Abstract
Although Cl− transport in fetal lung is important for fluid secretion and normal lung development, the role of Cl− transport in adult lung is not well understood. In physiological studies, the cystic fibrosis transmembrane regulator (CFTR) plays a role in fluid absorption in the distal air spaces of adult lung, and alveolar type II cells cultured for 5 days have the capacity to transport Cl−. Although both alveolar type I and type II cells express CFTR, it has previously not been known whether type I cells transport Cl−. We studied Cl− uptake in isolated type I cells directly, using either radioisotopic tracers or halide-sensitive fluorescent indicators. By both methods, type I cells take up Cl−. In the presence of β-adrenergic agonist stimulation, Cl− uptake can be inhibited by CFTR antagonists. Type I cells express both the Cl−/HCO3− anion exchanger AE2 and the voltage-gated Cl− channels CLC5 and CLC2. Inhibitors of AE2 also block Cl− uptake in type I cells. Together, these results demonstrate that type I cells are capable of Cl− uptake and suggest that the effects seen in whole lung studies establishing the importance of Cl− movement in alveolar fluid clearance may be, in part, the result of Cl− transport across type I cells.
Keywords: chloride transport, cystic fibrosis transmembrane regulator, CLC5
appropriate alveolar fluid homeostasis is important for maintaining efficient gas exchange. Excess alveolar fluid is removed from the alveolar space by active salt and water transport across the alveolar and distal airway epithelium (reviewed in Ref. 44). The link between Na+ transport and fluid clearance has been well established, leading to the generally accepted concept that alveolar fluid clearance is driven by an osmotic gradient created by active Na+ transport across the alveolar epithelium. While Cl− has long been considered the counterbalancing anion that accompanies Na+ movement to maintain electroneutrality, far less is known about the role of Cl− transport in the adult lung. In fetal lung, the alveolar epithelium in utero actively secretes Cl− into the developing air spaces, causing distention that is necessary for normal lung growth and development (1, 35, 48, 51). At birth, during the transition to an air environment, rapid fluid reabsorption occurs. It is not known to what extent and under which conditions active and/or passive Na+ or Cl− reabsorption occurs in the adult lung.
The alveolar epithelium is composed of two morphologically different cell types, alveolar type I (TI) cells, which cover 95–98% of the internal surface area of the lung, and alveolar type II (TII) cells, which cover the remaining 2–5%. Both alveolar TI and TII cells contain functional Na+ channels, allowing the transcellular transport of Na+ and fluid across the epithelium of both cell types. The role of Cl− transport in the adult lung is less clear, with controversy about both the directionality and the route of Cl− transport. Initial studies of Cl− transport across the alveolar epithelium suggested that Cl− moved via paracellular transport mechanisms, following the osmotic gradient generated by active Na+ transport (65). More recent studies involving the discovery of cAMP-stimulated Cl− uptake across apical Cl− channels in cultured alveolar rat TII epithelial cell monolayers have suggested transepithelial Cl− transport (29, 33, 38, 49). Other studies have suggested that adult alveolar epithelial cells can secrete Cl− (19, 30, 40, 50). The observations of inhibition of distal alveolar fluid clearance by the CFTR inhibitor glibenclamide in mouse whole lung and ex vivo normal human lung suggest a role for CFTR in modulating ion and fluid transport in the lung (20).
We have previously demonstrated that alveolar TI cells contain functional CFTR (31). In this communication, we report that TI cells take up Cl−, as measured by both uptake of radioisotopic Cl− and by fluorescent Cl− indicators. Cl− uptake can be stimulated by β-adrenergic agonists and inhibited both by the nonspecific chloride channel inhibitor NPPB and the CFTR-specific inhibitor CFTRinh172. Cl− uptake also occurs via AE2, a Cl−/HCO3− exchanger, which can be inhibited by the AE2 antagonist DIDS. Finally, we show that the voltage-gated chloride channels CLC5 and CLC2 are more prominently expressed in TI, rather than TII, cells, suggesting that anion transporters in addition to CFTR or AE2 may participate in alveolar TI cell Cl− transport.
METHODS
Alveolar epithelial cell isolation.
Adult Sprague-Dawley rats were anesthetized via intraperitoneal injection with pentobarbital (200 mg/kg) as per protocol approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco. TI cells were then isolated from the lungs by enzymatic digestion with porcine grade IV elastase, density gradient centrifugation, and negative and positive immunoselection with magnetic beads as previously described (14). For TI cell isolations, preparations containing <85% TI cells or >2% TII cells were discarded; >95% of the cells were viable as measured by a Live/Dead ratio kit (Invitrogen, Carlsbad, CA). TII cells were isolated by enzymatic digestion with porcine elastase and panned over IgG-coated plates followed by negative immunoselection with magnetic beads as described previously (15). Preparations of TII cells containing <85% TII cells or with >2% TI cells were discarded; viabilities were >95%. Each rat produced only one cell isolation, either TI or TII.
Measurement of Cl− transport using 36Cl.
Freshly isolated alveolar TI cells were resuspended in RPMI 1640 media/20% FBS, a sample of which was reserved for controls. Bumetanide (10−4 M; Sigma-Aldrich, St. Louis, MO), which blocks the NaK2Cl transporter and allows for the measurement of intracellular 36Cl accumulation over time, and 0.5 μCi/ml 36Cl (Amersham GE Pharmaceuticals, Piscataway, NJ) were added to both controls and experimental samples. At each time point for both the control and treated samples, 200-μl aliquots of the cell suspension were removed, and 0.1 μCi/ml U-14C-sucrose was added as a marker of extracellular space. After centrifugation through silica oil, triplicate 25-μl samples of the supernatant liquid were removed, and the liquid and pellet were separately dissolved in 1.0 ml of 0.1 N NaOH overnight. Radioactivity contained in the supernatant liquid and the pellets was measured using a beta scintillation counter (Beckman LS 5801). Protein contained in the cell pellets was measured by the bicinchoninic acid method (Pierce, Rockford, IL). The 36Cl content of the extracellular space was subtracted, and results were calculated as pmol Cl−/μg protein. In preliminary time-course experiments, 36Cl uptake for both control and cells treated with terbutaline (10−4 M, Sigma-Aldrich) was linear between 0 and 10 min and remained stable between 10 and 20 min. Because the number of cells is limiting in our experiments, we chose to measure 36Cl uptake at 5 min for the remaining conditions unless otherwise stated.
Measurement of Cl− uptake in the presence of Cl− channel agonists or inhibitors.
TI cells were isolated and divided into two portions, with one portion serving as the control. For studies of Cl− uptake in the presence of inhibitors, cells were pretreated for 10 min with NPPB [5-nitro-2-(3-phenylpropylamino) benzoate] (a nonselective Cl− channel inhibitor, 10−4 M); CFTRinh172 (a highly selective thiazolidinone CFTR inhibitor, 10 μM); or DIDS (an anion exchange inhibitor, 10−4 M). Bumetanide and 36Cl were then added to all samples, including the controls, before Cl− uptake was measured as described above. Control experiments measured Cl− uptake in the absence of the inhibitors. For studies aimed at measuring the effect of Cl− inhibitors in the presence of β-adrenergic agonist stimulation, TI cells were pretreated for 10 min with a chosen inhibitor (NPPB, CFTRinh172, or DIDS) before the addition of the β-adrenergic agonist terbutaline, bumetanide, and 36Cl; control cells were treated with terbutaline, bumetanide, and 36Cl only. Cl− uptake was measured at 5 min as described above. The results were calculated as pmol Cl−/μg protein.
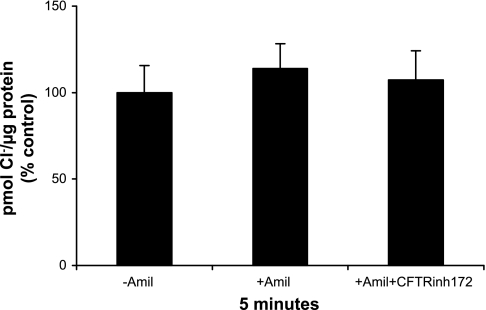
Measurement of Cl− uptake in the presence of amiloride.
Amiloride inhibits the amiloride-sensitive epithelial Na+ channel ENaC. To determine if Cl− uptake was dependent on Na+ uptake in TI cells, TI cells were isolated and divided into three portions. One portion served as the control and was not treated with amiloride. A second portion was pretreated with amiloride (10−4 M) for 10 min. A third was pretreated with both amiloride and CFTRinh172 (10 μM) for 10 min. Bumetanide and 36Cl were then added to all cell portions, and Cl− uptake was measured as described above. The results were calculated as pmol Cl−/μg protein.
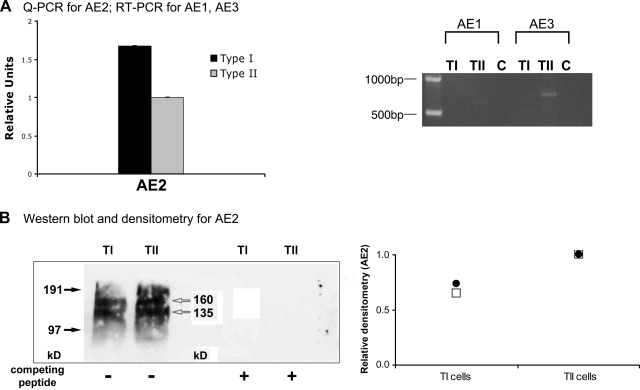
Analysis of AE and CLC mRNA expression.
Real-time quantitative PCR (Q-PCR) (ABI Prism 7700 Sequence Detection System, TaqMan, Applied Biosystems) was used to evaluate relative RNA expression of the Cl−/HCO3− anion exchanger 2 (AE2) mRNA in TI and TII cells. The primer-probe set for AE2 was purchased from Applied Biosystems and was not noted to be isoform specific. Relative amounts of mRNA (n = 3 separate cell isolations for each cell type) were then calculated using the comparative threshold method using 18S total RNA as the internal control. Reverse-transcriptase PCR was used to assay for AE1, AE3, CLC1, and CLC6 mRNA expression in TI and TII cells. Primers used for RT-PCR analyses were previously published: AE1 (sense 5′-GCT GAG GAC CTA AAG GAT CT-3′, antisense 5′-TCC TTT CCC CCG TCT AAT GC-3′); AE3 (sense 5′-GAT GAC AAG GAC AGT GGC TT-3′, antisense 5′-TCT TCA GAG GTT GCC TCG GA-3′) (54); CLC1 (sense 5′-ATA TCA TCT ATA AGA TCT TAC CAG G-3′, antisense 5′-TCT GGA GTA GGT TTC TTA GTT CC-3′) (5); CLC6 (sense 5′-GCT GAG AGC CAG CGA CAT CA-3′, antisense 5′-AGC GGA CGG AAT CGC TCC T-3′) (5). Ten nanograms of adult rat TI or TII cell cDNA were mixed with 1.25 units of Taq DNA polymerase (Fermentas, Ontario, Canada), 250 μM dNTPs (Sigma-Aldrich), and 1.0 mM MgCl2 and 200 nm of each primer (Operon, Huntsville, AL) in the appropriate buffers. For the CLC isoforms, the temperature cycling conditions were: 35 cycles of denaturation (95°C, 1 min), annealing (58°C, 1 min), and extension (72°C, 1 min). For AE1 and AE3, annealing occurred at 55°C. PCR products were run on a 2% agarose gel and visualized with UV fluorescence. The predicted sizes for the PCR products based on the primers used are as follows: CLC1, 351 bp; CLC6, 424 bp; AE1, 520 bp; AE3 (brain isoform), 410 bp.
Western blot analysis.
TI and TII cells and whole lung tissue were homogenized, and protein was extracted with a buffer containing 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and a cocktail of protease inhibitors (Sigma-Aldrich). Thirty micrograms of each protein were then resolved on a 4–12% Bis-Tris gel (Invitrogen) before transfer to a nitrocellulose membrane and overnight blocking with 5% powdered milk at 4°C. For detection of AE2, the blot was then incubated with rabbit polyclonal antibody against AE2 both in the absence and in the presence of the AE2 peptide antigen. The AE2 antibody and peptide antigen were kind gifts from Dr. Seth Alper. For detection of CLC5 and CLC2, separate blots were incubated with goat polyclonal antibody against CLC5 (Santa Cruz Biotechnology, Santa Cruz, CA) or a rabbit polyclonal antibody against CLC2 (Sigma-Aldrich). The membranes were then incubated with goat anti-rabbit IgG-HRP (Vector Laboratories, Burlingame, CA) for CLC2 or donkey anti-goat IgG-HRP (Santa Cruz Biotechnology) for CLC5 before incubation with ECL Plus chemiluminescent substrate (Pierce, Rockford, IL). Densitometric quantitation was performed by phosphorimage analysis (STORM scanner, ImageQuaNT software; Molecular Dynamics, Sunnyvale, CA). CLC blots were also incubated with β-actin antibody (Sigma-Aldrich) to normalize for protein loading.
Immunohistochemistry.
Immunohistochemistry was performed on both cytocentrifuged preparations of mixed lung cells and 2-μm cryostat sections of adult rat lung as previously described (32). The antibodies used for staining were the following: 1) goat polyclonal anti-CLC5 (Santa Cruz Biotechnology); 2) donkey anti-goat IgG-Alexa 594 (fluoresces red, Invitrogen); 3) either mouse monoclonal IgG anti-RTI40 (TI cell marker) or mouse monoclonal IgG anti-RTII70 (TII cell marker); and 4) goat anti-mouse IgG-Alexa 488 (fluoresces green, Invitrogen). Images were captured with a Leica DC500 camera on a Leica Orthoplan microscope.
Measurement of Cl− transport using MQAE, a fluorescent Cl− indicator.
MQAE [N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide] is a quinolinium salt-based halide-sensitive fluorescent indicator used to track Cl− flux into and out of cells (63). By measuring either the quenching (in the case of Cl− uptake) or dequenching (which occurs with Cl− secretion) of fluorescence in response to various stimuli, Cl− movement can be tracked. TI cells (∼100,000 cells/coverslip) were cultured on glass coverslips coated with Cell-Tak (BD Biosciences, San Jose, CA) in isotonic DMEM H-16 media (UCSF Cell Culture Facility) with 20% FBS for 24 h. The cells were then washed once with prewarmed serum-free fresh media containing 100 mM Cl− before adding 10 μM MQAE (Invitrogen) and incubating at 37°C in 10% CO2 for 4 h. The cells were washed again with media, and fluorescence was recorded using a time-lapse TE2000 Nikon epifluorescence microscope. Fluorescence measurements were taken at 0, 5, and 10 min in the absence and presence of CFTRinh172 and after the addition of terbutaline alone or terbutaline and CFTRinh172. Fluorescence intensity for each region of interest on each coverslip was recorded, and relative fluorescence was calculated as the fluorescence recorded at each time point (Ft) less the fluorescence recorded at the beginning of the experiment (F0). Background fluorescence was subtracted using Nikon imaging software before fluorescent measurements were obtained.
Data presentation and statistical analysis.
Cl− uptake was calculated as pmol Cl−/μg protein. The uptake figures are graphically represented as percent change from control (%control) ± SE. For comparisons between the control and treated groups, unpaired Student's t-test was employed. For comparisons of multiple groups, a one-way ANOVA followed by post hoc analysis using the Bonferroni t-test were conducted. Differences were considered significant if P ≤ 0.05.
RESULTS
Freshly isolated alveolar type I cells take up Cl−; uptake is augmented by stimulation with β-adrenergic agonists.
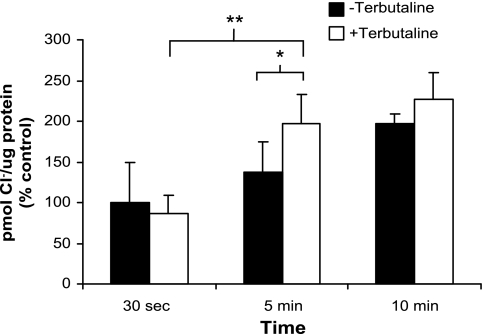
We measured Cl− uptake in freshly isolated rat TI cells in suspension using trace amounts of 36Cl. Bumetanide was added to NaK2Cl transporter to prevent simultaneous influx and efflux of Cl− to allow measurement of intracellular Cl−. Measurements were performed both in the presence and in the absence of the β-agonist terbutaline. Cl− uptake was measured in pmol Cl−/μg protein. The data are graphically represented for all uptake experiments as %change of control ± SE. As shown in Fig. 1, TI cells take up Cl−, and Cl− uptake is enhanced by β-agonist stimulation. At 5 min, terbutaline increased Cl− uptake in TI cells by 31% over cells not treated with terbutaline (P < 0.05). Cl− uptake 10 min after the addition of terbutaline was stimulated to a lesser extent (14%, not statistically significant) compared with unstimulated controls at the same 10-min time point.
Fig. 1.
Type I (TI) cells have the capacity to take up Cl−. Cl− uptake was measured in freshly isolated rat TI cells both in the absence and in the presence of terbutaline (10−4 M) at 30 s, 5 min, and 10 min. Cl− uptake increased between 30 s and 10 min in both groups, but at 5 min, Cl− uptake in terbutaline-treated cells was 31% greater than in untreated cells (n = 4 cell isolations, *P < 0.05). Uptake was measured in pmol Cl−/μg protein and graphically represented as %change of control ± SE. In these experiments, control was defined as Cl− uptake in unstimulated TI cells (no terbutaline) at 30 s. In terbutaline-stimulated cells, uptake increased significantly by 110% between 30 s and 5 min (**P < 0.05).
Bumetanide addition facilitates Cl− uptake measurements.
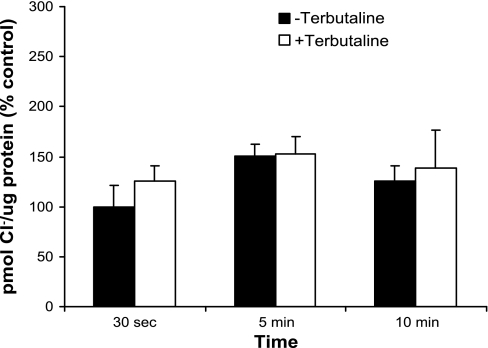
Other investigators have used bumetanide to facilitate measurement of Cl− flux in epithelial monolayers and whole lung studies. Our attempts to measure Cl− uptake in the absence of bumetanide resulted in nonlinear uptake with no difference in Cl− uptake at any of the measured time points either in the presence or absence of terbutaline (Fig. 2; n = 3 TI cell isolations). Specifically, at 5 min, the amount of Cl− was nearly identical either in the presence or absence of terbutaline (P = 0.81 when comparing samples not treated with terbutaline to samples treated with terbutaline). We conclude that inhibition by bumetanide is necessary for the measurement of net cellular Cl− uptake in freshly isolated cells.
Fig. 2.
Cl− uptake in the absence of bumetanide. In the absence of bumetanide, a NaK2Cl cotransport inhibitor, Cl− uptake was measured either with or without terbutaline. Cl− uptake was not linear, and at 5 min, there was an inability to detect differences in Cl− uptake in terbutaline-stimulated vs. nonstimulated cells (n = 3 cell isolations). Uptake is graphically represented as %change of control ± SE, with the control in these experiments defined as Cl− uptake in unstimulated TI cells at 30 s.
Cl− uptake in type I cells is inhibited by the Cl− channel inhibitor NPPB.
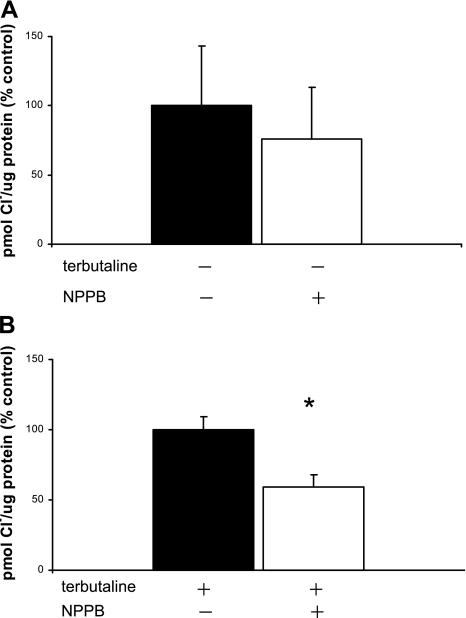
NPPB inhibits a variety of Cl− channels, including Ca+-activated and volume-sensitive Cl− channels and CFTR (9, 27, 57, 62). In the absence of terbutaline, NPPB inhibited Cl− uptake by ∼24%, but this degree of inhibition was not statistically significant (Fig. 3A; n = 3): Cl− uptake decreased from 176.1 pmol Cl−/μg protein (no NPPB) to 134.1 pmol Cl−/μg protein in the presence of NPPB. However, in terbutaline-stimulated cells, Cl− uptake decreased from 241.7 pmol Cl−/μg protein (in the absence of NPPB) to 119.4 pmol Cl−/μg protein in the presence of NPPB, demonstrating that NPPB significantly decreased terbutaline-stimulated Cl− uptake by 41% (Fig. 3B; n = 4, P < 0.03).
Fig. 3.
Cl− uptake is inhibited by NPPB. 36Cl uptake was measured in freshly isolated TI cells both in the absence and in the presence of the nonselective Cl− channel inhibitor NPPB. Results are expressed as %change from control in pmol Cl−/μg protein (%control) ± SE. There are separate controls for the experiments represented in A and B. In A, neither the controls nor cells with inhibitors received terbutaline. In B, both the controls and cells with inhibitors received terbutaline. Uptake data was obtained at 5 min. A: in the absence of terbutaline, Cl− uptake at 5 min in cells treated with NPPB was inhibited 24% compared with controls (−terbutaline, −NPPB), but this degree of inhibition was not significant (n = 3 cell isolations). B: in the presence of terbutaline, cells incubated with NPPB (+terbutaline, +NPPB) significantly reduced Cl− uptake by 41% compared with controls (+terbutaline, −NPPB) (n = 4, *P < 0.03).
CFTRinh172 inhibits Cl− uptake in type I cells.
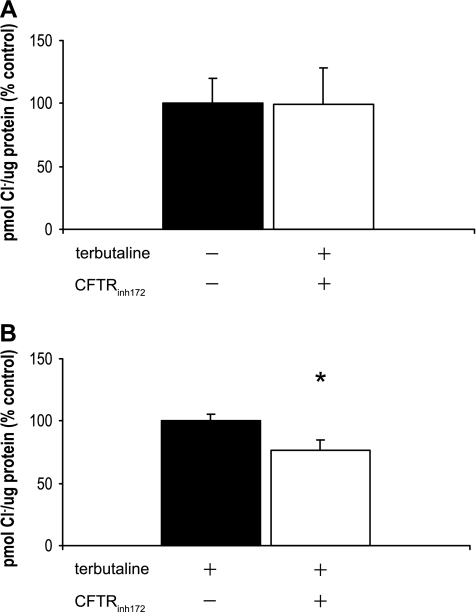
Since NPPB is a nonspecific inhibitor of CFTR, we measured Cl− uptake in the presence and in the absence of the specific CFTR inhibitor, CFTRinh172, to determine what portion of Cl− uptake in TI cells can be attributed to CFTR (42). Cl− uptake was measured as described above. In the absence of terbutaline, there was no appreciable inhibition of Cl− by CFTRinh172: 184.5 pmol Cl−/μg protein for cells without CFTRinh172 treatment, and 198.5 pmol Cl−/μg protein for cells treated with CFTRinh172 (Fig. 4A; n = 4). When terbutaline-stimulated TI cells were treated with CFTRinh172, Cl− uptake was inhibited by 23% compared with controls (control experiments for the terbutaline-stimulated cells were conducted in the presence of terbutaline and in the absence of CFTRinh172): 280.6 pmol Cl−/μg protein for controls, 214.9 pmol Cl−/μg protein for cells treated with CFTRinh172 (Fig. 4B; n = 5, P < 0.05).
Fig. 4.
CFTRinh172 blocks Cl− uptake in type I cells. 36Cl uptake was measured in freshly isolated TI cells both in the absence and in the presence of the CFTR-specific inhibitor CFTRinh172. Results are expressed as %change from control in pmol Cl−/μg protein (%control) ± SE. A: in the absence of terbutaline, there was no difference in Cl− uptake between cells that were treated with CFTRinh172 (−terbutaline, +CFTRinh172) and control cells (−terbutaline, −CFTRinh172) at 5 min (n = 4 cell isolations). B: in the presence of terbutaline, CFTRinh172 significantly inhibited Cl− uptake by 23% compared with controls (+terbutaline, −CFTRinh172) (n = 5, *P < 0.05).
Amiloride does not alter Cl− uptake in type I cells.
It has been suggested that Cl− flux in alveolar fluid clearance is contingent on Na+ transport, as Cl− passively follows the movement of Na+ to preserve electroneutrality. To test this hypothesis, we measured unstimulated Cl− uptake in TI cells that were treated with the ENaC inhibitor amiloride. There was no difference in Cl− uptake in cells treated with amiloride at 5 min compared with controls, suggesting that in TI cells, Cl− uptake is not entirely driven by amiloride-sensitive Na+ transport (Fig. 5). Additionally, there was no difference in Cl− uptake in cells treated with both amiloride and CFTRinh172. Lack of efficacy of CFTRinh172 may stem from the fact that the cells were not treated with terbutaline, thereby producing similar results as seen in experiments of cells treated with CFTRinh172 in the absence of terbutaline (no difference in Cl− uptake compared with controls).
Fig. 5.
Amiloride does not inhibit Cl− uptake. Freshly isolated TI cells were incubated with amiloride alone or amiloride and CFTRinh172. Cl− uptake was then measured at 5 min. There was no significant difference in Cl− uptake among control cells (−amiloride), cells treated with amiloride (+amiloride), and cells treated with both amiloride and CFTRinh172 (n = 3 cell isolations). Results are expressed as %change from control in pmol Cl−/μg protein (%control) ± SE.
Type I cells contain AE2 mRNA and protein.
TI and TII cells contain AE2 mRNA by Q-PCR (Fig. 6A); the AE1 isoform was not detected in either cell type, and the AE3 isoform was only detected in TII cells by RT-PCR. The primers used for AE3 predicted a PCR product of 410 bp; our gel detected a single band at ∼825 bp, suggesting that this may either represent a heteroduplex of the product or another splice variant of AE3 specific to the lung. By Western blotting, AE2 protein expression was shown to be present in both TI and TII cells (Fig. 6B). We detected bands consistent with the AE2a (∼170 kDa) and AE2c isoforms (∼135 kDa) in both TI and TII cells. The specificity of the antibody was demonstrated by competing with specific AE2 peptides (Fig. 6B). Two Western blots were performed; a representative blot is shown along with densitometry data normalized to the protein content of TII cells. Quantitative analysis of the Western blots showed that while TI cells contained ∼50% more AE2 message than TII cells, TII cells contained ∼25% more AE2 protein than their TI counterparts.
Fig. 6.
Anion exchanger expression in alveolar epithelial cells. A: Q-PCR results demonstrate the presence of AE2 mRNA in TI and type II (TII) cells. RT-PCR revealed no detectable AE1 mRNA in either cell type, but confirmed the presence of AE3 mRNA in TII cells. Controls (C) lacked reverse transcriptase. B: Western blots reveal both cell types contain AE2 and that antibody binding can be inhibited with AE2-specific competing peptides. Densitometry demonstrates that TII cells contain ∼25% more AE2 per microgram of total cellular protein than TI cells.
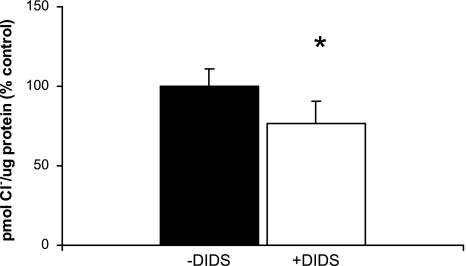
Cl− uptake in type I cells is inhibited by DIDS, an AE2 inhibitor.
To determine whether the Cl−/HCO3− anion exchanger AE2 is involved in Cl− uptake by TI cells, we measured Cl− uptake in the presence of DIDS, an AE2 inhibitor, and terbutaline (Fig. 7). Control experiments were conducted in the presence of terbutaline, but in the absence of DIDS. DIDS inhibited Cl− uptake in TI cells by 25% (n = 6, P < 0.05) suggesting that AE2 may have a role in Cl− uptake in terbutaline-stimulated TI cells.
Fig. 7.
The AE2 inhibitor DIDS decreases Cl− uptake in type I cells. 36Cl uptake was measured both in the absence and in the presence of DIDS (10−4 M) in terbutaline-treated TI cells. DIDS inhibited Cl− uptake by 25% compared with controls (n = 6 cell isolations, *P < 0.05). Results are expressed as %change from control in pmol Cl−/μg protein (%control) ± SE.
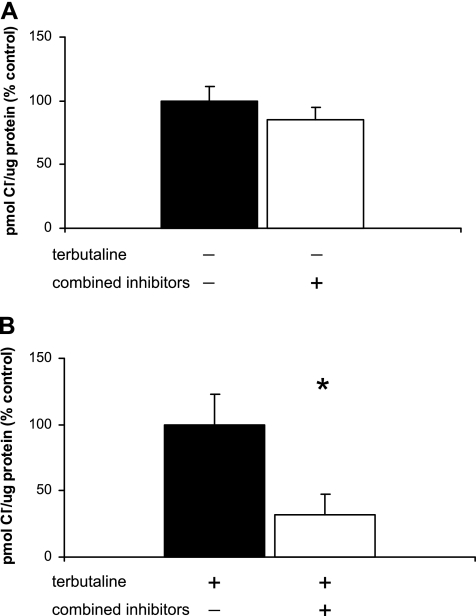
Terbutaline-stimulated Cl− uptake in type I cells is significantly reduced in the presence of multiple Cl− channel/transporter inhibitors.
In the absence of terbutaline, Cl− uptake is not significantly inhibited by the addition of NPPB, CFTRinh172, and DIDS: 212.9 pmol Cl−/μg protein for controls, 181.7 pmol Cl−/μg protein for cells treated with multiple inhibitors (Fig. 8A, n = 3). However, the degree of Cl− inhibition is additive with the same combination of inhibitors when the cells are treated with terbutaline. At 5 min, Cl− uptake was 305.9 pmol Cl−/μg protein in terbutaline-stimulated cells; the addition of multiple inhibitors decreased Cl− uptake to 97.3 pmol Cl−/μg protein, representing a 68% decline, which was statistically significant (Fig. 8B; n = 3, P < 0.02).
Fig. 8.
Cl− uptake is significantly inhibited in the presence of multiple Cl− inhibitors. 36Cl uptake was measured in freshly isolated TI cells in the presence of a combination of Cl− channel inhibitors, CFTRinh172 (10 μM), NPPB (10−4 M), and DIDS (10−4 M). Results are expressed as %change from control in pmol Cl−/μg protein (%control) ± SE. A: under unstimulated conditions, there was no significant inhibition of Cl− uptake at 5 min in cells exposed to multiple inhibitors (−terbutaline, +inhibitors) compared with controls (−terbutaline, −inhibitors) (n = 3 cell isolations). B: under terbutaline-stimulated conditions, there was significant inhibition of Cl− uptake, 68%, in TI cells treated with multiple Cl− channel antagonists (+terbutaline, +inhibitors) compared with controls (+terbutaline, −inhibitors) at 5 min (n = 3, *P < 0.02).
Cl− uptake in type I cells measured with the fluorescent indicator MQAE yields similar results to Cl− uptake measured by radioisotopes.
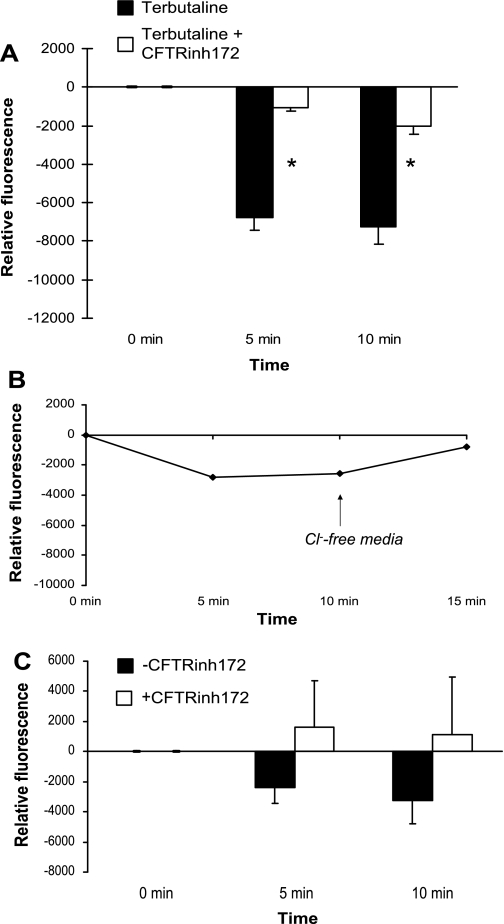
We also measured Cl− fluxes in type I cells by an alternative nonradioactive method using MQAE, a halide-sensitive fluorescent Cl− indicator (4, 36, 45, 61). Since Cl− ions quench MQAE fluorescence, the influx of Cl− into cells decreases the relative fluorescence. In the cells that were treated with terbutaline, fluorescence intensity decreased, indicating quenching of MQAE, from which Cl− influx into TI cells can be inferred. In the cells that were treated with terbutaline and CFTRinh172, overall fluorescence was decreased to a lesser extent, suggesting that CFTRinh172 inhibits terbutaline-stimulated Cl− influx in TI cells (Fig. 9A). Thus, the MQAE assay confirms results obtained in studies using radioisotopic tracers. The degree of inhibition of Cl− influx in TI cells as measured by this method was greater than that observed using radioisotopic methods, which may in part be due to the use of cultured cells in the MQAE experiments. Unlike cells in suspension, cultured cells establish polarity, which may alter their Cl− transport properties. The decrease in fluorescence that we measured was not due to photobleaching because the addition of media containing no Cl− after the completion of the experiments caused an increase in fluorescence intensity to baseline levels (Fig. 9B). In cells not treated with terbutaline, Cl− influx occurred in the absence of CFTRinh172, as exhibited by the decrease in fluorescence over time. However, in the presence of CFTRinh172, there was a slight increase in the relative fluorescence, suggesting Cl− efflux from the cells. The differences were not statistically significant (Fig. 9C).
Fig. 9.
A: CFTRinh172 attenuates Cl− influx into type I cells as measured by MQAE. TI cells were loaded with MQAE in 100 mM Cl−-containing media and then incubated with either terbutaline alone (n = 6 cell isolations) or terbutaline and CFTRinh172 (n = 6). Fluorescence measurements were taken at 0, 5, and 10 min. Relative fluorescence was calculated as the fluorescence recorded at each time point less the fluorescence recorded at the beginning of the experiment. Decreased fluorescence indicates Cl− influx into cells, i.e., Cl− uptake. TI cells demonstrate Cl− uptake as measured by decreasing MQAE fluorescence at 5 and 10 min. The relative fluorescence intensity in cells treated with both terbutaline and CFTRinh172 was brighter (less quenched) compared with similar measurements performed in the presence of terbutaline alone, suggesting that CFTRinh172 can inhibit Cl− uptake in TI cells. Mean values for the relative fluorescence ± SE for each experiment were plotted (*P < 0.05). B: decreased fluorescence intensity is not due to photobleaching. Addition of chloride-free media to cells treated with terbutaline and CFTRinh172 produced an increase in cell fluorescence to near-baseline levels, demonstrating that the decrease in fluorescence was not due to photobleaching. C: Cl− uptake in type I cells as measured by MQAE fluorescence in the absence of terbutaline. TI cells were loaded with MQAE as described in A; no terbutaline was added to any of the cells. CFTRinh172 was added and fluorescence measurements were taken at 0, 5, and 10 min. Relative fluorescence was calculated as described in A. The control cells (those not treated with inhibitor) demonstrated Cl− uptake (decrease in fluorescence) (n = 5 cell isolations). The addition of CFTRinh172 to the unstimulated cells (n = 5) produced a minimal increase in the relative fluorescence, suggesting Cl− efflux from the cells. The differences between experiments with and without CFTRinh172 for both the 5- and 10-min time points were not statistically significant. Mean values for the relative fluorescence ± SE for each experiment were plotted.
Type I cells contain the chloride channels CLC5 and CLC2.
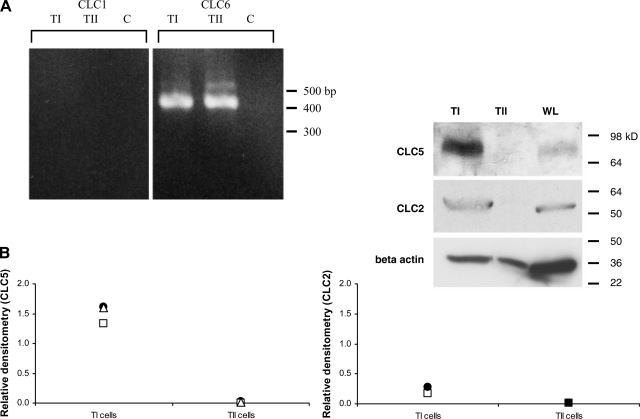
CLC5 and CLC2 have been previously described in the lung, but whether these channels were present in the alveolar epithelium was not clear (17, 47). Immunohistochemical staining of cytocentrifuged preparations of mixed lung cells and adult rat tissue (Figs. 10 and 11) and Western blot analysis (Fig. 12B, n = 3 blots for CLC5) revealed that CLC5 protein expression is predominantly in TI, rather than in TII, cells. Western blotting for CLC5 identified a band at ∼80 kDa, consistent with the predicted molecular weight of CLC5. Double staining of the tissue sections and mixed cell preparations revealed colocalization of CLC5 with the TI cell apical plasma membrane marker RTI40. In contrast, there was no colocalization of CLC5 with a plasma membrane marker of TII cells. By RT-PCR, we were unable to detect CLC1 in either cell type, but CLC6 was present in both TI and TII cells (Fig. 12A). By Western blotting, we found CLC2 principally in TI, rather than in TII, cells (Fig. 12B, n = 2 blots for CLC2). Western blotting with a commercially available antibody for CLC2 (Sigma) identified a band at ∼55 kDa in both TI and whole lung lysates. Band density was normalized to β-actin, and the individual data points were plotted for each blot.
Fig. 10.
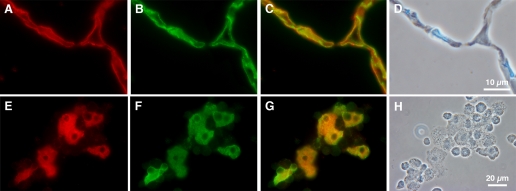
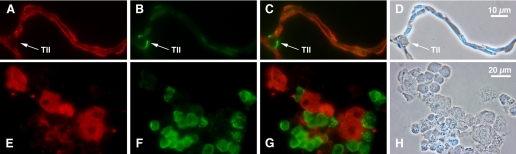
Immunohistochemical evidence of CLC5 in rat alveolar type I cells. Two-micrometer sections of adult rat lung (A–D) and cytocentrifuged preparations of adult rat mixed lung cells (E–H) were double stained with an antibody to CLC5 (red) and RTI40, an apical plasma membrane marker for TI cells (green). A: tissue section stained with CLC5 (red); B: same tissue section stained with RTI40 to display TI cells; C: merged image demonstrating colocalization of CLC5 and RTI40; D: phase-contrast image; of note, there are no TII cells in this section of tissue. E: mixed lung cell preparation stained with CLC5; F: cells stained with RTI40, which appear to be the same cells that stain positive for CLC5; G: merged image confirming colocalized signal; H: phase-contrast image of the cells; noteworthy are TII cells in the phase image are devoid of CLC staining in E. Results indicate the presence of CLC5 in TI cells.
Fig. 11.
Absence of CLC5 protein in rat alveolar type II cells. Employing the same double-staining methods mentioned earlier, rat lung sections and cytocentrifuged mixed cell preparations were stained with CLC5 (red) or RTII70, an antibody specific to the apical membrane of alveolar TII cells (green). A: tissue staining demonstrating CLC5 staining of the alveolar epithelium; arrow indicates a TII cell that is void of staining; B: same tissue section stained with the TII cell marker RTII70; C: merged image demonstrating no colocalized signal; D: phase-contrast image of the tissue section. E: mixed lung cell preparation stained with CLC5; the cells that are positively stained do not have the typical appearance of TII cells; F: cells stained with RTII70; G: merged image demonstrating no color shift, indicating the absence of colocalization of the 2 signals; H: phase-contrast image of the cells. These results demonstrate the relative absence of CLC5 protein in TII cells in both adult lung tissue and in mixed lung cell cytocentrifuged preparations.
Fig. 12.
A: CLC mRNA expression in alveolar epithelial cells. RT-PCR of the CLC1 and CLC6 isoforms demonstrates the absence of CLC1 in either TI or TII cells. CLC6, however, is present in both TI and TII cells (predicted PCR product size based on primers is 351 bp for CLC1 and 424 bp for CLC6). Our gel reveals an additional band at ∼520 in the TII cell lane for CLC6, which may represent an isoform of CLC6 derived from alternative splicing. B: CLC protein expression in alveolar epithelial cells. Western blots of TI and TII cell lysates reveal CLC5 and CLC2 are detectable in TI, but not TII, cells. WL, whole lung. Individual relative densitometric values normalized to β-actin for each blot are presented.
DISCUSSION
There is a dynamic balance between Na+ and Cl− secretion and absorption that is believed to be important in both establishing and maintaining fluid balance in the lung. In the fetal lung, Cl− secretion drives liquid secretion (51), causing lung distention that in turn promotes growth and development. In the peripartum period, the lung converts to Na+ absorption, presumably to clear the alveoli of fluid in preparation for survival in an air environment. The connection between active alveolar Na+ transport and alveolar fluid clearance has been extensively studied, but only recently has it been suggested that transport of ions other than Na+ may play a role in driving alveolar fluid uptake (20, 29, 40).
Previous physiological studies in mice demonstrated that β-adrenergic agonists administered in the air space in liquid containing radioactive tracers increased 36Cl uptake and alveolar fluid clearance (AFC), but produced no change in 22Na uptake, suggesting that fluid clearance may not be solely mediated by Na+ transport (20). CFTR inhibitors decreased 36Cl and fluid uptake, further suggesting that Cl− channels in the alveolar epithelium may be involved in AFC; however, the specific cell types involved in Cl− transport were not elucidated. Jiang et al. (28, 29) demonstrated that the β-adrenergic agonist terbutaline can activate Cl− channels in adult TII cells cultured for 5–7 days and that terbutaline-stimulated currents were inhibited in a dose-dependent fashion by the Cl− channel blockers like NPPB. These investigators also determined that the terbutaline-stimulated currents in TII cell monolayers were not inhibited by amiloride, suggesting that β-adrenergic agonists may directly activate Cl− channels. These studies underscore the importance of Cl− in the adult lung. Human alveolar TII cells cultured for 5 days can take up Cl−, but whether TI cells were also capable of Cl− uptake has remained unknown (21). Since TI cells cover >95% of the internal surface area of the lung (11) and this cell type contains functional CFTR channels (31), we hypothesized that TI cells were capable of Cl− uptake and could be responsible for a portion of the Cl− uptake seen in whole lung studies.
Our studies demonstrate that TI cells take up Cl− and that this uptake can be increased by β-adrenergic agonists. Freshly isolated TI cells were incubated with 36Cl and bumetanide, and Cl− uptake was measured in the presence and absence of terbutaline. Unstimulated TI cells (those not treated with terbutaline) took up Cl− in a linear fashion between 0 and 10 min; this uptake was enhanced in the presence of terbutaline. At 5 min, TI cells stimulated with terbutaline took up ∼30% more Cl− than did unstimulated TI cells.
Because freshly isolated cells in suspension lack polarity, an inhibitor of the NaK2Cl transporter (bumetanide) was added to facilitate the measurement of Cl− uptake so that chloride export would be inhibited. This is analogous to adding ouabain to inhibit Na+-K+-ATPase to facilitate the measurement of Na+ transport (7, 52, 55). To determine if bumetanide had any effect on our Cl− uptake measurements, we studied Cl− uptake in the absence of bumetanide. We found that in the absence of bumetanide, uptake was not linear, and there was no difference between unstimulated and stimulated Cl− uptake at any time point (Fig. 2). In comparison, cells that were treated with bumetanide, either in the presence or absence of terbutaline, demonstrated linear Cl− uptake over time. These studies suggest that in freshly isolated cells, inhibiting the NaK2Cl may stabilize Cl− flux and facilitate Cl− uptake measurement. Other investigators have used bumetanide in a similar fashion to prevent Cl− secretion across epithelial monolayers while studying the effects of Cl− and other ion channel agonists/antagonists in Ussing chamber experiments (10, 13, 25, 60) and in measurements of Cl− flux in whole lung studies (6); therefore, we added bumetanide to facilitate measurements of Cl− uptake.
We found that Cl− uptake in the presence of terbutaline could be significantly attenuated by the Cl− inhibitor NPPB (Fig. 3; 40% inhibition). In the absence of terbutaline, Cl− uptake decreased when the cells were treated with NPPB, but the difference was not statistically significant. NPPB inhibits a variety of channels, including CFTR, CLC channels (22, 64), K+ channels (34), and calcium-activated Cl− channels (39). The IC50 of NPPB in renal epithelia is ∼80 nM; however, higher concentrations of NPPB are required to inhibit transport in respiratory epithelia (10−4 to 10−3 M), lending to possible nonspecific inhibitory action at such doses (9, 42). The relatively large degree of inhibition in the presence of terbutaline may stem from the ability of NPPB to block multiple Cl− pathways that are activated by β-adrenergic agonists.
To determine the contribution of specific transporters to Cl− flux in TI cells, we measured Cl− uptake in the presence of CFTRinh172, a very potent and specific CFTR inhibitor (Ki = 300 nM). CFTRinh172 inhibits CFTR at submicromolar concentrations and does not affect other Cl− channels, including calcium-activated Cl− channels or volume-activated Cl− channels (42). In the absence of terbutaline, CFTRinh172 did not inhibit Cl− uptake (Fig. 4), but in the presence of terbutaline, Cl− uptake was significantly inhibited, suggesting that one mechanism by which Cl− uptake occurs is via CFTR. NPPB inhibited Cl− uptake in freshly isolated TI cells by ∼40%, whereas CFTRinh172 inhibited Cl− uptake by ∼20%, supporting the concept that Cl− channels or transporters in addition to CFTR participate in regulating Cl− movement.
The Cl−/HCO3− anion exchanger, AE2, believed to be ubiquitous in mammalian cells, is important in regulating intracellular pH (reviewed in Ref. 2). AE2 facilitates both Cl− absorption and secretion in various epithelial cells, including those of the intestine and kidney (3). Although AE2 is electroneutral, this exchanger is an excellent candidate for Cl− movement across the alveolar epithelium, given that it exchanges Cl− for HCO3−, which is readily available in the alveolar compartments as plasma HCO3− is converted to CO2 for exhalation. We were able to demonstrate the presence of both AE2 mRNA and protein in TI cells. AE3 (brain isoform) was previously detected in whole lung (54), thus we used the described primers to determine if AE3 was present in alveolar epithelial cells. We detected AE3 (brain isoform) only in TII cells; the PCR product was twice the size predicted, suggesting the possibility of heteroduplex formation or the presence of another splice variant of AE in TII cells (53). The AE1 isoform, found in red blood cells and in the renal collecting duct, was not present in either TI or TII cells. To determine the extent to which AE2 contributed to Cl− transport in TI cells, we measured Cl− uptake in the presence of the AE2 inhibitor DIDS, which has an IC50 for AE2 of 0.5–19 μM (23). Cyclic AMP has been reported to stimulate anion exchange activity in human airway (24) and human nasal (59) cells. In these studies, CFTR stimulation was shown to increase AE activity, which could be attenuated either by blocking AE activity or by blocking CFTR, suggesting that CFTR can mediate AE activity. We used terbutaline to measure the degree of inhibition achievable by DIDS. DIDS significantly inhibited Cl− uptake in TI cells treated with terbutaline, suggesting that AE2 may also contribute to Cl− uptake in TI cells.
To determine if the combination of different Cl− inhibitors would exert an additive effect on Cl− uptake in TI cells, we measured Cl− uptake in cells treated with multiple inhibitors. In the presence of terbutaline, the cells incubated with CFTRinh172 (10 μM), DIDS (10−4 M), and NPPB (10−4 M) exhibited a significant decrease in Cl− uptake compared with controls (Fig. 8, 68% inhibition). These results are compatible with our hypothesis that multiple Cl− pathways contribute to Cl− flux in TI cells.
The benefits of using freshly isolated cells in uptake studies rest on the premise that they have undergone fewer phenotypic changes than cultured cells [as exemplified by the phenotypic shift demonstrated by cultured TII cells (8, 15, 16)]. However, freshly isolated cells are in suspension and thus do not display polarity, so apical-to-basolateral ion transport cannot be established. Cells cultured to produce confluent monolayers with high transepithelial resistances (TER) can be studied in Ussing chambers to measure apical-to-basolateral ion transport, but there are technical difficulties with culturing TI cells. TI cells in culture form monolayers with gaps, preventing attainment of high TER needed for Ussing chamber experiments (unpublished observations). Therefore, we explored the use of the fluorescent Cl− indicator MQAE, which does not require establishment of confluent monolayers with high TER, and could be used to track Cl− influx or efflux from cultured cells that had established polarity. The most significant caveat to these experiments included the fact that there are no data on TI cells in culture for more than 24 h, so the use of such cultured cells could induce uncertainty in the interpretation of results. With this provision in mind, we performed our MQAE experiments both in an attempt to establish an alternative to radiotracer studies and to investigate a complementary method by which to measure Cl− flux.
We measured cellular Cl− influx in response to specific inhibitors or agonists by measuring relative fluorescence using MQAE. MQAE studies showed that in TI cells, terbutaline stimulates Cl− uptake and that in the presence of CFTRinh172, Cl− uptake can be inhibited. At both the 5- and 10-min time points, terbutaline-stimulated Cl− influx was markedly inhibited in the presence of CFTRinh172 compared with cells not treated with CFTRinh172. The degree of inhibition of Cl− influx in TI cells was significantly greater as measured by fluorescence studies than in our studies utilizing 36Cl uptake. When these studies were repeated in the absence of terbutaline, the cells not treated with CFTRinh172 took up Cl−, as evidenced by the decrease in fluorescence, but the degree of relative fluorescence was approximately one-half that seen in terbutaline-stimulated cells. However, in the presence of the CFTR-specific inhibitor, there was a slight increase in relative fluorescence, suggesting efflux of Cl− from the cells. One could theorize that CFTRinh172 in the absence of terbutaline could cause CFTR in TI cells to either secrete Cl− or could enable CFTR to activate other anion channels to promote Cl− secretion. Given the insignificance of the differences between the results obtained in the presence and absence of the inhibitor, we conclude that in the absence of terbutaline stimulation, CFTRinh172 does not inhibit Cl− uptake.
The more effective action of CFTRinh172 on Cl− uptake in our fluorescence studies may have been induced by culture conditions and the ability of cultured cells to establish polarity, which may enhance the ability of CFTR to establish directional Cl− flux, allowing greater inhibition by CFTRinh172. Additionally, uptake studies in cells in suspension are limited by the cell's attainment of a steady state between radiolabeled 36Cl and nonlabeled 35Cl in the face of blockers of Cl− extrusion (like bumetanide), which are necessary to allow measurement of total cellular Cl− uptake. One must also consider the possibility that cells in culture may exhibit a different anion channel profile than freshly isolated cells, or that the Cl− channels in cultured cells have altered sensitivities to inhibitors, enabling a more robust response to CFTRinh172. The appearance of decreased efficacy of CFTRinh172 over time in both cultured and freshly isolated cells may be due to multiple factors including recruitment of CFTR-independent methods of Cl− uptake, a waning of the ability of terbutaline over time to maintain activation of CFTR, or a decrease in the efficacy of CFTRinh172. Finally, while CFTRinh172 is a specific inhibitor of CFTR, and many studies use 10 μM to block the actions of CFTR, this dose may actually induce more nonspecific Cl− channel inhibition, and because we are better able to measure Cl− flux in cultured cells that have developed polarity, we may see greater inhibition of Cl− uptake than that seen in the radiolabeled uptake studies.
It is known that culture conditions can affect channel number and activity. For example, freshly isolated TII cells contain abundant highly selective cation (HSC) ENaC channels (composed of α-, β-, and γENaC subunits); in contrast, cultured TII cells contain more nonselective cation (NSC) channels (composed of the αENaC subunit alone) (26, 31). Alterations in channel content may be the case with TI cells as well, but studies have yet to be done comparing ion channel presence and function in cultured vs. freshly isolated TI cells. Our current findings of a similar decrease in trajectory of CFTRinh172 efficacy over time in both freshly isolated and cultured cells suggest that at least the CFTRinh172-sensitive channels in the cultured TI cells may retain some aspects of transporter function, despite being in culture; however, specific activity was not probed. We have previously shown that for HSC, NSC, and CFTR channels in TI cells, functional activity by patch-clamp analysis did not change after 24 h in culture (31). However, other anion transporters were not studied. Furthermore, NPPB and DIDS, when used at concentrations to inhibit respiratory epithelia, demonstrate autofluorescence, particularly with TI cells, making the measurement of MQAE fluorescence less reliable. Thus, due to concerns regarding phenotypic changes induced in culture that commonly occur in epithelial cells (15), we limited the number of experiments performed with MQAE.
The family of voltage-gated CLC channels has been described in respiratory epithelia and implicated in regulation of intracellular volume and pH, vesicular acidification, and transepithelial Cl− transport (reviewed in Refs. 12, 27, 43). Within the lung, CLC5 has been described in airways, but not in the alveoli, interstitium, or vascular spaces (17). CLC2 is expressed on the apical membranes of airway epithelia and is developmentally regulated, decreasing in expression with maturity (46, 47). There has been suggestion that CLC2 may serve as an alternate pathway for Cl− conduction in cystic fibrosis airway cells, as overexpression of CLC2 in such cells produced increased chloride conduction (58). Via immunohistochemistry and Western blotting, we demonstrated that CLC5 is not only present in alveolar epithelium, but that it is preferentially expressed in adult rat TI cells. By Western blotting, we were also able to show expression of CLC2 in adult TI cells, whereas expression in TII cells was much less prominent. Western blotting with a commercially available antibody for CLC2 (Sigma) identified a band at ∼55 kDa in both TI and whole lung lysates. While the published molecular weight for CLC2 has ranged in the literature from 80 to 110 kDa, the substrates studied were primarily fetal lung, renal, or ocular tissue; reports of CLC2 protein expression in adult rat lungs described minimal protein detection. It has been established that CLC2 has many isoforms and splice variants, which could explain the size of our detected protein (47). Thus, the findings of CLC5 and CLC2 broaden the possible role for TI cells in Cl− transport. By RT-PCR, we could not detect the CLC1 isoform, which is found in skeletal muscle (56). We could, however, detect CLC6 in both TI and TII cells. The predicted PCR product for CLC6 is 424 bp, but a faint second band was detected at ∼500, suggesting the presence of an isoform of CLC6 (18).
Evidence from the current studies, together with previously published reports (20, 28, 29, 33, 37, 40, 41, 51), suggest that anion transport, in addition to Na+ transport, may be important in regulating fluid fluxes in the adult lung. Animal studies with CFTR mutant mice suggest that Cl− transport and CFTR are important contributors to alveolar edema resolution. Cl− uptake in TII cells had been established, but it was not known if the cell type that covers 95% of the internal surface area of the lung, the TI cell, could likewise take up Cl−. We have been able to demonstrate not only that TI cells are capable of Cl− uptake, but that this uptake can be stimulated by β-adrenergic agonists and inhibited by Cl− channel antagonists. We have shown that Cl− uptake in TI cells is in part modulated by CFTR, but that other transporters, like AE2, may be responsible for Cl− uptake as well. Finally, we have evidence that the CLC family of channels is represented in TI cells and may be one way in which Cl− transport can be differentially regulated in the alveolar epithelium. Since the majority of Cl− uptake is independent of the Cl− channel inhibitors utilized in these studies, it is likely that additional Cl− channels contribute to Cl− transport in the lung. By providing evidence that TI cells can take up Cl−, we hope that further studies of alveolar anion transport will include investigation into the role of TI cells in this process, which may ultimately lead to important alternative treatments to address alveolar flooding and help us better understand how the lung maintains alveolar fluid balance.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-24075 and HL-57426 (L. Dobbs) and HL-088025 (M. Johnson) and the Robert Wood Johnson Foundation Amos Medical Faculty Development Program (M. Johnson).
ACKNOWLEDGMENTS
We thank Akash Desai and Zachary Tigue for assistance with the figures, Dr. Seth Alper for the gift of AE2 antibodies, Dr. Alan Verkman for the gift of CFTRinh172, and Dr. Kurt Thorn for microscopy assistance. The UCSF Nikon Imaging Center provided use of the time-lapse fluorescence microscopes. Portions of data have been presented in poster form at national meetings.
REFERENCES
- 1.Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat 123: 649–660, 1977 [PMC free article] [PubMed] [Google Scholar]
- 2.Alper SL. Molecular physiology of SLC4 anion exchangers. Exp Physiol 91: 153–161, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Alper SL, Darman RB, Chernova MN, Dahl NK. The AE gene family of Cl/HCO3− exchangers. J Nephrol 15, Suppl 5: S41–S53, 2002 [PubMed] [Google Scholar]
- 4.Andersson C, Dragomir A, Hjelte L, Roomans GM. Cystic fibrosis transmembrane conductance regulator (CFTR) activity in nasal epithelial cells from cystic fibrosis patients with severe genotypes. Clin Sci 103: 417–424, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Auzanneau C, Thoreau V, Kitzis A, Becq F. A novel voltage-dependent chloride current activated by extracellular acidic pH in cultured rat Sertoli cells. J Biol Chem 278: 19230–19236, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Basset G, Crone C, Saumon G. Significance of active ion transport in transalveolar water absorption: a study on isolated rat lung. J Physiol 384: 311–324, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland RD, Boyd CA. Cation transport in lung epithelial cells derived from fetal, newborn, and adult rabbits. J Appl Physiol 61: 507–515, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Borok Z, Hami A, Danto SI, Zabski SM, Crandall ED. Rat serum inhibits progression of alveolar epithelial cells toward the type I cell phenotype in vitro. Am J Respir Cell Mol Biol 12: 50–55, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Cabantchik ZI, Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol Cell Physiol 262: C803–C827, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Clerici C, Couette S, Loiseau A, Herman P, Amiel C. Evidence for Na-K-Cl cotransport in alveolar epithelial cells: effect of phorbol ester and osmotic stress. J Membr Biol 147: 295–304, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Crapo JD, Young SL, Fram EK, Pinkerton KE, Barry BE, Crapo RO. Morphometric characteristics of cells in the alveolar region of mammalian lungs. Am Rev Respir Dis 128: S42–S46, 1983 [DOI] [PubMed] [Google Scholar]
- 12.Devuyst O, Guggino WB. Chloride channels in the kidney: lessons learned from knockout animals. Am J Physiol Renal Physiol 283: F1176–F1191, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Diener M, Hug F, Strabel D, Scharrer E. Cyclic AMP-dependent regulation of K+ transport in the rat distal colon. Br J Pharmacol 118: 1477–1487, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobbs LG, Gonzalez R, Matthay MA, Carter EP, Allen L, Verkman AS. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc Natl Acad Sci USA 95: 2991–2996, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbs LG, Pian MS, Maglio M, Dumars S, Allen L. Maintenance of the differentiated type II cell phenotype by culture with an apical air surface. Am J Physiol Lung Cell Mol Physiol 273: L347–L354, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Dobbs LG, Williams MC, Brandt AE. Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim Biophys Acta 846: 155–166, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Edmonds RD, Silva IV, Guggino WB, Butler RB, Zeitlin PL, Blaisdell CJ. ClC-5: ontogeny of an alternative chloride channel in respiratory epithelia. Am J Physiol Lung Cell Mol Physiol 282: L501–L507, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Eggermont J, Buyse G, Voets T, Tytgat J, De Smedt H, Droogmans G, Nilius B. Alternative splicing of ClC-6 (a member of the ClC chloride-channel family) transcripts generates three truncated isoforms one of which, ClC-6c, is kidney-specific. Biochem J 325: 269–276, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Factor P, Mutlu GM, Chen L, Mohameed J, Akhmedov AT, Meng FJ, Jilling T, Lewis ER, Johnson MD, Xu A, Kass D, Martino JM, Bellmeyer A, Albazi JS, Emala C, Lee HT, Dobbs LG, Matalon S. Adenosine regulation of alveolar fluid clearance. Proc Natl Acad Sci USA 104: 4083–4088, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay M. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 119: 199–207, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 290: L242–L249, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Furukawa T, Ogura T, Katayama Y, Hiraoka M. Characteristics of rabbit ClC-2 current expressed in Xenopus oocytes and its contribution to volume regulation. Am J Physiol Cell Physiol 274: C500–C512, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Humphreys BD, Jiang L, Chernova MN, Alper SL. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am J Physiol Cell Physiol 267: C1295–C1307, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Illek B, Yankaskas JR, Machen TE. cAMP and genistein stimulate HCO3− conductance through CFTR in human airway epithelia. Am J Physiol Lung Cell Mol Physiol 272: L752–L761, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Inglis SK, Olver RE, Wilson SM. Differential effects of UTP and ATP on ion transport in porcine tracheal epithelium. Br J Pharmacol 130: 367–374, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain L, Chen XJ, Ramosevac S, Brown LA, Eaton DC. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. Am J Physiol Lung Cell Mol Physiol 280: L646–L658, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev 82: 503–568, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Ingbar DH, O'Grady SM. Adrenergic regulation of ion transport across adult alveolar epithelial cells: effects on Cl− channel activation and transport function in cultures with an apical air interface. J Membr Biol 181: 195–204, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Ingbar DH, O'Grady SM. Adrenergic stimulation of Na+ transport across alveolar epithelial cells involves activation of apical Cl− channels. Am J Physiol Cell Physiol 275: C1610–C1620, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Jin N, Kolliputi N, Gou D, Weng T, Liu L. A novel function of ionotropic gamma-aminobutyric acid receptors involving alveolar fluid homeostasis. J Biol Chem 281: 36012–36020, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 103: 4964–4969, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci USA 99: 1966–1971, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim KJ, Cheek JM, Crandall ED. Contribution of active Na+ and Cl− fluxes to net ion transport by alveolar epithelium. Respir Physiol 85: 245–256, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Kirkup AJ, Edwards G, Weston AH. Investigation of the effects of 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) on membrane currents in rat portal vein. Br J Pharmacol 117: 175–183, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitterman JA, Chapin CJ, Vanderbilt JN, Porta NF, Scavo LM, Dobbs LG, Ertsey R, Goerke J. Effects of oligohydramnios on lung growth and maturation in the fetal rat. Am J Physiol Lung Cell Mol Physiol 282: L431–L439, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Lamprecht G, Schaefer J, Dietz K, Gregor M. Chloride and bicarbonate have similar affinities to the intestinal anion exchanger DRA (down regulated in adenoma). Pflügers Arch 452: 307–315, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Lee SY, Maniak PJ, Ingbar DH, O'Grady SM. Adult alveolar epithelial cells express multiple subtypes of voltage-gated K+ channels that are located in apical membrane. Am J Physiol Cell Physiol 284: C1614–C1624, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Lee SY, Maniak PJ, Rhodes R, Ingbar DH, O'Grady SM. Basolateral Cl− transport is stimulated by terbutaline in adult rat alveolar epithelial cells. J Membr Biol 191: 133–139, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Liantonio A, Pusch M, Picollo A, Guida P, De Luca A, Pierno S, Fracchiolla G, Loiodice F, Tortorella P, Conte Camerino D. Investigations of pharmacologic properties of the renal CLC-K1 chloride channel co-expressed with barttin by the use of 2-(p-chlorophenoxy)propionic acid derivatives and other structurally unrelated chloride channels blockers. J Am Soc Nephrol 15: 13–20, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Lindert J, Perlman CE, Parthasarathi K, Bhattacharya J. Chloride-dependent secretion of alveolar wall liquid determined by optical-sectioning microscopy. Am J Respir Cell Mol Biol 36: 688–696, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lubman RL, Danto SI, Chao DC, Fricks CE, Crandall ED. Cl(−)-HCO3− exchanger isoform AE2 is restricted to the basolateral surface of alveolar epithelial cell monolayers. Am J Respir Cell Mol Biol 12: 211–219, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest 110: 1651–1658, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maduke M, Miller C, Mindell JA. A decade of CLC chloride channels: structure, mechanism, and many unsettled questions. Annu Rev Biophys Biomol Struct 29: 411–438, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 61: 627–661, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Munkonge F, Alton EW, Andersson C, Davidson H, Dragomir A, Edelman A, Farley R, Hjelte L, McLachlan G, Stern M, Roomans GM. Measurement of halide efflux from cultured and primary airway epithelial cells using fluorescence indicators. J Cyst Fibros 3, Suppl 2: 171–176, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Murray CB, Chu S, Zeitlin PL. Gestational and tissue-specific regulation of ClC-2 chloride channel expression. Am J Physiol Lung Cell Mol Physiol 271: L829–L837, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Murray CB, Morales MM, Flotte TR, McGrath-Morrow SA, Guggino WB, Zeitlin PL. CIC-2: a developmentally dependent chloride channel expressed in the fetal lung and downregulated after birth. Am J Respir Cell Mol Biol 12: 597–604, 1995 [DOI] [PubMed] [Google Scholar]
- 48.O'Brodovich H. Epithelial ion transport in the fetal and perinatal lung. Am J Physiol Cell Physiol 261: C555–C564, 1991 [DOI] [PubMed] [Google Scholar]
- 49.O'Grady SM, Jiang X, Ingbar DH. Cl-channel activation is necessary for stimulation of Na transport in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 278: L239–L244, 2000 [DOI] [PubMed] [Google Scholar]
- 50.O'Grady SM, Jiang X, Maniak PJ, Birmachu W, Scribner LR, Bulbulian B, Gullikson GW. Cyclic AMP-dependent Cl secretion is regulated by multiple phosphodiesterase subtypes in human colonic epithelial cells. J Membr Biol 185: 137–144, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Olver RE, Strang LB. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the foetal lamb. J Physiol 241: 327–357, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Planes C, Blot-Chabaud M, Matthay M, Couette S, Uchida T, Clerici C. Hypoxia and beta 2-agonists regulate cell surface expression of the epithelial sodium channel in native alveolar epithelial cells. J Biol Chem 277: 47318–47324, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Pushkin A, Kurtz I. SLC4 base (HCO3−, CO3 2−) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Renal Physiol 290: F580–F599, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Rajendran VM, Black J, Ardito TA, Sangan P, Alper SL, Schweinfest C, Kashgarian M, Binder HJ. Regulation of DRA and AE1 in rat colon by dietary Na depletion. Am J Physiol Gastrointest Liver Physiol 279: G931–G942, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Russo RM, Lubman RL, Crandall ED. Evidence for amiloride-sensitive sodium channels in alveolar epithelial cells. Am J Physiol Cell Physiol 262: C405–C411, 1992 [DOI] [PubMed] [Google Scholar]
- 56.Schnulle V, Antropova O, Gronemeier M, Wedemeyer N, Jockusch H, Bartsch JW. The mouse Clc1/myotonia gene: ETn insertion, a variable AATC repeat, and PCR diagnosis of alleles. Mamm Genome 8: 718–725, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Schwiebert EM, Flotte T, Cutting GR, Guggino WB. Both CFTR and outwardly rectifying chloride channels contribute to cAMP-stimulated whole cell chloride currents. Am J Physiol Cell Physiol 266: C1464–C1477, 1994 [DOI] [PubMed] [Google Scholar]
- 58.Schwiebert EM, Morales MM, Devidas S, Egan ME, Guggino WB. Chloride channel and chloride conductance regulator domains of CFTR, the cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 95: 2674–2679, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin JH, Son EJ, Lee HS, Kim SJ, Kim K, Choi JY, Lee MG, Yoon JH. Molecular and functional expression of anion exchangers in cultured normal human nasal epithelial cells. Acta Physiol 191: 99–110, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith MD, Penland CM, Van Scott MR. Differential effects of polycationic protein on Cl− secretory and Na+ absorptive airways. Am J Physiol Lung Cell Mol Physiol 272: L762–L771, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Stern M, Munkonge FM, Caplen NJ, Sorgi F, Huang L, Geddes DM, Alton EW. Quantitative fluorescence measurements of chloride secretion in native airway epithelium from CF and non-CF subjects. Gene Ther 2: 766–774, 1995 [PubMed] [Google Scholar]
- 62.Stutts MJ, Gatzy JT, Boucher RC. Activation of chloride conductance induced by potassium in tracheal epithelium. Pflügers Arch 411: 252–258, 1988 [DOI] [PubMed] [Google Scholar]
- 63.Verkman AS, Sellers MC, Chao AC, Leung T, Ketcham R. Synthesis and characterization of improved chloride-sensitive fluorescent indicators for biological applications. Anal Biochem 178: 355–361, 1989 [DOI] [PubMed] [Google Scholar]
- 64.Wei L, Xiao AY, Jin C, Yang A, Lu ZY, Yu SP. Effects of chloride and potassium channel blockers on apoptotic cell shrinkage and apoptosis in cortical neurons. Pflügers Arch 448: 325–334, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Zhu S, Yue G, Shoemaker RL, Matalon S. Adult alveolar type II cells lack cAMP and Ca2+-activated Cl-channels. Biochem Biophys Res Commun 218: 302–308, 1996 [DOI] [PubMed] [Google Scholar]