Abstract
Oxidative stress plays an important role in the pathogenesis of pulmonary fibrosis. Heme oxygenase-1 (HO-1) is a key antioxidant enzyme, and overexpression of HO-1 significantly decreases lung inflammation and fibrosis in animal models. Peroxisome proliferator-activated receptor-γ (PPARγ) is a transcription factor that regulates adipogenesis, insulin sensitization, and inflammation. We report here that the PPARγ ligands 15d-PGJ2 and 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO), which have potent antifibrotic effects in vitro, also strongly induce HO-1 expression in primary human lung fibroblasts. Pharmacological and genetic approaches are used to demonstrate that induction of HO-1 is PPARγ independent. Upregulation of HO-1 coincides with decreased intracellular glutathione (GSH) levels and can be inhibited by N-acetyl cysteine (NAC), a thiol antioxidant and GSH precursor. Upregulation of HO-1 is not inhibited by Trolox, a non-thiol antioxidant, and does not involve the transcription factors AP-1 or Nrf2. CDDO and 15d-PGJ2 contain an α/β unsaturated ketone that acts as an electrophilic center that can form covalent bonds with free reduced thiols. Rosiglitazone, a PPARγ ligand that lacks an electrophilic center, does not induce HO-1. These data suggest that in human lung fibroblasts, 15d-PGJ2 and CDDO induce HO-1 via a GSH-dependent mechanism involving the formation of covalent bonds between 15d-PGJ2 or CDDO and GSH. Inhibiting HO-1 upregulation with NAC has only a small effect on the antifibrotic properties of 15d-PGJ2 and CDDO in vitro. These results suggest that CDDO and similar electrophilic PPARγ ligands may have great clinical potential as antifibrotic agents, not only through direct effects on fibroblast differentiation and function, but indirectly by bolstering antioxidant defenses.
Keywords: 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid; myofibroblast; N-acetyl cysteine
pulmonary fibrosis is a group of disorders characterized by accumulation of scar tissue in the lung interstitium, resulting in loss of alveolar function, destruction of normal lung architecture, and respiratory distress (19, 52, 59). Lung fibroblasts are a key effector cell in pulmonary fibrosis; differentiation to myofibroblasts, proliferation of fibroblasts and myofibroblasts, and excess production of extracellular matrix proteins are important cellular events in the pathology of fibrosis (36, 46, 48). Recent research has demonstrated that reactive oxygen species (ROS) and reactive nitrogen species (RNS) play a role in many lung inflammatory and fibrotic diseases, particularly in patients with dust-induced fibrotic disease (18, 57). ROS and RNS are also elevated in animal models of bleomycin, silica, and asbestos-induced pulmonary fibrosis (1, 18, 45, 56). Decreasing the production of ROS and RNS has gained attention as a strategy for therapy of patients with pulmonary fibrosis (9, 23).
Heme oxygenase-1 (HO-1) is the inducible form of heme oxygenase and is the initial rate-limiting enzyme in the conversion of heme to equimolar concentrations of biliverdin, iron, and carbon monoxide (CO) (44). HO-1 has antioxidant and anti-inflammatory properties and has been shown to decrease apoptosis in pulmonary fibroblasts (35), modulate other inflammatory mediators, and decrease lipid peroxidation in alveolar epithelial cells (42). In the mouse and rat models of bleomycin-induced lung fibrosis, overexpression of HO-1 and treatment with CO or bilirubin significantly decreased inflammation, leading to a reduction in subsequent fibrosis (44, 54, 58, 62).
Glutathione (GSH) is the major cellular thiol and plays a significant role as an antioxidant. GSH acts as a substrate for glutathione peroxidase in a reaction that consumes hydrogen peroxide and lipid hydroperoxides (15). Increasing cellular GSH levels can be protective against oxidative stress (3), whereas reduced levels lead to increased cellular injury (6). N-acetyl-l-cysteine (NAC) is a GSH precursor as well as a direct ROS scavenger. NAC added to cell cultures in vitro increases intracellular GSH levels and protects against damage from oxidative stress (2, 3, 21, 33). It has therefore been hypothesized that NAC might be an effective treatment for fibrotic diseases (38, 47). A recent clinical trial investigated the effect of adding NAC to the therapeutic regime for patients with idiopathic pulmonary fibrosis (IPF) and demonstrated a small decrease in the deterioration of vital capacity (10), suggesting that NAC may help slow the progression of pulmonary fibrosis.
Peroxisome proliferator-activated receptor-γ (PPARγ) is a nuclear transcription factor that plays a role in cellular differentiation, insulin sensitization, and fat metabolism (12, 13, 16, 40). PPARγ ligands have anti-inflammatory properties that are increasingly the subject of study (25, 43). We and others (7, 14, 31) have reported that PPARγ agonists have antifibrotic effects both in vitro and in vivo, although many of the effects of PPARγ agonists appear to be independent of PPARγ-regulated transcription (7, 8, 14, 30, 40). It is particularly interesting that two PPARγ ligands that have the most potent antifibrotic effects, 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) and 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO), both have an α/β-unsaturated ketone ring with an electrophilic carbon that is highly susceptible to Michael addition reactions by nucleophiles of free sulfhydryls such as GSH (8, 40, 49, 50). This raised the possibility that these PPARγ agonists might inhibit fibrosis by upregulating cellular antioxidant defenses. We report here that 15d-PGJ2 and CDDO induce HO-1 in human lung fibroblasts via a glutathione-dependent, PPARγ-independent mechanism, but that HO-1 does not make a significant contribution to the antifibrotic properties of these ligands.
MATERIALS AND METHODS
Cells and reagents.
Normal human lung fibroblast cell strains were derived from tissue explants as described (4, 14) and maintained in MEM (Life Technologies, Gaithersburg, MD) supplemented with 10% FBS (Sigma Aldrich, St. Louis, MO), 2 mM l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin (0.25 μg/ml, Life Technologies) at 37°C in 7% CO2. Patient samples were obtained with informed consent under the approval of the Institutional Review Board of the University of Rochester. The PPARγ agonists 15d-PGJ2 (Biomol, Plymouth Meeting, PA) and CDDO were prepared as 10 mM stock in DMSO and added to cell cultures to the final concentrations indicated. Rosiglitazone and GW9662 (Cayman Pharmaceuticals, Ann Arbor, MI) was prepared in the same manner. For pretreatment with antioxidants, NAC (Sigma Aldrich) was used at a concentration at 5 mM, and 6-hydroxyl-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox; Calbiochem, San Diego, CA) was used at 500 μM. For some experiments, the AP-1 inhibitor SP600125 (Calbiochem) was added at a concentration of 0.1 mM 2 h before treatment with PPARγ ligands. Recombinant human TGF-β1 was purchased from R&D Systems (Minneapolis, MN).
Western blots.
Primary human lung fibroblasts were plated in triplicate in six-well plates (Falcon/Becton Dickson, Franklin Lakes, NJ) at 100,000 cells well, were allowed to grow for 24–48 h, and were treated as described. Cell lysates were prepared using a Nonidet P-40-based lysis buffer. Lysates containing 2 or 5 μg of protein were separated by 10% SDS-PAGE under reducing conditions, transferred to a nitrocellulose membrane, and examined for expression of α-SMA (Sigma), calponin, fibronectin (Dako, Carpinteria, CA), HO-1, and HO-2 (Stressgen, Ann Arbor, MI). GAPDH (Abcam, Cambridge, MA) was used as a loading control. Western blots were visualized with Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer, Wellesley, MA), and densitometry was performed using Kodak Molecular Imaging Software (Rochester, NY).
Glutathione assay.
Total intracellular glutathione was measured in fibroblast lysates as described (39, 53) and was reported as nanomoles of glutathione per milligram of protein.
Lentiviral vectors.
A plasmid encoding a Flag-tagged dominant-negative PPARγ (pcDNA-Flag-PPARγ1-L466A/E469A) was a kind gift from V. K. K. Chatterjee (Univ. of Cambridge, UK). The cDNA was subcloned into a lentiviral expression system (System Bioscience, Mountain View, CA) as described (14). This vector was named LV-PPARγ-DN, and the vector without PPARγ cDNA was termed LV-empty. Primary human lung fibroblasts were infected at a multiplicity of infection of 1, with polybrene at a concentration of 8 μg/ml. Cells were passaged as needed and evaluated for their level of GFP after each passage. Cells were plated in six-well tissue culture plates (as above) at 100,000 cells/well. After 24 h, the cells were treated with either TGF-β alone (5 ng/ml) or TGF-β plus either 15d-PGJ2 (5 μM), CDDO (1 μM), or rosiglitazone (20 μM). Lysates were harvested 24 h after treatment, and HO-1 expression was evaluated by Western blot.
AP-1 luciferase reporter assay.
Primary lung fibroblasts cultured in six-well plates were cotransfected using Fugene6 (Roche Applied Science, Indianapolis, IN) with an AP-1 luciferase reporter (a gift from Dr. Sanjay Maggirwar, Univ. of Rochester) (29) and a CMV-β-galactosidase control construct (a gift from Dr. T. Gasiewicz, Univ. of Rochester) (60). After 24 h, the cells were washed and treated with 1 μM CDDO, 5 μM 15d-PGJ2, or 20 μM rosiglitazone, with or without 5 mM NAC. After a further 24-h incubation, luciferase activity in lysates was measured using a luciferase assay system (Promega, Madison, WI) with a luminometer (Packard Instruments, Meriden, CT) and normalized to β-galactosidase activity, determined by a colorimetric assay (Promega). The experiments were carried out in triplicate wells.
Immunofluorescence.
Equal numbers (1 × 104) of primary human lung fibroblasts were cultured to subconfluence on glass chamber slides under standard conditions as described above. Fibroblasts were then exposed to DMSO (control) or to the PPARγ ligands CDDO (1 μM) or 15d-PGJ2 (5 μM) for 6 h, and Nrf2 was detected by immunofluorescence as previously described (5). Briefly, fibroblasts were incubated with the antibody against Nrf2 (1:200, Santa Cruz Biotechnology), followed by a biotinylated anti-rabbit antibody (1:200) and streptavidin-FITC (1:1,000). The cells were incubated for 10 min with the nucleic acid stain Hoechst (1:1,000 in PBS, Invitrogen) and then coverslipped with Immu-Mount (Shandon, Pittsburgh, PA). Fluorescence was viewed with an Olympus BX51 microscope (New Hyde Park, NY) and photographed with a SPOT Pursuit camera (Diagnostic Instruments, Sterling Heights, MI).
Statistics.
All data are expressed as means ± SD. A Student's unpaired t-test and ANOVA were used to establish statistical significance. Results were considered significant if P < 0.05.
RESULTS
PPARγ ligands induce expression of HO-1 in a concentration-dependent manner.
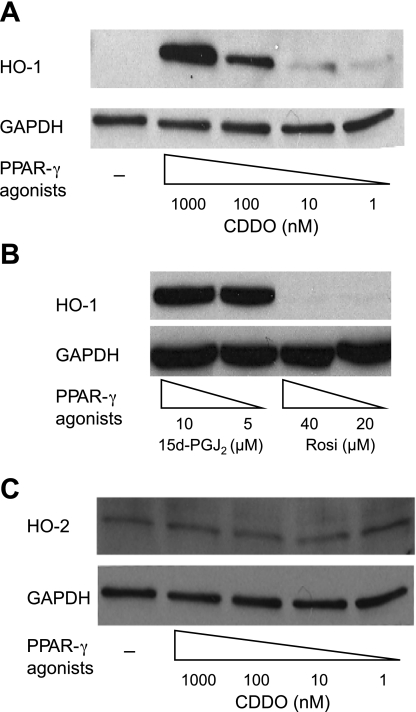
Primary human lung fibroblasts were treated with PPARγ agonists, and protein expression was analyzed by Western blot. HO-1 was induced in a concentration- and time-dependent manner by CDDO and 15d-PGJ2 but not rosiglitazone (Fig. 1, A and B). At 24 h, CDDO potently induced HO-1 at 1 μM (1,000 nM) and 100 nM, and weakly at 10 or 1 nM. 15d-PGJ2 induced HO-1 at 5 and 10 μM. Interestingly, 20 μM rosiglitazone, which is approximately as effective as 5 μM 15d-PGJ2 at inhibiting fibroblast-to-myofibroblast differentiation (14), had no effect on HO-1 expression (Fig. 1B). HO-2, the constitutively expressed isoform of heme oxygenase, is not induced by CDDO (Fig. 1C), 15d-PGJ2, or rosiglitazone (data not shown).
Fig. 1.
2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO) and 15d-PGJ2 but not rosiglitazone induce heme oxygenase-1 (HO-1). Primary human lung fibroblasts were treated with the indicated concentrations of CDDO (A) or 15d-PGJ2 (B) or rosiglitazone. After 24 h, cells were harvested, and HO-1 expression was determined by Western blot. GAPDH was used as a loading control. C: cell lysates were also analyzed for expression of HO-2.
Induction of HO-1 is PPARγ independent.
CDDO and 15d-PGJ2 activate PPARγ-dependent gene transcription, but their ability to inhibit TGF-β-stimulated myofibroblast differentiation is largely PPARγ independent (14). To investigate the PPARγ dependency of HO-1 induction, we used both pharmacological and genetic approaches. GW9662 is a small molecule inhibitor of PPARγ, which binds to a lysine residue in the ligand binding pocket, permanently inhibiting the binding of other ligands (17). PPARγ agonist-driven adipogenesis is PPARγ dependent and is completely inhibited by GW9662 (13). Primary human lung fibroblasts were pretreated for 4 h with 5μM GW9662 and then treated with PPARγ agonists, and HO-1 expression was analyzed by Western blot. GW9662 did not inhibit the induction of HO-1 by CDDO (Fig. 2A) or 15d-PGJ2 (data not shown).
Fig. 2.
Induction of HO-1 by CDDO and 15d-PGJ2 is peroxisome proliferator-activated receptor-γ (PPARγ) independent. A: primary human lung fibroblasts were pretreated with 5 μM GW9662 for 4 h or left untreated and were then treated with 1 μM CDDO for 24 h. HO-1 expression was determined by Western blot and evaluated by densitometry normalized to GAPDH. RE, relative expression of HO-1 normalized to GAPDH with untreated cells = 1. B: lung fibroblast cultures were infected with a lentiviral vector that coexpresses GFP and a dominant negative PPARγ (LV-PPARγ-DN) or the GFP-expressing vector only (LV-Empty). Twenty-four hours after infection, the media was changed, and CDDO (1 μM), 15d-PGJ2 (5 μM), or rosiglitazone (20 μM) was added. After a further 24 h, the cells were harvested, and HO-1 expression was determined by Western blot. PPARγ overexpression is demonstrated in cells infected with LV-PPARγ-DN using an antibody to PPARγ (Abcam). Endogenous PPARγ expression is detected in cells infected with the control virus upon longer exposure. RE, relative expression of HO-1 normalized to GAPDH with untreated cells = 1. Results shown are representative of 2 independent experiments, each performed on duplicate wells.
We then overexpressed a dominant negative PPARγ (LV-PPARγ-DN) in primary human lung fibroblasts using a lentiviral vector that results in stable coexpression of the DN PPARγ and GFP. Greater than 90% of cells infected with the construct expressed GFP and the Flag-tagged DN PPARγ (Fig. 2B and data not shown). Overexpression of LV-PPARγ-DN did not inhibit the induction of HO-1 by CDDO or 15d-PGJ2 (Fig. 2B). These data indicate that the effects of CDDO and 15d-PGJ2 on HO-1 induction are independent of PPARγ.
NAC but not Trolox inhibits CDDO- and 15d-PGJ2-induced HO-1 expression.
HO-1 is induced by oxidative stress and has antioxidant function (11, 44). HO-1 can also be induced by reductions in the intracellular levels of glutathione, a potent scavenger of ROS (5, 26). Interestingly, both 15d-PGJ2 and CDDO contain an α/β unsaturated ketone that can form covalent bonds with the free sulfhydryl group in glutathione via a Michael addition reaction (55) (Fig. 3A). To investigate whether glutathione might be involved in the upregulation of HO-1, intracellular glutathione levels were measured in human lung fibroblasts treated with CDDO and 15d-PGJ2. Both CDDO and 15d-PGJ2 significantly reduced glutathione levels after 3 and 24 h (Fig. 3B). Additionally, lung fibroblasts were pretreated with NAC, an antioxidant and glutathione precursor (38), or Trolox, a non-thiol antioxidant (37), then treated with CDDO, and expression of HO-1 was determined. NAC completely inhibited induction of HO-1 by CDDO, whereas Trolox had no effect (Fig. 3C), suggesting that upregulation of HO-1 is GSH or thiol dependent and not a consequence of generalized oxidative stress.
Fig. 3.
CDDO induces HO-1 by a glutathione-dependent mechanism. A: the structures of CDDO and glutathione (GSH) are illustrated. The electrophilic centers of CDDO are circled. CDDO and GSH undergo a Michael addition reaction that results in the formation of a covalent bond between the reduced thiol of GSH and either of the electrophilic centers of CDDO. B: primary human lung fibroblasts were treated with CDDO (1 μM) or 15d-PGJ2 (5 μM), and intracellular GSH levels were measured 3 and 24 h after treatment. Results shown are representative of 2 independent experiments, each performed in triplicate. *P < 0.05; **P < 0.01, ANOVA. C: primary lung fibroblasts were pretreated for 2 h with 5 mM N-acetyl-l-cysteine (NAC) or 500 μM 6-hydroxyl-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) before addition of 1 μM CDDO. Cells were harvested, and HO-1 was determined by Western blot after 24 h. RE, relative expression of HO-1 normalized to GAPDH with untreated cells = 1. Results shown are representative of 3 independent experiments.
HO-1 is not induced by AP-1.
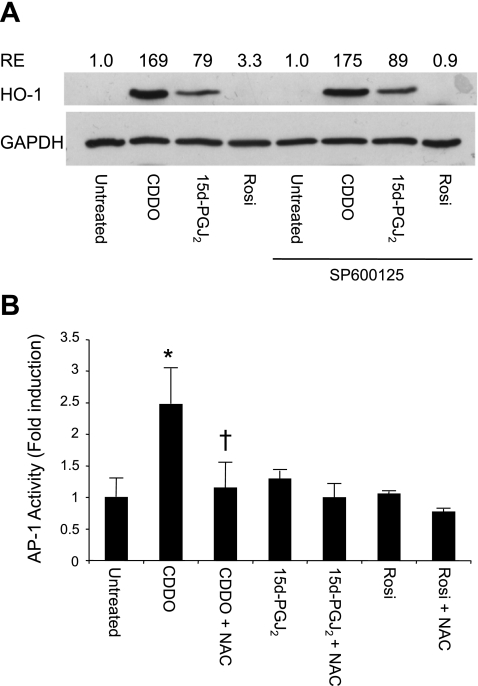
CDDO and 15d-PGJ2, but not rosiglitazone, induce AP-1 activity in human lung fibroblasts (14). It has been reported that AP-1 upregulates HO-1 (5, 11), and, conversely, that HO-1 can upregulate AP-1 (28). To determine whether induction of HO-1 by PPARγ agonists involved AP-1, human lung fibroblasts were pretreated with SP600125, an AP-1 inhibitor (20), and then treated with PPARγ ligands and evaluated for HO-1 expression. SP600125 had no effect on the upregulation of HO-1 by CDDO and 15d-PGJ2, suggesting that AP-1 activation is not required for upregulation of HO-1 in human lung fibroblasts treated with PPARγ agonists (Fig. 4A). To confirm this, lung fibroblasts were transfected with an AP-1 reporter construct and treated with PPARγ ligands with and without pretreatment with NAC. The induction of AP-1 activity by CDDO was completely inhibited by NAC (Fig. 4B). Together, these results suggest that AP-1 activation by CDDO and 15d-PGJ2 is dependent on upregulation of HO-1.
Fig. 4.
Induction of HO-1 by PPARγ ligands is upstream of AP-1 induction. A: primary lung fibroblasts were pretreated with the AP-1 inhibitor SP600125 (0.1 mM) and then treated with CDDO (1 μM), 15d-PGJ2 (5 μM), or rosiglitazone (20 μM) for 24 h. HO-1 expression was evaluated by Western blot. RE, relative expression of HO-1 normalized to GAPDH with untreated cells = 1. Results shown are representative of 2 independent experiments. B: primary human lung fibroblasts were cotransfected with an AP-1 reporter construct and a β-galactosidase control construct and then treated with CDDO (1 μM), 15d-PGJ2 (5 μM), or rosiglitazone (20 μM) with or without 5 mM NAC. After 24 h, luciferase activity was determined and normalized to β-galactosidase activity. Results shown are representative of 2 independent experiments, each performed on triplicate cultures. *Significant increase compared with untreated; †significant decrease compared with CDDO alone, P < 0.05 (ANOVA).
HO-1 upregulation is not dependent on Nrf2.
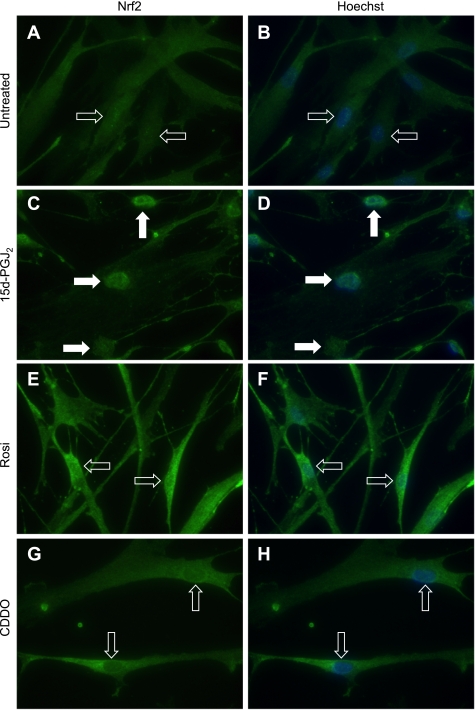
The transcription factor Nrf2 is an important regulator of the response to oxidative stress. It has been reported in bovine aortic endothelial cells that 15d-PGJ2 covalently modifies the Nrf2 partner Keap1, resulting in nuclear translocation of Nrf2 and upregulation of Nrf2-dependent genes including HO-1 (34). To investigate this possible mechanism of HO-1 induction by PPARγ ligands, immunofluorescence was performed to determine the intracellular distribution of Nrf2 following ligand treatment. Nrf2 localization in untreated fibroblasts was primarily cytoplasmic, whereas 15d-PGJ2 induced a marked nuclear localization of Nrf2 6 h after treatment (Fig. 5, A and C). However, neither rosiglitazone (Fig. 5E) nor CDDO (Fig. 5G) induced nuclear localization of Nrf2.
Fig. 5.
PPARγ ligands do not induce nuclear translocation of Nrf2 in human lung fibroblasts. Primary human lung fibroblasts were untreated (DMSO only) or were treated with CDDO (1 μM), rosiglitazone (Rosi; 20 μM), or 15d-PGJ2 (5 μM) for 6 h, and the localization of Nrf2 was determined by immunofluorescence. Nuclei were observed by Hoechst staining (blue). Nrf2 localization in fibroblasts that were untreated (A and B) was primarily cytoplasmic (open arrows). 15d-PGJ2 induces strong nuclear localization of Nrf2 (solid arrows) (C and D). However, rosiglitazone (E and F) and CDDO (G and H) do not induce nuclear localization, with a broad distribution of NRf2 similar to untreated cells (open arrows). Original magnification, ×400.
Induction of HO-1 by PPARγ ligands is independent of myofibroblast differentiation.
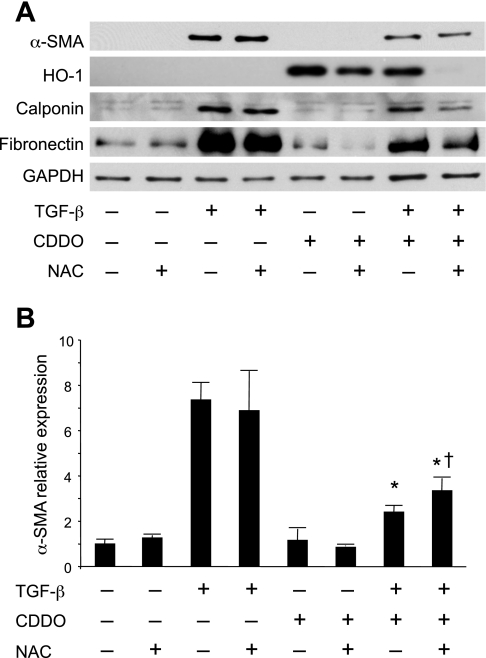
PPARγ agonists, including CDDO, are potent inhibitors of TGF-β-stimulated fibroblast-to-myofibroblast differentiation in vitro (7, 14, 31). To determine whether inhibition of differentiation is dependent on HO-1, human lung fibroblasts were treated with TGF-β and CDDO, with and without NAC, for 3 days to induce myofibroblast differentiation (Fig. 6). TGF-β alone induces expression of the myofibroblast markers α-smooth muscle actin (α-SMA), calponin, and fibronectin, all of which are significantly inhibited by CDDO. CDDO also upregulates HO-1 expression. As expected, NAC inhibits CDDO-induced HO-1 expression. In cells treated with NAC, CDDO inhibits TGF-β-stimulated expression of α-SMA with 80% efficiency compared with CDDO without NAC. CDDO also inhibits expression of calponin and fibronectin in the presence of NAC. This suggests that induction of HO-1 is not necessary for the antifibrotic properties of CDDO, although it may play a minor role.
Fig. 6.
HO-1 is not directly involved in the inhibition of myofibroblast differentiation by CDDO. Primary lung fibroblasts were pretreated with or without 5 mM NAC and then treated with 5 ng/ml TGF-β and 1 μM CDDO as indicated. After 72 h, protein expression was determined by Western blot. A: representative lanes are shown. B: expression of α-SMA was analyzed by densitometry of triplicate cultures and normalized to GAPDH, with untreated cells = 1. CDDO potently induced HO-1 and inhibited TGF-β-driven α-SMA expression as previously shown. NAC inhibited induction of HO-1 by CDDO, but had only a minor effect on the ability of CDDO to block α-SMA expression. *Significant reduction compared with TGF-β alone and TGF-β + CDDO; †significant increase compared with TGF-β + CDDO, P < 0.05 (ANOVA). Results shown are representative of 2 independent experiments.
DISCUSSION
HO-1 is a potential therapeutic target in pulmonary fibrosis. HO-1 and its products CO and bilirubin have antioxidant effects and are effective in reducing lung fibrosis in the mouse and rat bleomycin models (54, 58, 62). Therefore, it is of great interest that 15d-PGJ2 and CDDO, two PPARγ agonists with potent antifibrotic effects in vitro, strongly induce HO-1 in primary human lung fibroblasts. This induction of HO-1 is PPARγ independent, as it is not inhibited by GW9662, a pharmacological inhibitor of PPARγ, or by overexpression of a dominant negative PPARγ (Fig. 2). This parallels our recent report that 15d-PGJ2 and CDDO exert their antifibrotic action via a PPARγ-independent mechanism (14). Rosiglitazone does not induce HO-1 in human lung fibroblasts, in contrast to human umbilical venous endothelial cells (HUVEC) and human vascular smooth muscle cells (VSMCs), in which rosiglitazone does induce HO-1 (24). This appears to be a cell type-specific difference in regulation, as induction of HO-1 in HUVEC and HVSMC cells is dependent on transcriptional activation of PPARγ and on the peroxisome proliferator response element (PPRE) in the HO-1 promoter. Our data indicate that induction of HO-1 in human lung fibroblasts is PPARγ independent and rosiglitazone has little if any PPARγ-independent activity (7, 14).
There are a number of mechanisms by which 15d-PGJ2 and CDDO might upregulate HO-1. HO-1 can be upregulated by reductions in cellular GSH levels (5, 26), by the transcription factor AP-1 (11), and by oxidative stress via the oxidative stress-responsive transcription factor Nrf2 (22). Both 15d-PGJ2 and CDDO can form covalent bonds with free thiols, such as GSH, suggesting that these PPARγ ligands might reduce the amount of intracellular GSH, triggering GSH-dependent upregulation of HO-1. Indeed, treatment of human lung fibroblasts with 15d-PGJ2 and CDDO leads to a reduction in intracellular GSH (Fig. 3B). Treatment with NAC, a thiol antioxidant and GSH precursor, completely inhibits the induction of HO-1 (Fig. 3C). Interestingly, human lung fibroblasts treated with 15d-PGJ2 or CDDO do not appear to be under generalized oxidative stress, as Trolox, a non-thiol antioxidant, did not inhibit HO-1 expression, suggesting that the effects of 15d-PGJ2 and CDDO are thiol specific.
CDDO strongly induces AP-1 transcriptional activity in human lung fibroblasts (14), but this effect could be upstream or downstream of HO-1. Here we report that an AP-1 inhibitor has no effect on the induction of HO-1 but that NAC, which inhibits the upregulation of HO-1, also inhibits CDDO-induced AP-1 activation (Fig. 4). Therefore, AP-1 upregulation is a downstream consequence of HO-1 induction and is not the mechanism for HO-1 induction by PPARγ ligands. Finally, it has been reported in mice and in human cancer cells that CDDO activates the transcription factor Nrf2 with subsequent upregulation of genes containing antioxidant response elements such as HO-1 (22, 27). Interestingly, 15d-PGJ2 but not CDDO promoted nuclear localization of Nrf2 (Fig. 5). Since both compounds upregulate HO-1, this suggests that upregulation of HO-1 by PPARγ ligands is not dependent on Nrf2. Together, these results suggest that in primary human lung fibroblasts, the PPARγ agonists 15d-PGJ2 and CDDO (but not rosiglitazone) induce HO-1 via a glutathione-dependent mechanism involving the formation of covalent bonds between the electrophilic α/β unsaturated ketones in 15d-PGJ2 and CDDO and the free reduced thiol of GSH. NAC completely inhibits induction of HO-1 either by acting as a GSH precursor to raise intracellular GSH levels or as a thiol decoy, or both.
To determine whether induction of HO-1 was responsible for the antifibrotic effects of CDDO in vitro, human lung fibroblasts were treated with TGF-β and CDDO, with and without NAC. As previously reported (14), CDDO inhibits TGF-β-stimulated myofibroblast differentiation, with reduced expression of α-SMA, fibronectin, and calponin. Cotreatment with NAC completely blocked the induction of HO-1 but only blocked 20% of the inhibitory effect of CDDO on myofibroblast differentiation and did not block the inhibition of fibronectin or calponin (Fig. 6). As rosiglitazone did not induce HO-1 in our lung fibroblasts, together these results demonstrate that induction of HO-1 is independent of the antifibrotic properties of PPARγ ligands in vitro.
We have previously reported that PPARγ ligands have potent antifibrotic effects in vitro (7, 14). Our present results suggest that certain PPARγ ligands containing electrophilic centers, such as CDDO, may access a second anti-inflammatory and antifibrotic pathway mediated by HO-1. It has recently been reported that an imidazole derivative of CDDO with a longer biological half-life protected mice from cigarette smoke-induced emphysema, via upregulation of HO-1 and other oxidative stress response genes in the lungs (51). CDDO was also effective in protecting mice from liver damage due to oxidative stress (41). There is evidence that the pathology of lung fibrosis in humans involves ROS and RNS (18, 57). HO-1 also has important cytoprotective properties against inflammatory mediators involved in recruiting cells to injured tissue (32, 61). We hypothesize that CDDO and other PPARγ ligands have significant clinical potential as fibrosis therapies acting through a dual mechanism involving direct effects on fibroblast differentiation and indirect effects via upregulation of HO-1. It is interesting to consider the potential interaction between PPARγ ligands and NAC, another proposed antioxidative stress treatment for lung fibrosis. A clinical trial of NAC in IPF showed modest improvement in some outcome measures (10). Although NAC does not block the inhibition by CDDO of myofibroblast differentiation, it does block the upregulation of HO-1. Thus, combined therapy with CDDO and NAC would be contra-indicated.
GRANTS
This research was supported in part by National Institutes of Health Grants HL-095402, HL-75432, DE-011390, ES-01247, T32-HL-001752, EY-017123, HL-066988, and T32-ES-07026. C. J. Baglole was supported by a Parker B. Francis Fellowship and by a research grant from the American Thoracic Society.
REFERENCES
- 1.Albrecht C, Knaapen AM, Becker A, Hohr D, Haberzettl P, van Schooten FJ, Borm PJ, Schins RP. The crucial role of particle surface reactivity in respirable quartz-induced reactive oxygen/nitrogen species formation and APE/Ref-1 induction in rat lung. Respir Res 6: 129, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonicelli F, Brown D, Parmentier M, Drost EM, Hirani N, Rahman I, Donaldson K, MacNee W. Regulation of LPS-mediated inflammation in vivo and in vitro by the thiol antioxidant Nacystelyn. Am J Physiol Lung Cell Mol Physiol 286: L1319–L1327, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Baglole CJ, Bushinsky SM, Garcia TM, Kode A, Rahman I, Sime PJ, Phipps RP. Differential induction of apoptosis by cigarette smoke extract in primary human lung fibroblast strains: implications for emphysema. Am J Physiol Lung Cell Mol Physiol 291: L19–L29, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Baglole CJ, Reddy SY, Pollock SJ, Feldon SE, Sime PJ, Smith TJ, Phipps RP. Isolation and phenotypic characterization of lung fibroblasts. Methods Mol Med 117: 115–127, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Baglole CJ, Sime PJ, Phipps RP. Cigarette smoke-induced expression of heme oxygenase-1 in human lung fibroblasts is regulated by intracellular glutathione. Am J Physiol Lung Cell Mol Physiol 295: L624–L636, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett EG, Johnston C, Oberdorster G, Finkelstein JN. Silica-induced chemokine expression in alveolar type II cells is mediated by TNF-α-induced oxidant stress. Am J Physiol Lung Cell Mol Physiol 276: L979–L988, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1146–L1153, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Chintharlapalli S, Papineni S, Konopleva M, Andreef M, Samudio I, Safe S. 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid and related compounds inhibit growth of colon cancer cells through peroxisome proliferator-activated receptor gamma-dependent and -independent pathways. Mol Pharmacol 68: 119–128, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Day BJ. Antioxidants as potential therapeutics for lung fibrosis. Antioxid Redox Signal 10: 355–370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 353: 2229–2242, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Elbirt KK, Bonkovsky HL. Heme oxygenase: recent advances in understanding its regulation and role. Proc Assoc Am Physicians 111: 438–447, 1999 [PubMed] [Google Scholar]
- 12.Elte JW, Blickle JF. Thiazolidinediones for the treatment of type 2 diabetes. Eur J Intern Med 18: 18–25, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Feldon SE, O'Loughlin CW, Ray DM, Landskroner-Eiger S, Seweryniak KE, Phipps RP. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol 169: 1183–1193, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson HE, Kulkarni A, Lehmann GM, Garcia-Bates TM, Thatcher TH, Huxlin KR, Phipps RP, Sime PJ. Electrophilic peroxisome proliferator activated receptor-{gamma} (PPAR{gamma}) ligands have potent anti-fibrotic effects in human lung fibroblasts. Am J Respir Cell Mol Biol . In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flohe L, Jaeger T, Pilawa S, Sztajer H. Thiol-dependent peroxidases care little about homology-based assignments of function. Redox Rep 8: 256–264, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83: 803–812, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Fu M, Zhang J, Zhu X, Myles DE, Willson TM, Liu X, Chen YE. Peroxisome proliferator-activated receptor gamma inhibits transforming growth factor beta-induced connective tissue growth factor expression in human aortic smooth muscle cells by interfering with Smad3. J Biol Chem 276: 45888–45894, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med 34: 1507–1516, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Gauldie J, Kolb M, Sime PJ. A new direction in the pathogenesis of idiopathic pulmonary fibrosis? Respir Res 3: 1, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto K, Farrow BJ, Evers BM. Activation and role of MAP kinases in 15d-PGJ2-induced apoptosis in the human pancreatic cancer cell line MIA PaCa-2. Pancreas 28: 153–159, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Liu X, Wang H, Kohyama T, Kobayashi T, Wen FQ, Romberger DJ, Abe S, MacNee W, Rahman I, Rennard SI. Glutathione prevents inhibition of fibroblast-mediated collagen gel contraction by cigarette smoke. Am J Physiol Lung Cell Mol Physiol 283: L409–L417, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kim HR, Kim S, Kim EJ, Park JH, Yang SH, Jeong ET, Park C, Youn MJ, So HS, Park R. Suppression of Nrf2-driven heme oxygenase-1 enhances the chemosensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer 60: 47–56, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Koli K, Myllarniemi M, Keski-Oja J, Kinnula VL. Transforming growth factor-β activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal 10: 333–342, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Kronke G, Kadl A, Ikonomu E, Bluml S, Furnkranz A, Sarembock IJ, Bochkov VN, Exner M, Binder BR, Leitinger N. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol 27: 1276–1282, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Lakatos HF, Thatcher TH, Kottmann RM, Garcia TM, Phipps RP, Sime PJ. The role of PPARs in lung fibrosis. PPAR Res 2007: 71323, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lautier D, Luscher P, Tyrrell RM. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis 13: 227–232, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res 65: 4789–4798, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem 282: 20621–20633, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Maggirwar SB, Ramirez S, Tong N, Gelbard HA, Dewhurst S. Functional interplay between nuclear factor-kappaB and c-Jun integrated by coactivator p300 determines the survival of nerve growth factor-dependent PC12 cells. J Neurochem 74: 527–539, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Melichar B, Konopleva M, Hu W, Melicharova K, Andreeff M, Freedman RS. Growth-inhibitory effect of a novel synthetic triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, on ovarian carcinoma cell lines not dependent on peroxisome proliferator-activated receptor-gamma expression. Gynecol Oncol 93: 149–154, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Milam JE, Keshamouni VG, Phan SH, Hu B, Gangireddy SR, Hogaboam CM, Standiford TJ, Thannickal VJ, Reddy RC. PPAR-γ agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L891–L901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA 98: 8798–8803, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 18: 1897–1899, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Oh JY, Giles N, Landar A, Darley-Usmar V. Accumulation of 15-deoxy-delta(12,14)-prostaglandin J2 adduct formation with Keap1 over time: effects on potency for intracellular antioxidant defence induction. Biochem J 411: 297–306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-α-induced apoptosis in cultured fibroblasts. Am J Physiol Lung Cell Mol Physiol 278: L312–L319, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Phan SH. The myofibroblast in pulmonary fibrosis. Chest 122: 286S–289S, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Qanungo S, Wang M, Nieminen AL. N-acetyl-l-cysteine enhances apoptosis through inhibition of nuclear factor-kappaB in hypoxic murine embryonic fibroblasts. J Biol Chem 279: 50455–50464, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal 7: 42–59, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1: 3159–3165, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Ray DM, Akbiyik F, Phipps RP. The peroxisome proliferator-activated receptor gamma (PPARgamma) ligands 15-deoxy-delta12,14-prostaglandin J2 and ciglitazone induce human B lymphocyte and B cell lymphoma apoptosis by PPARgamma-independent mechanisms. J Immunol 177: 5068–5076, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Reisman SA, Buckley DB, Tanaka Y, Klaassen CD. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol Appl Pharmacol 236: 109–114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiter TA, Pang B, Dedon P, Demple B. Resistance to nitric oxide-induced necrosis in heme oxygenase-1 overexpressing pulmonary epithelial cells associated with decreased lipid peroxidation. J Biol Chem 281: 36603–36612, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Rizzo G, Fiorucci S. PPARs and other nuclear receptors in inflammation. Curr Opin Pharmacol 6: 421–427, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid Redox Signal 9: 2157–2173, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Schins RP, Borm PJ. Mechanisms and mediators in coal dust induced toxicity: a review. Ann Occup Hyg 43: 7–33, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest 132: 1311–1321, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP., 3rd Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs 64: 405–430, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol 99: 308–319, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol 28: 551–558, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, Maue RA, Place AE, Porter DM, Spinella MJ, Williams CR, Wu G, Dannenberg AJ, Flanders KC, Letterio JJ, Mangelsdorf DJ, Nathan CF, Nguyen L, Porter WW, Ren RF, Roberts AB, Roche NS, Subbaramaiah K, Sporn MB. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res 59: 336–341, 1999 [PubMed] [Google Scholar]
- 51.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA 106: 250–255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thannickal VJ, Toews GB, White ES, Lynch JP 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27: 502–522, 1969 [DOI] [PubMed] [Google Scholar]
- 54.Tsuburai T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Hasiba T, Ueda A, Ikehara K, Matsuse T, Ishigatsubo Y. Adenovirus-mediated transfer and overexpression of heme oxygenase 1 cDNA in lung prevents bleomycin-induced pulmonary fibrosis via a Fas-Fas ligand-independent pathway. Hum Gene Ther 13: 1945–1960, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Uchida K, Shibata T. 15-Deoxy-delta(12,14)-prostaglandin J2: an electrophilic trigger of cellular responses. Chem Res Toxicol 21: 138–144, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Upadhyay D, Panduri V, Ghio A, Kamp DW. Particulate matter induces alveolar epithelial cell DNA damage and apoptosis: role of free radicals and the mitochondria. Am J Respir Cell Mol Biol 29: 180–187, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Walters DM, Cho HY, Kleeberger SR. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2. Antioxid Redox Signal 10: 321–332, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Wang HD, Yamaya M, Okinaga S, Jia YX, Kamanaka M, Takahashi H, Guo LY, Ohrui T, Sasaki H. Bilirubin ameliorates bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med 165: 406–411, 2002 [DOI] [PubMed] [Google Scholar]
- 59.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol 201: 343–354, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willey JJ, Stripp BR, Baggs RB, Gasiewicz TA. Aryl hydrocarbon receptor activation in genital tubercle, palate, and other embryonic tissues in 2,3,7,8-tetrachlorodibenzo-p-dioxin-responsive lacZ mice. Toxicol Appl Pharmacol 151: 33–44, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Zampetaki A, Minamino T, Mitsialis SA, Kourembanas S. Effect of heme oxygenase-1 overexpression in two models of lung inflammation. Exp Biol Med 228: 442–446, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Zhou Z, Song R, Fattman CL, Greenhill S, Alber S, Oury TD, Choi AM, Morse D. Carbon monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol 166: 27–37, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]