Abstract
The release of reactive oxygen species (ROS) and cytokines by alveolar macrophages has been demonstrated in asbestos-induced pulmonary fibrosis, but the mechanism linking alveolar macrophages to the pathogenesis is not known. The GTPase Rac1 is a second messenger that plays an important role in host defense. In this study, we demonstrate that Rac1 null mice are protected from asbestos-induced pulmonary fibrosis, as determined by histological and biochemical analysis. We hypothesized that Rac1 induced pulmonary fibrosis via generation of ROS. Asbestos increased TNF-α and ROS in a Rac1-dependent manner. TNF-α was elevated only 1 day after exposure, whereas ROS generation progressively increased in bronchoalveolar lavage cells obtained from wild-type (WT) mice. To determine whether ROS generation contributed to pulmonary fibrosis, we overexpressed catalase in WT monocytes and observed a decrease in ROS generation in vitro. More importantly, administration of catalase to WT mice attenuated the development of fibrosis in vivo. For the first time, these results demonstrate that Rac1 plays a crucial role in asbestos-induced pulmonary fibrosis. Moreover, it suggests that a simple intervention may be useful to prevent progression of the disease.
Keywords: macrophages
pulmonary fibrosis is a progressive disease characterized by focal acute lung injury and associated inflammation that induces aberrant repair. This is typified by increased extracellular matrix deposition by proliferating fibroblasts and myofibroblasts that ultimately results in remodeling and destruction of the normal architecture of lung tissue. Focal lung injury can result from a variety of stimuli, including allergens, persistent infections, autoimmune responses, chemical insults, radiation, and inorganic dusts and fibers, such as asbestos. Although the use of asbestos is now regulated, cases of asbestosis continue to be diagnosed at a rate of ∼200,000 per year, and 4,000 people die each year from asbestos-induced lung fibrosis (2). Asbestos, therefore, remains an important environmental cause of pulmonary fibrosis.
Asbestos fibers may remain in alveolar spaces for extended periods of time. Fiber retention in respiratory bronchioles and alveoli leads to sequential lung injury with an associated inflammatory response. The release of proinflammatory/profibrogenic mediators and reactive oxygen species (ROS) by inflammatory cells, such as neutrophils, macrophages, and monocytes, has been demonstrated in the lungs of patients with asbestosis (18, 34). In fact, a characteristic feature of these cells obtained from patients with asbestos-induced fibrosis is spontaneous release of these factors (36, 45).
The Rho family of GTP-binding proteins comprises 20 members, one of which is Rac1. The GTPase Rac1, which is expressed in all cell types, is an upstream second messenger that plays an important role in host defense. Specifically, Rac1 is known to regulate the assembly of the actin cytoskeleton and NADPH oxidase, the cellular transformation initiated by Ras oncogenes, and the migration, adhesion, and differentiation of cells. Its expression has been shown to be increased in activated fibroblasts and in various tissues with some degree of fibrosis (1, 9, 10, 24). However, the link between Rac1 activity in inflammatory cells and the development of fibrosis has not been evaluated.
Several cytokines have been implicated in the development of complex lung diseases, such as pulmonary fibrosis. TNF-α, which has been shown to increase and decrease production of collagen (5, 13), is one proinflammatory cytokine that has been studied extensively with regard to pulmonary fibrosis. It is clear that TNF-α expression in the lung is increased after asbestos exposure, and TNF-α receptor knockout mice have been shown to be protected from developing fibrosis secondary to bleomycin, silica, and asbestos exposure (27, 29, 32). However, the role of TNF-α in collagen deposition in the later stages of the disease has not been determined. TNF-α may be regulated by Rac1 via activation of the p38 mitogen-activated protein kinase, which we have shown to be necessary for asbestos-induced TNF-α expression in monocytes (8, 39).
In addition to regulating cytokine expression, Rac1 modulates the generation of ROS. Rac is an essential component of the NADPH oxidase, an important source of superoxide anion and its prooxidant derivatives (19). Increased nonphagocytic NADPH oxidase activity and ROS have been observed after Rac1 activation in fibrotic tissues of several major organs, such as the kidney, liver, and heart (1, 9, 10). Mitigation of oxidative stress has been shown to have a beneficial impact on the progression of fibrosis (23). A recent study revealed that administration of N-acetylcysteine, together with traditional anti-inflammatory agents, improved lung function in patients with idiopathic pulmonary fibrosis (11). It is not known whether inhibition of Rac1 prevents pulmonary fibrosis by interfering with ROS generation and/or producing cytokines.
Asbestos-induced lung disease has a long latency period, with the disease developing 15–30 yr after exposure. Once exposure has occurred, progression of lung disease is common, and no effective treatment is available. Thus a better understanding of the pathogenesis may provide a therapeutic option to prevent the progression of lung disease and may shed light on why some workers develop progressive disease after asbestos exposure and others do not.
MATERIALS AND METHODS
Materials.
Catalase was purchased from Worthington Biochemical (Lakewood, NJ). Chrysotile asbestos was provided by the North American Insulation Manufacturers Association Fiber Repository. TiO2 was obtained from Sigma-Aldrich (St. Louis, MO); dichlorofluorescin diacetate (DCFH-DA) from Invitrogen (Carlsbad, CA); mouse monoclonal antibodies to Rac1 and β-actin from Upstate Biotechnology, Millipore (Billerica, MA) and Sigma (St. Louis, MO), respectively; goat anti-mouse IgG-horseradish peroxidase (HRP) from Santa Cruz Biotechnology (Santa Cruz, CA); Rac1 activation assay kit (GLISA) from Cytoskeleton (Denver, CO); and TNF-α, IL-1β, and transforming growth factor (TGF)-β ELISA Duo Set kits from R & D Systems (Minneapolis, MN).
Mice.
Wild-type (WT) and Rac1 null C57BL/6 mice were used in these studies, and all protocols were approved by the University of Iowa Institutional Animal Care and Use Committee. Rac1 null mice were generated by selective disruption of the Rac1 gene in cells of the granulocyte/monocyte lineage, as described previously (17). A bolus dose of 100 μg of chrysotile asbestos or TiO2 in 50 μl of normal saline was administered intratracheally to 6- to 24-wk-old mice after 2–5 min of anesthesia with 3% isoflurane via a precision Fortec vaporizer (Cyprane, Keighley, UK). After 1 and 21 days, mice were euthanized with an overdose of isoflurane, and bronchoalveolar lavage (BAL) was performed. BAL cells were used for determination of total and differential cell number and ROS generation. BAL fluid was used for determination of cytokine and hydroxyproline concentrations. The lungs were removed and stained for collagen fibers using Masson's trichrome.
Hydroxyproline determination.
On the basis of evidence that pulmonary fibrosis is associated with increased BAL fluid collagen levels (3), BAL fluid was digested for 24 h at 110°C with hydrochloric acid at a final concentration of 6 N, and hydroxyproline content was determined as previously described (14).
Histological scoring.
Sections of right lungs that were stained with Masson's trichrome were scored for fibrosis by a pathologist who was blinded to the treatment groups. Separate fields associated with aggregates of inflammatory cells in each section were scored as follows: 0 for normal, 1 for peribronchial fibrosis, 2 for parenchymal fibrosis, 3 for peribronchial and parenchymal fibrosis, and 4 for widespread fibrosis.
Cells.
The human monocyte THP-1 cell line (ATCC, Manassas, VA) were maintained in RPMI 1640 containing 10 mM HEPES, 1 mM sodium pyruvate, 4.5 g/l glucose, 2 mM l-glutamine, 10% fetal bovine serum, and gentamicin. Mouse bone marrow-derived monocytes isolated from WT or Rac1 null mice, as previously described (40), were immortalized using a retrovirus (105–106 neomycin colony-forming units/ml) containing SV40 large T antigen and a neomycin-resistant cassette. Cell culture medium was removed 24 h after infection. After 2 days, the cells were incubated with medium containing 500 μg/ml gentacin for selection. Individual clones were selected by a standard limiting dilution culture method. SV40 large T antigen expression was confirmed by RT-PCR with the primer pair 5′-AGAGGAATCTTTGCAGCTAA-3′ (forward) and 5′-CTAAACACAGCATGACTCAA-3′ (reverse). Rac1 deletion was confirmed in Rac1 null cells by RT-PCR with the primer pair 5′-CACCACTGTCCCAATACTCCT-3′ (forward) and 5′-GGGCTCCGACATTTACAACA-3′ (reverse) and Western blot analysis using hypothalamus as the positive control. The cells were maintained in RPMI 1640 supplemented with 10% newborn calf serum, 50 μM β-mercaptoethanol, and penicillin-streptomycin.
Adenoviral vectors.
Cells were infected for 48 h with Ad5.CMV vectors in medium supplemented with 0.5% newborn calf serum. The catalase vector (44), constitutive active V12-Rac1 (37), and dominant-negative N17-Rac1 (15) were obtained from the Vector Core at the University of Iowa and used at 500 multiplicity of infection. Cells were exposed to chrysotile asbestos for the last 24 h of the 48-h infection period.
Cytokine determinations.
TNF-α, IL-1β, and TGF-β in BAL fluid and conditioned media of cell culture experiments were measured by cytokine-specific ELISA Duo Set kits. BAL cytokines were normalized to total protein levels that were determined using the Micro-BCA kit (Pierce, Rockford, IL).
Quantitative real-time PCR.
Total RNA from BAL cells and cells grown in culture was isolated by TRIzol and, after DNase treatment, subjected to reverse transcription using the reverse transcriptase kit Iscript (Bio-Rad Laboratories, Hercules, CA). TNF-α and β-actin mRNA transcripts were determined by quantitative real-time PCR using SYBR Green (Bio-Rad Laboratories) and respective primers on an IQ5 real-time PCR machine (Bio-Rad Laboratories). The following primer sets were used: 5′-CCACATCTCCCTCCAGAAA-3′ (forward) and 5′-CACTTGGTGGTTTGCTACGA-3′ (reverse) for TNF-α and 5′-AGAGGGAAATCGTGCGTGAC-3′ (forward) and 5′-CAATAGTGATGATGACCTGGCCGT-3′ (reverse) for β-actin. Data were calculated by the cycle threshold (ΔΔCT) method. TNF-α mRNA was normalized to β-actin mRNA and expressed as arbitrary units.
Rac1 activation.
Cells were exposed to asbestos for the indicated times, and Rac1 activity was determined using the GLISA kit according to the manufacturer's instructions. GLISA is an ELISA-based assay that uses wells precoated with PAK-PBD to bind activated Rac1. Cells were lysed, and 10 μg of protein were added to each well. In this assay, only the activated Rac1 binds to PAK-PBD. This complex was then detected after sequential incubations with Rac1-specific monoclonal antibody and anti-mouse IgG-HRP secondary antibody. Activity of HRP was then measured enzymatically, with luminescence as the final end point. Results are expressed as relative light units normalized to control values at 0 min of incubation with asbestos.
Determination of ROS.
BAL cells and bone marrow-derived monocytes from WT and Rac1 null mice were plated in six-well plates in 1 ml of phenol red-free RPMI 1640 containing 0.5% newborn calf serum. Cells were allowed to adhere for 1 h and then treated for 20 min with 20 μM DCFH-DA. Cells were trypsinized, centrifuged for 10 min at 216 g, and resuspended in 350 μl of Hanks' balanced salt solution. By adding Hoechst 33258 (4.8 μl of 250 μg/ml) to each tube, we were able to distinguish live from dead cells. Fluorescence due to oxidation of DCFH to DCF and Hoechst reagent in cells was determined by flow cytometry (model LSR II, Becton Dickinson, Franklin Lakes, NJ).
Western blot analysis.
Cell lysis was performed as previously described (7). Western blot analysis was performed with anti-Rac1 monoclonal antibody (1:2,500 dilution). Equal loading was demonstrated by stripping membranes with Restore Western blot stripping buffer (Pierce) for 15 min at room temperature and performing a Western blot analysis for β-actin using the β-actin monoclonal antibody (1:25,000 dilution).
Statistical analysis.
Statistical comparisons were performed using an unpaired, one-tailed t-test. Values are means ± SE. P < 0.05 was considered to be significant.
RESULTS
Rac1 null mice are protected from developing pulmonary fibrosis.
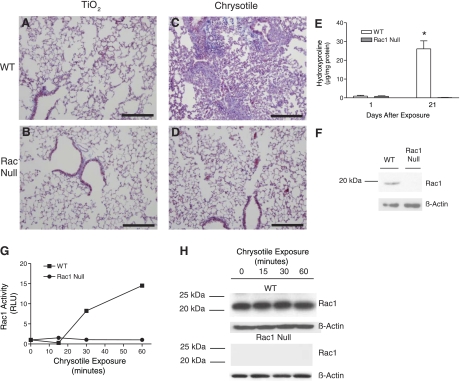
The small GTPase Rac1 is an upstream second messenger that plays an important role in host defense (4, 6). The initial response to asbestos exposure is the recruitment of inflammatory cells, which are a significant source of cytokines and ROS in the lung (21). We hypothesized that inflammatory cells containing Rac1 play an important role in the development of pulmonary fibrosis. To determine whether Rac1 has a critical role in the lung, we exposed C57BL/6 WT mice and mice with Rac1 selectively deleted in cells of the granulocyte/monocyte lineage to chrysotile asbestos or to TiO2 as a negative control. After exposure to TiO2 or asbestos, animals were euthanized and lungs were excised and processed with Masson's trichrome stain to visualize collagen deposition. Lungs from WT and Rac1 null animals exposed to TiO2 demonstrated normal peribronchiolar and alveolar architecture (Fig. 1, A and B). In lungs of WT mice exposed to asbestos, deposition of collagen was extensive in peribronchial and parenchymal areas (Fig. 1C). In marked contrast to WT mice, asbestos-exposed Rac1 null mice displayed normal lung architecture, similar to mice exposed to TiO2 (Fig. 1D). These mice showed no evidence of epithelial damage or abnormal collagen deposition.
Fig. 1.
Rac1 null mice are protected from developing asbestos-induced pulmonary fibrosis. TiO2 (A and B) or chrysotile asbestos (C and D) was administered intratracheally at 100 μg in 50 μl of normal saline to wild-type (WT) and Rac1 null C57BL/6 mice, and animals were euthanized 21 days later. Lungs were removed and processed for collagen deposition using Masson's trichrome stain. Representative micrographs of 1 of 5 animals are shown. Scale bars, 200 μm. E: 1 and 21 days after chrysotile asbestos exposure, WT and Rac1 null mice were subjected to bronchoalveolar lavage (BAL), and hydroxyproline concentration was determined in BAL fluid. *Significantly different from Rac1 null at 21 days. F: equivalent amounts of protein from WT and Rac1 null mouse monocytes were separated by SDS-PAGE, and Western blot analysis was performed with Rac1 and β-actin monoclonal antibodies to determine the presence and equal loading of the proteins, respectively. G: WT and Rac1 null monocytes were exposed to chrysotile asbestos (10 μg/cm2) for 0–60 min. Activated Rac1 was determined by binding of Rac1 to PAK-PBD beads immobilized in a 96-well plate using GLISA. Rac1 activation is expressed as relative light units (RLU) at each time point normalized to control values. H: Rac1 protein levels and equal loading of proteins in lysates from each time point in F were determined by Western blot analysis using Rac1 and β-actin monoclonal antibodies, respectively.
Next, using a biochemical method, we assessed collagen deposition in the lungs of these mice. Increased levels of collagen in BAL fluid obtained from animals and humans with asbestosis have been shown to be directly associated with histological evidence of lung fibrosis (3). Hydroxyproline, derived from proline, is preferentially found in collagen, and its concentration is used as an estimate of collagen content. Therefore, to assess whether collagen content was greater in the peribronchiolar and alveolar spaces of asbestos-exposed WT than Rac1 null mice, we determined hydroxyproline content in BAL fluid. There was no difference in the hydroxyproline concentration in BAL fluid obtained from WT and Rac1 null mice 1 day after asbestos exposure (Fig. 1E). However, in BAL of WT mice, hydroxyproline concentration dramatically increased 21 days after asbestos exposure to 25 times the values detected 1 day after exposure. In contrast, there was no increase in hydroxyproline concentration in BAL fluid from Rac1 null mice. Rac1 protein was detected in inflammatory cells isolated from bone marrow of WT, but not Rac1 null, mice (Fig. 1F). These results demonstrate that the presence of Rac1 in inflammatory cells is necessary for the development of pulmonary fibrosis after exposure to asbestos.
Since the absence of Rac1 in inflammatory cells protects mice from asbestos-induced pulmonary fibrosis, we determined whether asbestos induced Rac1 activity. Monocytes from WT and Rac1 null mice were exposed to chrysotile asbestos for 0–60 min. Rac1 activation in WT monocytes dramatically increased 30 min after exposure to asbestos, and after 60 min it increased to ∼15 times the baseline control (Fig. 1G). As expected, Rac1 activity and protein were not detected in the Rac1 null monocytes at any time after asbestos exposure. The increase in activity in WT cells was not due to higher levels of total Rac1, inasmuch as similar amounts of the protein were evident at the indicated times (Fig. 1H). Taken together, these results demonstrate that asbestos-induced Rac1 activation in inflammatory cells is necessary for the subsequent development of pulmonary fibrosis.
Rac1 activation is required for TNF-α expression and ROS generation.
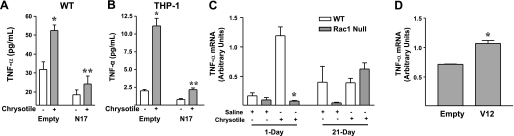
To determine the role of Rac1 in the development of fibrosis, we utilized an in vitro approach to investigate two possible mechanisms based on what is known about Rac1 activity and asbestos-induced pulmonary fibrosis. We previously showed that monocytes express TNF-α when they are exposed to asbestos (39). The significance of this observation is that several studies have shown that alveolar macrophages from patients with asbestosis spontaneously release cytokines, including TNF-α (36, 45). Studies in animal models of asbestosis demonstrating the prevention of pulmonary fibrosis following inhibition of TNF-α underscore its importance in this disease (25). Thus we first examined the role of Rac1 in regulating TNF-α expression. WT monocytes were infected with an adenoviral construct containing an empty vector or a dominant-negative N17-Rac1 vector. Asbestos increased TNF-α expression in cells infected with empty vector, but overexpression of N17-Rac1 in these cells decreased TNF-α expression below control levels (Fig. 2A). Similar results were obtained with the human monocytic THP-1 cell line (Fig. 2B).
Fig. 2.
Rac1 activation is necessary for asbestos-induced TNF-α production. A and B: WT mouse monocytes and THP-1 cells were infected for 48 h with Ad5.CMV containing either empty vector or dominant-negative N17-Rac1 vector at 500 multiplicity of infection (moi). For the last 24 h, cells were exposed to chrysotile asbestos (10 μg/cm2). Conditioned medium was analyzed for TNF-α by ELISA. Values are means ± SE; n = 2. *Significantly different from chrysotile(−) Empty. **Significantly different from chrysotile(+) Empty. C: BAL cells were collected from WT and Rac1 null mice 1 and 21 days after saline or chrysotile asbestos exposure, and TNF-α mRNA was determined by real-time PCR. Values are means ± SE; n = 2. *Significantly different from WT 1 day. D: Rac1 null monocytes were infected for 48 h with Ad5.CMV containing either empty vector or Ad5.V12-Rac1 vector at 500 moi, and TNF-α mRNA was determined by real-time PCR. Values are means ± SE; n = 2. *Significantly different from Empty.
To demonstrate differences in TNF-α expression in WT and Rac1 null monocytes, we determined TNF-α mRNA in BAL cells collected from WT and Rac1 null mice. WT cells expressed significantly more TNF-α mRNA than Rac1 null cells 1 day after exposure to asbestos (Fig. 2C). In fact, TNF-α expression was similar in cells obtained from asbestos- and saline-exposed Rac1 null mice. However, no difference was observed between these strains of mice at 21 days. Overexpression of constitutively active V12-Rac1 augmented TNF-α expression in Rac1 null cells. Rac1 null cells infected with V12-Rac1 expressed significantly more TNF-α mRNA than cells infected with empty vector (Fig. 2D). These data demonstrate that Rac1 regulates TNF-α expression in murine and human monocytes exposed to asbestos.
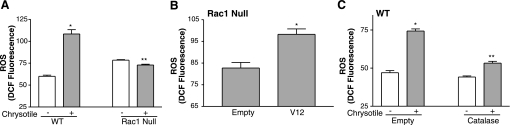
The second mechanism investigated was the role of Rac1 in the generation of ROS. The presence of oxidized proteins in lavage fluid obtained from patients with asbestosis supports the relevance of evaluating ROS in cells obtained from BAL (26). WT and Rac1 null monocytes were exposed to asbestos, and ROS generation was detected by oxidation of DCFH. After 24 h of exposure, asbestos caused a significant increase in ROS in WT, but not Rac1 null monocytes (Fig. 3A). However, infection of Rac1 null monocytes with constitutively active V12-Rac1 restored their ability to generate ROS in response to asbestos (Fig. 3B).
Fig. 3.
Rac1 activation is necessary for asbestos-induced generation of reactive oxygen species (ROS). Dichlorofluorescin diacetate (DCFH-DA, 20 μM) was added to cells during the last 20 min, and intracellular ROS levels were determined by counting the number of cells in which DCFH was oxidized to its fluorescent analog DCF by flow cytometry. A: WT and Rac1 null monocytes were exposed to chrysotile asbestos (10 μg/cm2) for 24 h, and ROS generation was determined by measurement of cells labeled with DCF by flow cytometry. Values are means ± SE of relative fluorescence of DCF; n = 3 wells per treatment. *Significantly different from chrysotile(−) WT. **Significantly different from chrysotile(+) WT. B: Rac1 null monocytes were infected with Ad5.CMV containing either empty vector or constitutive active V12-Rac1 vector at 500 moi. After 24 h, cells were exposed to chrysotile asbestos (10 μg/cm2) for 24 h, and ROS generation was determined as described in A. Values are means ± SE; n = 3. *Significantly different from Empty. C: WT monocytes were infected for 48 h with Ad5.CMV containing either empty vector or a catalase vector at 500 moi. Cells were exposed to chrysotile asbestos (10 μg/cm2) for the last 24 h, and ROS generation was determined as described in A. Values are means ± SE; n = 3. *Significantly different from chrysotile(−) Empty. **Significantly different from chrysotile(+) Catalase.
We previously showed that H2O2 is generated in monocytes exposed to asbestos (8), so we evaluated whether increased oxidation of DCFH in WT monocytes was due to H2O2 generation. WT monocytes were infected with an adenoviral construct containing either an empty vector or a catalase vector. After 24 h, cells were exposed to asbestos for an additional 24 h, and DCFH oxidation was determined. As expected, asbestos increased ROS generation in WT cells expressing the empty vector (Fig. 3C). In contrast, after asbestos exposure, ROS generation in cells overexpressing catalase was near control levels. In aggregate, these results demonstrate that Rac1 activity in inflammatory cells is required for asbestos-induced ROS production. Furthermore, these data suggest that H2O2 is a significant component of the ROS generated in WT mice.
Total cell recruitment and cell differential are similar in WT and Rac1 null mice exposed to asbestos.
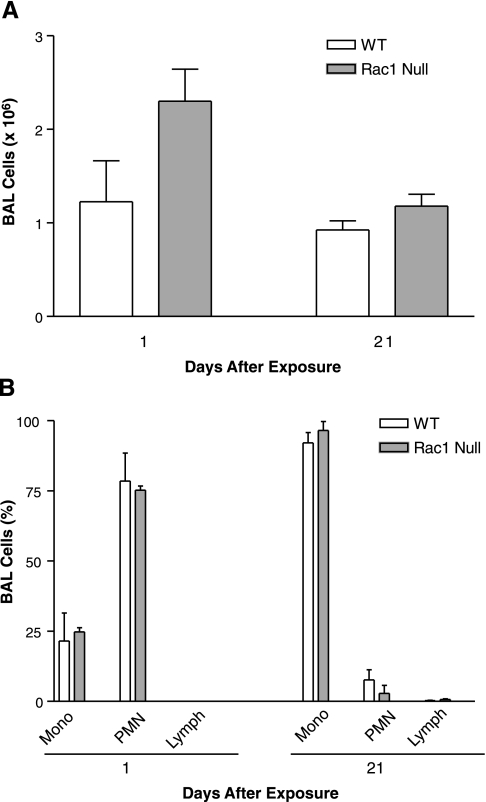
Since our in vitro data demonstrated that Rac1 was necessary for TNF-α expression and ROS generation, we determined whether these observations were reproduced in vivo. We first evaluated whether Rac1 had a role in cell recruitment. To examine whether asbestos increased the recruitment of total inflammatory cells, WT and Rac1 null mice were exposed to chrysotile asbestos or to TiO2 as a negative control. The total number of cells was measured in the BAL collected 1 and 21 days after exposure. The total cell number was highest in the BAL collected 1 day after asbestos exposure, but there was no difference between WT and Rac1 null mice 1 or 21 days after exposure (Fig. 4A). BAL total cell number was not elevated at 1 or 21 days after exposure to TiO2 (data not shown).
Fig. 4.
BAL cell counts (A) and cell differential (B) are similar in WT and Rac1 null mice after asbestos exposure. WT or Rac1 null mice were exposed to 100 μg of chrysotile asbestos in 50 μl of saline. After 1 and 21 days, animals were euthanized, and BAL was performed. BAL cells were counted to determine total cell count and cell differential was determined with Wright-Giemsa stain. Values are means ± SE; n = 3 animals per group. Mono, mononuclear; PMN, polymorphonuclear; Lymph, lymphocytes.
The relative distribution of monocytes, polymorphonuclear cells, and lymphocytes in the BAL differed between 1 and 21 days after exposure to asbestos. Polymorphonuclear cells constituted the majority of cells in BAL collected 1 day after exposure, whereas monocytic cells predominated 21 days after exposure (Fig. 4B). At 1 and 21 days, lymphocytes were <10% of the total cell number. The relative distribution of cell types was not different in WT and Rac1 null mice 1 or 21 days after asbestos exposure. These data show that neither the total cell number nor the cell type recruited to the lung after exposure to asbestos is regulated by Rac1.
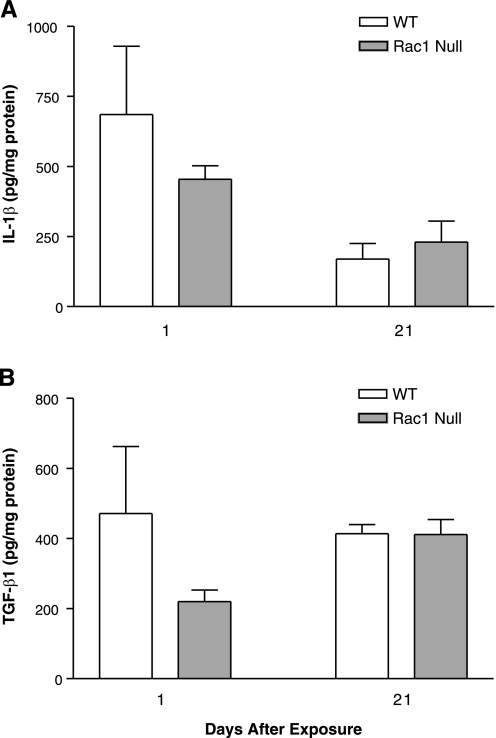
IL-1β and TGF-β1 in BAL are not regulated by Rac1.
Although our data indicate that Rac1 regulates TNF-α expression, we evaluated whether it also regulates the expression of IL-1β and TGF-β1 in vivo on the basis of previous evidence demonstrating their involvement in the development of pulmonary fibrosis (16, 25). IL-1β and TGF-β1 were measured in BAL obtained from WT and Rac1 null mice exposed to asbestos or TiO2. There was no significant cytokine expression in mice exposed to TiO2 (data not shown). Although IL-1β was elevated in BAL fluid collected from WT and Rac1 null mice 1 day after exposure, the concentrations were similar in both strains of mice (Fig. 5A). The presence of active TGF-β1 in the BAL fluid was similar in WT and Rac1 null mice 1 day after exposure, and the concentrations remained at the same level 21 days after exposure (Fig. 5B). These data indicate that these cytokines do not have a significant role in the development of asbestos-induced pulmonary fibrosis with regard to Rac1 activity in cells of granulocyte/monocyte lineage.
Fig. 5.
IL-1β and transforming growth factor (TGF)-β1 in BAL are not regulated by Rac1. Mice were exposed to 100 μg of chrysotile asbestos, and BAL was performed 1 and 21 days later. BAL fluid was analyzed for IL-1β (A) and TGF-β1 (B) by ELISA. Values, normalized to BAL total protein, are means ± SE; n = 3 animals per group.
Rate of development of pulmonary fibrosis after asbestos exposure.
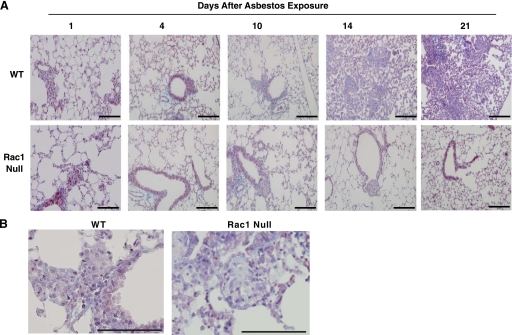
The pattern of cytokine expression prompted us to question whether its temporal variation correlated with the rate of fibrosis development. WT and Rac1 null mice were exposed to chrysotile asbestos. The mice were euthanized 1, 4, 10, 14, and 21 days later, and their lungs were removed and stained for collagen deposition. Pathological collagen deposition was evident at 14 days and continued to progress until 21 days after exposure (Fig. 6A). This collagen deposition was dense and extensive and included the alveolar spaces. Lungs from WT and Rac1 null mice demonstrated an increase in aggregates of inflammatory cells (Fig. 6B), but in contrast to WT mice, there was no associated collagen deposition in Rac1 null mice at any time after asbestos exposure.
Fig. 6.
Rate of development of pulmonary fibrosis after asbestos exposure. A: WT or Rac1 null mice were exposed to 100 μg of asbestos in 50 μl of saline and euthanized 1, 4, 10, 14, and 21 days later. Lungs were removed and processed for collagen deposition using Masson's trichrome stain. Representative serial sections from 1 of 3 animals per time point are shown. Scale bars, 200 μm. B: high-power (×40) field of inflammatory cell aggregates in WT and Rac1 null mice. Scale bars, 100 μm.
Increased ROS generation in WT mice mediated development of pulmonary fibrosis.
Since Rac1 regulated asbestos-induced ROS generation in vitro, we next evaluated the role of Rac1 in regulating ROS generation in vivo. BAL cells were collected from WT and Rac1 null mice 1, 4, 10, 14, and 21 days after asbestos exposure. Cells were incubated with DCFH for determination of ROS. ROS generation was similar in WT and Rac1 null mice until 14 days after asbestos exposure (Table 1). At this time, ROS in inflammatory cells isolated from WT mice increased significantly by almost threefold compared with Rac1 null mice. The generation of ROS increased in both strains of mice 21 days after exposure, but no significant differences were observed between the groups. Several ROS and reactive nitrogen species are capable of oxidizing DCFH, including H2O2, HO•, ROO•, and ONOO−. HO•, ROO•, and ONOO− are highly reactive and cause immediate oxidation of molecules that they encounter. Since Rac1 null mice do not develop asbestos-induced pulmonary fibrosis, we expect that the increase in oxidized DCFH at 21 days in these mice represents one of these unstable species. In contrast, a progressive increase in oxidized DCFH in BAL cells in WT mice suggests that these mice generate a more stable ROS species, such as H2O2. This notion is also supported by the catalase inhibition of DCFH oxidation in vitro in WT monocytes.
Table 1.
Chrysotile asbestos increases ROS in BAL cells
| Genotype | ROS (DCF Fluorescence) |
|---|---|
| Day 1 | |
| WT | 47.53±18.41 |
| Rac1 null | 49.61±11.83 |
| Day 4 | |
| WT | 16.39±5.21 |
| Rac1 null | 15.97±3.68 |
| Day 10 | |
| WT | 23.45±2.51 |
| Rac1 null | 20.99±2.87 |
| Day 14 | |
| WT | 95.17±19.44 |
| Rac1 null | 33.46±5.67* |
| Day 21 | |
| WT | 134.97±15.35 |
| Rac1 null | 184.82±20.42 |
Values are means ± SE of 3 animals per time point. Wild-type (WT) and Rac1 null mice were exposed to 100 μg of chrysotile asbestos in 50 μl of saline and euthanized 1, 4, 10, 14, and 21 days later. Bronchoalvaolar lavage (BAL) was performed, and reactive oxygen species (ROS) generation was determined by oxidation of dichlorofluorescin (DCFH) using flow cytometry and expressed in relative light units (RLU).
Significantly different from WT at day 14.
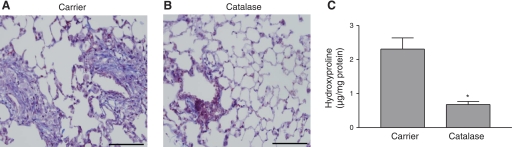
To elucidate whether H2O2 was the ROS that induced pulmonary fibrosis in WT mice, we questioned whether inhibition of H2O2 generation would attenuate its development. WT mice were exposed to chrysotile asbestos, and for the next 20 days they were treated with carrier (water) alone or 2,000 U of catalase delivered intratracheally in 50 μl of water. At 21 days, mice were euthanized, and the lungs were removed and stained for collagen deposition. As expected, the mice that received the carrier alone developed significant pulmonary fibrosis (Fig. 7A). In contrast, Masson's trichrome stain showed significantly less collagen deposition in mice treated with catalase (Fig. 7B). Hydroxyproline concentrations were three times greater in BAL fluid collected from carrier-treated than from catalase-treated mice (Fig. 7C).
Fig. 7.
Exogenous catalase prevents pulmonary fibrosis. WT mice were exposed to asbestos, and, on each day for the next 20 days, carrier (A) or catalase (2,000 U; B) was administered intratracheally. Animals were euthanized on day 21, and lungs were removed and processed for collagen deposition using Masson's trichrome stain. Representative sections of 1 of 6 mice are shown. Scale bars, 200 μm. C: hydroxyproline concentrations determined in BAL fluid collected from mice treated with carrier (water) or catalase for 20 days after chrysotile asbestos exposure. Values are means ± SE; n = 4. *Significantly different from carrier.
To further quantify the development of pulmonary fibrosis, we used histological scoring in lung sections obtained from WT and Rac1 null mice and WT mice that received the carrier or catalase for 20 days after asbestos exposure. We consistently found scores of 3–4 in asbestos-exposed WT mice and scores of 0–1 in Rac1 null and WT mice that received catalase (Table 2). The results of the histological scoring corroborate the hydroxyproline data. These data demonstrate for the first time that inhibition of extracellular ROS, specifically H2O2, attenuates pulmonary fibrosis and may be one mechanism by which Rac1 null mice are protected from developing the disease. These data also suggest a potential target to use as a therapeutic option to prevent the development of pulmonary fibrosis after asbestos exposure.
Table 2.
Histological scoring of trichrome-stained lung sections
Values are means ± SE; n = 4. WT and Rac1 null mice were intratracheally administered a bolus dose of 100 μg of chrysotile asbestos in 50 μl of normal saline. WT animals were then administered carrier (water) or 2,000 units/mouse of catalase intratracheally or nothing every day for the next 20 days. Lungs were removed and stained for collagen with Masson's trichrome stain. After blinding, areas of each section were scored as described in materials and methods.
WT vs. Rac1 null,
WT + carrier vs. WT + catalase.
DISCUSSION
Results from this study clearly demonstrate the crucial role of Rac1 in the development of pulmonary fibrosis and show for the first time that ROS mediate the effects of Rac1 on the development of asbestos-induced pulmonary fibrosis. In addition, deletion of Rac1 from inflammatory cells or administration of catalase prevented lung fibrosis in asbestos-exposed animals.
Rac1 is a member of the Rho family of small GTPases. It is known to regulate actin polymerization, ROS generation, cell adhesion, and cell differentiation (17, 38, 41). Rac1 and Rac2 isoforms share 92% identity, and both have been demonstrated to regulate the activity of the NADPH oxidase, an important source of ROS (4). Nevertheless, the two isoforms exhibit cell type-dependent differences in their expression and have nonoverlapping functions. Rac1 is ubiquitously expressed, whereas Rac2 expression is restricted to cells of the hematopoietic lineage, with both isoforms present in equivalent amounts in murine neutrophils (12). Although Rac2 is required for NADPH activity in neutrophils, Rac1 regulates the enzyme in nonphagocytic cells (19). In monocytes and macrophages, which express significantly more Rac1 than Rac2, both isoforms are utilized for the assembly of NADPH oxidase depending on the nature of the initiating stimulus (43, 46). Rac1 also regulates actin polymerization and lamellipodium extension and spreading (17, 33, 43). Although our data implicate ROS, especially H2O2, in the development of asbestos-induced pulmonary fibrosis, Rac1 null mice may also have been protected from developing fibrosis because of ineffective migration of monocytes and macrophages to asbestos fibers in the lung (19).
Rac1 activation is known to increase H2O2 generation (22, 30, 31, 42). Several different ROS have been detected in the lung after asbestos exposure. We have shown that H2O2 is important in mediating the effects of asbestos in monocytes (8). H2O2 generation is biologically relevant on the basis of previous data demonstrating the presence of oxidized proteins in the lavage fluid of patients with asbestosis (26). ROS, including H2O2, oxidize several cellular constituents such as DNA, proteins, and lipids. As a result, ROS stimulate a wide range of biological processes, such as apoptosis, cell proliferation, and signal transduction events, that result in lung injury and aberrant wound healing.
One important regulator of H2O2 is catalase, which converts H2O2 to water. In one previous study, polyethylene glycol-catalase was administered subcutaneously in rats, and changes in collagen deposition were determined by measurement of hydroxyproline content (28). It is unclear which cells were targeted by catalase administered in this manner. Since H2O2 can freely cross the cell membrane, we administered catalase into the alveolar space to directly target deposition of extracellular matrix. The rationale for this approach was based on previous reports demonstrating matrix remodeling by ROS (23). Extracellular ROS have been shown to increase matrix deposition, a characteristic of later stages of pulmonary fibrosis. Our results demonstrate that asbestos induced an increase in ROS generation in the later stages of the disease. This is important because collagen, when oxidized, is more resistant to degradation (35).
Previous work demonstrated an extensive assortment of peroxide-generating and peroxide-removing enzymes, including Cu,Zn-SOD, Mn-SOD, catalase, and glutathione peroxidase, in the lung. Cu,Zn-SOD and Mn-SOD catalyze the dismutation of O2•− to H2O2, and catalase and glutathione peroxidase catalyze the conversion of H2O2 to water. This delay in ROS generation in WT mice may be secondary to the known early expression and activity of these antioxidant enzymes, which are not sustained at later times after exposure (20). Although total ROS increased in Rac1 null mice after 21 days of exposure, it is likely that this was due to the formation of unstable species, such as HO•, ROO•, and ONOO−, rather than H2O2. These species, in contrast to H2O2, are unlikely to induce the development of asbestosis because of their short half-life. This notion is supported by the observation that administration of catalase, which converts H2O2 to water, prevented the development of fibrosis in WT mice. Since Rac1 is necessary for regulation of actin polymerization and lamellipodium extension and spreading, an additional mechanism by which Rac1 null mice may be protected from developing fibrosis is the inability of monocytes and macrophages to migrate to areas of asbestos deposition.
Our novel study reveals that Rac1 activity in inflammatory cells is necessary for the development of pulmonary fibrosis. Since Rac1 is ubiquitously expressed, it is not the optimal target for therapeutic intervention. We demonstrate that the effects of Rac1 are mediated by ROS and that the disease can be significantly attenuated by its removal. These results suggest that ROS, instead of Rac1, may be a more effective target for therapeutic intervention in asbestos-induced pulmonary fibrosis. Pulmonary fibrosis is a progressive disease. Although the disease after exposure may not be prevented, limitation of its progression could diminish the morbidity of the disease. The results demonstrated in this study not only provide insight into mechanisms by which asbestos-induced pulmonary fibrosis develops, but they also suggest potential therapeutic strategies for intervention.
GRANTS
This work was supported by National Institutes of Health grants ES-015981 and ES-014871 (both to A. B. Carter) and P30-05605.
ACKNOWLEDGMENTS
We thank the Flow Cytometry and Central Microscopy Research Facilities at the University of Iowa for assistance with flow cytometry and tissue processing, respectively. We thank Gary Hunninghake, Joseph Zabner, and Douglas Spitz for critical review of the manuscript.
REFERENCES
- 1.Adam O, Frost G, Custodis F, Sussman MA, Schafers HJ, Bohm M, Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol 50: 359–367, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 170: 691–715, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Begin R, Martel M, Desmarais Y, Drapeau G, Boileau R, Rola-Pleszczynski M, Masse S. Fibronectin and procollagen 3 levels in bronchoalveolar lavage of asbestos-exposed human subjects and sheep. Chest 89: 237–243, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol 15: 163–171, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Brenner DA, O'Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-α. Nature 337: 661–663, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Cantrell DA. GTPases and T cell activation. Immunol Rev 192: 122–130, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression. The role of TATA-binding protein (TBP). J Biol Chem 274: 30858–30863, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Carter AB, Tephly LA, Venkataraman S, Oberley LW, Zhang Y, Buettner GR, Spitz DR, Hunninghake GW. High levels of catalase and glutathione peroxidase activity dampen H2O2 signaling in human alveolar macrophages. Am J Respir Cell Mol Biol 31: 43–53, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Abair TD, Ibanez MR, Su Y, Frey MR, Dise RS, Polk DB, Singh AB, Harris RC, Zent R, Pozzi A. Integrin α1β1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol Cell Biol 27: 3313–3326, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys 462: 266–272, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 353: 2229–2242, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Didsbury J, Weber RF, Bokoch GM, Evans T, Snyderman R. Rac, a novel Ras-related family of proteins that are botulinum toxin substrates. J Biol Chem 264: 16378–16382, 1989 [PubMed] [Google Scholar]
- 13.Dubin W, Martin TR, Swoveland P, Leturcq DJ, Moriarty AM, Tobias PS, Bleecker ER, Goldblum SE, Hasday JD. Asthma and endotoxin: lipopolysaccharide-binding protein and soluble CD14 in bronchoalveolar compartment. Am J Physiol Lung Cell Mol Physiol 270: L736–L744, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ, Fattman CL, Ramsgaard L, Richards TJ, Loutaev I, Nawroth PP, Kasper M, Bierhaus A, Oury TD. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol 172: 583–591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fjeld CC, Rice AE, Kim Y, Gee KR, Denu JM. Mechanistic basis for catalytic activation of mitogen-activated protein kinase phosphatase 3 by extracellular signal-regulated kinase. J Biol Chem 275: 6749–6757, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, Ryffel B, Couillin I. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 117: 3786–3799, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol 170: 5652–5657, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Goodglick LA, Kane AB. Role of reactive oxygen metabolites in crocidolite asbestos toxicity to mouse macrophages. Cancer Res 46: 5558–5566, 1986 [PubMed] [Google Scholar]
- 19.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9: 690–701, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Janssen YM, Marsh JP, Absher MP, Hemenway D, Vacek PM, Leslie KO, Borm PJ, Mossman BT. Expression of antioxidant enzymes in rat lungs after inhalation of asbestos or silica. J Biol Chem 267: 10625–10630, 1992 [PubMed] [Google Scholar]
- 21.Kamp DW, Weitzman SA. The molecular basis of asbestos induced lung injury. Thorax 54: 638–652, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science 280: 898–902, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med 172: 417–422, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen K, Tufvesson E, Malmstrom J, Morgelin M, Wildt M, Andersson A, Lindstrom A, Malmstrom A, Lofdahl CG, Marko-Varga G, Bjermer L, Westergren-Thorsson G. Presence of activated mobile fibroblasts in bronchoalveolar lavage from patients with mild asthma. Am J Respir Crit Care Med 170: 1049–1056, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lasky JA, Brody AR. Interstitial fibrosis and growth factors. Environ Health Perspect 108Suppl 4: 751–762, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenz AG, Costabel U, Maier KL. Oxidized BAL fluid proteins in patients with interstitial lung diseases. Eur Respir J 9: 307–312, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Liu JY, Brass DM, Hoyle GW, Brody AR. TNF-α receptor knockout mice are protected from the fibroproliferative effects of inhaled asbestos fibers. Am J Pathol 153: 1839–1847, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mossman BT, Marsh JP, Sesko A, Hill S, Shatos MA, Doherty J, Petruska J, Adler KB, Hemenway D, Mickey R, et al. Inhibition of lung injury, inflammation, and interstitial pulmonary fibrosis by polyethylene glycol-conjugated catalase in a rapid inhalation model of asbestosis. Am Rev Respir Dis 141: 1266–1271, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Ortiz LA, Lasky J, Lungarella G, Cavarra E, Martorana P, Banks WA, Peschon JJ, Schmidts HL, Brody AR, Friedman M. Upregulation of the p75 but not the p55 TNF-α receptor mRNA after silica and bleomycin exposure and protection from lung injury in double receptor knockout mice. Am J Respir Cell Mol Biol 20: 825–833, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Ozaki M, Deshpande SS, Angkeow P, Suzuki S, Irani K. Rac1 regulates stress-induced, redox-dependent heat shock factor activation. J Biol Chem 275: 35377–35383, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, Rhee SG, Bae YS. Sequential activation of phosphatidylinositol 3-kinase, β-Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol 24: 4384–4394, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piguet PF, Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respir J 7: 515–518, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 10: 183–196, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Rom WN, Bitterman PB, Rennard SI, Cantin A, Crystal RG. Characterization of the lower respiratory tract inflammation of nonsmoking individuals with interstitial lung disease associated with chronic inhalation of inorganic dusts. Am Rev Respir Dis 136: 1429–1434, 1987 [DOI] [PubMed] [Google Scholar]
- 35.Rucklidge GJ, Milne G, McGaw BA, Milne E, Robins SP. Turnover rates of different collagen types measured by isotope ratio mass spectrometry. Biochim Biophys Acta 1156: 57–61, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Schwartz DA, Galvin JR, Frees KL, Dayton CS, Burmeister LF, Merchant JA, Hunninghake GW. Clinical relevance of cellular mediators of inflammation in workers exposed to asbestos. Am Rev Respir Dis 148: 68–74, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Sulciner DJ, Irani K, Yu ZX, Ferrans VJ, Goldschmidt-Clermont P, Finkel T. Rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-κB activation. Mol Cell Biol 16: 7115–7121, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun CX, Downey GP, Zhu F, Koh AL, Thang H, Glogauer M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood 104: 3758–3765, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Tephly LA, Carter AB. Asbestos-induced MKP-3 expression augments TNF-α gene expression in human monocytes. Am J Respir Cell Mol Biol 39: 113–123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Lebowitz D, Sun C, Thang H, Grynpas MD, Glogauer M. Identifying the relative contributions of Rac1 and Rac2 to osteoclastogenesis. J Bone Miner Res 23: 260–270, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J Cell Sci 117: 1259–1268, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Woo CH, You HJ, Cho SH, Eom YW, Chun JS, Yoo YJ, Kim JH. Leukotriene B4 stimulates Rac-ERK cascade to generate reactive oxygen species that mediates chemotaxis. J Biol Chem 277: 8572–8578, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi A, Kim C, Li S, Marchal CC, Towe J, Atkinson SJ, Dinauer MC. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol 173: 5971–5979, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Zhang HJ, Drake VJ, Xu L, Hu J, Domann FE, Oberley LW, Kregel KC. Redox regulation of adenovirus-induced AP-1 activation by overexpression of manganese-containing superoxide dismutase. J Virol 76: 355–363, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Lee TC, Guillemin B, Yu MC, Rom WN. Enhanced IL-1β and tumor necrosis factor-α release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J Immunol 150: 4188–4196, 1993 [PubMed] [Google Scholar]
- 46.Zhao X, Carnevale KA, Cathcart MK. Human monocytes use Rac1, not Rac2, in the NADPH oxidase complex. J Biol Chem 278: 40788–40792, 2003 [DOI] [PubMed] [Google Scholar]