+ with LDLR−/− mice. We then crossed the resultant ob/+;LDLR−/− mice, which yielded only 1 in 4 pups with the desired ob/ob;LDLR−/− genotype. Therefore, for this study, we used 19–20 lean LDLR−/− mice per group but only 5–7 ob/ob;LDLR−/− mice per group. At 2 to 3 mo of age, these mice were fed a high fat diet (AIN-93G diet; 39% of kJ from fat, 0.5% cholesterol, Dyets) supplemented with 6% olive oil or menhaden oil (fish oil containing 140 mg EPA and 95 mg DHA per gram of oil) for 6 wk. An olive oil supplemented diet has been shown to increase dyslipidemia and insulin resistance in rats (23). Moreover, our previous study demonstrated that a fish oil diet improved several features of metabolic syndrome compared with a diet supplemented with olive oil in LDLR−/− mice (8). Therefore, in this study we used an olive oil–enriched diet to compare the effects of fish oil in modulating metabolic syndrome in the presence or absence of obesity. The diet contained a fat mixture which provided all essential fatty acids, as well as added vitamins and minerals, so that all macro- and micro-nutrient needs of the animals were met during the course of the diet study. A detailed description of the diet has been published previously (8). The fat mix (209 g/kg) contained 45% coconut oil, 30% olive oil, 15% corn oil, and 10% soybean oil. To prevent oxidation, t-BHQ was added at 0.02% of total fat and diets were stored at 4°C until provided to mice. At the end of 6 wk, the mice were food-deprived for 5 h and killed by isoflurane overdose followed by cervical dislocation. All animal care procedures were performed with approval from the Institutional Animal Care and Use Committee of Vanderbilt University.
Measurement of plasma metabolic variables.
At the end of 6 wk, the mice were anesthetized with isoflurane and 500 μL of blood was collected via the retro-orbital plexus, into EDTA, using heparinized capillary tubes, following a 5 h food deprivation. After centrifugation (600 × g; 20 min), plasma was collected, aliquoted, frozen, and then used for nonesterified fatty acids (NEFA), total cholesterol (TC), and TG measurements. Whole blood glucose was measured using a Lifescan glucometer from Johnson and Johnson. Plasma insulin measurements were performed using an insulin assay kit (Linco Research). The homeostasis model assessment of insulin resistance (HOMA-IR) was used as a measure of insulin resistance and was calculated using the following equation: HOMA-IR = fasting serum insulin (μU/mL) × fasting serum glucose (mg/dL)/405. Plasma TC and TG were measured using kits from Raichem. NEFA were measured with a NEFA C kit from Wako Chemicals. Plasma leptin measurements were performed at the Hormone Assay Core Laboratory of the Vanderbilt Mouse Metabolic Phenotyping Center (MMPC), using a mouse leptin radioimmunoassay kit from Millipore. Plasma adiponectin was analyzed by nondenaturing gel electrophoresis followed by western blotting as described previously (8).
Oil Red O staining of liver sections.
Liver samples were embedded in optimal cutting temperature compound (Tissue-Tek), and were frozen at −20°C. Liver sections were cut and stained with Oil Red O for 4 h. Sections were counterstained with hematoxylin for 3 min. Images were captured using a Q-Imaging Micropublisher camera mounted on an Olympus upright microscope.
Liver lipid analyses.
Lipids were extracted from liver samples using the method of Folch–Lees. Individual lipid fractions such as TG, cholesterol esters, phospholipids, NEFA and unesterified cholesterol were analyzed by the Lipid Core Laboratory of the Vanderbilt MMPC using gas chromatography as previously described (24).
Western blotting.
Liver samples were homogenized in lysis buffer containing 20 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 0.5% NP-40, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L sodium orthovanadate and protease inhibitor cocktail (Roche). A modified Lowry protein estimation was performed and equal concentrations of protein were electrophoresed through 4–12% SDS gels (Invitrogen), transferred to nitrocellulose membranes, and immunoblotted for phosphorylated and unphosphorylated forms of AMP activated protein kinase (AMPK). To confirm equal loading, blots were also probed with β-actin antibody. The antibodies for AMPK, phospho AMPK (Thr 172), and β-actin were purchased from Cell Signaling. Detection of immunoreactive proteins was performed using enhanced chemiluminescence (Amersham Pharmacia Biotech) and signal was quantified using Quantity One software from BioRad. Plasma adiponectin concentrations were detected using a rabbit polyclonal anti-adiponectin antibody (ABCAM).
Statistical analysis.
Statistical analyses were performed using Graphpad PRISM. Data are presented as the mean ± SEM. To specifically analyze the effects of olive and fish oil diets in the lean LDLR−/− and in the obese LDLR−/− groups, we performed unpaired Student's t tests. A statistical probability of P < 0.05 was considered significant. Values from both males and females were combined in both LDLR−/− and ob/ob;LDLR−/− groups unless otherwise indicated.
Results
Metabolic variables in LDLR−/− and ob/ob;LDLR−/− mice.
The baseline values for body weight, TC, TG, NEFA, and glucose were measured in lean LDLR−/− and ob/ob;LDLR−/− mice (Table 1). After 6 wk of feeding the high fat diets supplemented with either fish oil or olive oil (control), the body weight and the perigonadal adipose tissue mass of the mice did not differ between diet groups in either lean LDLR−/− or ob/ob;LDLR−/− mice (Table 2). In LDLR−/− mice fed the fish oil diet, plasma TC concentrations were 21% (P < 0.01), TG concentrations were 29% (P < 0.01), and NEFA concentrations were 32% (P < 0.001) lower than in the olive oil fed mice (Table 2). However, these lipid concentrations were not altered in fish oil fed ob/ob;LDLR−/− mice. The plasma glucose concentrations were not significantly altered by fish oil in either lean LDLR−/− or ob/ob;LDLR−/− mice. Plasma insulin concentrations and HOMA-IR were not modulated by fish oil feeding in LDLR−/− mice, whereas both were lower in ob/ob;LDLR−/− mice fed fish oil than in those fed olive oil (P < 0.05). Plasma leptin concentrations did not differ between the olive oil– and fish oil–fed LDLR−/− mice when data for males and females were analyzed together. However, leptin concentrations in female LDLR−/− mice were higher (P < 0.05) in the fish oil fed mice (13.4 ± 2.6 μg/L, n = 8–10) compared with olive oil fed controls (7.0 ± 0.8 μg/L, n = 8–10). The perigonadal adipose tissue mass in fish oil fed female LDLR−/− mice (2.29 ± 0.31% of body weight, n = 10) was also higher than in olive oil fed mice (1.31 ± 0.14% body weight, n = 10, P < 0.01), similar to data we reported earlier (8).
TABLE 1.
Body weight, plasma lipid, and glucose concentrations at baseline in LDLR−/− and ob/ob;LDLR−/− mice12
| Measurements | LDLR−/− | ob/ob;LDLR−/− |
|---|---|---|
| n (male/female) | 39 (20/19) | 12 (4/8) |
| Body weight, g | 21.0 ± 0.5 | 33.0 ± 1.6 |
| TC, mg/dL | 190 ± 5 | 1062 ± 103 |
| TG, mg/dL | 101 ± 7 | 629 ± 92 |
| NEFA, mmol/L | 0.49 ± 0.04 | 1.32 ± 0.13 |
| Glucose, mg/dL |
113 ± 3 |
234 ± 17 |
Data are the mean ± SEM.
To convert from mg/dL to mmol/L, multiply by the following: 0.02586 for TC and 0.0113 for TG, and divide by 18 for glucose.
TABLE 2.
Body and adipose tissue weights and plasma biochemistry of LDLR−/− and ob/ob;LDLR−/− mice fed high fat diets containing 6% olive oil or fish oil for 6 wk12
| LDLR−/− |
ob/ob;LDLR−/− |
|||
|---|---|---|---|---|
| Measurements | Olive Oil | Fish Oil | Olive Oil | Fish Oil |
| n (male/female) | 19 (9/10) | 20 (10/10) | 5 (2/3) | 7 (2/5) |
| Body weight, g | 26.8 ± 1.3 | 27.9 ± 1.1 | 53.0 ± 1.0 | 52.7 ± 1.0 |
| Perigonadal adipose tissue weight, g | 0.66 ± 0.11 | 0.77 ± 0.09 | 3.40 ± 0.25 | 3.43 ± 0.19 |
| Perigonadal adipose tissue weight, % body wt | 2.25 ± 0.28 | 2.62 ± 0.23 | 6.39 ± 0.39 | 6.50 ± 0.35 |
| TC, mg/dL | 603 ± 36 | 476 ± 22† | 2263 ± 116 | 2339 ± 104 |
| TG, mg/dL | 112 ± 13 | 70 ± 7† | 445 ± 76 | 436 ± 30 |
| NEFA, mmol/L | 0.82 ± 0.06 | 0.56 ± 0.04‡ | 1.73 ± 0.22 | 1.93 ± 0.29 |
| Glucose, mg/dL | 117 ± 6 | 118 ± 5 | 115 ± 17 | 87 ± 10 |
| Insulin, μg/L | 0.92 ± 0.15 | 0.68 ± 0.09 | 4.86 ± 0.79 | 2.98 ± 0.35* |
| HOMA-IR | 7.2 ± 1.4 | 5.2 ± 0.8 | 32.8 ± 7.9 | 16.0 ± 2.2* |
| Leptin, μg/L |
13.6 ± 3.0 |
14.2 ± 2.2 |
n.d3 |
n.d. |
Data are presented as mean ± SEM. *Different from corresponding olive oil ob/ob;LDLR−/−, P < 0.05; †different from corresponding olive oil LDLR−/−, P < 0.01; ‡different from corresponding olive oil LDLR−/−, P < 0.001.
To convert from mg/dL to mmol/L multiply by the following: 0.02586 for TC and 0.0113 for TG, to convert from mg/dL (glucose) to mmol/L, divide by 18, and to convert from μg/L (insulin) to pmol/L, multiply by 173.
Assay not conducted.
Plasma adiponectin concentrations in LDLR−/− and ob/ob;LDLR−/− mice.
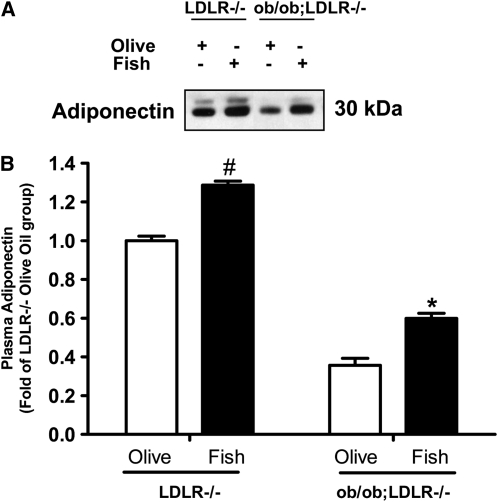
Plasma adiponectin concentrations were elevated by 30% in fish oil fed LDLR−/− mice (P < 0.01), and by 70% in ob/ob;LDLR−/− (P < 0.05) mice compared with their respective LDLR−/− or ob/ob;LDLR−/− olive oil fed controls (Fig. 1).
FIGURE 1 .
Plasma adiponectin in lean LDLR−/− and ob/ob;LDLR−/− mice fed high fat diets containing 6% olive oil or fish oil for 6 wk. Representative bands for plasma adiponectin are shown (A). Band intensities were quantified and plotted as fold of the lean LDLR−/−, olive oil control (B). Data are means ± SEM, n = 4–7. #Different from corresponding olive oil LDLR−/−, P < 0.01; *different from corresponding olive oil ob/ob;LDLR−/−, P < 0.05.
Liver weight and lipids in LDLR−/− and ob/ob;LDLR−/− mice.
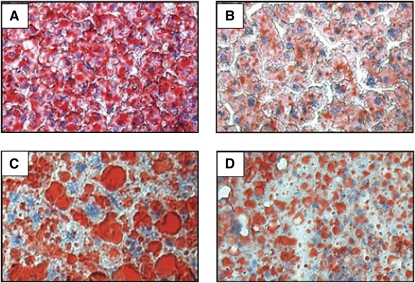
Relative liver weight was lower (P < 0.001) in fish oil (3.50 ± 0.12% body weight, n = 19) compared with olive oil fed LDLR−/− mice (4.51 ± 0.15% body weight, n = 17), whereas it was not altered by fish oil feeding in ob/ob;LDLR−/− mice (6.41 ± 0.32 vs. 7.09 ± 0.60% body weight, fish oil vs. olive oil fed mice, respectively, n = 5–7). Oil red O staining of liver sections for neutral lipids showed that fish oil feeding remarkably reduced neutral lipid accumulation in both LDLR−/− and ob/ob;LDLR−/− mice (Fig. 2A–D).
FIGURE 2 .
Hepatic neutral lipid accumulation in lean LDLR−/− (A,B) and ob/ob;LDLR−/− (C,D) mice fed high fat diets containing 6% olive oil (A,C) or fish oil (B,D) for 6 wk. Images are shown at 10× magnification.
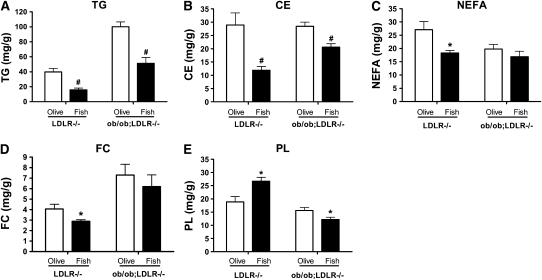
Assessing the lipid profile in liver samples by gas chromatography revealed that fish oil feeding resulted in 60% (P < 0.01) and 49% (P < 0.01) lower liver TG in LDLR−/− and ob/ob;LDLR−/− mice, respectively, compared with their respective olive oil fed controls (Fig. 3A). Similarly, the cholesterol ester concentrations were 59% (P < 0.01) and 27% (P < 0.01) lower in fish oil fed LDLR−/− and ob/ob;LDLR−/− mice, respectively (Fig 3B). Fish oil diet led to lower concentrations of NEFA and free cholesterol (P < 0.05) only in LDLR−/− livers (Fig. 3C,D). On the other hand, in line with our previous report (8), liver phospholipids concentrations were higher upon fish oil feeding in LDLR−/− mice (P < 0.01), whereas a reduction was noted in ob/ob;LDLR−/− mice (P < 0.05, Fig. 3E).
FIGURE 3 .
Hepatic content of TG (A), cholesterol ester (B), NEFA (C), free cholesterol (D), and phospholipids (E) in lean LDLR−/− and ob/ob;LDLR−/− mice fed high fat diets containing 6% olive oil or fish oil for 6 wk. Data are plotted as means ± SEM, n = 5–10. Cholesterol ester (CE), free cholesterol (FC), and phospholipids (PL). To convert from mg/g to μmol/g tissue, multiply by the following: 1.1295 for TG, 2.5933 for CE and FC, 3.5398 for NEFA, and 1.4166 for PL. *Different from corresponding olive oil control, P < 0.05; #different from corresponding olive oil control, P < 0.01.
Hepatic AMPK phosphorylation in LDLR−/− and ob/ob;LDLR−/− mice.
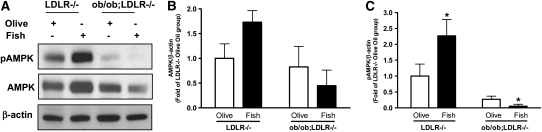
To determine whether fish oil feeding modulates hepatic AMPK signaling, western blot analysis for phosphorylated and unphosphorylated forms of AMPK was performed on liver homogenates (Fig. 4). Higher concentrations of phosphorylated AMPK was noted in livers of LDLR−/− mice (Fig. 4C, P < 0.05). Interestingly, AMPK phosphorylation was very low in the olive oil fed control ob/ob;LDLR−/− mice and was nearly abolished upon fish oil feeding.
FIGURE 4 .
Representative blots and band intensities of phosphorylated AMPK (A,C, respectively) and unphosphorylated AMPK (A,B, respectively) in liver of lean LDLR−/− and ob/ob;LDLR−/− mice fed high fat diets containing 6% olive oil or fish oil for 6 wk. Equal protein loading was confirmed with β-actin. Phosphorylated AMPK (pAMPK) and unphosphorylated AMPK (AMPK). AMPK and pAMPK were quantified and normalized to β-actin and plotted as fold of lean LDLR−/− olive oil control. Data are the means ± SEM, n = 4–7. *Different from corresponding olive oil control, P < 0.05.
Discussion
In this study, we have demonstrated that fish oil exerts distinct effects on lipid metabolism in plasma and liver of lean LDLR−/− and obese leptin deficient ob/ob;LDLR−/− mice (diagram in Supplemental Fig. 1). In lean mice, fish oil supplementation exerted a hypolipidemic effect (lower plasma TG, TC, and NEFA); however, in obese mice, fish oil feeding improved insulin sensitivity. Fish oil supplementation reduced hepatic lipid content in both models. Furthermore, we have demonstrated that fish oil increases hepatic phospho AMPK in lean LDLR−/− but not in ob/ob;LDLR−/− mice. Thus, whereas fish oil improved metabolic outcomes in both models, there were distinct differences, providing some insight into mechanisms by which fish oils may mediate their effects.
An interesting finding of our study is that the hypolipidemic effect of fish oil was abolished in the ob/ob;LDLR−/− mice. Fish oil is a well-known lipid-lowering agent and is used clinically to reduce plasma TG. However, the mechanisms by which fish oil mediates its lipid-lowering effect are not well understood. The elevations in plasma TC and TG concentrations were severe in the ob/ob;LDLR−/− mice after high fat diet feeding (Table 2); thus, it cannot be ruled out that the impact of fish oil on plasma lipids was too subtle to detect in these animals. However, a more intriguing possibility is that the presence of leptin is required to mediate the effects of fish oil. There are several reasons to speculate that leptin plays a role in mediating the hypolipidemic effects of fish oil. First, leptin plays a crucial role in hepatic lipid metabolism, and fish oil feeding has been associated with increased plasma leptin concentrations in a number of studies (9,16,17). Second, fish oil feeding increases hepatic AMPK activity and promotes lipid metabolism (25), and leptin is an important regulator of the AMPK pathway in the liver (26). Third, Itoh et al. (11) reported that an EPA-supplemented diet did not reduce plasma TG concentrations in leptin-deficient ob/ob mice. In line with these reports, our current data demonstrate that fish oil potently reduced dyslipidemia in lean LDLR−/− mice, and this effect was absent in leptin deficient ob/ob;LDLR−/− mice.
Another important finding of this study is that fish oil increased plasma adiponectin concentrations in both LDLR−/− and ob/ob;LDLR−/− mice. Whereas the increased adiponectin concentrations were not associated with a reduction in dyslipidemia in ob/ob;LDLR−/− mice, they were associated with an improvement in insulin sensitivity. As noted in our data, the insulin sensitizing effects of fish oil were evident in the ob/ob;LDLR−/− mice. Several lines of evidence suggest that cross-talk between leptin and insulin occurs in liver cells (27,28) and that leptin can modify insulin-induced changes in gene expression in vitro and in vivo (28). Therefore, our data suggest that the insulin-sensitizing effects of fish oil may be independent of leptin.
We have demonstrated that fish oil reduced hepatic steatosis in both LDLR−/− and ob/ob;LDLR−/− mice, as evidenced from lower TG and cholesterol ester concentrations. Because adiponectin concentrations were increased in both mouse models, and because adiponectin can also promote hepatic fatty acid metabolism, it is possible that adiponectin may help to ameliorate hepatic steatosis upon fish oil feeding. This notion is corroborated by the observation that mice deficient for both leptin and adiponectin exhibit an increase in hepatic TG, relative to mice that are only leptin deficient (29), suggesting the role of adiponectin in reducing hepatic TG concentrations even in the absence of leptin. Adiponectin may reduce hepatic TG accumulation via activating peroxisome proliferators activated receptor α (PPARα), a nuclear transcription factor that is well known to promote fatty acid oxidation. It should be noted that not only adiponectin, but also the (n-3) fatty acids EPA and DHA, can directly function as the ligands for PPARα (30). Thus, the hepatic lipid lowering effect of fish oil may be mediated through PPARα, via activation by adiponectin or by the (n-3) fatty acids themselves. Further investigation is required to distinguish between these possibilities.
The mechanism by which fish oil reduces hepatic TG without altering plasma TG in ob/ob;LDLR−/− mice is unclear. One possible reason for the sustained dyslipidemia seen in the leptin deficient mice is impaired TG clearance. This notion is strengthened by our previous report that the persistent dyslipidemia exhibited by ob/ob;LDLR−/− mice may be a result of impaired clearance of TG-rich VLDL from plasma (22). It is also possible that the de novo fatty acid synthesis is not inhibited efficiently in fish oil fed ob/ob;LDLR−/− mice, as it is in lean LDLR−/− mice. In fact, a mild increase in NEFA concentrations was noted in ob/ob;LDLR−/− mice upon fish oil feeding (Table 2). Aside from lipoprotein production and clearance, and de novo fatty acid synthesis, processes such as dietary fat absorption and bile acid synthesis may be affected by fish oil supplementation differently in lean than in obese mice.
As noted, the reduced hepatic steatosis in fish oil fed mice is accompanied by a reduction of not only hepatic TG but also cholesterol esters. The reduced cholesterol ester accumulation may be a result of the partitioning of hepatic cholesterol for bile acid synthesis. Indeed, fish oil has been reported to promote hepatic cholesterol metabolism via bile acid formation (31). However, whereas TG and cholesterol ester concentrations were reduced upon fish oil feeding, the phospholipid concentrations were increased in fish oil fed LDLR−/− mice. Puri et al. (32) have performed a lipidomic analysis of nonalcoholic fatty liver disease and have shown that hepatic steatosis is associated with an increase in TG and cholesterol esters and a decrease in phospholipid content. Thus, an increase in hepatic phospholipids by fish oil feeding appears to be beneficial in ameliorating hepatic steatosis. However, this effect of fish oil is impaired in the absence of leptin, and, in fact, an opposite trend was noted in fish oil fed ob/ob;LDLR−/− mice. It is not clear why the hepatic phospholipids were reduced by fish oil in ob/ob;LDLR−/− mice. Further studies are needed to determine the mechanisms by which fish oil regulates phospholipid homeostasis in the liver.
Finally, we have demonstrated that hepatic phospho AMPK was higher in fish oil fed lean LDLR−/− mice than in their olive oil fed controls. Interestingly, the liver abundance of phospho AMPK was drastically reduced in ob/ob;LDLR−/− mice and even further reduced upon fish oil feeding. Of note, the impaired hepatic AMPK signaling in ob/ob;LDLR−/− mice was associated with sustained Dyslipidemia, even after fish oil feeding. As mentioned earlier, the persistent dyslipidemia in fish oil fed ob/ob;LDLR−/− mice may be due to impaired TG clearance and increased lipogenesis in liver. With regard to the role of AMPK, previous studies have shown that AMPK regulates the expression or activity of lipoprotein lipase (33), which plays a crucial role in TG clearance from plasma. Thus, it is plausible that impaired AMPK signaling in the absence of leptin may lead to perturbations in plasma TG clearance, which in turn, may result in sustained hyperlipidemia in fish oil-fed ob/ob;LDLR−/− mice. It should also be noted that AMPK has been shown to increase fatty acid oxidation and reduce fatty acid synthesis in liver [reviewed in (34)]. Thus, in addition to lipoprotein clearance, reduced AMPK phosphorylation may also lead to increased de novo fatty acid synthesis which, in turn, could result in sustained dyslipidemia in these mice. Further studies are needed to determine the link between leptin and AMPK in modulating dyslipidemia upon fish oil feeding.
In conclusion, our data demonstrate that fish oil has hypolipidemic effects in lean mice and insulin sensitizing effects in obese mice. However, the effects of fish oil in reducing hepatic steatosis were apparent in both models. The extreme obesity and its downstream metabolic consequences may account for the lack of hypolipidemic effects of fish oil in ob/ob;LDLR−/− mice, although a direct role for leptin in mediating the hypolipidemic effects of fish oil remains possible. Our findings have implications for considering fish oil as a dietary supplement to ameliorate components of metabolic syndrome, such as leptin resistance and hepatic steatosis.
Supplementary Material
Acknowledgments
V.S., and A.H.H. designed research; J.D.M. provided essential materials; V.S., and A.H.H. wrote the paper and approved the final manuscript.
Supported by a grant from the NIH (HL089466) to A. H. Hasty and by NIH grants GM015431 and ES013125 to J. D. Morrow. A. H. Hasty is also supported by a Career Development Award from the American Diabetes Association (1-07-CD-10). V. Saraswathi is supported by an American Heart Association Scientist Development Grant (0930335N). Hepatic lipid profiles and plasma leptin concentrations were determined at the Lipid Core Laboratory and Hormone Core laboratory, respectively, of the Mouse Metabolic Phenotyping Center at Vanderbilt University (DK59637).
Author disclosures: V. Saraswathi, J. D. Morrow, and A. H. Hasty, no conflicts of interest.
Supplemental Figure 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AMPK, AMP activated protein kinase; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HOMA-IR, homeostasis model assessment of insulin resistance; LDLR−/−, LDL receptor deficient; MMPC, Mouse Metabolic Phenotyping Center; NEFA, nonesterified fatty acids; ob/ob, leptin deficient; PPAR, peroxisome proliferator-activated receptor; TC, total cholesterol; TG, triglycerides.
References
- 1.Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65(5, Suppl)1645S–54S. [DOI] [PubMed] [Google Scholar]
- 2.Roche HM, Gibney MJ. Postprandial triacylglycerolaemia: the effect of low-fat dietary treatment with and without fish oil supplementation. Eur J Clin Nutr. 1996;50:617–24. [PubMed] [Google Scholar]
- 3.Jacobson TA. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am J Clin Nutr. 2008;87:1981S–90S. [DOI] [PubMed] [Google Scholar]
- 4.Bays HE, Tighe AP, Sadovsky R, Davidson MH. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev Cardiovasc Ther. 2008;6:391–409. [DOI] [PubMed] [Google Scholar]
- 5.Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17:387–93. [DOI] [PubMed] [Google Scholar]
- 6.Huff MW, Telford DE, Barrett PH. Dietary fish oil plus lovastatin decreases both VLDL and LDL apo B production in miniature pigs. Arterioscler Thromb. 1992;12:902–10. [DOI] [PubMed] [Google Scholar]
- 7.Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res. 2003;44:455–63. [DOI] [PubMed] [Google Scholar]
- 8.Saraswathi V, Gao L, Morrow JD, Chait A, Niswender KD, Hasty AH. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J Nutr. 2007;137:1776–82. [DOI] [PubMed] [Google Scholar]
- 9.Rossi AS, Lombardo YB, Lacorte JM, Chicco AG, Rouault C, Slama G, Rizkalla SW. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R486–94. [DOI] [PubMed] [Google Scholar]
- 10.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, Gillum M, Shulman GI. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–8. [DOI] [PubMed] [Google Scholar]
- 11.Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27:1918–25. [DOI] [PubMed] [Google Scholar]
- 12.Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, Jebb SA. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond). 2006;30:1535–44. [DOI] [PubMed] [Google Scholar]
- 13.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–9. [DOI] [PubMed] [Google Scholar]
- 14.Jang IS, Hwang DY, Chae KR, Lee JE, Kim YK, Kang TS, Hwang JH, Lim CH, Huh YB, Cho JS. Role of dietary fat type in the development of adiposity from dietary obesity-susceptible Sprague-Dawley rats. Br J Nutr. 2003;89:429–38. [DOI] [PubMed] [Google Scholar]
- 15.Ukropec J, Reseland JE, Gasperikova D, Demcakova E, Madsen L, Berge RK, Rustan AC, Klimes I, Drevon CA, Sebokova E. The hypotriglyceridemic effect of dietary n-3 FA is associated with increased beta-oxidation and reduced leptin expression. Lipids. 2003;38:1023–9. [DOI] [PubMed] [Google Scholar]
- 16.Peyron-Caso E, Taverna M, Guerre-Millo M, Veronese A, Pacher N, Slama G, Rizkalla SW. Dietary (n-3) polyunsaturated fatty acids up-regulate plasma leptin in insulin-resistant rats. J Nutr. 2002;132:2235–40. [DOI] [PubMed] [Google Scholar]
- 17.Cha MC, Jones PJ. Dietary fat type and energy restriction interactively influence plasma leptin concentration in rats. J Lipid Res. 1998;39:1655–60. [PubMed] [Google Scholar]
- 18.Perez-Matute P, Marti A, Martinez JA, Fernandez-Otero MP, Stanhope KL, Havel PJ, Moreno-Aliaga MJ. Eicosapentaenoic fatty acid increases leptin secretion from primary cultured rat adipocytes: role of glucose metabolism. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1682–8. [DOI] [PubMed] [Google Scholar]
- 19.Silver DL, Jiang X–C, Tall AR. Increased high density lipoprotein (HDL), defective hepatic catabolism of apoA-I and apoA-II, and decreased apoA-I mRNA in ob/ob mice. J Biol Chem. 1999. ds4140;2744140–6. [DOI] [PubMed] [Google Scholar]
- 20.Hasty AH, Shimano H, Osuga J, Namatame I, Takahashi A, Yahagi N, Perrey S, Iizuka Y, Tamura Y, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J Biol Chem. 2001;276:37402–8. [DOI] [PubMed] [Google Scholar]
- 21.Gruen ML, Saraswathi V, Nuotio-Antar AM, Plummer MR, Coenen KR, Hasty AH. Plasma insulin levels predict atherosclerotic lesion burden in obese hyperlipidemic mice. Atherosclerosis. 2006;186:54–64. [DOI] [PubMed] [Google Scholar]
- 22.Coenen KR, Gruen ML, Hasty AH. Obesity causes very low density lipoprotein clearance defects in low-density lipoprotein receptor-deficient mice. J Nutr Biochem. 2007;18:727–35. [DOI] [PubMed] [Google Scholar]
- 23.Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, Bollheimer LC. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol. 2006;36:485–501. [DOI] [PubMed] [Google Scholar]
- 24.Saraswathi V, Hasty AH. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J Lipid Res. 2006;47:1406–15. [DOI] [PubMed] [Google Scholar]
- 25.Suchankova G, Tekle M, Saha AK, Ruderman NB, Clarke SD, Gettys TW. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun. 2005;326:851–8. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47:2012–21. [DOI] [PubMed] [Google Scholar]
- 27.Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science. 1996;274:1185–8. [DOI] [PubMed] [Google Scholar]
- 28.Szanto I, Kahn CR. Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc Natl Acad Sci USA. 2000;97:2355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yano W, Kubota N, Itoh S, Kubota T, Awazawa M, Moroi M, Sugi K, Takamoto I, Ogata H, et al. Molecular mechanism of moderate insulin resistance in adiponectin-knockout mice. Endocr J. 2008;55:515–22. [DOI] [PubMed] [Google Scholar]
- 30.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19:242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonkers IJ, Smelt AH, Princen HM, Kuipers F, Romijn JA, Boverhof R, Masclee AA, Stellaard F. Fish oil increases bile acid synthesis in male patients with hypertriglyceridemia. J Nutr. 2006;136:987–91. [DOI] [PubMed] [Google Scholar]
- 32.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–90. [DOI] [PubMed] [Google Scholar]
- 33.An D, Pulinilkunnil T, Qi D, Ghosh S, Abrahani A, Rodrigues B. The metabolic “switch” AMPK regulates cardiac heparin-releasable lipoprotein lipase. Am J Physiol Endocrinol Metab. 2005;288:E246–53. [DOI] [PubMed] [Google Scholar]
- 34.Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, et al. AMPK: Lessons from transgenic and knockout animals. Front Biosci. 2009;14:19–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.