Abstract
Peroxiredoxin 6 (Prdx6), an enzyme with glutathione peroxidase and PLA2 (aiPLA2) activities, is highly expressed in respiratory epithelium, where it participates in phospholipid turnover and antioxidant defense. Prdx6 has been localized by immunocytochemistry and subcellular fractionation to acidic organelles (lung lamellar bodies and lysosomes) and cytosol. On the basis of their pH optima, we have postulated that protein subcellular localization determines the balance between the two activities of Prdx6. Using green fluorescent protein-labeled protein expression in alveolar epithelial cell lines, we showed Prdx6 localization to organellar structures resembling lamellar bodies in mouse lung epithelial (MLE-12) cells and lysosomes in A549 cells. Localization within lamellar bodies/lysosomes was in the luminal compartment. Targeting to lysosome-like organelles was abolished by the deletion of amino acids 31–40 from the Prdx6 NH2-terminal region; deletion of the COOH-terminal region had no effect. A green fluorescent protein-labeled peptide containing only amino acids 31–40 showed lysosomal targeting that was abolished by mutation of S32 or G34 within the peptide. Studies with mutated protein indicated that lipid binding was not necessary for Prdx6 targeting. This peptide sequence has no homology to known organellar targeting motifs. These studies indicate that the localization of Prdx6 in acidic organelles and consequent PLA2 activity depend on a novel 10-aa peptide located at positions 31–40 of the protein.
Keywords: phospholipase A2, lung lamellar bodies, lipid binding, protein targeting motif, lipase motif
peroxiredoxin 6 (Prdx6) is a unique member of the peroxiredoxin family of ubiquitous thiol-specific antioxidant enzymes. The protein is a bifunctional enzyme with glutathione peroxidase and Ca2+-independent PLA2 (aiPLA2) activities and is highly expressed in human respiratory epithelium (17, 21). Prdx6 can reduce a broad spectrum of peroxides and has been shown to play a major role in antioxidant defense (21, 34). In addition, Prdx6 participates in lung surfactant phospholipid turnover. Lung surfactant phospholipids that are endocytosed by alveolar epithelial cells are degraded in lysosomal compartments, where PLA2 plays a major role (2). Based on use of a specific inhibitor, Prdx6 was shown to be the PLA2 primarily responsible for degradation of internalized dipalmitoylphosphatidylcholine (DPPC), the major phospholipid component of lung surfactant (7). PLA2 activity generates lysophosphatidylcholine, the substrate for resynthesis of DPPC by an acyltransferase. Thus targeted deletion of Prdx6 in a mouse model markedly diminished aiPLA2 activity, lung surfactant phospholipid degradation, and the resynthesis of DPPC by the reacylation pathway (8), whereas overexpression of the enzyme led to an increased phospholipid degradation and resynthesis (10). The PLA2 activity of Prdx6 is known to require an acidic pH (17).
In our previous studies, we used immunocytochemistry, immunogold electron microscopy, and subcellular fractionation analysis to detect Prdx6 in lung lysosomes and lamellar bodies of lung alveolar type II cells (1, 10, 17, 35). This protein localization is consistent with the low pH requirement for its PLA2 activity. We have postulated that Prdx6 targeting to the specific subcellular compartments may rely on discrete signals, sorting “tags,” located within the amino acid sequence of the protein, which can be recognized by specific receptors, molecular chaperones, or signaling molecules. A number of such tags that can direct proteins to the intracellular compartment consistent with their biological function have been identified (3, 4). Targeting signals may represent a linear sequence of amino acids or a stretch of residues (signal patches) that are exposed in the correct three-dimensional conformation for protein-protein interactions and initiate protein translocation across limiting membranes. Neither the nature of the Prdx6 subcellular sorting mechanism nor possible signals that target this protein to lamellar body and lysosomal compartments have been defined.
Our studies implicate the sequence between amino acids 31 and 40 in the Prdx6 NH2-terminal region as being essential for protein localization to organellar structures such as lamellar bodies and lysosomes. We determined that the role of this sequence in targeting is separate from its role in phospholipid binding. Thus this sequence appears to represent a unique signaling tag for determination of protein subcellular targeting.
METHODS
DNA constructs.
The mammalian expression plasmid encoding NH2-terminal (GFP:Prdx6) green fluorescent protein (GFP)-tagged full-length Prdx6 has been described previously (24). The COOH-terminal (Prdx6:GFP)-tagged full-length protein was constructed by similar methods in the pGFP-N1 vector.
Deletion mutagenesis.
A set of Prdx6:GFP COOH-terminal deletion mutants and one NH2-terminal deletion mutant were constructed using Pfu Turbo DNA polymerase, as described previously (33). Briefly, we used a pair of HPLC-purified 5′-phosphorylated inverse primers, separated by the region to be deleted, similar in size and melting temperature. The primer pairs used for mutagenesis are presented in Table 1. A mixture containing 150 ng of each primer, 50 ng of GFP:Prdx6 as a template DNA, 200 μM dNTPs, and 2.5 U of Pfu Turbo polymerase (Stratagene, La Jolla, CA) in a total volume of 50 μl was subjected to the PCR, characterized by the following conditions: denaturation at 95°C for 3 min, followed by 18 cycles of denaturation at 95°C for 45 s, annealing at 60–70°C, and extension at 68°C for 1 min/kb template. A portion of the reaction mixture (10 μl) was then examined by agarose gel electrophoresis to determine whether the correct-sized product had been obtained. The rest of the reaction mixture was treated with 20 U of Dpn I restriction enzyme for 2 h at 37°C to digest parental DNA. The PCR product was purified from a 1% agarose gel using the QIAEX II gel extraction kit (Qiagen, Valencia, CA), ligated, and transformed into 50 μl of XL1-Blue supercompetent cells (Stratagene). The Δ1–30 aa GFP:Prdx6 deletion mutant was used as template DNA to generate the GFP-tagged 31–40 aa construct.
Table 1.
Pairs of oligonucleotides used to generate GFP-tagged Prdx6 deletion and point mutants by PCR
| Mutant | PCR Primers | Template Plasmid |
|---|---|---|
| Prdx6 deletion mutants | ||
| 1–70 aa | [Phos]-5′-TAAAAGCTTCGAATTCTGCAGTCGACGGTACCGCGG-3′ | FL |
| [Phos]-5′-AGCAATCAACTTAACATTCCTCTTGGCAAACTCTGG-3′ | ||
| 1–40 aa | [Phos]-5′-TAAAAGCTTCGAATTCTGCAGTCGACGGTACCGCGG-3′ | FL |
| [Phos]-5′-TGGGTGGGAAAAGAGAATGCCCCATGAATCTCCTAG-3′ | ||
| 1–30 aa | [Phos]-5′-TAAAAGCTTCGAATTCTGCAGTCGACGGTACCGCGG-3′ | FL |
| [Phos]-5′-TCCTAGGAAATCGTGGAAGCGGATGTGGCCGATGGT-3′ | ||
| 31–224 aa | [Phos]-5′-GATTCATGGGGCATTCTCTTTTCCCACCCACGGGAC-3′ | FL |
| [Phos]-5′-CATGGCTCGAGATCTGAGTCCGGACTTGTATAGTTC-3′ | ||
| 1–70 aa | [Phos]-5′-CGGGACTTTACCCCAGTGTGCACCACAGAACTTGGC-3′ | FL |
| Δ(31–39) aa | [Phos]-5′-TCCTAGGAAATCGTGGAAGCGGATGTGGCCGATGGT-3′ | |
| 31–40 aa | [Phos]-5′-TAAAAGCTTCGAATTCTGCAGTCGACGGTACCGCGG-3′ | 31–224 aa |
| [Phos]-5′-TGGGTGGGAAAAGAGAATGCCCCATGAATCCATGGC-3′ | ||
| 31–34 aa | [Phos]-5′-GCCCCATGAATCCATGGCTCGAGATCTGAGTCCGGA-3′ | 31–40 aa |
| [Phos]-5′-TGGGTGGGAAAAGAGAATGCCCCATGAATCCATGGC-3′ | ||
| Prdx6 point mutants | ||
| H26A | 5′-CGGCCACATCCGCTTCGCCGATTTCCTAGGAGATTC-3′ | FL |
| 5′-GAATCTCCTAGGAAATCGGCGAAGCGGATGTGGCCG-3′ | ||
| S32A | 5′-CACGATTTCCTAGGAGATGCATGGGGCATTCTCTTTTCC-3′ | FL |
| 5′-GGAAAAGAGAATGCCCCATGCATCTCCTAGGAAATCGTG-3′ | ||
| G30L, G34L | 5′-CGCTTCCACGATTTCCTACTAGATTCATGGCTCATTCTCTTTTCCCACCCA-3′ | FL |
| 5′-GGGTGGGAAAAGAGAATGAGCCATGAATCTAGTAGGAAATCGTGGAAGCG-3′ | ||
| 31–40 aa, S32A | 5′-AGATCTCGAGCCATGGATGCATGGGGCATTCTCTTTTCC-3′ | 31–40 aa |
| 5′-GGAAAAGAGAATGCCCCATGCATCCATGGCTCGAGATCT-3′ | ||
| 31–40 aa, S38L | 5′-TCATGGGGCATTCTCTTTCTGCACCCATAAAAGCTTCGA-3′ | 31–40 aa |
| 5′-TCGAAGCTTTTATGGGTGCAGAAAGAGAATGCCCCATGA-3′ | ||
| 31–40 aa, S38G | 5′-TCATGGGGCATTCTCTTTGGCCACCCATAAAAGCTTCGA-3′ | 31–40 aa |
| 5′-TCGAAGCTTTTATGGGTGGCCAAAGAGAATGCCCCATGA-3′ |
GFP, green fluorescent protein; Prdx6, peroxiredoxin 6; FL, full-length Prdx6.
Site-directed mutagenesis.
Site-directed mutations were performed using the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's protocol using pairs of HPLC-purified primers (Table 1). In separate mutants of the full-length protein, S32 or H26 was substituted by alanine, or G30 and G34 were mutated to leucines. In the truncated 31–40 aa peptide, S32 was mutated to alanine or S38 was mutated to glycine or leucine.
Cell culture and transfections.
Lung epithelial cell lines MLE-12 (CRL-2110) and A549 (CCL-185) were obtained from the American Type Culture Collection (Manassas, VA). MLE-12 cells (11), a mouse lung alveolar epithelial cell line, were cultured in HITES medium [Ham's F-12 medium (50:50 mixture) with 0.005 mg/ml insulin, 0.01 mg/ml transferrin, 30 nM sodium selenite, 10 nM hydrocortisone, 10 nM β-estradiol, 10 mM HEPES buffer, and 2 mM l-glutamine] supplemented with 2% fetal bovine serum and antibiotics. These cells contain organelles with some of the ultrastructural characteristics of lamellar bodies and secrete phospholipids. We have termed these organelles “lamellar body-like” (LBL) structures. MLE-12 cells (3 × 106) were transiently transfected with 3 μg of wild-type or mutant GFP:Prdx6 with use of the normal human bronchial epithelial Nucleofector kit (Amaxa, Gaithersburg, MD) according to the manufacturer's protocol. After electroporation, cells in growth medium were plated on coverslips in six-well plates and cultured for 48 h before experimental treatments.
A549 cells, a human lung carcinoma cell line (20), were grown in DMEM (GIBCO Laboratories, Grand Island, NY) supplemented with 10% fetal bovine serum and antibiotics. Although these cells possibly originated from an alveolar epithelial cell tumor, their lysosomes have relatively few of the characteristics of lamellar bodies. Cells were maintained in 5% CO2 at 37°C. For transient expression of GFP-tagged constructs in A549 cells, 95% confluent cell layers in six-well plates were transfected with 3 μg of each expression plasmid in 10 μl of Lipofectamine 2000 reagent (Invitrogen) per well according to the manufacturer's protocol. Cells were subjected to experimental treatments 48 h after transfection.
Immunofluorescence and confocal microscopy.
Cells cultured on glass coverslips were rinsed with PBS and either permeabilized by fixation with cold ethanol-acetone [1:1 (vol/vol)] for 5 min on ice or fixed with 3% paraformaldehyde for 10 min at room temperature and then permeabilized with 1% Triton X-100 for 10 min. Both methods gave similar results. The subcellular distribution of GFP:Prdx6 and its mutants in MLE-12 and/or A549 cells was observed under a confocal microscope (Bio-Rad) at ×60 magnification.
Nile Red and LysoTracker Red staining.
Nile Red, a stain for lipid-rich organelles, was used to stain LBL structures in MLE-12 cells fixed in 3% paraformaldehyde (5). A saturated solution (0.1 mg/ml) of Nile Red (Sigma, St. Louis, MO) was prepared in acetone and stored with protection from light at −20°C. A working solution of the dye was prepared by addition of 1 ml of a 75:25 glycerol-water mixture to Nile Red stock solution (0.5 μl). Fixed GFP:Prdx6-transfected cells were subjected to 5 min of incubation at room temperature with 25 μl of the Nile Red working solution. LysoTracker Red, a stain for acidic compartments, was used as a lysosomal marker in A549 cells. LysoTracker Red (Invitrogen) was added to the GFP:Prdx6-transfected cells 30 min before fixation according to the manufacturer's protocol. After they were stained, the cells were fixed, mounted on a slide, and observed with a confocal microscope using a ×60 objective.
Isolation of lysosome-like organelles.
LBL structures from MLE-12 cells and lysosomes from A549 cells were isolated in isotonic sucrose as described previously (13). Briefly, pelleted cells (0.5 g) were washed once with PBS, resuspended in 5 ml of buffer containing 0.25 M sucrose in 10 mM Tris·HCl (pH 7.4), and homogenized with 10–15 strokes in a Teflon Dounce homogenizer. The cell debris was centrifuged at 12,000 g for 10 min at 4°C. CaCl2 was added to the cytosolic supernatant to a final concentration of 8 mM, and the sample was recentrifuged at 25,000 g for 15 min at 4°C. The pellet containing lysosomes or LBL structures was washed in 4 ml of 150 mM KCl in 10 mM Tris·HCl buffer (pH 7.4), and the organelles were resedimented by a final centrifugation at 25,000 g for 15 min at 4°C. Lamellar bodies also were isolated from mouse lung by sucrose gradient centrifugation as described previously (6). Prdx6 localization was analyzed by Western blot and flow cytometry analysis.
Flow cytometry analysis.
Flow cytometry analysis of Prdx6 expression was performed in freshly isolated vesicles with a four-color dual-laser FACSCalibur system (Becton Dickinson, San Jose, CA) using CellQuest software as described previously (15, 19). Prdx6 was detected using a polyclonal primary antibody (17, 24) and FITC-conjugated goat anti-rabbit IgG secondary antibody (Jackson Laboratories, Bar Harbor, ME). Assays were developed in experimental pairs (surface and luminal Prdx6 expression) as single-color staining (26). For assay of luminal Prdx6, the freshly isolated organellar fraction was treated with 0.1% paraformaldehyde for 20 min, washed three times in PBS, and lightly permeabilized with 0.1% Triton X-100 at room temperature. Samples were incubated with antibodies for 1 h on ice and then washed three times in PBS before flow cytometry. Extraluminal (surface) Prdx6 was detected by staining without prior permeabilization. Both unstained vesicles and incubation without the primary antibody were evaluated as negative controls.
Western blot analysis.
Isolated lamellar bodies or lysosomal vesicles were permeabilized with 0.1% Triton X-100 for 30 min on ice to extract luminal proteins. Vesicular membranes were pelleted at 100,000 g and then treated with 0.2 M Na2CO3 for an additional 30 min on ice to detach proteins loosely associated with lysosomal membranes. Luminal and membrane fractions were subjected to Western blot analysis using the two-color Odyssey LI-COR (Lincoln, NE) technique as previously described (25). The secondary antibody IrDye 800 goat anti-rabbit (Rockland, Gilbertsville, PA) was used for imaging in the green 800-nm channel.
Liposome preparation.
Unilamellar liposomes consisting of DPPC-egg phosphatidylcholine-cholesterol-phosphatidylglycerol (50:25:15:10, mol/mol) were prepared by extrusion under pressure, as described previously (7). This lipid mixture was chosen to mimic the composition of lung surfactant. Fluorescently labeled liposomes were prepared by replacement of 2 mol% of egg phosphatidylcholine with N-(5-dimethylaminonaphthalene-1-sulfonyl)-sn-glycero-3-phosphatidylethanolamine (N-DNS-PE). Analysis by dynamic light scattering (DLS 90 Plus Particle Size Analyzer, Brookhaven Instruments, Holtsville, NY) showed a homogeneous population of 100- to 120-nm-diameter liposomes, which represented >95% of total vesicles.
Fluorescence measurements.
N-DNS-PE-labeled phospholipids were used to study the interaction between phospholipids and the Prdx6 31–40 aa region, which was measured as the change in fluorescence emission as described previously (22). All fluorescence studies were done at 22 ± 0.5°C with use of a water bath temperature-controlled sample holder. The ratio of fluorescence emission at 415 nm to fluorescence emission at 505 nm was measured with a spectrofluorometer equipped with a single-photon counting system for fluorescence intensity detection, dual fluorescence, and absorbance channels with use of excitation and emission slits of 1 and 2 nm, respectively (Photon Technology International, Lawrenceville, NJ).
Binding of Prdx6 peptides to liposomes.
Synthetic decamer peptides, corresponding to the Prdx6 25–34 aa and 31–40 aa sequences, were produced and purified by ELIM Biopharmaceuticals (San Francisco, CA). Four peptides were synthesized: two corresponded to the wild-type sequences for amino acids 25–34 (FHDFLGDSWG) and amino acids 31–40 (DSWGILFSHP), and the others were the H26A and S32A mutants of these peptides. The COOH-terminal amino acid (proline) in the 31–40 aa sequence was substituted with lysine. Peptides were NH2 terminally acetylated. Real-time determination of the binding of Prdx6 wild-type and mutant peptides (100 μM final concentration) to unilamellar liposomes [100 μM total phospholipids in 40 mM potassium phosphate buffer (pH 7.4) or in 40 mM sodium acetate buffer (pH 4.0)] was performed in a time-based ratiometric mode of the fluorometer, with recording of one measurement per second for 15 min before and 45 min after the addition of the peptide as described previously (22). Excitation was set at 340 nm, and fluorescence emission was measured at 415 and 505 nm, with constant stirring of the sample before and after the addition of the peptide. The data were plotted as the ratio of fluorescence emission at 415 nm to fluorescence emission at 505 nm and analyzed using standard sigmoidal curve fitting (4 parameters) with SigmaPlot version 9 software (SPSS, Chicago, IL). R2 for all fits was >0.98.
Statistical analysis.
Values are means ± SE. Statistical significance was assessed with SigmaStat software (Jandel Scientific, San Jose, CA). Group differences were evaluated by one-way ANOVA or by Student's t-test as appropriate. Differences between mean values were considered statistically significant at P < 0.05.
RESULTS
Prdx6 localization in lysosome-like organelles.
Our previous studies demonstrated Prdx6 expression in lysosomes and lamellar bodies of lung alveolar type II cells (1, 10, 35). To further investigate Prdx6 subcellular localization, we isolated lamellar bodies from mouse lung homogenate, LBL organelles from MLE-12 cells derived from mice, and lysosomes from human lung (A549) epithelial cells (Fig. 1). We have termed the isolates from MLE-12 cells LBL structures and those from A549 cells lysosomes, although they have similarities and may represent different stages in development of the secretory bodies. Prdx6 staining, as assessed by flow cytometry, increased significantly after light permeabilization, indicating that it is within the organelles (Fig. 1A). Identity of the organelles was confirmed by staining with lysosome-associated membrane protein (LAMP-1; Fig. 1B), a marker of lysosome-related compartments (14). The organelles were treated with Na2CO3 and then subjected to Western blot analysis to differentiate proteins that were loosely associated with the membrane (luminal proteins) from intrinsic membrane proteins. These studies showed that Prdx6 is predominantly localized to the lumen of the lysosomal vesicles (Fig. 1B).
Fig. 1.
Expression of endogenous peroxiredoxin 6 (Prdx6) in lysosome-related compartments in lung epithelial cells studied by flow cytometry and Western blotting. A: flow cytometry analysis of Prdx6 content in lysosome-related organelles before and after light permeabilization. Gray peak, surface staining; black peak, luminal expression; dashed peak, negative control; au, arbitrary units. B: Western blot analysis of Prdx6 expression in lysosomes freshly isolated from mouse lung epithelial (MLE-12) and A549 cells and in mouse lung lamellar bodies. Organelles were treated with Na2CO3 to differentiate between luminal and intrinsic membrane protein. Lysosome-associated membrane protein (LAMP-1) was used as a lysosomal membrane marker.
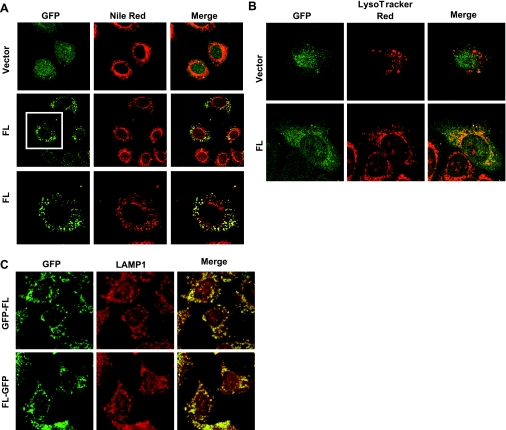
Prdx6:GFP constructs were prepared for study of the mechanism of Prdx6 targeting. As a first step, we expressed these constructs or GFP alone in MLE-12 and A549 cells, cell lines derived from mouse and human lung epithelium, respectively, and evaluated their targeting to lysosome-related compartments. In the MLE-12 and A549 cells, the expression of GFP alone was diffuse, compatible with cytoplasmic localization (Fig. 2, A and B, top panels). On the other hand, GFP:Prdx6 colocalized with Nile Red, a marker for lamellar bodies, in MLE-12 cells (Fig. 2A, middle and bottom panels) and with the lysosomal marker LysoTracker Red in A549 cells (Fig. 2B). Targeting was similar for the GFP:Prdx6 and the Prdx6:GFP constructs, indicating that location of the tag did not affect localization (Fig. 2C). These results indicate that an element in Prdx6 can direct the Prdx6:GFP fusion complex to acidic organelles.
Fig. 2.
Expression of green fluorescence protein (GFP)-tagged Prdx6 in lysosome-related compartments of MLE-12 and A549 lung epithelial cells. Left panels: GFP full-length Prdx6 fusion construct (FL) and GFP control (vector) expressed in transfected MLE-12 (A) and A549 (B) cells. Middle panels: lamellar bodies (A), stained with Nile Red, and lysosomes (B), stained with LysoTracker Red. Right panels: colocalization, detected by yellow color, in the merged image. Bottom panels in A show a magnified image of the cell in the boxed field. C: colocalization of full-length GFP-tagged Prdx6 (green) with lysosome-related organelles stained with LAMP-1 (red) in MLE-12 cells. GFP:FL, GFP tag on the NH2 terminus of Prdx6; FL:GFP, GFP tag on the COOH terminus of Prdx6.
Prdx6 lysosomal targeting signal.
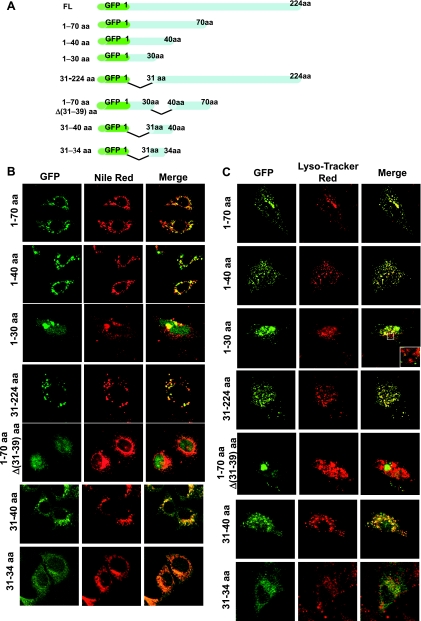
To identify a possible amino acid sequence responsible for targeting of Prdx6 to lamellar bodies and lysosomes, we generated a set of GFP-tagged Prdx6 deletion mutants (Fig. 3A). In MLE-12 (Fig. 3B) and A549 (Fig. 3C) cells, deletion of the COOH-terminal region had no effect, as indicated by normal targeting of the peptides composed of amino acids 1–70 and 1–40 of Prdx6. On the other hand, a peptide of amino acids 1–30 fused to GFP did not show targeting. This peptide appeared to be toxic for MLE-12 and A549 cells and resulted in the formation of large aggregations (compatible with aggresomes) that stained positively with Nile Red and LysoTracker Red (Fig. 3, B and C). However, regions that were devoid of aggregations failed to show colocalization between the GFP and the organellar markers (Fig. 3C, inset). Furthermore, deletion of the initial 30 aa from the NH2 terminus of the full-length protein had no effect on targeting. These data suggest that the Prdx6 31–40 aa region contains a targeting signal that is necessary for its localization to lysosome-related compartments in MLE-12 and A549 cells. Indeed, deletion of the 31–39 aa segment from the 1–70 aa peptide eliminated targeting, whereas a GFP-tagged peptide with only the 31–40 aa segment (DSWGILFSHP) showed targeting similar to the full-length protein (Fig. 3, B and C). The ability of the GFP-tagged Prdx6 31–40 aa peptide to localize to lysosome-like structures in MLE-12 and A549 cells confirmed that this region is not only necessary, but also sufficient, for Prdx6 targeting to lysosome-related compartments. A construct of GFP tagged to a peptide composed of only the four amino acids at positions 31–34 did not show organellar targeting (Fig. 3, B and C).
Fig. 3.
Expression of GFP-tagged Prdx6 deletion mutants in MLE-12 and A549 lung epithelial cells. A: schematic representation of GFP:Prdx6 deletion constructs. B: colocalization of each of the GFP:Prdx6 deletion mutants with Nile Red in MLE-12 cells. C: colocalization of Prdx6 with LysoTracker Red in A549 cells. Left panels: fusion constructs expressed in transfected cells. Middle panels: lamellar bodies (B) and lysosomes (C), shown in red. Right panels: colocalization, detected by yellow color, in the merged image. Inset in C demonstrates the absence of colocalization of GFP:Prdx6 (1–30 aa) in lysosomes.
Site-directed mutagenesis.
The Prdx6 30–40 aa region contains a sequence GDSWG at amino acids 30–34 (Fig. 4A) that fits the consensus of a lipase motif, GXSXG (31). Our previous studies indicated that S32 is essential for phospholipid substrate binding to Prdx6 (22). S32 in this motif also constitutes an important component of the catalytic triad (H26-S32-D140) that is required for the PLA2 activity of Prdx6 (22). The GDSWG sequence in Prdx6 also fits the consensus of a GXXXG/S-type motif (glycine “zipper”), which has been previously shown to target proteins to the plasma membrane (12). A second similar sequence within the 30–40 aa region is contained at amino acids 34–38 (GXXXS). On the basis of these observations, we tested point mutations of these key amino acids (S32, G34, and S38) within the 31–40 aa sequence to determine whether they affected Prdx6 subcellular localization. We generated GFP-tagged Prdx6 mutants with an S32-to-alanine mutation (S32A), an S38-to-leucine or -glycine mutation (S38L/G), or a double-glycine (G30/34)-to-leucine (GDSWG-to-LDSWL) mutation (Fig. 4B). The S32 and G30/34 mutations within the full-length protein resulted in the loss of Prdx6 lamellar body targeting in MLE-12 cells (Fig. 4C). These same point mutations within the 1–40 aa peptide, as well as the S32A mutation in the GFP:31–40 aa fusion peptide, also abolished targeting to lysosomal organelles in MLE-12 cells (Fig. 4C). Of course, the G30 amino acid is not contained in the 31–40 aa targeting sequence. Thus these results indicate that S32 and G34 within the 31–40 aa targeting sequence are required for Prdx6 subcellular localization. The S38L (Fig. 4D) and S38G (not shown) mutations had no effect, and thus this moiety does not play a role in targeting. We chose these substituents for the S38 mutation, because serine and glycine appear to be interchangeable in the putative targeting motif (thereby serving as a control) whereas the leucine mutation of G34 resulted in loss of targeting.
Fig. 4.
Mutations in Prdx6 30–40 aa region affect protein targeting to lysosome-related compartments in MLE-12 cells. A: sequence of the Prdx6 30–40 aa NH2-terminal region. One 5-aa sequence at top indicates the lipase motif (18, 31), and two 5-aa sequences at bottom indicate potential glycine “zippers” (29). B: schematic representation of point mutations in GFP:Prdx6 constructs used in these studies. C: effect of point mutations on colocalization of full-length GFP:Prdx6 and a GFP:Prdx6 peptide (31–40 aa) with Nile Red in MLE-12 cells. Point mutations S32A and G30L/G34L were studied in the full-length protein and S32A and S38L were studied in the peptide. Left panels: fusion construct expressed in the transfected cells. Middle panels: lysosome-related organelles stained with Nile Red. Right panels: colocalization, detected by yellow color, in the merged image. D: same as C, but with H26A mutation in the full-length GFP:Prdx6 construct. LAMP-1 was used as the lysosome-related organelle marker.
H26, like S32, in Prdx6 is a component of the PLA2 catalytic triad and also has been shown to participate in binding of Prdx6 to phospholipid substrate (22). To evaluate the possible role of phospholipid binding in Prdx6 targeting, we determined the effect of mutating H26 (H26A) in the full-length protein. This mutation had no effect on targeting, indicating that phospholipid binding is not required for Prdx6 organellar localization (Fig. 4D).
Binding of synthetic peptides to liposomes.
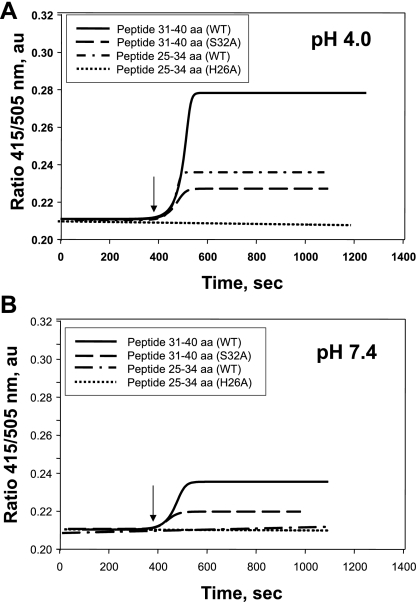
To provide additional evidence related to the negative results for a role of phospholipid binding, we evaluated the binding of peptides to phospholipids in vitro. The N-DNS-PE probe was used to label the lipid shell of liposomes, and the binding of Prdx6 25–34 aa and 31–40 aa peptides to liposomes was studied by recording the real-time changes in the 415-nm to 505-nm fluorescence emission ratio (Fig. 5). These studies were done at pH 4.0 and 7.4 on the basis of our previous results with the full-length protein (22, 23). Shift of the fluorescence emission maximum with the N-DNS-PE probe from 505 to 415 nm in the presence of the peptide reflects the change in the polarity of its surroundings, indicating the shielding of the DNS chromophore by bound ligand (22). Thus an increase in the 415-nm to 505-nm fluorescence emission ratio indicates binding of the Prdx6 peptide to the liposomal surface. There was significant binding of the wild-type peptide to N-DNS-PE at pH 4.0 but significantly less binding at pH 7.4 (Fig. 5, A and B). The 25–34 aa peptide bound to a lesser degree at pH4 and did not bind at pH 7.4. The effect on binding of the H26A and S32A mutations in the Prdx6 25–34 and 31–40 aa sequences was studied. Compared with the respective wild-type peptides, both mutant peptides showed a significant reduction in binding (Fig. 5, Table 2). These data regarding the effect of pH and the effect of the S32 and H26 mutations on phospholipid binding are similar to our previous findings for the full-length protein (22, 23).
Fig. 5.
Binding of Prdx6 25–34 aa and 31–40 aa peptides to N-(5-dimethylaminonaphthalene-1-sulfonyl)-sn-glycero-3-phosphatidylethanolamine (N-DNS-PE) unilamellar liposomes. Liposomes (100 μM total phospholipid) were suspended in 40 mM sodium acetate buffer (pH 4.0) or 40 mM potassium phosphate buffer (pH 7.4). Binding was determined by measurement of the ratio of fluorescence emission at 415 nm to fluorescence emission at 505 nm. Binding curves of wild-type (WT) peptides and S32A and H26A mutants (100 μM) to N-DNS-PE liposomes at pH 4.0 and pH 7.4 are shown by a sigmoidal curve fit. Binding is shown in real time by detection of the DNS fluorescence ratio (415/505 nm). Arrow indicates addition of peptides to the liposome mixture.
Table 2.
Phospholipid binding of wild-type and mutant peptides at pH 4.0 and 7.4
| Peptide | Fluorescence Emission Ratio (415/505 nm), % |
|
|---|---|---|
| pH 4.0 | pH 7.4 | |
| 31–40 aa | ||
| WT | 100 | 33.7±1.8 |
| S32A | 28.2±1.5 | 14.1±0.1 |
| 25–34 aa | ||
| WT | 42.8±1.6 | 0 |
| H26A | 0 | 0 |
Values are means ± SE. WT, wild type.
DISCUSSION
The goal for this study was to evaluate mechanisms that regulate the subcellular targeting of Prdx6 to lysosome-related compartments in lung type II epithelial cells. We studied the MLE-12 and A549 cell lines derived from lung epithelium as readily transfectable cells that express Prdx6. These cell lines express endogenous Prdx6 in the luminal compartment of their LBL structures (MLE-12) and lysosomes (A549) similar to their localization in type II epithelial cells. Furthermore, the Prdx6:GFP fusion protein (with the GFP tag on the NH2 or COOH terminus of Prdx6) in these cell lines is targeted to organelles that co-stain with markers for lysosome-like structures. This targeting was not a default pathway, since it was abolished by site-specific mutations in the protein.
Our initial focus was on the primary sequence of Prdx6, inasmuch as it is known that discrete domains located within the amino acid sequence of proteins can determine subcellular localization. We determined that deletion of amino acids 31–40 prevented the lysosomal localization of Prdx6 and that this 10-aa peptide by itself could drive lysosomal targeting of GFP. Thus this peptide appears to be necessary and sufficient for Prdx6 subcellular localization. A smaller fragment (amino acids 31–34) was ineffective as a targeting signal. The 31–40 aa sequence is 100% identical in human, rat, mouse, and bovine Prdx6 (9, 17).
Surfactant protein C (SP-C) is synthesized by type II alveolar epithelial cells and, similar to Prdx6, is targeted to lysosome-like organelles/lamellar bodies. However, in contrast to the luminal Prdx6, SP-C is an integral membrane protein, and its topography has an important role in its targeting (27). Also in contrast to Prdx6, the “purpose” for targeting of SP-C is for further processing before secretion, whereas the role of Prdx6 targeting is expression of enzymatic activity within the organelles. The targeting sequence for SP-C has been identified as a 9-aa peptide, MESPPDYSA, present at the NH2 terminus of the protein (16). Neither this specific sequence nor homologous domains are present in Prdx6.
Previous studies in cells from other organs have shown that protein targeting to post-Golgi compartments such as endosomes, lysosomes, or lysosome-like organelles may involve tyrosine-based YXXϕ-type motifs, where ϕ is a hydrophobic group amino acid, such as glycine, alanine, valine, leucine, or isoleucine (30, 32). [D/E]XXXL[L/I] and DXXLL-type sequences also have been shown to mediate the rapid internalization and targeting of proteins to endosomes and lysosomes (30). These dileucine sorting motifs require the presence of two consecutive leucines or a leucine-isoleucine pair. Within the Prdx6 amino acid sequence, a YXXϕ-type motif, YNGA, is located between amino acids 89 and 92 and a dileucine motif can be found between amino acids 107 and 112. However, in this study, we demonstrate that the deletion of all residues beyond amino acid 40 and before amino acid 30 of Prdx6 has no effect on targeting. Therefore, these tyrosine-based and dileucine motifs are unlikely to be responsible for lysosomal localization of Prdx6.
Three recognized motifs within the Prdx6 30–40 aa sequence (GDSWGILFSHP) could be involved with targeting. The first is a consensus lipase motif (GXSXG) (18, 31), which is necessary for PLA2 activity of the protein and appears to be crucial for Prdx6 binding to phospholipid substrate (22, 23). In previous studies, Prdx6 bound to phospholipids in mixed unilamellar liposomes at pH 4.0 but bound poorly at pH 7.4, which is likely to be closer to the pH of the organelles (presumably, the Golgi) where targeting is initiated. The present studies using a synthetic peptide (amino acids 31–40) containing the lipase motif gave results for binding that are similar to previous observations with the full-length protein. Binding was greatly diminished by mutation of S32 within the lipase motif (22, 23). Mutation of S32 also abolished targeting, raising the possibility that binding of Prdx6 to lipids may be the mechanism for organellar localization. Lipid binding by the protein also is diminished by mutation of H26, another component of the catalytic triad that is necessary for PLA2 activity (22). The present results with a synthetic peptide (Fig. 5) confirmed a role for H26 in binding. However, this mutation had no effect on targeting of the full-length protein (Fig. 4). This result indicates that lipid binding is not necessary for Prdx6 targeting.
The 30–40 aa sequence of Prdx6 also contains two signature GXXXG/S-type motifs, known as glycine zippers [GDSWG (amino acids 30–34) and GILFS (amino acids 34–38)]. A GXXXG-type signal has been demonstrated to serve as the framework for dimerization of transmembrane α-helices (29), thus serving to target proteins to the plasma membrane (12). A similar mechanism was proposed to mediate the function of membrane receptors, where GXXXG-like motifs were found to be present in transmembrane domains of many G protein-coupled receptors. These include the α-factor receptor of various yeast species, class A amine and cannabinoid receptors, class B secretin-like receptors, and class C metabotropic receptors (28). Mutation within the GXXXG motif was shown to reduce expression and increase receptor retention in intracellular compartments such as the endoplasmic reticulum. The mechanisms for this effect are unknown. Mutation of G34, along with G30, did abolish targeting of the GFP:Prdx6 construct; however, the effective targeting sequence was amino acids 31–40. Thus the first glycine (G30) is not essential for Prdx6 targeting. With respect to the second glycine zipper motif, mutation of S38 had no effect on targeting. These results indicate that the glycine zipper signaling sequences per se do not have a role in Prdx6 subcellular targeting.
In summary, we have evaluated mechanisms of Prdx6 targeting to specific acidic subcellular locations (lysosomes and lamellar bodies) that are compatible with the PLA2 activity of the protein. Our data indicate that protein localization in lysosome-related compartments depends on the 31–40 aa NH2-terminal region of Prdx6. Within this sequence, the amino acids S32 and G34 and at least one or more of amino acids 35–40 play an essential role. On the basis of these results, the minimal Prdx6 lysosomal targeting motif could be as small as SxGx, whereas the maximal motif is xSxG(x)3X(x)2, where x represents amino acids that have not yet been studied for their essentiality and X is any amino acid. This study has not evaluated mechanisms for the cytosolic localization of Prdx6, which presumably is necessary for its glutathione peroxidase activity. We expect that Prdx6 synthesis begins on free ribosomes in the cytosol. We propose that much of the newly synthesized Prdx6 remains cytosolic, whereas some fraction is internalized into the lysosomal matrix, possibly through interaction with a chaperone protein. Although the present results indicate the signal sequence necessary for lysosomal localization, further studies are necessary for an understanding of the mechanism for regulation of Prdx6 distribution between cytoplasmic and lysosomal compartments.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-19737 and HL-79063.
ACKNOWLEDGMENTS
We thank Drs. Yefim Manevich and Sandra Bates (University of Pennsylvania) for critical review of the manuscript, Drs. Michael Marks and Michael Beers for valuable discussion, Dr. Madesh Muniswamy and Kevin Yu for assistance with confocal microscopy, Chandra Dodia and Jain-Qin Tao for technical assistance, and Susan Turbitt for typing the manuscript.
This study was presented in part at the Experimental Biology Annual Meetings, San Francisco, CA, April 2006, and Washington, DC, April-May 2007.
REFERENCES
- 1.Akiba S, Dodia C, Chen X, Fisher AB. Characterization of acidic Ca2+-independent phospholipase A2 of bovine lung. Comp Biochem Physiol B Biochem Mol Biol 120: 393–404, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Batenburg JJ, Haagsman HP. The lipids of pulmonary surfactant: dynamics and interactions with proteins. Prog Lipid Res 37: 235–276, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Bonifacino JS, Marks MS, Ohno H, Kirchhausen T. Mechanisms of signal-mediated protein sorting in the endocytic and secretory pathways. Proc Assoc Am Physicians 108: 285–295, 1996 [PubMed] [Google Scholar]
- 4.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72: 395–447, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Brown WJ, Sullivan TR, Greenspan P. Nile red staining of lysosomal phospholipid inclusions. Histochemistry 97: 349–354, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Chander A, Johnson RG, Reicherter J, Fisher AB. Lung lamellar bodies maintain an acidic internal pH. J Biol Chem 261: 6126–6131, 1986 [PubMed] [Google Scholar]
- 7.Fisher AB, Dodia C. Lysosomal-type PLA2 and turnover of alveolar DPPC. Am J Physiol Lung Cell Mol Physiol 280: L748–L754, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Fisher AB, Dodia C, Feinstein SI, Ho YS. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J Lipid Res 46: 1248–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Fisher AB, Dodia C, Manevich Y, Chen JW, Feinstein SI. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem 274: 21326–21334, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Fisher AB, Dodia C, Yu K, Manevich Y, Feinstein SI. Lung phospholipid metabolism in transgenic mice overexpressing peroxiredoxin 6. Biochim Biophys Acta 1761: 785–792, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Gazdar AF, Linnoila RI, Kurita Y, Oie HK, Mulshine JL, Clark JC, Whitsett JA. Peripheral airway cell differentiation in human lung cancer cell lines. Cancer Res 50: 5481–5487, 1990 [PubMed] [Google Scholar]
- 12.Gimpelev M, Forrest LR, Murray D, Honig B. Helical packing patterns in membrane and soluble proteins. Biophys J 87: 4075–4086, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidcamp WH. Cell biology laboratory manual [Online]. Gustavus Adolphus College, Saint Peter, MN. http://homepages.gac.edu/∼cellab/chpts/chpt7/ex7–1.html[1996]
- 14.Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J 26: 313–324, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobberger JW. Increasing the power of cytometry. Nat Methods 3: 343–344, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Johnson AL, Braidotti P, Pietra GG, Russo SJ, Kabore A, Wang WJ, Beers MF. Post-translational processing of surfactant protein-C proprotein: targeting motifs in the NH2-terminal flanking domain are cleaved in late compartments. Am J Respir Cell Mol Biol 24: 253–263, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Kim TS, Dodia C, Chen X, Hennigan BB, Jain M, Feinstein SI, Fisher AB. Cloning and expression of rat lung acidic Ca2+-independent PLA2 and its organ distribution. Am J Physiol Lung Cell Mol Physiol 274: L750–L761, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Kim TS, Sundaresh CS, Feinstein SI, Dodia C, Skach WR, Jain MK, Nagase T, Seki N, Ishikawa K, Nomura N, Fisher AB. Identification of a human cDNA clone for lysosomal type Ca2+-independent phospholipase A2 and properties of the expressed protein. J Biol Chem 272: 2542–2550, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Krutzik PO, Nolan GP. Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat Methods 3: 361–368, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer 17: 62–70, 1976 [DOI] [PubMed] [Google Scholar]
- 21.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med 38: 1422–1432, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Manevich Y, Reddy KS, Shuvaeva T, Feinstein SI, Fisher AB. Structure and phospholipase function of peroxiredoxin 6: identification of the catalytic triad and its role in phospholipid substrate binding. J Lipid Res 48: 2306–2318, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Manevich Y, Shuvaeva T, Dodia C, Kazi A, Feinstein SI, Fisher AB. Binding of peroxiredoxin 6 to substrate determines differential phospholipid hydroperoxide peroxidase and phospholipase A2 activities. Arch Biochem Biophys 485: 139–149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci USA 99: 11599–11604, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milovanova T, Chatterjee S, Manevich Y, Kotelnikova I, Debolt K, Madesh M, Moore JS, Fisher AB. Lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species. Am J Physiol Cell Physiol 290: C66–C76, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Milovanova T, Manevich Y, Haddad A, Chatterjee S, Moore JS, Fisher AB. Endothelial cell proliferation associated with abrupt reduction in shear stress is dependent on reactive oxygen species. Antioxid Redox Signal 6: 245–258, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Mulugeta S, Beers MF. Processing of surfactant protein C requires a type II transmembrane topology directed by juxtamembrane positively charged residues. J Biol Chem 278: 47979–47986, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Overton MC, Chinault SL, Blumer KJ. Oligomerization of G-protein-coupled receptors: lessons from the yeast Saccharomyces cerevisiae. Eukaryot Cell 4: 1963–1970, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol 296: 911–919, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Sandoval IV, Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol 4: 292–297, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Sato T, Aoki J, Nagai Y, Dohmae N, Takio K, Doi T, Arai H, Inoue K. Serine phospholipid-specific phospholipase A that is secreted from activated platelets. A new member of the lipase family. J Biol Chem 272: 2192–2198, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol 9: 129–161, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Wilkinson MF. Deletion mutagenesis of large (12-kb) plasmids by a one-step PCR protocol. Biotechniques 31: 722–724, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Feinstein SI, Fisher AB. Peroxiredoxin 6 as an antioxidant enzyme: protection of lung alveolar epithelial type II cells from H2O2-induced oxidative stress. J Cell Biochem 104: 1274–1285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu YZ, Manevich Y, Baldwin JL, Dodia C, Yu K, Feinstein SI, Fisher AB. Interaction of surfactant protein A with peroxiredoxin 6 regulates phospholipase A2 activity. J Biol Chem 281: 7515–7525, 2006 [DOI] [PubMed] [Google Scholar]