Abstract
The ability of transforming growth factor-β1 (TGF-β1) to induce epithelial-mesenchymal transition (EMT) in alveolar epithelial cells (AEC) in vitro and in vivo, together with the demonstration of EMT in biopsies of idiopathic pulmonary fibrosis (IPF) patients, suggests a role for TGF-β1-induced EMT in disease pathogenesis. We investigated the effects of N-acetylcysteine (NAC) on TGF-β1-induced EMT in a rat epithelial cell line (RLE-6TN) and in primary rat alveolar epithelial cells (AEC). RLE-6TN cells exposed to TGF-β1 for 5 days underwent EMT as evidenced by acquisition of a fibroblast-like morphology, downregulation of the epithelial-specific protein zonula occludens-1, and induction of the mesenchymal-specific proteins α-smooth muscle actin (α-SMA) and vimentin. These changes were inhibited by NAC, which also prevented Smad3 phosphorylation. Similarly, primary alveolar epithelial type II cells exposed to TGF-β1 also underwent EMT that was prevented by NAC. TGF-β1 decreased cellular GSH levels by 50–80%, whereas NAC restored them to ∼150% of those found in TGF-β1-treated cells. Treatment with glutathione monoethyl ester similarly prevented an increase in mesenchymal marker expression. Consistent with its role as an antioxidant and cellular redox stabilizer, NAC dramatically reduced intracellular reactive oxygen species production in the presence of TGF-β1. Finally, inhibition of intracellular ROS generation during TGF-β1 treatment prevented alveolar EMT, but treatment with H2O2 alone did not induce EMT. We conclude that NAC prevents EMT in AEC in vitro, at least in part through replenishment of intracellular GSH stores and limitation of TGF-β1-induced intracellular ROS generation. We speculate that beneficial effects of NAC on pulmonary function in IPF may be mediated by inhibitory effects on alveolar EMT.
Keywords: alveolar epithelium, lung injury, idiopathic pulmonary fibrosis, transforming growth factor-β, glutathione, reactive oxygen species
myofibroblasts are central to the process of fibrosis in the lung. They function as the primary source of excess extracellular matrix (ECM) deposition during lung remodeling after injury, are the primary effectors of alveolar architectural destruction, and figure prominently in the pathogenesis of chronic interstitial lung diseases such as idiopathic pulmonary fibrosis (IPF) (31). Replacement of functional lung alveoli with collagen-rich ECM leads to irreversible loss of lung function resulting in a rapid and severe decrease in lung compliance with a postdiagnosis survival for IPF of 2–5 years (27, 28). The development of effective therapies has to this point been thwarted at least in part by a poor understanding of the cellular origin of the myofibroblast itself. In this regard, epithelial-mesenchymal transition (EMT) of alveolar epithelial cells (AECs) has recently been implicated as an important source of myofibroblasts during fibrogenesis (19, 21, 43). Elucidation of the mechanisms of alveolar EMT and the development of therapies directed towards modulating EMT are therefore clearly needed.
To date, the only successful clinical trial demonstrating preservation of lung function in patients with IPF utilized oral N-acetylcysteine (NAC) (10). NAC is a powerful antioxidant shown to react specifically with several reactive oxygen species (ROS), as well as serving as a precursor for intracellular glutathione synthesis (3). While the mechanism(s) underlying the clinical efficacy of NAC are not understood, glutathione is critical to maintaining intracellular redox states and reduces intracellular ROS production. Patients with IPF have severely decreased tissue glutathione levels and high levels of hydrogen peroxide in epithelial lining fluid (5, 24), and increased levels of oxidized glutathione are seen in IPF patients, a direct indicator of oxidative stress (25). Cellular ROS production and a limited ROS scavenging capability due to reduced glutathione stores activates a variety of signaling molecules, including MAPK/ERK, p38 MAPK, Smad proteins, and the transcription factors HIF-1 and AP-1, which may induce EMT and propagate fibrosis (1, 15, 23). In addition, the predominant effector of EMT, transforming growth factor (TGF)-β1, inhibits γ-glutamylcysteine synthase, the rate-limiting enzyme in glutathione synthesis (2, 17, 33), while also directly inducing cellular ROS production (16, 30). Together, these findings suggest that the interaction of TGF-β and cellular ROS generation may contribute to the induction of alveolar EMT and subsequent parenchymal fibrosis.
In this study, we investigated the hypothesis that NAC inhibits TGF-β1-induced EMT. We induced EMT in vitro in both an alveolar epithelial cell line (RLE-6TN) and primary alveolar epithelial type II cells and assessed the effects of NAC on cell morphology, loss of epithelial markers, acquisition of mesenchymal markers, and phosphorylation of Smad3 during TGF-β1-induced EMT. Effects of NAC on intracellular glutathione content and cellular ROS production were evaluated, and the efficacy of NAC in upregulating intracellular glutathione content was compared with direct provision of glutathione in the form of glutathione monoethyl ester (GME). Finally, the effects of direct treatment of AECs with H2O2 as well as the effects of inhibition of NADH-oxidase during TGF-β-induced EMT were assessed to distinguish whether TGF-β-specific ROS generation is necessary for induction of EMT or whether general oxidant stress and depletion of intracellular glutathione is sufficient to induce EMT.
MATERIALS AND METHODS
Culture of RLE-6TN cells.
RLE-6TN cells, a rat type II alveolar epithelial cell line purchased from American Type Culture Collection (Manassas, VA), were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA) medium supplemented with 5% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and nonessential amino acids. Cells were plated in six-well dishes or on chamber slides and allowed to attach overnight in media alone, after which cells were maintained in either media alone or media supplemented with 1 ng/ml rh-TGF-β1 (R&D Systems, Minneapolis, MN) with or without addition of 5 mM NAC (Sigma, St. Louis, MO) for 5 days. A dose response experiment done early in our work (data not shown) showed that 5 mM was more effective than 0.5 mM, but that 10 mM or higher was toxic, leading us to utilize 5 mM as the dose in all of our experiments. Media and additives were replaced every 24 h. Cultures were maintained at 37°C and 5% CO2.
Primary cell isolation and culture.
All animal studies were approved by the Institutional Animal Care and Use Committee at St. Joseph's Hospital and Medical Center. Type II AEC were isolated from Sprague-Dawley rats, ∼40 days of age, as previously described (6). Cells were plated on 0.4-μm pore polycarbonate Transwell filters (Corning, Acton, MA), and initial cell purity was consistently 92–97% as determined by surfactant protein C staining. Fibroblasts were eliminated by supplementing media with 100 μg/ml cis-OH-proline for the first 48 h after plating, as previously described (44). Following the initial period in cis-OH-proline, cells were maintained in minimally defined serum-free media (6) with or without addition of 1 ng/ml TGF-β1, 5 mM NAC, and/or 50 μM H2O2 for up to 10 days.
Immunoblotting.
Cell cultures were lysed with 1% SDS lysis buffer on ice for 30 min and briefly sonicated. Protein sample concentrations were determined using a standard protein concentration assay (Bio-rad, Hercules, CA). Samples were resolved on 10% acrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked in 5% nonfat dry milk in Tris-buffered saline with Tween (TBS-T; pH 7.4) either overnight at 4°C or for 1 h at room temperature (RT). Incubation with primary antibodies was carried out overnight at 4°C, and HRP-conjugated secondary antibody incubation was at RT for 2 h. Primary antibodies for α-smooth muscle actin (α-SMA), vimentin, and β-actin were obtained from Sigma, ZO-1 antibody was purchased from Zymed (San Francisco, CA), phospho-Smad3, total Smad antibodies, and all secondary antibodies were from Cell Signaling (Danvers, MA). Blots were imaged on a Kodak Image Station equipped with Molecular Imaging software version 4.05 and analyzed with Adobe Photoshop CS3 (Adobe Systems, San Jose, CA).
Immunofluorescence.
Cells were fixed for 30 min in either methanol or 4% paraformaldehyde. Monolayers were then permeabilized with 0.2% Triton X-100 and blocked for up to 1 h at RT with CAS block (Invitrogen-Zymed). Slides and filters were incubated with primary antibodies overnight at 4°C and incubated with Alexa Fluor (Invitrogen-Molecular Probes)-conjugated secondary antibodies at RT for up to 2 h. Mouse (Chemicon, Billerica, MA) and rabbit IgG (Upstate, Lake Placid, NY) were used as isotype controls. Slides were mounted with Vectashield with DAPI (Vector Labs, Burlingame, CA) and coverslipped. Slides were imaged on a Zeiss Axioscope utilizing Axiovision software (Zeiss, Thornwood, NY).
Determination of intracellular glutathione content.
Cells were cultured as described above and lysed on day 5 of treatment. Total glutathione levels were determined by measuring the conversion of 5,5′-dithiobis(2-nitrobenzoic acid) in the presence of GSH to 2-nitro-5-thiobenzoic acid with a colorimetric assay (Dojindo, Rockville, MD). Sample absorbance was read at 415 nm. Cellular glutathione levels were normalized to total protein, as determined by a standard protein assay (Bio-Rad).
Treatment with GME.
To compare the efficacy of NAC with that of direct glutathione replenishment, RLE-6TN cells were treated with GME (400 μM-2.5 mM) with or without TGF-β1 for 5 days. Concentrations of GME (Calbiochem) were based on a dose response curve generated by measuring replenishment of intracellular glutathione levels detected by the method described above (data not shown). Media were changed every 24 h, and cells were lysed at the end of treatment.
Intracellular ROS levels.
The effect of NAC on TGF-β1-induced ROS production was assessed by the rate of conversion of 5-carboxy-2′,7′-dichorodihydrofluorescein diacetate (carboxy-H2DCFDA) to its reduced fluorescent configuration. RLE-6TN cells were cultured in 96-well plates with or without TGF-β1, NAC, or both. As a positive control for intracellular ROS production, cells were treated with 100 μM tert-butyl hydroperoxide. At each time point, cells were incubated with carboxy-H2DCFDA (Molecular Probes). Nuclei were stained with Hoechst dye. ROS levels were quantified by measuring excitation/emission at 495/529 nm on a 96-well plate reader (Beckman Coulter, Fullerton, CA). Total cell counts were obtained and utilized to normalize ROS level readings. Live cell ROS production and cell count images were acquired on a Zeiss Axioscope using standard fluorescence filters.
Inhibition of NADH oxidase.
The contribution of intracellular ROS generation to the process of TGF-β-induced EMT was assessed by treatment of AECs with diphenyliodonium (DPI), an inhibitor of NADH oxidase. AEC were cultured for 2 days and then treated with 1 ng/ml TGF-β1 with and without DPI at 5 μM. Cells were then lysed, and protein was harvested for analysis by immunoblotting as described above.
Statistics.
Data are expressed as means ± SE. Raw densitometry data were normalized to control levels and expressed as fold change. Immunoblot densitometry, glutathione, and ROS data were analyzed by ANOVA followed by Newman-Keuls posttest. Significance was defined as P < 0.05.
RESULTS
Inhibition of TGF-β1-induced EMT by NAC.
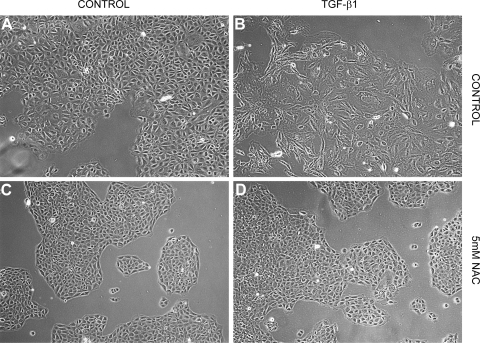
Effects of NAC on TGF-β1-induced EMT were assessed by changes in cell morphology and expression of a panel of mesenchymal and epithelial protein markers. Control RLE-6TN cells maintained in media alone exhibited a rounded cobblestone appearance (Fig. 1A). After treatment with TGF-β1, RLE-6TN cells displayed an increase in overall size, loss of cell-cell contacts, and assumed a fibroblast-like morphology (Fig. 1B). Cells treated with 5 mM NAC in the presence or absence of TGF-β1 retained their rounded shape and cobblestone appearance consistent with retention of the epithelial phenotype (Fig. 1, C and D).
Fig. 1.
N-acetylcysteine (NAC) prevents transforming growth factor-β1 (TGF-β1)-induced changes in epithelial morphology in an alveolar epithelial cell (AEC) line (RLE-6TN). A: control cells in media alone demonstrate a cobblestone appearance and good cell-cell contacts consistent with an epithelial morphology. B: cells treated with TGF-β1 exhibit a fibroblast-like morphology with cellular elongation and reduction of cell-cell contacts. C: treatment with NAC alone causes no appreciable changes in cell morphology. D: NAC prevents changes induced by TGF-β1 and preserves an epithelial morphology.
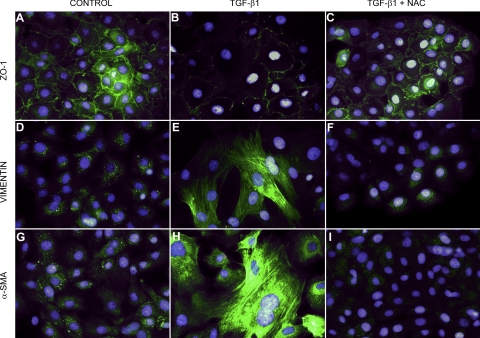
Immunofluorescence microscopy in control RLE-6TN cells demonstrated localized expression of the epithelial marker ZO-1 at cell borders and relatively low levels of expression of the mesenchymal markers α-SMA and vimentin (Fig. 2, A, D, and G). TGF-β1 treatment reduced membrane-associated expression of ZO-1 with loss of expression at cell borders, and concomitant increases in expression of α-SMA and vimentin in a fibril-associated pattern (Fig. 2, B, E, and H). Cells treated concurrently with NAC and TGF-β1 retained high levels of localized expression of epithelial ZO-1 and showed no increase in mesenchymal markers (Fig. 2, C, F, and I). Notably, control cells treated with NAC alone exhibited even lower levels of α-SMA and vimentin than the relatively low levels detected in untreated control cells (data not shown).
Fig. 2.
NAC prevents aspects of alveolar epithelial-mesenchymal transition (EMT) in RLE-6TN cells. Control cells express significant amounts of membrane-associated ZO-1, but little vimentin and α-smooth muscle actin (α-SMA) (A, D, G). Cells treated with TGF-β1 alone lose membrane-associated ZO-1 and demonstrate dramatically increased expression of vimentin and α-SMA (B, E, H). Cells treated with TGF-β1 and NAC appear similar to control cells, with preservation of membrane-associated ZO-1 and no upregulation of vimentin and α-SMA (C, F, I).
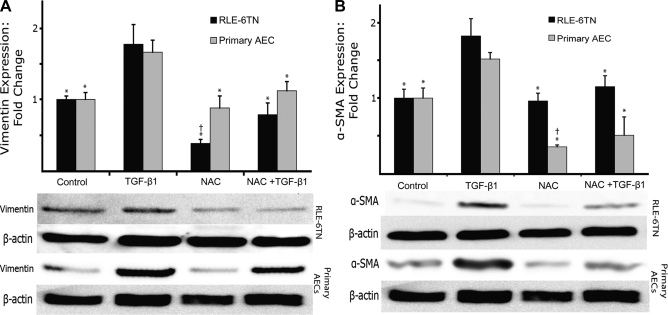
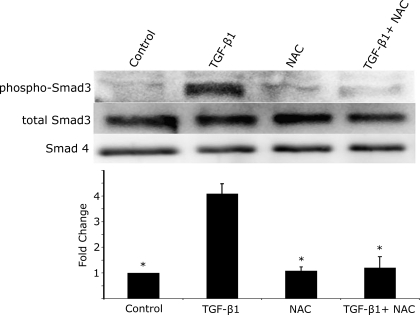
Immunoblot analysis of expression levels of mesenchymal markers corroborated the immunofluorescence data (Fig. 3). Expression of α-SMA and vimentin in TGF-β1-treated RLE-6TN cells increased ∼75% compared with control cells. In cells treated with NAC and TGF-β1, both α-SMA and vimentin expression levels were unchanged compared with untreated cells. To ensure that these findings were not specific to this cell line, we also determined expression levels in freshly isolated rat alveolar type II cells that underwent the same series of treatments. Primary AECs demonstrated similar relative changes in expression of α-SMA and vimentin as seen in RLE-6TN cells following treatment with TGF-β1. Following treatment with NAC in both control and TGF-β1-treated cells, levels of α-SMA were maintained below control levels, and vimentin levels were cut to nearly one-half of those of control cells. NAC also significantly inhibited the increase in the phosphorylation of Smad3 seen with TGF-β1 treatment (Fig. 4).
Fig. 3.
NAC prevents EMT in both an AEC line (RLE-6TN) and primary AEC. A: NAC prevents TGF-β1-induced increases in the mesenchymal marker vimentin in both RLE-6TN cells (black bars) and primary AEC (gray bars). B: NAC also prevents TGF-β1-induced increases in the myofibroblast marker α-SMA in both RLE-6TN cells (black bars) and primary AEC (gray bars). *Significantly different from TGF-β1. †Significantly different from control. N = 3–5.
Fig. 4.
NAC prevents TGF-β1-mediated phosphorylation of Smad3. RLE-6TN cells demonstrate significant phosphorylation of Smad3 2 h after treatment with TGF-β1. NAC alone did not induce Smad3 phosphorylation. NAC treatment at 5 mM concurrently with TGF-β1 treatment completely inhibits Smad3 phosphorylation. Protein loading was normalized to total cellular protein, and total cellular levels of Smad3 and Smad4 were assessed as a loading control and used for normalization of phospho-Smad3 levels. *Significantly different from TGF-β1.
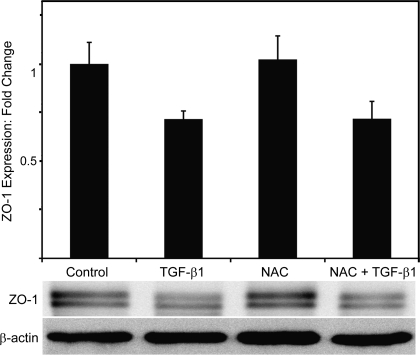
Interestingly, while NAC preserved the membrane localization of ZO-1 and appeared to prevent its removal from cell borders following TGF-β treatment, the overall expression levels of ZO-1 in primary AEC were not significantly changed by NAC (Fig. 5). Treatment with TGF-β1 caused a significant decrease in cellular expression levels of ZO-1, but levels of ZO-1 were not restored to baseline levels in NAC-treated cells in the presence of TGF-β1.
Fig. 5.
NAC does not prevent the reduction in total expression of ZO-1 induced by TGF-β1 in RLE-6TN cells. Despite the dramatic preservation of membrane-associated ZO-1 expression noted with immunofluorescent staining, NAC had no significant effect on total cellular ZO-1 expression. β-actin levels were assessed as a loading control. N = 4.
Mechanisms of ROS-mediated alveolar EMT.
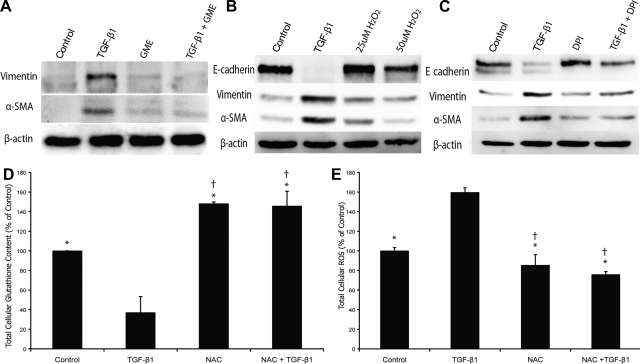
NAC functions to reduce intracellular ROS levels in a variety of ways, but is generally thought to predominantly provide a substrate for glutathione generation. To compare our findings with NAC with directly providing glutathione, we treated cells with GME, a cell-permeable glutathione precursor in place of NAC, and evaluated the effects on TGF-β1-induced EMT. Similar to NAC, GME was able prevent TGF-β1 induction of α-SMA and vimentin (Fig. 6A) at doses as low as 400 μM.
Fig. 6.
A: direct supplementation with glutathione prevents aspects of EMT. Treatment with glutathione monoethyl ester (GME) prevents the increase in vimentin and α-SMA in RLE-6TN cells seen during TGF-β1-induced alveolar EMT in a manner similar to that seen with NAC treatment. B: treatment of RLE-6TN cells with H2O2 does not induce mesenchymal markers. Immunoblots of total cellular vimentin and α-SMA after treatment with 25 and 50 μM H2O2 for 5 days results in no increase in vimentin or α-SMA. TGF-β1-treated cells are used as a positive control. C: inhibition of intracellular ROS generation inhibits aspects of alveolar EMT. Treatment of RLE-6TN cells with diphenyliodonium (DPI) preserves E-cadherin expression and prevents expression of the mesenchymal markers vimentin and α-SMA. D: NAC restores AEC intracellular glutathione content in RLE-6TN cells. Cells treated with TGF-β1 demonstrate a >60% reduction in intracellular glutathione content. Treatment with NAC prevents this reduction and increases intracellular glutathione levels beyond those seen in control cells. E: NAC abrogates TGF-β1-induced increases in intracellular ROS in RLE-6TN cells. TGF-β1 significantly increases cellular ROS content in RLE-6TN cells. NAC treatment prevents this increase and reduces ROS levels below those seen in controls. *Significantly different from TGF-β1. †Significantly different from control. β-actin was used as a loading control in all blots. N = 3–5.
To determine if extracellular oxidative stress alone is sufficient to induce alveolar EMT, AEC were treated with H2O2, and effects on epithelial and mesenchymal markers were assessed. A range of doses of up to 100 μM was utilized (10–100 μM) until cells were no longer viable. The maximum viable dose of 50 μM was used to treat AEC for 5 days. H2O2 treatment had no effect on the expression of α-SMA, vimentin, or E-cadherin despite the fact that it is well known to induce oxidative stress and deplete cellular antioxidants (Fig. 6B).
To determine if the induction of EMT by TGF-β1 required the generation of intracellular ROS, AEC were treated with an inhibitor of NADH oxidase (DPI) concurrently with TGF-β1. DPI significantly decreased the TGF-β1-mediated induction of α-SMA and vimentin (by 70 and 82%, respectively) and preserved the expression of E-cadherin (Fig. 6C). These findings indicate that the effects of glutathione on TGF-β-induced EMT are likely not just the result of restoration of cellular glutathione levels but also the inhibition of the effects of TGF-β on intracellular ROS production.
Intracellular glutathione levels were also compared in RLE-6TN cells with and without TGF-β1 and NAC and normalized to total protein concentrations (Fig. 6D). TGF-β1 treatment reduced intracellular glutathione to less than one-half of that in control cells, whereas glutathione levels in cells treated with both NAC and TGF-β1 were maintained above that of control cells.
Finally, we measured total cellular ROS in RLE-6TN cells treated with and without TGF-β1 and NAC (Fig. 6E). TGF-β1 treatment resulted in an increase in ROS production of 60% compared with control (P < 0.05). Treatment with NAC in the presence of TGF-β1 prevented this increase and maintained intracellular ROS levels at ∼20% below that of control cells.
DISCUSSION
In the present study, we demonstrate that treatment of both primary rat alveolar epithelial cells and an alveolar epithelial cell line (RLE-6TN) with NAC inhibits aspects of TGF-β1-induced EMT. NAC preserved epithelial cell morphology, maintaining cell size, shape, and cell-cell contacts, and prevented the TGF-β1-induced transition to a fibroblast-like phenotype. NAC also dramatically reduced TGF-β1-induced expression of the mesenchymal markers α-SMA and vimentin, as well as phosphorylation of Smad3. Additionally, membrane localization of ZO-1 was preserved with NAC treatment compared with TGF-β1 alone. Whereas with TGF-β1 treatment we saw a decrease in overall expression of ZO-1 and a steady loss from cellular borders, NAC preserved ZO-1 membrane localization, although not overall expression. Glutathione levels were preserved in the presence of NAC, reinforcing its role as a source of cysteine and a glutathione precursor. In addition, TGF-β1-induced increases in cellular ROS were significantly reduced with NAC treatment. The effects of NAC were recapitulated by treatment with GME. Furthermore, inhibition of the intracellular generation of ROS during TGF-β1 treatment by DPI also prevented alveolar EMT. Finally, treatment with H2O2 alone did not induce EMT, suggesting that oxidant-mediated depletion of glutathione stores alone is not sufficient to induce EMT. Together, our findings suggest that NAC inhibits TGF-β1-induced EMT through replenishment of intracellular glutathione stores and prevention of the generation of intracellular ROS.
Despite intensive efforts over many years to determine the causes of and investigate potential treatments for fibrotic diseases of the lung such as IPF, few effective therapies exist for this disease, and our understanding of its pathogenesis is limited at best (27). Our group (44) and others (45) have recently demonstrated that EMT can be induced in AECs in vitro and that EMT may be highly prevalent in the epithelium during fibrotic lung injury (22). Given these findings, and the knowledge that inflammation may not play a major role in the propagation of fibrosis once it is established (11), we have been interested in investigating “epithelio-centric” therapies. Any intervention that could target the epithelium, promote “appropriate” epithelial survival, proliferation, and differentiation, reduce intracellular epithelial oxidative stress, and inhibit alveolar EMT may have great potential to improve epithelial integrity and promote repair after lung injury.
NAC is a commonly used and readily available agent that in addition to effects on other cell types, e.g., fibroblasts (34), may have potential as a pulmonary epithelium-preserving intervention. NAC is a precursor in the glutathione synthetic pathway, and its administration results in replenishment of intracellular glutathione stores and an increase in free radical scavenging, thereby protecting cells from oxidative damage (13). Both inhaled and oral NAC attenuate lung fibrosis induced by bleomycin in mice (14, 38) and rats (46). Glutathione administration inhibited oxidant-induced changes in alveolar epithelial permeability (36) and reversed the oxidant/antioxidant imbalance found in the lungs of patients with IPF (5). Most importantly, oral NAC administered to patients with IPF preserved clinical pulmonary function over a period of 1 year (10). This latter finding represents the first intervention identified to date that had a significant effect on the course of IPF. However, the mechanism by which NAC attenuates fibrosis in the lung and to some extent maintains pulmonary function is largely unknown. Given the dramatic effects of NAC on alveolar EMT in vitro demonstrated in this study, we hypothesize that effects of NAC on the alveolar epithelium, specifically NAC-mediated inhibition of alveolar EMT, may play an important role in its salutary clinical effects in pulmonary fibrosis. However, the specific mechanistic role of NAC in alveolar EMT is completely unknown.
TGF-β1 is the primary cytokine initiating fibrosis in many organs including the lung (39). Interestingly, the generation of intracellular ROS mediates or accentuates many of the myriad signaling events induced by TGF-β1. TGF-β1 induces the activation of a hydrogen peroxide-generating NADH oxidase in pulmonary fibroblasts, with a subsequent dramatic increase in the intracellular ROS burden (41). ROS generation is required for the activation of MAPK/ERK and the transcription factor AP-1 by TGF-β1 in human lung fibroblasts (20) leading to fibroblast activation and the generation of myofibroblasts. Finally, the activation of MAPK/ERK, p38 MAPK, and Smad proteins by TGF-β1 was effectively inhibited by antioxidants during TGF-β1-induced EMT in renal tubular epithelial cells (35). Together, these findings suggest that TGF-β1-induced induction of cellular ROS result in the activation of fibrosis-promoting pathways in both fibroblasts and epithelial cells. In addition, it is clear that both Smad-mediated and non-Smad-mediated signaling pathways, likely through the generation of intracellular ROS, play critical roles in the induction of EMT (35, 42). The use of NAC to reduce ROS production in epithelial cells may therefore be effective in the inhibition of TGF-β1-initiated signaling cascades that lead to alveolar EMT and the propagation of fibrosis through this pathway.
In addition to promoting pathogenetic signaling cascades relevant to EMT, oxidative stress has additional directly deleterious effects on the pulmonary epithelium. Hydrogen peroxide increases epithelial permeability (37) and inhibits epithelial wound repair (12). Cigarette smoke-induced oxidative stress results in growth arrest and apoptosis of AEC (34), and ROS production has been demonstrated to be elevated in phagocytes and AEC from patients with pulmonary fibrosis (7, 24). Clearly, ROS related to both intracellular signaling and direct cellular damage play significant roles in a variety of pulmonary diseases that are characterized by derangements of the alveolar epithelium.
Glutathione is used as a primary defense against oxidative stress in many cell types and is dramatically reduced in the lungs of patients with IPF (33). We found in the current study that intracellular glutathione levels were dramatically reduced after treatment with TGF-β1 and that NAC treatment restored levels to those found in control cells. Depletion of intracellular glutathione was previously demonstrated to occur in AECs after treatment with TGF-β1 and was postulated to underlie the deleterious effects of TGF-β1 during acute lung injury (32). In a recent small study, ARDS patients with null polymorphisms of glutathione-S-transferase demonstrated an increased mortality compared with controls (29), suggesting that an inability to regenerate glutathione is critical to cellular physiological defenses during lung injury. Of note, it has been shown that relative intracellular glutathione levels can determine whether intracellular ROS act as second messengers or induce direct oxidative stress (9). Our data indicate that the generation of intracellular ROS by TGF-β1 is necessary for the induction of EMT, given that DPI effectively prevented TGF-β1-induced EMT. This is in agreement with previous studies demonstrating that ROS mediate the activation of a variety of downstream mediators and transcription factors, including NF-κB, Smad proteins, PAI-1, and others, some of which may themselves promote EMT (4, 8, 18). Interestingly, treatment with H2O2 did not induce EMT in AEC, suggesting that critical oxidant levels are necessary but not sufficient to induce EMT. Together, our data suggest that TGF-β1-induced elevations in intracellular ROS mediate the downstream activation of signaling mediators, including Smad3, that lead to EMT. NAC likely serves to limit the generation of intracellular ROS as well as to maintain a critical threshold of intracellular glutathione, effectively preventing downstream TGF-β1 signaling events and EMT.
Our study does have some limitations. The study was performed using in vitro culture models, and as such it remains to be determined whether NAC can inhibit EMT in vivo in animal models of lung injury. Further studies examining the use of NAC during in vivo induction of fibrosis to determine the extent of protection against alveolar EMT are clearly needed. One potential limitation of the application of NAC therapy is the relatively high dose needed in our studies to prevent EMT, although some effect was noted at lower concentrations (data not shown). While concentrations of thiols in bronchoalveolar lavage fluid can be obtained that are similar to those needed in our current study (26), and other recent in vivo studies have used similar doses (40), the achievement of such levels consistently in animals or in patients may be difficult without creative strategies for delivery and release of glutathione specifically in the lung. Finally, while we have demonstrated that NAC reduces cellular ROS and preserves cellular glutathione and that the prevention of intracellular ROS generation prevents EMT, a deeper investigation of the specific signaling pathways that may be inhibited is also clearly needed.
In conclusion, our results demonstrate that NAC is an effective inhibitor of EMT in AECs in vitro, through stabilization of AEC intracellular redox state and preservation of intracellular glutathione levels. Combined with the recent clinical findings demonstrating the unique effectiveness of NAC in stabilization of lung function in patients with IPF, and the potential pathogenetic importance of alveolar EMT in pulmonary fibrosis, further in vivo studies of NAC-mediated inhibition of alveolar EMT are needed.
GRANTS
B. C. Willis is supported by the St. Joseph's Hospital Foundation. Z. Borok is funded by National Heart, Lung, and Blood Institute Grants HL-38578, HL-62569, and HL-89445 and the Hastings Foundation.
ACKNOWLEDGMENTS
Z. Borok holds the Ralph Edgington Chair in Medicine.
REFERENCES
- 1.Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene 18: 6104–6111, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Arsalane K, Dubois CM, Muanza T, Begin R, Boudreau F, Asselin C, Cantin AM. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol 17: 599–607, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6: 593–597, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Black D, Lyman S, Qian T, Lemasters JJ, Rippe RA, Nitta T, Kim JS, Behrns KE. Transforming growth factor beta mediates hepatocyte apoptosis through Smad3 generation of reactive oxygen species. Biochimie 89: 1464–1473, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borok Z, Buhl R, Grimes GJ, Bokser AD, Hubbard RC, Holroyd KJ, Roum JH, Czerski DB, Cantin AM, Crystal RG. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet 338: 215–216, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Borok Z, Danto SI, Zabski SM, Crandall ED. Defined medium for primary culture de novo of adult rat alveolar epithelial cells. In Vitro Cell Dev Biol Anim 30A: 99–104, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Cantin AM, North SL, Fells GA, Hubbard RC, Crystal RG. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest 79: 1665–1673, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins-Underwood JR, Zhao W, Sharpe JG, Robbins ME. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Radic Biol Med 45: 929–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bleser PJ, Xu G, Rombouts K, Rogiers V, Geerts A. Glutathione levels discriminate between oxidative stress and transforming growth factor-beta signaling in activated rat hepatic stellate cells. J Biol Chem 274: 33881–33887, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 353: 2229–2242, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Geiser T. Idiopathic pulmonary fibrosis–a disorder of alveolar wound repair? Swiss Med Weekly 133: 405–411, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Geiser T, Ishigaki M, van Leer C, Matthay MA, Broaddus VC. H2O2 inhibits alveolar epithelial wound repair in vitro by induction of apoptosis. Am J Physiol Lung Cell Mol Physiol 287: L448–L453, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Gillissen A, Nowak D. Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir Med 92: 609–623, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med 162: 225–231, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Hoshino Y, Mishima M. Redox-based therapeutics for lung diseases. Antioxid Redox Signal 10: 701–704, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Hu T, Ramachandrarao SP, Siva S, Valancius C, Zhu Y, Mahadev K, Toh I, Goldstein BJ, Woolkalis M, Sharma K. Reactive oxygen species production via NADPH oxidase mediates TGF-β-induced cytoskeletal alterations in endothelial cells. Am J Physiol Renal Physiol 289: F816–F825, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CS, Chang LS, Anderson ME, Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem 268: 19675–19680, 1993 [PubMed] [Google Scholar]
- 18.Ichikawa T, Sugiura H, Koarai A, Yanagisawa S, Kanda M, Hayata A, Furukawa K, Akamatsu K, Hirano T, Nakanishi M, Matsunaga K, Minakata Y, Ichinose M. Peroxynitrite augments fibroblast-mediated tissue remodeling via myofibroblast differentiation. Am J Physiol Lung Cell Mol Physiol 295: L800–L808, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS, Lee TH, Bae YS, Ha KS, Lee ZW, Rhee SG, Choi I. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol 165: 2190–2197, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 103: 13180–13185, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinnula VL. Redox imbalance and lung fibrosis. Antioxid Redox Signal 10: 249–252, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Kinnula VL, Myllarniemi M. Oxidant-antioxidant imbalance as a potential contributor to the progression of human pulmonary fibrosis. Antioxid Redox Signal 10: 727–738, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Koli K, Myllarniemi M, Keski-Oja J, Kinnula VL. Transforming growth factor-beta activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal 10: 333–342, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Lailey AF, Upshall DG. Thiol levels in rat bronchio-alveolar lavage fluid after administration of cysteine esters. Hum Exp Toxicol 13: 776–780, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J 30: 835–839, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis 3: 8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moradi M, Mojtahedzadeh M, Mandegari A, Soltan-Sharifi MS, Najafi A, Khajavi MR, Hajibabayee M, Ghahremani MH. The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine. Respir Med 103: 434–441, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Perez LM, Milkiewicz P, Ahmed-Choudhury J, Elias E, Ochoa JE, Sanchez Pozzi EJ, Coleman R, Roma MG. Oxidative stress induces actin-cytoskeletal and tight-junctional alterations in hepatocytes by a Ca2+-dependent, PKC-mediated mechanism: protective effect of PKA. Free Radic Biol Med 40: 2005–2017, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Phan SH. The myofibroblast in pulmonary fibrosis. Chest 122: 286S–289S, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest 107: 1537–1544, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 16: 534–554, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Rennard SI, Togo S, Holz O. Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc 3: 703–708, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, Lee HB. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol 16: 667–675, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Roum JH, Aledia AS, Carungcong LA, Kim KJ, Borok Z. Extracellular glutathione inhibits oxygen-induced permeability changes in alveolar epithelial monolayers. J Appl Physiol 91: 748–754, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Seeger W, Hansen T, Rossig R, Schmehl T, Schutte H, Kramer HJ, Walmrath D, Weissmann N, Grimminger F, Suttorp N. Hydrogen peroxide-induced increase in lung endothelial and epithelial permeability–effect of adenylate cyclase stimulation and phosphodiesterase inhibition. Microvasc Res 50: 1–17, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Shahzeidi S, Sarnstrand B, Jeffery PK, McAnulty RJ, Laurent GJ. Oral N-acetylcysteine reduces bleomycin-induced collagen deposition in the lungs of mice. Eur Respir J 4: 845–852, 1991 [PubMed] [Google Scholar]
- 39.Sime PJ, O'Reilly KMA. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol 99: 308–319, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Sugiura H, Ichikawa T, Liu X, Kobayashi T, Wang XQ, Kawasaki S, Togo S, Kamio K, Mao L, Ann Y, Ichinose M, Rennard SI. N-acetyl-l-cysteine inhibits TGF-beta(1)-induced profibrotic responses in fibroblasts. Pulm Pharmacol Ther . In press [DOI] [PubMed] [Google Scholar]
- 41.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 270: 30334–30338, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 293: L525–L534, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc 3: 377–382, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 166: 1321–1332, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao HW, Xie QM, Chen JQ, Deng YM, Tang HF. TGF-β1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci 76: 29–37, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Yildirim Z, Kotuk M, Iraz M, Kuku I, Ulu R, Armutcu F, Ozen S. Attenuation of bleomycin-induced lung fibrosis by oral sulfhydryl containing antioxidants in rats: erdosteine and N-acetylcysteine. Pulm Pharmacol Ther 18: 367–373, 2005 [DOI] [PubMed] [Google Scholar]