Abstract
We recently reported that vasa vasorum expansion occurs in the pulmonary artery (PA) adventitia of chronically hypoxic animals and that extracellular ATP is a pro-angiogenic factor for isolated vasa vasorum endothelial cells (VVEC). However, the sources of extracellular ATP in the PA vascular wall, as well as the molecular mechanisms underlying its release, remain elusive. Studies were undertaken to explore whether VVEC release ATP in response to hypoxia and to determine signaling pathways involved in this process. We found that hypoxia (1–3% O2) resulted in time- and O2-dependent ATP release from VVEC. Preincubation with the inhibitors of vesicular transport (monensin, brefeldin A, and N-ethylmaleimide) significantly decreased ATP accumulation in the VVEC conditioned media, suggesting that hypoxia-induced ATP release occurs through vesicular exocytosis. Additionally, both hypoxia and exogenously added ATP resulted in the activation of PI3K and accumulation of GTP-bound RhoA in a time-dependent manner. Pharmacological inhibition of PI3K and ROCK or knockout of RhoA by small interfering RNA significantly abolished hypoxia-induced ATP release from VVEC. Moreover, RhoA and ROCK play a critical role in ATP-induced increases in VVEC DNA synthesis, migration, and tube formation, indicating a functional contribution of PI3K, Rho, and ROCK to both the autocrine mechanism of ATP release and ATP-mediated angiogenic activation of VVEC. Taken together, our findings provide novel evidence for the signaling mechanisms that link hypoxia-induced increases in extracellular ATP and vasa vasorum expansion.
Keywords: vasa vasorum, hypoxia, angiogenesis, ATP release, phosphatidylinositol 3-kinase, Rho, ROCK
vasa vasorum is a microvascular network that supplies adventitia and media of large blood vessels with oxygen and nutrients. It was established that angiogenic expansion of the vasa vasorum network contributes to the progression of both pulmonary and systemic vascular diseases (14, 36, 41, 44). Our laboratory recently demonstrated that marked expansion of the vasa vasorum network occurs in the pulmonary artery (PA) adventitia of chronically hypoxic calves with severe pulmonary hypertension. However, little is known regarding molecular mechanisms that may be involved in this angiogenic process (14, 17, 52).
Endogenous autocrine and paracrine soluble factors, released under hypoxic conditions from the cells of vasculature and circulating blood cells, are important contributors to angiogenesis. An autocrine mechanism for the regulation of vascular cell growth has been demonstrated for several vasoactive compounds, including TGF-α, VEGF, prostaglandin E2, and adrenomedulin (26, 27, 33). Extracellular nucleotides (ATP, ADP, AMP, and adenosine) are also recognized as important regulators of vascular cell proliferation, migration, permeability, and inflammation (1, 9). Endothelial cells (EC) from various vascular beds are known to be potent sources of adenine nucleotides (31). In addition, vascular cells express multiple purinergic receptors and ecto-nucleotidases (ecto-NTPDases) that provide fine-tuned regulatory mechanisms for triggering and regulating nucleotide-mediated signaling (1, 62). Our earlier studies demonstrated that extracellular ATP might play an autocrine role in hypoxia-induced mitogenic activation of adventitial fibroblasts mediated by the activation of ERK1/2 pathways and the transcription factor Egr-1 (18). Importantly, we recently found that extracellular ATP exerts dramatic effects on proliferation, migration, and tube formation in vasa vasorum EC (VVEC) isolated from the PA adventitia (17). However, the cellular sources of extracellular ATP in the PA vascular wall and the mechanisms of its release remain unknown. The exaggerated responses to extracellular ATP observed in angiogenic VVEC suggests that these microvascular EC are both sources and targets of adenine nucleotides in the PA wall.
ATP release in vascular cells can be observed in response to various stimuli including fluid shear stress, low osmolarity, inflammation, and hypoxia (4, 7, 18, 25, 30). Several studies demonstrated that shear stress-induced ATP release from EC is the major physiological response regulating vascular tone though sequential changes in membrane deformation, increase of intracellular Ca2+, and NO production (21, 59). However, despite the strong evidence of ATP release from vascular cells, the molecular and signaling mechanisms involved in this process remain poorly understood. It has been shown that EC may release ATP through volume-regulated anion channels (VRACs), connexin hemichannels (Cx), and vesicular exocytosis (19, 20, 30, 39). Studies on human umbilical vein EC (HUVEC) and bovine aortic EC demonstrated that VRAC mediate hypotonic stress-induced ATP release by a mechanism that also involves actin cytoskeleton reorganization and activation of Rho/ROCK and focal adhesion kinase (FAK)/paxillin pathways (21, 30, 39). Agonist-dependent ATP release has been observed in EC in response to bradykinin, acetylcholine, and serotonin, but the mechanisms underlying this response have not been addressed (61). Other potential mechanisms of ATP release involve ATP-binding cassette transporters, the P-glycoprotein, voltage-dependent anion channels, and cell surface ATP synthase. However, very limited information exists regarding the molecular mechanisms regulating ATP release in vascular EC in response to hypoxia (4, 6, 15, 47, 60).
Activation of PI3K, Rho, and ROCK pathways and reorganization of actin cytoskeleton were defined as intracellular signaling events associated with proliferative, contractile, and secretory responses in vasculature (10, 16, 43, 55). The mechanisms that link PI3K activation to exocytosis involve direct interaction of 3-d-phosphoinositides with Rho family guanine nucleotide exchange factors (GEFs), and FYVE zinc finger domain proteins, which serve various functions in membrane trafficking, cytoskeleton regulation, and signal transduction pathways (55, 58). Rho proteins play important roles in angiogenesis, development, and tumor growth through regulation of the activity and the expression levels of cyclin D1, cyclin A1, p21Cip1, and p27 Kip1 (8, 13, 34, 35, 40, 57). Elevated levels of Rho proteins and their effectors, ROCK I and ROCK II, are commonly observed in human cancers and are often associated with more invasive and metastatic phenotypes (13, 43, 53). In a rat model of pulmonary hypertension, it has been shown that ROCK is involved in hypoxia-induced capillary angiogenesis, suggesting that ROCK may be widely involved in angiogenic responses in microvascular EC (24).
To further elucidate the potential mechanisms responsible for hypoxia-induced vasa vasorum neovascularization, we performed studies to determine whether VVEC release ATP in response to hypoxia and to identify the molecular mechanisms contributing to this response. Our data demonstrate that VVEC represent a potent source of the pro-angiogenic molecule ATP. We also provide evidence that PI3K and RhoA/ROCK are critically important to ATP release and ATP-induced angiogenic responses in VVEC, suggesting that, under chronic hypoxic conditions, these pathways constitute an autocrine/paracrine loop of ATP release and signaling.
MATERIALS AND METHODS
Cultures of VVEC from PA adventitia.
Pulmonary arteries were obtained from male Holstein calves that had been exposed to hypobaric hypoxia for 2 wk (barometric pressure = 430 mmHg). Adventitia was dissected from the media, extensively washed in PBS, and enzymatically digested for 1.5–2 h at 37°C in a mixture containing collagenase type 2 (0.5 mg/ml, Worthington Biochemical, Lakewood, NJ), elastase (0.5 mg/ml, Worthington Biochemical), bovine albumin (2 mg/ml, Worthington Biochemical), and soybean trypsin inhibitor (0.02 mg/ml, Worthington Biochemical). Dispersed cell mixtures were filtered through a 100 μM nylon cell strainer (BD Biosciences, San Diego, CA), plated on 6-well plates, and grown in DMEM media supplemented with 10% FBS and endothelial growth supplement (Upstate Biotechnology, Charlottesville, VA). VVEC were purified from the co-cultures with adventitial fibroblasts using cloning rings and trypsinization techniques. Isolated VVEC have been shown to express endothelial markers, including vWF, eNOS, and PECAM-1; binding of the lectin Licopercsicon esculentum; and incorporate acetylated low-density lipoproteins labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate (DiI-Ac-LDL) (14). All studies were performed on cells between passages 2 and 7.
Transfection with RhoA small interfering RNA oligos.
VVEC were seeded in a 6-well plate at 2.4 × 105 cells per well and grown to 80% confluence. RhoA small interfering RNA (siRNA) (target sequence: 5′GGAAGAAACUGGUGAUUGUUU 3′), custom designed and synthesized by Dharmacon (Lafayette, CO) from the bovine accession number NM_176645, was used at 100 nM. Nonsilencing siRNA scramble “Negative #4” was used as a negative control (Ambion, Austin, TX) at 100 nM. siRNAs were delivered to the cells with DharmaFECT1 transfection reagent (Dharmacon) for 6 h at a 1:2 ratio of siRNA to transfection reagent, according to manufacturer's specifications. The cells were allowed to recover in DMEM/10%FBS overnight after transfection and then maintained in a serum-free medium for 48 h.
Cell extracts and Western blot analysis.
Fourty-eight hours after siRNA transfection, VVEC were washed twice with ice-cold PBS and lysed with Tris·HCl buffer (40 mM pH 7.5, 4°C), containing 0.1% Triton X-100, 0.25 M sucrose, 3 mM EGTA, 3 mM EDTA, 50 μM β-mercaptoethanol, 1 mM PMSF, and complete protease inhibitor cocktail (Calbiochem, San Diego, CA). Cell lysates were centrifuged at 7,500 g for 10 min at +4°C. Equivalent amounts of total cell protein (20–40 μg) were subjected to 10% SDS-PAGE. Proteins were transferred to polyvinylidene fluoride membranes and probed with mouse monoclonal antibodies against RhoA (Santa Cruz Biotechnology, Santa Cruz, CA). After being washed with TBS-Tween buffer, membranes were incubated with donkey anti-mouse peroxidase-conjugated IgG, 1:20,000 dilution (Amersham Bioscienses, Piscataway, NJ), for 1 h at room temperature. Immunoreactive bands were detected by ECL kit (Renaissance, NEN Life Science Product, Boston, MA) followed by exposure to Hyperfilm. In all experiments, equivalent sample loading and transfer were verified by staining polyvinylidene fluoride membrane with Ponceau or probing with antibodies against β-actin.
Measurement of ATP release.
Concentration of ATP in the conditioned media was detected with luciferase-luciferin kit (ENLITEN ATP Assay System, Promega, Madison, WI) using 2020n Luminometer (Turner Biosystems, Sunnyvale, CA). Cells were plated at a density of 500 × 103 cells/cm2, growth arrested in DMEM without serum for 72 h, and exposed to either normoxic (21% O2, 5% CO2, remainder N2) or hypoxic (3% O2 or 1% O2, 5% CO2, remainder N2) conditions. Aliquots of conditioned media (75–100 μl) after 10, 30, and 60 min of incubation were collected into chilled polypropylene tubes (Sigma, St. Louis, MO) and centrifuged at 12,000 g for 10 min to remove any cell debris. Supernatants were heated at 95°C for 1 min and used for luciferin-luciferase assay according to manufacturer's specifications. The sampled luminescence was compared with an ATP standard curve performed in each individual experiment. In the experiments designed to evaluate a role of PI3K, Rho, and ROCK in hypoxia-stimulated ATP release, cells were preincubated with PI3K inhibitors LY294002 (10 μM, 60 min) and wortmannin (100 nM, 120 min); ROCK inhibitor Y27632 (10 μM, 60 min) or cells were transfected with RhoA siRNA or scrambled oligos (100 nM, 6 h; followed by recovery in DMEM/10% FBS overnight and growth arrest in a serum-free medium for 48 h). Then cells were exposed to hypoxic conditions (1% O2, 30 min) before ATP was measured. In the experiments designed to evaluate a role of vesicular transport in hypoxia-stimulated ATP release, cells were treated with monensin (1 μM), brefeldin A (20 μM), or N-ethylmaleimide (NEM; 4 μM), or vehicle for 30 min followed by exposure to hypoxic conditions (1% O2, 30 min). According to the cell viability test with trypan blue staining, none of the inhibitors used had a toxic effect on VVEC.
In vitro PI3K activity assay.
EC were cultured to 80% confluence in 100-mm2 dishes, growth-arrested in DMEM without serum for 72 h, and then stimulated with hypoxia (3% and 1% O2), ATP (100 μM), or FBS (10%) for 30 min. After stimulation, cells were washed with ice-cold PBS containing 0.2 mM activated orthovanadate and incubated in 750 μl of lysis buffer containing 137 mM NaCl, 20 mM Tris·HCl, pH 7.5, 1 mM MgCl2, 1 mM CaCl2, 10% glycerol, 1% Nonidet P-40, 1 mM sodium orthovanadate, 1 mM PMSF, and complete protease inhibitor cocktail (Calbiochem) and incubated for 20 min at +4°C. Cell lysates were then centrifuged at 10,000 g for 10 min to sediment insoluble material. Supernatants were normalized for protein content, and PI3K was immunoprecipitated using anti-p85 or anti-phospho-tyrosine antibody and Catch and Release immunoprecipitation system (Upstate Biotechnology). The lipid kinase activity in the immunoprecipitates was measured with l-α-phosphatidylinositol as a substrate as previously described (17).
Rho GTP pull-down assay.
The level of activated, GTP-bound Rho was assessed utilizing GST-Rhotekin Pulldown Assay (Pierce Biotechnology, Rockford, IL). Growth-arrested VVEC were stimulated with hypoxia (1% O2) or extracellular ATP (100 μM) for 10, 30, and 60 min. After incubation, cells were washed twice with ice-cold PBS and solubilized in 500 μl of lysis buffer. Total cell lysates (500–750 μg) were incubated with GST-tagged recombinant Rho-binding domain of Rhotekin for 45 min at 4°C on a rotating shaker. Precipitated GTP-bound Rho was detected by Western blotting using RhoA antibody. Total cell lysates from unstimulated cells were preincubated with GDP and GTPγS and were used as negative and positive controls, respectively.
DNA synthesis.
Cells were plated in 24-well plates at a density of 1.2 × 104 cells/well in DMEM supplemented with 10% FBS. On the next day, cells were rinsed with PBS and incubated in DMEM without serum for 72 h. Cells were stimulated with extracellular ATP (10−9 to 10−3 M) in the presence of 0.125 μCi of [methyl-3H]thymidine (NEN Life Science Products, Boston, MA) for 24 h. In the experiments designed to evaluate a role of RhoA and ROCK pathways, cells were preincubated with C3 exoenzyme from C. botulinum (BTC3; 0.5 μg/ml and 1.0 μg/ml, 2–4 h) (Cytoskeleton, Denver, CO) and Y27632 (10 μM, 60 min) (Cell Signaling, Danvers, MA) and then stimulated with ATP (100 μM) in the presence of 0.125 μCi of [methyl-3H]thymidine for 24 h. Incorporated radioactivity was determined as previously described (17). The experiments were conducted under normoxic (21% O2, 5% CO2, remainder N2) conditions.
Migration assay.
Growth-arrested VVEC (1.0 × 105 cells/well) were plated in 200 μl of serum-free DMEM in permeable cell culture inserts (8.0 μM pore size, Costar, Milpitas, CA) precoated with 0.1% gelatin (Sigma, St. Louis, MO). ATP (100 μM) was added in the lower chamber containing 800 μl of serum-free DMEM. To evaluate the contribution of RhoA and ROCK to VVEC migration, cells in transwells were preincubated with cell-permeable ADP-ribosyltransferase, BTC3 (0.5 μg/ml and 1.0 μg/ml, 2–4 h), Y27632 (10 μM, 60 min), or transfected with RhoA siRNA oligos. After 24-h incubation under normoxic (21% O2, 5% CO2, remainder N2) conditions, cells remaining on the upper surface of the filter were wiped off, and migrated cells were fixed with methanol for 15 min and stained with 0.2% crystal violet in 2% (vol/vol) ethanol for a minimum of 15 min. Cells migrated through the filter were photographed under ×40 magnification of a phase contrast microscope in six random fields.
Tube formation assay.
Growth-arrested VVEC (1.25 × 105 cells/well in 24-well plate) were preincubated with BTC3 (1.0 μg/ml, 4 h) or Y27632 (10 μM, 60 min) or transfected with RhoA siRNA oligos and plated on growth factor reduced Matrigel in serum-free DMEM either with or without ATP (100 μM). Cells were incubated for 8–10 h, and photographs were taken in three fields with a phase contrast microscope at ×10 magnification. Images were analyzed using S.CORE Image Analysis (S.CO LifeScience).
Visualization of filamentous actin.
Cells were plated on Lab-Tek Chamber slides (Nunc, Rochester, NY) at 1.5 × 105 cells/well and growth arrested in DMEM for 72 h. BTC3 cell-permeable toxin was added at a concentration of 0.5–2 μg/ml for 2 h. In parallel, Y27632 (Calbiochem) was added at a concentration of 1–10 μM for 60 min. Cells were then washed with PBS and fixed with 4% paraformaldehyde for 30 min. Fixed cells were permeabilized with 0.1% Triton-X100 for 5 min at room temperature and incubated with 100 nM rhodamine phalloidin (Cytoskeleton) for 30 min. Images were taken with a Zeiss fluorescent microscope using the AxioVision digital imaging system at ×100 and ×400 magnification (Carl Zeiss MicroImaging, Thornwood, NY). Images were analyzed using AxioVision software.
Statistical analysis.
For the analysis of variances between groups of data, Dunnett test or Bonferroni test followed by one-way ANOVA were performed using GraphPad Prism 3.0 (GraphPad Software). Density of radioactive spots on TLC chromatography and protein bands from Western blot images were quantified using NIH ImageJ program. Data are expressed as means ± SE; n equals the number of replicates in one experiment or a number of observations in independent experiments. A P value of <0.05 was considered statistically significant.
RESULTS
Hypoxia stimulates ATP release from VVEC in a time- and concentration-dependent manner.
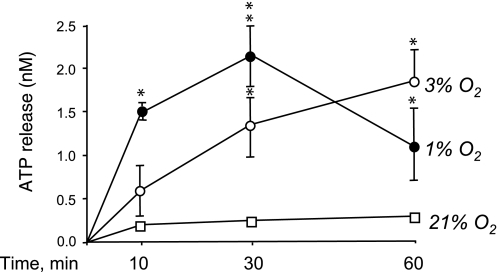
Since our laboratory recently reported that extracellular ATP exerts dramatic angiogenic effects on VVEC (17), we sought to determine whether hypoxia induces ATP release from VVEC. As shown in Fig. 1, stimulation of VVEC with 3% O2 and 1% O2 resulted in a time-dependent accumulation of ATP in the conditioned media. In 1% O2-stimulated cells, the maximal response (∼10-fold over normoxic control) was observed at 30 min of hypoxic exposure followed by a decrease in ATP accumulation in the media at 60 min of exposure. In 3% O2-stimulated cells, more prolonged ATP accumulation in the conditioned media was observed with the maximal effect (∼9.3-fold) at 60 min of hypoxic exposure. In contrast, under normoxic conditions (21% O2), no significant ATP accumulation was detected in the conditioned media during the course of incubation.
Fig. 1.
Hypoxia stimulates ATP release from vasa vasorum endothelial cells (VVEC). VVEC were growth arrested (72 h) in serum-free media and exposed to either normoxic (21% O2) or hypoxic (3% O2 and 1% O2) conditions. ATP concentration was measured in the aliquots of conditioned media from VVEC at 0, 30, and 60 min exposure using ENLITEN ATP Assay System. Data are means ± SE (*P < 0.05, **P < 0.01 vs. normoxic controls) from three to five independent experiments conducted on two distinct cell populations.
The inhibitors of vesicular transport attenuate hypoxia-induced ATP release from VVEC.
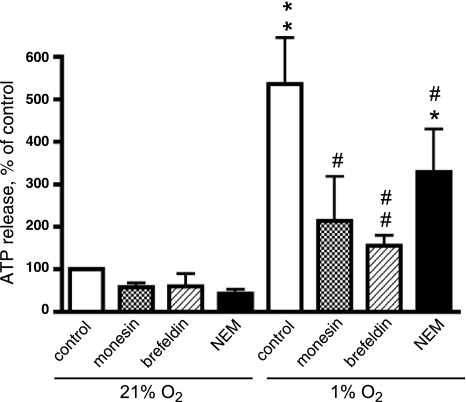
In addition to voltage-regulated anion channels and connexins, vesicular exocytosis has been proposed as a potential mechanism of ATP release in vascular endothelium (31, 39). To test this possibility, we examined the effect of pharmacological compounds known to act at different stages of vesicular transport on hypoxia-induced ATP release. As shown in Fig. 2, treatment of VVEC with monensin (1 μM), an inhibitor of vesicular transport at the level of Golgi complex, reduced hypoxia-stimulated ATP release by 65%. A similar inhibitory (up to 79%) effect was observed when cells were treated with brefeldin A (20 μM), an inhibitor of vesicular trafficking from the endoplasmic reticulum to the Golgi complex. In addition, we found that NEM (4 μM), an inhibitor of vesicle fusion with plasma membrane, attenuated hypoxia-induced ATP release by 46%. Together, these observations suggest that vesicular exocytosis is likely involved in endothelial response to hypoxia and, therefore, may contribute to ATP-mediated autocrine signaling in VVEC angiogenesis.
Fig. 2.
Inhibitors of vesicular transport attenuate hypoxia-induced ATP release from VVEC. Growth arrested cells (72 h, serum-free DMEM) were treated with the inhibitors of vesicular transport, monensin (1 μM), brefeldin A (20 μM), N-ethylmaleimide (4 μM), or vehicle for 30 min and were exposed to hypoxic conditions (1% O2, 30 min). Extracellular ATP was determined in the aliquots of the conditioned media using luciferin-luciferase assay. A value of 100% indicates ATP release under normoxic conditions [21% O2, 30 min (0.34 nM ATP)]. Data are means ± SE of at least three independent experiments performed in triplicates on three distinct VVEC populations. **Significant difference vs. nonstimulated control (P < 0.01). Significant difference vs. hypoxia-stimulated cells: #P < 0.05; ##P < 0.01; *P < 0.05.
Hypoxia and extracellular ATP activate PI3K and Rho in VVEC.
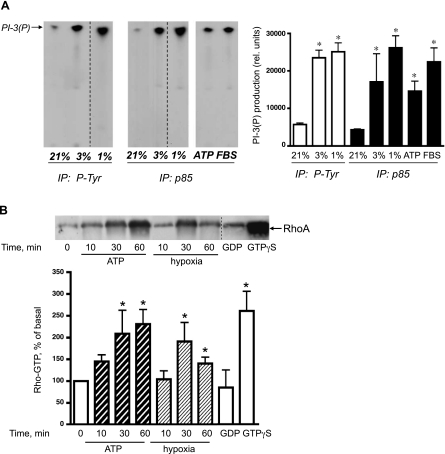
PI3K and Rho/ROCK participate in vesicular transport and cytoskeleton remodeling in a wide variety of cell types (43, 55). Since in vascular cells PI3K and Rho/ROCK are sensitive to hypoxic stimulation, we examined whether these pathways were activated by hypoxia in VVEC and whether the activation of these pathways contributes to hypoxia-induced ATP release. To determine the effect of hypoxia on PI3K activity, we used an in vitro PI3K activity assay. Using this approach, we found that 30-min exposure of growth-arrested VVEC to hypoxia (3% and 1% O2) resulted in a significant activation of PI3K (Fig. 3A). The degree of hypoxia-induced PI3K activation was measured as accumulation of phosphatidylinositol-3′-phosphate in phospho-tyrosine (P-Tyr) and PI3K p85 regulatory subunit (p85) immunoprecipitated and reached 4.2- and 3.9-fold, respectively, in response to 3% O2; and 4.5- and 5.8-fold, respectively, in response to 1% O2. These responses were comparable in magnitude to the responses elicited by extracellular ATP (100 μM) and 10% FBS (3.3-fold and 5.1-fold, respectively) used in these studies as positive controls.
Fig. 3.
Hypoxia and extracellular ATP induce activation of PI3K and Rho/ROCK pathways in VVEC. A: hypoxia and extracellular ATP increase PI3K activity in VVEC. Growth-arrested VVEC (72 h, serum-free DMEM) were stimulated with hypoxia (3% and 1% O2), ATP (100 μM), or 10% FBS for 30 min. Equivalent amounts of total cell protein (500–750 μg) were immunoprecipitated with rabbit polyclonal antibodies against phospho-tyrosine (P-Tyr) or against the p85 regulatory subunit of PI3K. Activity was measured in in vitro kinase assay with a l-α-phosphatidylinositol (PtdIns) as a substrate. Arrow indicates the accumulation of PtdIns(3)P, a product of PI3K activity; dashed lines on the composed image separate samples from the same experiment. B: hypoxia and extracellular ATP increase Rho-GTP accumulation in VVEC. Growth-arrested cells were exposed to hypoxia (1% O2) or extracellular ATP (100 μM) for 10, 30, and 60 min. Equivalent amounts of total cell protein (500–650 μg) were subjected to Rhotekin Rho-GTP pull-down assay as described in materials and methods. GDP is “negative” control; lysates of nonstimulated cells were incubated with 100 μM GDP (30 min, 30°C) followed by pull-down assay. GTPγS is “positive” control; lysates of nonstimulated cells were incubated with 100 μM GTPγS (30 min, 30°C) followed by pull-down assay. Top: the data from one representative experiment. Bottom: the results from four independent experiments. *Significant difference vs. nonstimulated control (P < 0.05).
The products of PI3K activity, 3-d-phosphoinositides, can directly interact with and activate Rho family GEFs, facilitating a transition of Rho proteins to the active GTP-bound form (55). Using a Rho pull-down assay and Western blot analysis, we found that stimulation of growth-arrested VVEC with hypoxia (3% and 1% O2) resulted in a time-dependent accumulation of GTP-bound Rho in rhotekin-agarose precipitates. Significant effects were observed at 30 and 60 min of hypoxic exposure with a greater response at 30 min (Fig. 3B). Using the same experimental approach, we found that stimulation of growth-arrested VVEC with extracellular ATP (100 μM) also resulted in the accumulation of GTP-bound Rho (Fig. 3B). Similar to hypoxia, extracellular ATP induced significant increases in the accumulation of Rho-GTP at 30 and 60 min; however, a greater response was observed at 60 min.
PI3K and Rho/ROCK pathways are involved in hypoxia-induced ATP release from VVEC.
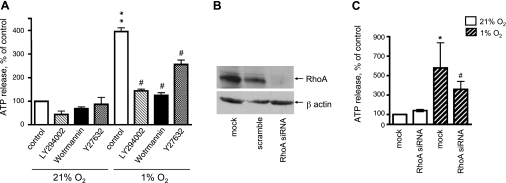
The evidence that hypoxia induces both ATP release and activation of the PI3K and Rho/ROCK pathways in VVEC suggests that PI3K, Rho, and ROCK may be involved in hypoxia-induced ATP release. To test this hypothesis, growth-arrested VVEC were treated with PI3K inhibitors LY294002 (20 μM, 60 min) and wortmannin (100 nM, 120 min) and the ROCK inhibitor Y27632 (10 μM, 60 min) and exposed to hypoxic conditions. Hypoxia-induced accumulation of ATP in conditioned media was significantly attenuated by LY294002, wortmannin, and Y27632 by 66, 80, and 41%, respectively, pointing out that PI3K and ROCK play a role in hypoxia-induced ATP release (Fig. 4A). To dissect a role of Rho in this process, we designed siRNA. Transfection of VVEC with siRNA suppresses endogenous RhoA in VVEC up to 95% (Fig. 4B) and decreased hypoxia-induced ATP release by ∼56% (Fig. 4C). The greater inhibitory effects of wortmannin and LY294002 compared with Y27632 and RhoA siRNA on hypoxia-induced ATP release suggest that, in VVEC, PI3K may contribute to ATP release through RhoA and ROCK as well as through other, as yet unknown, intracellular targets.
Fig. 4.
PI3K and Rho/ROCK pathways play a critical role in hypoxia-induced ATP release from VVEC. A: growth arrested VVEC were preincubated with LY294002 (10 μM, 60 min), wortmannin (100 nM, 120 min), Y27632 (10 μM, 60 min), or vehicle and exposed to hypoxic conditions (1% O2, 30 min). Accumulation of extracellular ATP was measured in the samples of conditioned media using luciferin-luciferase assay (**P < 0.01 vs. nonstimulated control; #P < 0.05, vs. hypoxia-stimulated cells). 100% (control) indicates ATP release under normoxic conditions [21% O2, 30 min (0.93 nM ATP)]. B–C: cells were transfected with RhoA siRNA, scramble oligos (100 nM, 6 h), or remained mock-transfected. Fourty-eight hours posttransfected growth-arrested cells were exposed to hypoxic conditions (1% O2, 30 min), and samples were processed for ATP measurements. Western blot analysis of Rho expression in total cell lysates demonstrates the efficacy of RhoA siRNA knockout technique in VVEC. Data are means ± SE of six independent experiments performed in triplicate on two distinct cell populations. *Significant difference vs. nonstimulated control (P < 0.05). #Significant difference vs. hypoxia-stimulated cells (P < 0.05).
Rho and ROCK play a critical role in extracellular ATP-induced DNA synthesis in VVEC.
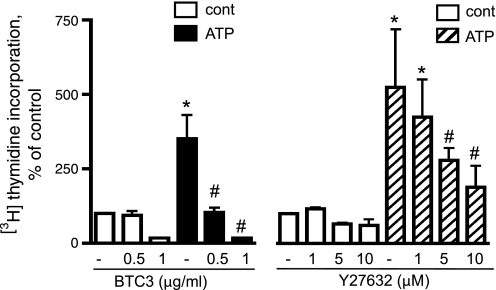
We recently reported a critical role of PI3K in extracellular ATP-induced VVEC proliferation and migration (17). Because extracellular ATP also dramatically activates the Rho/ROCK pathway, we further examined whether Rho and/or ROCK contributes to ATP-induced mitogenic responses in VVEC. As shown in Fig. 5, extracellular ATP dramatically increased DNA synthesis in growth-arrested VVEC. Pretreatment of VVEC with BTC3 (0.5–1.0 μM, 2–4 h), which inactivates Rho through ADP-ribosylation, completely attenuated ATP-induced DNA synthesis. BTC3 has been used in these studies as a tool to inactivate RhoA protein, since it was found that transfection of VVEC with RhoA siRNA significantly decreases cell attachment when cells were plated at a density lower than 1.2 × 104/cm2. Pretreatment of VVEC with ROCK inhibitor Y27632 (1–10 μg/ml, 60 min) attenuated ATP-induced DNA synthesis by 34–72% (Fig. 5), implying involvement of ROCK in mediating the mitogenic effects of extracellular ATP in these cells.
Fig. 5.
Rho and ROCK pathways play a critical role in extracellular ATP-induced DNA synthesis in VVEC. Growth-arrested VVEC were preincubated with either Y27632 (1–10 μM, 60 min) or BTC3 (0.5–1 μg/ml, 2–4 h) followed by stimulation with ATP (100 μM) in the presence of 0.125 μCi [3H]thymidine for 24 h. Results are expressed as percentage of basal thymidine incorporation in control cells. Data are means ± SE from three to five independent experiments conducted on two distinct cell populations. *Significant difference vs. nonstimulated control (P < 0.05). #Significant difference vs. ATP-stimulated cells (P < 0.05).
It should be mentioned that, although inactivation of Rho and ROCK proteins can induce apoptosis in several cell types including endothelium, using an Annexin V binding assay we found that not more than 12% and 10% of apoptotic cells can be detected after VVEC were treated with BTC3 (1 μg/ml, 4 h) and Y27632 (10 μM, 24 h), respectively. Under the same conditions, caspase-3 immunostaining revealed 14% and 8.5% of apoptotic cells in the VVEC population (supplementary Fig. 1 available online at the AJP Lung website). These observations suggest that inhibitory effects of BTC3 and Y27632 on ATP-stimulated angiogenic responses are not explained by VVEC apoptosis.
Rho and ROCK are key signaling components in extracellular ATP-induced VVEC migration.
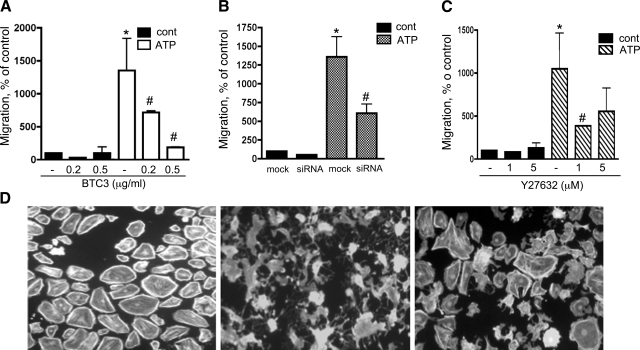
To further evaluate the role of Rho/ROCK in angiogenic responses in VVEC, we determined the contribution of this pathway to ATP-induced VVEC migration, a process that includes cytoskeletal remodeling and changes in cell shape. As shown in Fig. 6, extracellular ATP (100 μM) stimulates VVEC migration up to 11.5-fold. Treatment with 0.2 and 0.5 μg/ml BTC3 for 4 h decreased ATP-induced migration by 46% and 93%, respectively (Fig. 6A). Consistent with these observations, transfection of VVEC with RhoA siRNA (100 nM, 6 h) decreased ATP-induced VVEC migration by 67% (Fig. 6B). Treatment of VVEC with the ROCK inhibitor Y27632 (1 and 5 μg/ml, 60 min) decreased ATP-induced migratory responses in VVEC by 70% and 46%, respectively, indicating that changes in cell contractility and/or cytoskeleton rearrangements mediated by ROCK play a critical role in controlling endothelial cell migration (Fig. 6C). The effect of BTC3 (0.5 μg/ml, 4 h) and Y27632 (5 μM, 1 h) treatment on actin cytoskeleton remodeling in VVEC was visualized using rhodamine phalloidin staining (Fig. 6D). Treatment with BTC3 and Y27632 resulted in disassembly of actin filaments and visible morphological changes in VVEC. The effect of BTC3 on VVEC morphology was more dramatic than the effect of Y27632. In agreement with a previously reported role for actin cytoskeleton rearrangements in regulating DNA synthesis and migration in vascular smooth muscle cells, these observations indicate that both Rho and ROCK are critically involved in ATP-induced angiogenic responses in VVEC.
Fig. 6.
Rho and ROCK pathways play a critical role in extracellular ATP-induced VVEC migration. A–C: growth-arrested VVEC (1.0 × 105cells/well) were plated on top of inserts in serum free DMEM. Cells were preincubated with either Y27632 (10 μM, 60 min) or BTC3 (1–2 μg/ml, 2–4 h) or transfected with RhoA siRNA or scrambled oligos (100 nM, 6 h) as described in materials and methods. Cell migration was stimulated by adding ATP (100 μM) in the lower transwell compartment. At the end of incubation, cells on the bottom of filters were fixed and counted in three fields at ×10 magnification. Quantitative data for each experimental condition represent the means ± SE. Similar results were reproduced in at least three experiments on three distinct cell populations. *Significant difference vs. nonstimulated control (P < 0.05). #Significant difference vs. ATP-stimulated cells (P < 0.05). D: treatment of VVEC with Rho inhibitor BTC3 (0.5 μg/ml, 2 h) and ROCK inhibitor Y27632 (5 μM, 60 min) results in disassembly of actin cytoskeleton. Actin fibers were visualized by rhodamine phalloidin staining.
RhoA and ROCK play a key role in extracellular ATP-induced VVEC tube formation on matrigel.
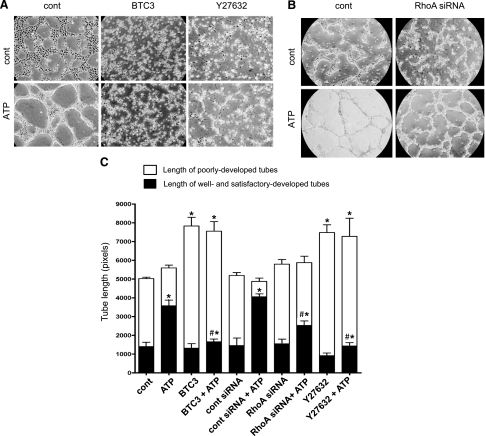
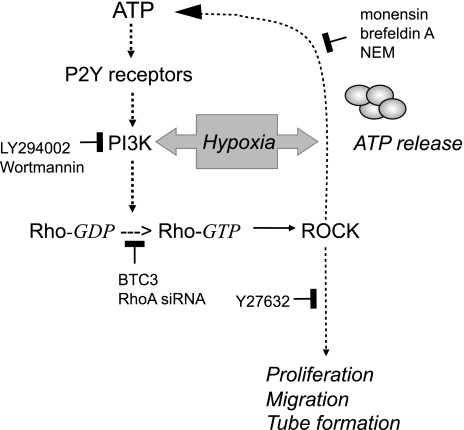
Morphogenetic changes in EC are necessary to neovessel formation. To further delineate a signaling role of RhoA and ROCK in ATP-induced angiogenic responses in VVEC, we examined whether RhoA and ROCK contribute to VVEC tube formation in matrigel. For these studies, growth-arrested VVEC were treated with cell-permeable BTC3 (1.0 μg/ml, 4 h) and Y27639 (10 μM, 1 h) or transfected with RhoA siRNA (100 nM, 6 h). As shown in Fig. 7, plating VVEC on growth factor-reduced (GFR) matrigel in a serum-free medium in the presence of extracellular ATP (100 μM) resulted in profound cell rearrangements into tube-like networks within 6–10 h. In response to stimulation with extracellular ATP, the ratios between the length of well and satisfactorily developed tubes and the length of poorly developed tubes were markedly increased (“cont” vs. “ ATP” and “cont siRNA” vs. “cont siRNA + ATP”); however, no significant changes in total tube length were observed. Inactivation of Rho and ROCK by treatment with BTC3 or Y27632 prevented ATP-induced formation of well and satisfactorily developed tubes, whereas higher numbers of poorly developed tubes were observed under both basal and ATP-stimulated conditions. We also found that treatment with BTC3 or Y2763 resulted in increases in total number of tubes under both basal and ATP-stimulated conditions, consistent with VVEC rearrangements into tubular networks characterized by multiple, poorly developed cellular interconnections (Fig. 7A). Inhibition of RhoA by siRNA had significant but lesser inhibitory effect on ATP-induced tube formation and did not result in the increased numbers of poorly developed tubular networks. Together, these observations suggest that Rho- and ROCK-mediated cytoskeleton rearrangement and possibly contractile responses play a critical role in ATP-induced tube formation and that, in addition to RhoA, the other Rho protein family members may contribute to VVEC morphogenesis (Fig. 8).
Fig. 7.
Extracellular ATP induces VVEC tube-like network formation through activation of Rho and ROCK. A and B: growth-arrested VVEC (1.25 × 105 cells/well) were plated on growth factor-reduced Matrigel in serum-free DMEM. Cells were preincubated with Y27632 (10 μM, 60 min) or BTC3 (1 μg/ml, 4 h) or transfected with RhoA siRNA or scramble oligos (100 nM, 6 h) as described in materials and methods. Formation of tube-like networks was stimulated by the addition ATP (100 μM). At the end of incubation, images were captured in three fields using AxioVision program. The data show one representative image for each experimental condition. C: quantitative tube formation evaluation using S.CORE Image Analysis. Data represent means ± SE of three to five independent experiments for each experimental conditions. *Significant difference vs. nonstimulated control (P < 0.05). #Significant difference vs. ATP-stimulated cells (P < 0.05).
Fig. 8.
Schematic diagram illustrating a role of PI3K, Rho, and ROCK pathways in hypoxia-induced ATP release and ATP-mediated angiogenic effects in VVEC. Activation of PI3K/Rho/ROCK pathway in response to hypoxia results in regulated ATP release from VVEC. In turn, extracellular ATP triggers/initiates P2 receptor-dependent activation of PI3K/Rho/ROCK pathway, leading to angiogenic responses in VVEC such as proliferation, migration, and tube formation.
DISCUSSION
We previously reported that extracellular ATP is a potent regulator of the angiogenic activity of VVEC derived from the PA adventitia of chronically hypoxic animals. However, whether VVEC themselves represent a source of extracellular ATP was unknown. Our present study demonstrates that hypoxia induces ATP release in VVEC, and it is very likely that this process may involve vesicular exocytosis and the activation of PI3K and Rho/ROCK pathways. We found that, in addition to ATP release, PI3K and Rho/ROCK are also involved in ATP-induced angiogenic responses in VVEC. This observation provides novel evidence for the molecular mechanisms that link hypoxia-induced elevated extracellular ATP levels and the vasa vasorum neovascularization observed in chronically hypoxic animals.
Accumulating evidence suggests that extracellular nucleotides play an autocrine/paracrine role in a variety of cellular systems. It was found that, under physiological and pathological conditions, a majority of intact endothelial and epithelial cells constitutively release ATP in the absence of cell lysis and that the rate of nonlytic ATP release can be gradually increased by a variety of external stimuli (31). EC in the pulmonary and systemic vasculature have been characterized as a rich source of extracellular ATP (49). Whereas it has been shown that fluid shear stress and low osmolarity are major and potent physiological stimuli capable of inducing ATP release in EC (4, 30), very limited information exists regarding the effect of hypoxia on this response. It has been reported that shear stress and hypoxia may act synergistically to stimulate ATP release from HUVEC (4), although signaling mechanisms underlying this response have not been elucidated. Here, we show for the first time that VVEC isolated from the PA adventitia represent a potent source of extracellular ATP. Moreover, hypoxia-induced ATP release is regulated in a time- and O2-dependent manner.
The predominant mechanisms of ATP release in vascular EC involve VRACs, connexins, and vesicular exocytosis (15, 19, 22, 38, 39). Vesicular exocytosis is the mechanism of Weibel-Pallade body and P-selectin secretion in EC in responses to vascular damage and inflammation (32). Vesicular ATP release has also been demonstrated in nonvascular cell types, including astrocytes, osteoblasts, and pancreatic acini (12, 46, 48, 51). Intriguingly, regulated exocytosis is utilized by neurons as the mechanism of synaptic neurotransmission. The existing parallel growth pattern of nerves and blood vessels and their ability to form network structures suggests that both vascular and nerve cells may have developed similar or overlapping regulatory mechanisms for mediator release that may promote signal transmission along the vascular and nerve tree. The observed inhibitory effects of monensin, brefeldin A, and NEM (the pharmacological agents that block different stages of vesicular transport) suggest that hypoxia-induced ATP release in VVEC most likely occurs through regulated exocytosis, which involves ATP mobilization from Golgi-derived secretory vesicles and is regulated by a N-ethylmaleimide-sensitive factor-dependent mechanism. Using the fluorescent dye quinacrine, we also found that, in VVEC, intracellular ATP stores are localized around the nuclei and to the cytosol (Gerasimovskaya EV, unpublished observations). Similarly, vesicular ATP stores have been identified in HUVEC and were reduced in response to shear stress (5). Together, these data support the idea that ATP exocytosis may be a common mechanism utilized by EC from different vascular beds. Recently, it has been demonstrated that Cx43 hemichannels contribute to the basal level of ATP release in confluent, not growth-arrested, lung microvascular EC. Under such conditions, Cx43-mediated ATP release was inhibited by hypoxia (15). These findings point out that regulation of ATP release through different cellular mechanisms occurs in a stimulus- and cell-type-specific manner. A variety of experimental conditions may create an apparent variability of ATP release responses observed in vitro. However, it cannot be excluded that ATP release from EC through exocytosis and Cx may be differentially regulated by hypoxia and may utilize discrete intracellular ATP “pools” or compartments.
Our studies demonstrate that both hypoxia and extracellular ATP activate PI3K and Rho/ROCK pathways in VVEC. Similar to our previous observations in PA adventitial fibroblasts (18), data presented here suggest an autocrine-paracrine role of ATP in mediating hypoxia-induced angiogenic activation of VVEC. In addition to the previously reported role of PI3K in VVEC angiogenesis (17), our data show that PI3K is essential for hypoxia-induced ATP release from VVEC. Complementary to our findings, studies by others demonstrated that PI3K contributes to volume-sensitive ATP release from biliary epithelial cells (32), TNF-α-stimulated elastase release from neutrophils (4), and allergen-IgE-induced cytokine release from mast cells (2). In cultured chromaffin cells, PI3K is involved in regulation of exocytosis through subplasmalemmal actin rearrangements necessary for catecholamine secretion (10). As mentioned above, the involvement of PI3K in exocytosis is mediated by the Rho family GEFs and FYVE zinc finger domain proteins, which serve various functions in membrane trafficking, cytoskeletal regulation, and signal transduction (55, 58). The results of our study show that stimulation of growth-arrested VVEC with hypoxia and extracellular ATP leads to accumulation of activated RhoA. Inhibition of RhoA by siRNA and inhibition of ROCK by its selective inhibitor Y27632 resulted in significant attenuation of hypoxia-induced ATP release, pointing out that, in VVEC, RhoA and ROCK play a key role in hypoxia-induced ATP release. The lesser inhibitory effect of RhoA siRNA than BTC3 on hypoxia-induced ATP release may indicate that other members of the Rho protein family may be involved in hypoxia-induced ATP release. Complementary to our observations, studies on bovine and human EC showed that PI3K, ROCK, FAK, and tyrosine kinases are critical signaling components of the ATP release pathway in response to osmotic stress (21, 30). Similar to EC, in astrocyte cell line 132N1, activation of Rho-GTPases was found to contribute to thrombin- and LPS-stimulated ATP release (3).
Previous studies have demonstrated that cytoskeletal rearrangements are important for EC organization during angiogenesis (23). HUVEC overexpressing RhoA exhibited higher migratory and tube-forming activity than nontransfected cells, linking Rho activation and morphogenetic responses associated with angiogenesis (63). Moreover, suppression of the Rho/ROCK pathways inhibited angiogenesis both in vitro and in vivo (54). Importantly, activation of PI3K/Rho/ROCK signaling cascade has been demonstrated for hypoxia-induced c-Myc-dependent, but HIF-1α-independent, expression of VEGF, as well as the involvement of RhoA and ROCK in VEGF-induced microvascular EC migration and angiogenesis (35, 56). Rho and ROCK have been shown to contribute to EC proliferation mediated by VRAC activation and also to thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration (37, 50). Our findings demonstrate that both hypoxia and extracellular ATP result in a considerable activation of PI3K, Rho, and ROCK in VVEC. In addition to their role in ATP release, Rho and ROCK are essential to extracellular ATP-induced DNA synthesis, migration, and VVEC morphogenic responses (Fig. 8). Eliminating RhoA expression by siRNA or Rho inactivation by BTC3 significantly attenuated angiogenic responses in VVEC. Inhibition of ROCK had slightly lesser inhibitory effect on all angiogenic responses, suggesting that, in VVEC, Rho proteins may operate through additional target proteins. According to the role of actin cytoskeleton remodeling in endothelial cell morphogenesis, proliferative, and migratory responses, our studies show that disassembly of VVEC actin fibers by treatment with BTC3 and Y27632 correlated with the inhibitory effect of these compounds on ATP-induced angiogenic responses. There is also one study demonstrating an involvement of Rho and ROCK in TNF-α-induced inhibition of HUVEC proliferation. However, no effect of TNF-α on actin remodeling has been observed (29).
The nature of molecular mechanisms underlying ATP release and ATP-induced angiogenic responses in VVEC requires further investigation. For example, studies addressing interaction between Rho/ROCK, ERK1/2, and FAK may provide a link between proliferative, morphogenetic, and secretory responses in VVEC (45). Of particular interest are studies demonstrating that ATP release and its autocrine feedback signaling via P2Y2 and adenosine A3 receptors create signal amplification and chemotactic gradient sensing during neutrophil migration (11). Recently, it has been shown that, in migrating fibroblasts, exocytosis occurs at the leading edge, providing a membrane surface for forward protrusion (28). With this in mind, it is quite possible that, under hypoxic conditions, ATP exocytosis in migrating VVEC may occur toward a hypoxic gradient and would direct neovessel growth through ATP autocrine/paracrine signaling.
In summary, our findings emphasize the biological significance of ATP as a fundamental signaling molecule in hypoxia-induced vasa vasorum expansion. We demonstrate that VVEC represent a potent source of extracellular ATP in the PA wall and that PI3K and Rho/ROCK pathways are the critical signaling components of the autocrine/paracrine signaling loop linking ATP release and ATP-induced angiogenic responses in VVEC.
GRANTS
This work was funded by American Heart Association Grant 0665464Z (to E. V. Gerasimovskaya), by National Heart, Lung, and Blood Institute Grants R01 HL-086783 (to E. V. Gerasimovskaya), PPG HL-14985 (to K. R. Stenmark), and T32-HL-007171-32 (to A. Anwar).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Stephen Hofmeister for the help with lung tissue collection. We thank Prof. John Stewart for the critical reading of the manuscript.
Preliminary results were presented in abstracts at Aspen Lung Conference, 47th Annual Meeting, Aspen, CO, 2004; Vascular Biology Developmental Workshop, Asilomar, CA, 2004; and the Experimental Biology Meeting, San Diego, CA, 2004.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature 431: 1007–1011, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Blum AE, Joseph SM, Przybylski RJ, Dubyak GR. Rho-family GTPases modulate Ca2+-dependent ATP release from astrocytes. Am J Physiol Cell Physiol 295: C231–C241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodin P, Burnstock G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Experientia 51: 256–259, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol 38: 900–908, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem 71: 537–592, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Bours MJL, Swennen ELR, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Therapeutics 112: 358–404, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Bryan BA, D'Amore PA. Rho-GTPase control of angiogenesis. Cell Mol Life Sci 64: 2053–2065, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol 22: 364–373, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Chasserot-Golaz S, Hubert P, Thiersé D, Dirrig S, Vlahos CJ, Aunis D, Bader MF. Possible involvement of phosphatidylinositol 3-kinase in regulated exocytosis: studies in chromaffin cells with inhibitor LY294002. J Neurochem 70: 2347–2356, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem 278: 1354–1362, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Croft DR, Sahai E, Mavria G, Li S, Tsai J, Lee WM, Marshall CJ, Olson MF. Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res 64: 8994–9001, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol 168: 1793–1807, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One 3: e2801, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatof D, Kilic G, Fitz JG. Vesicular exocytosis contributes to volume-sensitive ATP release in biliary cells. Am J Physiol Gastrointest Liver Physiol 286: G538–G546, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Gerasimovskaya EV, Woodward HN, Tucker DA, Stenmark KR. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis 11: 169–182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J Biol Chem 277: 44638–44650, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci 46: 1208–1218, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol 4: 285–294, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Hirakawa M, Oike M, Karashima Y, Ito Y. Sequential activation of RhoA and FAK/paxillin leads to ATP release and actin reorganization in human endothelium. J Physiol 558: 479–488, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M. Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J Gen Physiol 119: 511–520, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoang MV, Whelan MC, Senger DR. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci USA 101: 1874–1879, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Idzko M, Hammad H, van Nimwegen Kool M M, Willart MAM, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Jr, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nature Med 13: 913–919, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Jumblatt MM. Autocrine regulation of corneal endothelium by prostaglandin E2. Invest Ophthalmol Vis Sci 35: 2783–2790, 1994 [PubMed] [Google Scholar]
- 27.Kato H, Shichiri M, Marumo F, Hirata Y. Adrenomedullin as an autocrine/paracrine apoptosis survival factor for rat endothelial cells. Endocrinology 138: 2615–2620, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Kay RR, Langridge P, Traynor D, Hoeller O. Changing directions in the study of chemotaxis. Nat Rev Mol Cell Biol 9: 455–463, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Kishore R, Qin G, Luedemann C, Bord E, Hanley A, Silver M, Gavin M, Yoon YS, Goukassian D, Losordo DW. The cytoskeletal protein ezrin regulates EC proliferation and angiogenesis via TNF-alpha-induced transcriptional repression of cyclin A. J Clin Invest 115: 1785–1796, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyama T, Oike M, Ito Y. Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol 532: 759–769, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785–795, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med 15: 302–308, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Mayer H, Bertram Lindenmaier W H, Korff Weber T, H, Weich H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem 95: 827–839, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Merajver SD, Usmani SZ. Multifaceted role of Rho proteins in angiogenesis. J Mammary Gland Biol Neoplasia 10: 291–298, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Mizukami Y, Fujiki K, Duerr EM, Gala M, Jo WS, Zhang X, Chung DC. Hypoxic regulation of vascular endothelial growth factor through the induction of phosphatidylinositol 3-kinase/Rho/ROCK and c-Myc. J Biol Chem 281: 13957–1363, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol 44: 2293–2300, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Nilius B, Voets T, Prenen J, Barth H, Aktories K, Kaibuchi K, Droogmans G, Eggermont J. Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. J Physiol 516: 67–74, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilius B, Eggermont J, Droogmans G. Inhibition of angiogenesis by blockers of volume-regulated anion channels. Cell Physiol Biochem 10: 313–320, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Oike M, Droogmans G, Ito Y. ATP release pathways in vascular endothelial cells. Nippon Yakurigaku Zasshi 123: 403–411, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Olson MF, Paterson HF, Marshall CJ. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature 394: 295–259, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Paik DC, Fu C, Bhattacharya J, Tilson MD. Ongoing angiogenesis in blood vessels of the abdominal aortic aneurysm. Exp Mol Med 36: 524–533, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem 282: 28749–28758, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol 11: 471–477, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res 75: 649–658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renshaw MW, Toksoz D, Schwartz MA. Involvement of the small GTPase rho in integrin-mediated activation of mitogen-activated protein kinase. J Biol Chem 271: 21691–21694, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Romanello M, Codognotto A, Bicego M, Pines A, Tell G, D'Andrea P. Autocrine/paracrine stimulation of purinergic receptors in osteoblasts: contribution of vesicular ATP release. Biochem Biophys Res Commun 331: 1429–1438, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Rostovtseva T, Colombini M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. M Biophys J 72: 1954–1962, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadhu C, Dick K, Tino WT, Staunton DE. Selective role of PI3K delta in neutrophil inflammatory responses. Biochem Biophys Res Commun 308: 764–769, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Schwiebert LM, Rice WC, Kudlow BA, Taylor AL, Schwiebert EM. Extracellular ATP signaling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am J Physiol Cell Physiol 282: C289–C301, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Seasholtz TM, Majumdar M, Kaplan DD, Brown JH. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ Res 84: 1186–1193, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem 276: 32925–32932, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Tang Y, Olufemi L, Wang MT, Nie D. Role of Rho GTPases in breast cancer. Front Biosci 13: 759–776, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Uchida S, Watanabe G, Shimada Y, Maeda M, Kawabe A, Mori A, Arii S, Uehata M, Kishimoto T, Oikawa T, Imamura M. The suppression of small GTPase rho signal transduction pathway inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun 269: 633–640, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res 253: 239–254, 1999 [DOI] [PubMed] [Google Scholar]
- 56.van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VW. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol 23: 211–217, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Weber JD, Hu W, Jefcoat SC, Jr, Raben DM, Baldassare JJ. Ras-stimulated extracellular signal-related kinase 1 and RhoA activities coordinate platelet-derived growth factor-induced G1 progression through the independent regulation of cyclin D1 and p27. J Biol Chem 272: 32966–32971, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Wurmser AE, Gary JD, Emr SD. Phosphoinositide 3-kinases and their FYVE domain-containing effectors as regulators of vacuolar/lysosomal membrane trafficking pathways. J Biol Chem 274: 9129–9132, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, Ando J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol 285: H793–H803, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto K, Shimizu N, Obi S, Kumagaya S, Taketani Y, Kamiya A, Ando J. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am J Physiol Heart Circ Physiol 293: H1646–H1653, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Yang S, Cheek DJ, Westfall DP, Buxton IL. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res 74: 401–407, 1994 [DOI] [PubMed] [Google Scholar]
- 62.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783: 673–694, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Zhao L, Xu G, Zhou J, Xing H, Wang S, Wu M, Lu YP, Ma D. The effect of RhoA on human umbilical vein endothelial cell migration and angiogenesis in vitro. Oncol Rep 15: 1147–1152, 2006 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.