Abstract
The reversible acetylation of histones is an important mechanism of gene regulation. During prostate cancer progression, specific modifications in acetylation patterns on histones are apparent. Targeting the epigenome, including the use of histone deacetylase (HDAC) inhibitors, is a novel strategy for cancer chemoprevention. Recently, drugs classified as HDAC inhibitors have shown promise in cancer clinical trials. We have previously found that sulforaphane (SFN), a compound found in cruciferous vegetables, inhibits HDAC activity in human colorectal and prostate cancer cells. Based on the similarity of SFN metabolites and other phytochemicals to known HDAC inhibitors, we previously demonstrated that sulforaphane acted as an HDAC inhibitor in the prostate, causing enhanced histone acetylation, derepression of P21 and Bax, and induction of cell cycle arrest/apoptosis, leading to cancer prevention. The ability of SFN to target aberrant acetylation patterns, in addition to effects on phase 2 enzymes, may make it an effective chemoprevention agent. These studies are important because of the potential to qualify or change recommendations for high-risk prostate cancer patients and thereby increase their survival through simple dietary choices incorporating easily accessible foods into their diets. These studies also will provide a strong scientific foundation for future large-scale human clinical intervention studies.
Introduction: Epigenetics and cancer development
Epigenetics is the study of the regulation of gene activity that is not dependent on nucleotide sequence; this may include heritable changes in gene activity and expression but also long-term alterations in the transcriptional potential of a cell that are not heritable. These features are potentially reversible and may affect genomic stability and expression of genes. In recent years, epigenetics researchers have made great strides in understanding the many molecular sequences and patterns that determine which genes can be turned on and off. This work has made it increasingly clear that in addition to genetic changes, the epigenome is just as critical as the DNA to healthy human development. More importantly, dietary factors and specific nutrients can modulate epigenetic alterations and alter susceptibility to disease. The classic view of cancer etiology is that genetic alterations (via genotoxic agents) damage DNA structure and induce mutations resulting in nonfunctional proteins that lead to disease progression. More recently, the role of epigenetic alterations during development and chronic disease development has gained increasing attention and has resulted in a paradigm shift in our understanding of mechanisms leading to disease susceptibility. A major focus in this review is the identification of dietary agents that target histone modifications and the mechanisms leading to cancer prevention.
Use of histone deacetylase inhibitors in prostate cancer
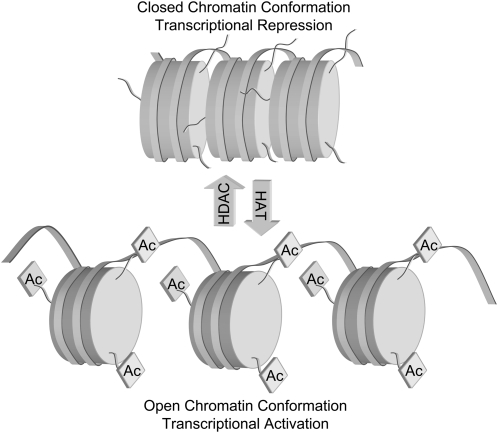
The reversible acetylation of nuclear histones is an important mechanism of gene regulation. In general, addition of acetyl groups to histones by histone acetyltransferases (HAT)8 results in an “open” chromatin conformation, facilitating gene expression by allowing transcription factors access to DNA. Removal of acetyl groups by histone deacetylases (HDACs) results in a “closed” conformation, which represses transcription (Fig. 1). A tightly regulated balance exists in normal cells between HAT and HDAC activities, and when this balance is disrupted, cancer development can ensue. The HDACs can be divided into 3 classes based on their structure and sequence homology: class I consists of HDACs 1, 2, 3, 8, and 11; class II includes HDACs 4, 5, 6, 7, 9, and 10; and class III enzymes are HDACs originally found in yeast and include Sir2-related proteins. Increased HDAC activity and expression are common in many cancers and can result in repression of transcription that results in a deregulation of differentiation, cell cycle, and apoptotic mechanisms. Moreover, tumor suppressor genes, such as p21 appear to be targets of HDACs and are “turned off” by deacetylation. Prostate cancer cells also exhibit aberrant acetylation patterns. The use of class I and class II HDAC inhibitors in cancer chemoprevention and therapy has gained substantial interest. Several clinical trials are currently ongoing aimed at establishing the chemotherapeutic efficacy of HDAC inhibitors, based on evidence that cancer cells undergo cell cycle arrest, differentiation, and apoptosis in vitro and that tumor volume and/or tumor number may be reduced in animal models. HDAC inhibitors have been shown to increase global acetylation as well as acetylation associated with specific gene promoters. Although the equilibrium is shifted toward greater histone acetylation after treatment with HDAC inhibitors, the expression of only a relatively small number of genes is altered in an upward or downward direction (1). Importantly, only neoplastically transformed cells appear to respond to increased acetylation by undergoing differentiation, cell cycle arrest, or apoptosis; normal cells, despite the increased acetylation, do not respond in this manner to HDAC inhibitors (2). Thus, effects of HDAC inhibitors on apoptosis and antiproliferation appear to be selective to cancer, not normal cells, although the mechanism is poorly understood.
FIGURE 1 .
Modulation of chromatin conformation and transcriptional status by acetylation of lysine tails in histone core proteins. HDAC, histone deacetylase; HAT, histone acetyltransferase.
Increases in HDACs and decreases in histone acetylation have been found in several types of cancer. In the case of prostate cancer, for example, it has been shown that HDAC activity increases in metastatic cells compared with prostate hyperplasia (3), and overexpression of HDAC1 in PC-3 cells results in an increase in cell proliferation and an overall decrease in cell differentiation (4). Increased expression of HDACs may be of particular importance in the progression to androgen independence because accumulation of HDAC4 coincides with loss of androgen sensitivity (5). In human patient samples, global decreases in histone acetylation state corresponded with increased grade of cancer and risk of prostate cancer recurrence (6). Importantly, inhibitors of HDAC, including suberoylanilide hydroxamic acid (SAHA), valproic acid, depsipeptide, and sodium butyrate have been demonstrated to be effective against prostate cancer cell lines and xenograft models (7,8). Thus, alterations in HDAC activity and histone acetylation status could act as future biomarkers for prostate cancer progression. The identification of other novel dietary HDAC inhibitors to target aberrant HDAC activity is an important area of research.
Sulforaphane and HDAC inhibition—a new paradigm
Isothiocyanates (ITCs) are found in cruciferous vegetables such as broccoli, Brussels sprouts, cauliflower, and cabbage. Sulforaphone (SFN) is an ITC derived from cruciferous vegetables and is especially high in broccoli and broccoli sprouts (9). In broccoli and broccoli sprouts, SFN exists as the glucosinolate precursor glucoraphanin. When the plant is consumed, plant myrosinases or microbial hydrolases present in gut bacteria convert glucoraphanin to SFN. SFN is an effective chemoprotective agent in carcinogen-induced animal models (9–11) as well as in xenograft models of prostate cancer (12). Recent work has implicated multiple mechanisms of SFN action, with the majority of studies focusing on SFN as a potent Phase 2 enzyme inducer and additional evidence for cell cycle arrest and apoptosis. Early research focused on Phase 2 enzyme induction by SFN as well as on the inhibition of enzymes involved in carcinogen activation, but there has been growing interest in other mechanisms of chemoprotection by SFN. The “blocking activity” of SFN has received substantial attention, focused on nuclear factor E2-related factor-2 (Nrf2) signaling and antioxidant response element-driven gene expression. Thus, chemoprotective effects of SFN have been attributed to its ability to upregulate heme oxygenase and Phase 2 detoxification systems such as NAD(P)H:quinone reductase (NQO1), epoxide hydrolase, and γ-glutamylcysteine synthetase (rate-limiting enzyme in glutathione synthesis), via antioxidant response element sites in the 5′-flanking region of the corresponding genes. Upregulation of Phase 2 metabolism is likely a critical mechanism leading to cancer prevention by SFN in the “initiation” phase, helping to more rapidly eliminate genotoxins from the body.
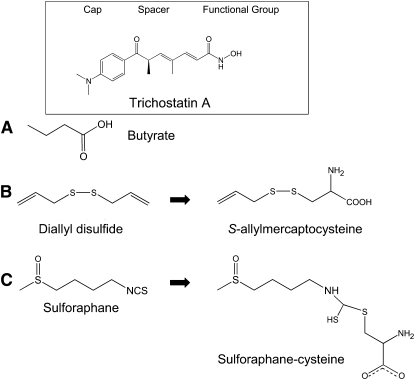
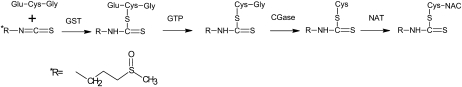
Recent studies also suggest that SFN offers protection against tumor development during the “postinitiation” phase, and mechanisms for “suppression” effects of SFN are of particular interest. In the course of studying “suppression” mechanisms, we discovered that SFN is an inhibitor of HDAC. The general structure of HDAC inhibitors is comprised of a functional group at one end that interacts with a zinc atom and neighboring amino acids at the base of the HDAC active site, a spacer that fits into the channel of the active site, and a cap group, which is hypothesized to interact with external amino acid residues (13). Based on the similarity of SFN metabolites to the conserved structure of HDAC inhibitors (Fig. 2), we hypothesized that SFN could effectively inhibit HDAC. SFN is metabolized via the mercapturic acid pathway, starting with glutathione (GSH) conjugation by glutathione-S-transferase (GST) and subsequent steps generate SFN-cysteine (SFN-Cys) followed by SFN-N-acetylcysteine (SFN-NAC) (14) (Fig. 3). Based on modeling and in vitro work (15), we hypothesized that SFN-NAC or SFN-Cys are the active HDAC inhibitors. The identification of novel dietary HDAC inhibitors to target aberrant histone status is an important area of research and aligns with the NIH Roadmap priority area “epigenetics.”
FIGURE 2 .
Structural similarities between known pharmacological HDAC inhibitor (trichostatin A) and dietary HDAC inhibitors: butyrate (A), allyl disulfide metabolites (B), and SFN-cysteine (C), a metabolite of SFN.
FIGURE 3 .
Metabolism of SFN via the mercapturic acid pathway. GST, glutathione-S-transferase; GTP, γ-glutamyltranspeptidase; CGase, cysteinylglycinase; NAT, N-acetyltransferase.
Biochemical assays showed that SFN metabolites did indeed inhibit HDAC activity in vitro, the greatest inhibition involving SFN-NAC and SFN-Cys. Molecular modeling in the active site of an HDAC enzyme provided evidence that SFN-Cys is acting as a competitive inhibitor (15). In BPH1, PC3, and LnCap prostate cancer cells, SFN inhibited HDAC activity with a concomitant increase in global histone acetylation, increased acetylated histone H4 interactions with the P21 and Bax promoter, and induction of p21 and Bax mRNA and protein levels (16). HDAC inhibition coincided with the induction of G2/M phase cell cycle arrest and apoptosis as indicated by multicaspase activation (16). HDAC inhibition by SFN has also been established in several other cancer cell lines including breast and colon (15,17), suggesting the effects are not specific to the prostate. The same effects observed in prostate cell lines were seen in HCT116 human colorectal cancer cells treated with SFN, namely HDAC inhibition, increased global histone acetylation, and selective increase in histone acetylation at the p21 promoter (15). HT-29 colon cancer cells, which lack endogenous Nrf2 protein, and Nrf2−/− mouse embryonic fibroblasts both exhibited an HDAC inhibitory response to SFN treatment (R. H. Dashwood, unpublished results). These results indicated the possibility of a separate SFN chemoprevention pathway distinct from the classic Nrf2 pathway (18). Importantly, the effects of SFN do appear to be tumor cell specific. We have found that 3–15 μmol/L SFN induces potent HDAC inhibition and G2/M arrest in PC3 cancer cells but have no effect on normal prostate epithelial cells (J. D. Clarke and E. Ho, unpublished data). These data support the hypothesis that HDAC inhibition may be an important mechanism of chemoprevention for SFN and similar pharmacological HDAC inhibitors: the cytotoxic effects are specific to cancer, not normal cells.
In PC3 xenograft studies, dietary SFN supplementation resulted in slower tumor growth and significant HDAC inhibition in the xenografts as well as in the prostate and circulating peripheral blood mononuclear cells (19). In other dietary studies examining colon cancer, Apcmin mice were fed ∼6 μmol SFN/d for 10 wk. In these experiments a significant decrease in intestinal polyps and an increase in global acetylated histones H3 and H4 were observed, with specific increases at the Bax and p21 promoters (20). From these studies it can be concluded that HDAC inhibition represents a novel chemoprevention mechanism by which SFN might promote cell cycle arrest and apoptosis in vivo.
Bioavailability and human studies
The ability of SFN to be distributed throughout the body and reach target tissues has been investigated in vitro, in mouse models, and in human subjects. In the human small intestine, SFN can be efficiently absorbed and conjugated to GSH. Human perfusion experiments showed that 74 ± 29% of SFN from broccoli extracts can be absorbed in the jejunum and that a portion of that returns to the lumen of the jejunum as SFN-GSH (21). Pharmacokinetic studies in both rats and humans also support that SFN can be distributed in the body and reach micromolar concentrations in the blood. In rats, following a 50 μmol gavage of SFN, detectable SFN was evident after 1 h and peaked at ∼20 μmol/L at 4 h, with a half life of ∼2.2 h (22). Broccoli sprouts contain up to 50 times higher concentrations of the SFN precursor glucoraphanin than mature broccoli. Thus, in humans, the majority of studies have used broccoli sprouts as a source of high SFN. In human subjects given single doses of 200 μmol broccoli sprouts ITC preparation, ITC plasma concentrations peaked between 0.943 and 2.27 μmol/L 1 h after feeding, with half-life times of 1.77 ± 0.13 h (23).
To date, the bioavailability of SFN to the prostate is unknown and is an important area of future research. However, in a recent pilot study in human mammary tissue, an oral dose of broccoli sprout preparation containing 200 μmol SFN 1 h prior to tissue removal showed mean accumulation of 1.45 ± 1.12 pmol/mg tissue in the right breast and 2.00 ± 1.95 pmol/mg in the left breast. In these tissues the induction of detoxification genes NQO1 and heme oxygenase-1 as biomarkers of SFN activity were also detected (24). Collectively, the published data indicate that SFN concentrations reach micromolar concentrations in the blood and reach target tissues.
To date, very few human clinical trials have evaluated the effects of SFN on cancer outcome; however, several pilot and phase 1 human SFN trials have been conducted utilizing different sources of SFN. In our laboratory, a small preliminary human study was performed in the interest of determining if the HDAC inhibition effects observed in cell culture and mice could be translated into humans. In clinical trials using pharmacological HDAC inhibitors such as SAHA, alterations in acetylated histone status in peripheral blood cell samples are used as biomarkers for HDAC inhibitory efficacy. In normal healthy volunteers, 3–6 h after the ingestion of 68 g of broccoli sprouts, a >50% significant decrease in HDAC activity was evident in peripheral blood mononuclear cells with a concomitant increase in acetylated histones H3 and H4 (19). HDAC activity was restored by 24 h. These data give preliminary evidence for the ability of dietary SFN to inhibit HDAC in humans.
Conclusions
In summary, prostate cancer is the second leading cause of cancer death in men in the United States, exceeded only by lung cancer. Despite its being so common, very little is known at the present time about the cellular and molecular events associated with its pathogenesis. Targeting the epigenome, including the use of HDAC inhibitors, is an evolving strategy for cancer chemoprevention, and both have shown promise in cancer clinical trials. We have found that SFN, an isothiocyanate derived from cruciferous vegetables, inhibits HDAC activity in prostate cancer cells, in mouse xenografts, and in human peripheral blood mononuclear cells. The ability of SFN to target aberrant epigenetic patterns, in addition to effects on phase 2 enzymes, may make it an effective chemoprevention agent at multiple stages of the carcinogenesis pathway. The identification of dietary HDAC inhibitors and their use either alone or in combination, may increase the efficacy of anticancer therapies/prevention strategies without side effects. These translational studies provide the important link to human relevance for SFN as a promising anticancer agent and provide a strong scientific foundation for future trials to identify effective dietary intervention strategies that are broadly applicable to public health recommendations and will greatly reduce the burden of prostate cancer.
Other articles in the supplement include references (25–28).
Acknowledgments
E.H., J.D.C., and R.H. wrote the paper, and E.H. had primary responsibility for final content. All authors read and approved the final manuscript.
Presented as part of the symposium entitled “Nutrients and Epigenetic Regulation of Gene Expression” at the Experimental Biology 2009 meeting, April 20, 2009, in New Orleans, LA. This symposium was sponsored the American Society for Nutrition (ASN) and had no outside support declared. The Guest Editor for this symposium publication was Kevin Schalinske. Guest Editor disclosure: no relationships to disclose.
A contribution of the Oregon Agricultural Experiment Station, supported in part by funds provided through the Hatch Act. Additional support was provided by NIH grants CA090890, CA065525, CA122906, CA122959, and Environmental Health Sciences Center (National Institute of Environmental Health Sciences) P30 ES00210.
Abbreviations used: GSH, glutathione; GST, glutathione-S-transferase; HAT, histone acetyltransferase; HDAC, histone deacetylase; ITC, isothiocyanate; NAC, N-acetylcysteine; Nrf2, nuclear-related factor 2; SAHA, suberoylanilide hydroxamic acid; SFN, sulforaphane.
References
- 1.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, Akiyama M, Chauhan D, Munshi N, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci USA. 2004;101:540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkmann H, Dahler AL, Popa C, Serewko MM, Parsons PG, Gabrielli BG, Burgess AJ, Saunders NA. Histone hyperacetylation induced by histone deacetylase inhibitors is not sufficient to cause growth inhibition in human dermal fibroblasts. J Biol Chem. 2001;276:22491–9. [DOI] [PubMed] [Google Scholar]
- 3.Patra SK, Patra A, Dahiya R. Histone deacetylase and DNA methyltransferase in human prostate cancer. Biochem Biophys Res Commun. 2001;287:705–13. [DOI] [PubMed] [Google Scholar]
- 4.Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate. 2004;59:177–89. [DOI] [PubMed] [Google Scholar]
- 5.Halkidou K, Cook S, Leung HY, Neal DE, Robson CN. Nuclear accumulation of histone deacetylase 4 (HDAC4) coincides with the loss of androgen sensitivity in hormone refractory cancer of the prostate. Eur Urol. 2004;45:382–9, author reply 9. [DOI] [PubMed] [Google Scholar]
- 6.Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–6. [DOI] [PubMed] [Google Scholar]
- 7.Fronsdal K, Saatcioglu F. Histone deacetylase inhibitors differentially mediate apoptosis in prostate cancer cells. Prostate. 2005;62:299–306. [DOI] [PubMed] [Google Scholar]
- 8.Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60:5165–70. [PubMed] [Google Scholar]
- 9.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–91. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. [DOI] [PubMed] [Google Scholar]
- 13.Furumai R, Komatsu Y, Nishino N, Khochbin S, Yoshida M, Horinouchi S. Potent histone deacetylase inhibitors built from trichostatin A and cyclic tetrapeptide antibiotics including trapoxin. Proc Natl Acad Sci USA. 2001;98:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kassahun K, Davis M, Hu P, Martin B, Baillie T. Biotransformation of the naturally occurring isothiocyanate sulforaphane in the rat: identification of phase I metabolites and glutathione conjugates. Chem Res Toxicol. 1997;10:1228–33. [DOI] [PubMed] [Google Scholar]
- 15.Myzak MC, Karplus PA, Chung F-L, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64:5767–74. [DOI] [PubMed] [Google Scholar]
- 16.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27:811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6:1013–21. [DOI] [PubMed] [Google Scholar]
- 18.Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: From cells to mice to man. Semin Cancer Biol. 2007;17:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med. 2007;232:227–34. [PMC free article] [PubMed] [Google Scholar]
- 20.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006;20:506–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petri N, Tannergren C, Holst B, Mellon FA, Bao Y, Plumb GW, Bacon J, O'Leary KA, Kroon PA, et al. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab Dispos. 2003;31:805–13. [DOI] [PubMed] [Google Scholar]
- 22.Hu R, Hebbar V, Kim BR, Chen C, Winnik B, Buckley B, Soteropoulos P, Tolias P, Hart RP, Kong AN. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J Pharmacol Exp Ther. 2004;310:263–71. [DOI] [PubMed] [Google Scholar]
- 23.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. [DOI] [PubMed] [Google Scholar]
- 24.Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen M-SA, Stierer T, Garrett-Mayer E, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–90. [DOI] [PubMed] [Google Scholar]
- 25.Ho E, Zempleni J. Overview to symposium “Nutrients and Epigenetic Regulation of Gene Expression.”. J Nutr. 2009;139:2387–8. [DOI] [PubMed] [Google Scholar]
- 26.Zempleni J, Chew YC, Bao B, Pestinger V, Wijeratne SSK. Repression of transposable elements by histone biotinylation. J Nutr. 2009;139:2389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkland JB. Niacin status impacts chromatin structure. J Nutr. 2009;139:2397–401. [DOI] [PubMed] [Google Scholar]
- 28.Stover PJ. One-carbon metabolism–genome interactions in folate-associated pathologies. J Nutr. 2009;139:2402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]