Abstract
Accumulating evidence suggests a pivotal role of the calcitonin receptor-like receptor (CRLR) signaling pathway in preventing damage of the lung by stabilizing pulmonary barrier function. Intermedin (IMD), also termed adrenomedullin-2, is the most recently identified peptide targeting this receptor. Here we investigated the effect of hypoxia on the expression of IMD in the murine lung and cultured murine pulmonary microvascular endothelial cells (PMEC) as well as the role of IMD in regulating vascular permeability. Monoclonal IMD antibodies were generated, and transcript levels were assayed by quantitative RT-PCR. The promoter region of IMD gene was analyzed, and the effect of hypoxia-inducible factor (HIF)-1α on IMD expression was investigated in HEK293T cells. Isolated murine lungs and a human lung microvascular endothelial cell monolayer model were used to study the effect of IMD on vascular permeability. IMD was identified as a pulmonary endothelial peptide by immunohistochemistry and RT-PCR. Hypoxia caused an upregulation of IMD mRNA in the murine lung and PMEC. As shown by these results, HIF-1α enhances IMD promoter activity. Our functional studies showed that IMD abolished the increase in pressure-induced endothelial permeability. Moreover, IMD decreased basal and thrombin-induced hyperpermeability of an endothelial cell monolayer in a receptor-dependent manner and activated PKA in these cells. In conclusion, IMD is a novel hypoxia-induced gene and a potential interventional agent for the improvement of endothelial barrier function in systemic inflammatory responses and hypoxia-induced vascular leakage.
Keywords: endothelial permeability, edema
the pulmonary vascular endothelium forms a continuous, semipermeable dynamic barrier for water, solutes, and plasma proteins between the intravascular space and underlying tissues. Impairment of the barrier function results in vascular leakage and pulmonary edema formation. Inflammatory cytokines (22, 36), viruses (17, 30), biophysical forces such as stretch and shear stress (25, 29), and hypoxia (3, 7, 27, 40) can lead to increased endothelial cell permeability, thus disrupting the segregation between blood plasma and interstitial fluid. Accumulating evidence suggests a pivotal role of the calcitonin receptor-like receptor (CRLR) signaling pathway in stabilizing this barrier function and preventing pulmonary damage. This class B G protein-coupled receptor binds peptides of the calcitonin gene-related peptide (CGRP) family, and its activation results in a marked increase in cytosolic cAMP and subsequent activation of protein kinase A, supposing a coupling to adenylyl cyclase (19, 33). Notably, CRLR alone exhibits little affinity to its ligands and becomes ligand selective only when associated with one of the three receptor activity-modifying proteins, RAMP1–3. CGRP activates the CRLR/RAMP1 complex, adrenomedullin (AM) appears to act as an agonist for CRLR/RAMP2 and RAMP3 (8), and intermedin (IMD; synonym adrenomedullin-2) signals primarily through CRLR associated with either RAMP1 or RAMP3 but also CRLR/RAMP2 heteromers (9, 33).
The pronounced protective pulmonary effects of AM in inflammation and hypoxia are well documented. It counteracts endothelial hyperpermeability induced by hydrogen peroxide, thrombin, or Escherichia coli hemolysin (19, 20), dilates rat pulmonary artery rings during hypoxia (44), and is upregulated in hypoxia via the hypoxia-inducible factor (HIF)-1α pathway (16). On the receptor side, however, hypoxia induces upregulation of RAMP1 and RAMP3, but not RAMP2, in the rat lung (32), suggesting preferential IMD signaling. Two mature IMD peptides of 47 and 40 amino acids, sharing in mice ∼33% sequence homology to AM, were identified in 2004 by Roh et al. (33) and Takei et al. (37). Current knowledge on its vascular and pulmonary effects is limited but points toward a potential beneficial role in pathophysiological conditions: IMD is cardioprotective in ischemia-reperfusion injury (2, 43), its intravenous application decreases blood pressure (1, 14, 15), and it dilates the rat pulmonary vascular bed (5, 23).
Hence, we reasoned that IMD might represent an endogenous pulmonary peptide being regulated by hypoxia and contributing to vascular homeostasis under inflammatory and hypoxic conditions. To test this hypothesis, we conducted experiments to identify hypoxia-responsive elements in the IMD promoter, to characterize hypoxia-induced alterations in IMD expression, and to elucidate the role of IMD in regulating endothelial hyperpermeability.
MATERIALS AND METHODS
Animals.
All experiments conformed to National Institutes of Health guidelines on the care and use of experimental animals and were approved by Regierungspäsidium Giessen. FVB mice of either sex (n = 4 for mRNA analysis, n = 5 for immunofluorescence) were exposed to normobaric hypoxia [inspired O2 fraction (FiO2) = 0.10] in a ventilated chamber for 15 h. The level of hypoxia was held constant by an autoregulatory control unit (model 4010 O2 controller, Labotec, Göttingen, Germany). Control animals (n = 4 for mRNA analysis, n = 4 for immunofluorescence) were exposed to normobaric normoxia in a similar chamber at an FiO2 of 0.21.
Antibodies.
Monoclonal anti-IMD antibodies were obtained by screening of the MorphoSys Human Combinatorial Antibody Library (HuCAL) GOLD library (AbD Serotec, Martinsried, Germany). In this library, more than 15 billion functional human antibody specificities in Fab format are prefabricated, and they were screened by ELISA for reactivity against a synthetic peptide (RPAGRRDSAPVDPSSPHSY) corresponding to amino acid residues 131–149 (accession no. NP_891558) of mouse IMD. At the same time, they were tested for nonreactivity to the homologous peptide AM with the synthetic peptide TDKDKDGMAPRNKISPQGY, which corresponds to amino acid residues 126–144 (accession no. NP_033757) of mouse AM. Monovalent Fab fragments that fulfilled these criteria (n = 9) were converted into a bivalent Mini-Antibody format (AbD Serotec) and used for immunofluorescence. Cy3-conjugated anti-human IgG F(ab)2 from goat was from Dianova (Hamburg, Germany). Monoclonal anti-mouse CD31 (clone MEC 13.3) antibody was from BD Biosciences (Heidelberg, Germany). Monoclonal anti-phospho-vasodilator-stimulated phosphoprotein (VASP) Ser157 (PKA phosphorylation site) was purchased from Nanotools (Teningen, Germany). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG antibody was from Amersham Biosciences (Heidelberg, Germany). HRP-conjugated goat anti-human IgG was from Dianova.
Immunofluorescence.
Mice were killed by inhalation of an overdose of isoflurane (Abbott, Wiesbaden, Germany), and lungs were filled via a tracheal cannula with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Specimens were cryoprotected with 18% sucrose in 0.1 M phosphate buffer, frozen, and sectioned at 12-μm thickness. Nonspecific protein binding sites were saturated by incubation (1 h) in 10% horse serum, 0.5% Tween 20, 0.1% bovine serum albumin in 0.005 M phosphate buffer with 4.48 g/l NaCl, and primary antibodies (clones AbD06980.1 and AbD06988.1) were then applied at 0.4 μg/ml overnight. Bound antibodies were tagged by 1-h incubation with Cy3-conjugated anti-human IgG F(ab)2 (diluted 1:500), and sections were washed, coverslipped in 1:1 carbonate-buffered glycerol at pH 8.4, and evaluated with an epifluorescence microscope (Axioplan 2, Zeiss, Jena, Germany). Specificity of the primary antibody was controlled by preabsorption (1 h at room temperature) with either synthetic mouse IMD(1–47) (Phoenix Pharmaceuticals, Belmont, CA) or a synthetic peptide corresponding to amino acid residues 126–144 of mouse AM precursor (AbD Serotec) or synthetic rat CGRP (Acris, Hiddenhausen, Germany), each at a concentration of 5 μg/ml, before application to the sections. Specificity of the secondary antibody was tested by omission of the primary antibody. Endothelial cells were identified in double-labeling experiments using a biotinylated rat monoclonal anti-mouse CD31 antibody applied at 1 μg/ml simultaneously with anti-IMD antibody. This biotinylated antibody was detected with fluorescein isothiocyanate-conjugated streptavidin (1:500, 1 h; Sigma, Deisenhofen, Germany).
Anti-IMD Western blot and dot blot assay.
Mouse lung tissue homogenized in extraction buffer [7 M urea, 10% glycerol, 10 mM Tris·HCl pH 6.8, 1% sodium dodecyl sulfate (SDS), 5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 1× concentrated Complete Mini Protease Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany)] was resolved by tricine-SDS-15% polyacrylamide gel electrophoresis. Proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Schwalbach, Germany) by semidry blotting, and after incubation with blocking buffer [Tris-buffered saline (TBS), 0.01% Tween 20, 10% milk powder] for 1 h at room temperature the membrane was covered overnight with human anti-IMD clone AbD06988.1 (1.52 μg/ml) or AbD06980.1 (1.3 μg/ml) in TBS, 0.01% Tween 20, 5% milk powder. After the membrane was washed with TBS-0.01% Tween 20 it was incubated with HRP-conjugated goat anti-human IgG (1:10,000 in TBS, 0.01% Tween 20, 2.5% milk powder). Bound antibody was visualized by enhanced chemiluminescence using 9 volumes of Super Signal West Pico Substrate mixed with 1 volume of Super Signal West Dura Extended Duration Substrate (both from Pierce/Perbio Science Deutschland, Bonn, Germany). For the dot blot assay dilution series of IMD peptide (aa residues 131–149) and AM peptide (aa residues 126–144) were pipetted onto nitrocellulose membrane. After drying, the membrane was blocked with blocking buffer and handling was continued as described for Western blotting.
Determination of VASP phosphorylation.
To determine the phosphorylated forms of VASP, human lung microvascular endothelial cells were lysed in Laemmli buffer (24) and equal amounts of lysed cellular proteins (30 μg protein/well) were separated by SDS-10% polyacrylamide gel electrophoresis followed by protein transfer onto nitrocellulose membrane. The membrane was probed with an anti-phospho-VASP Ser157 antibody (0.2 μg/ml), which recognizes the phosphorylated form of VASP at Ser157, or anti-actin. Signals were visualized with a peroxidase-conjugated anti-rabbit antibody (0.1 μg/ml), and bands were densitometrically evaluated.
Lung isolation, perfusion, and ventilation.
The model of isolated, perfused mouse lungs has been described previously (41, 42). FVB mice were deeply anesthetized by intraperitoneal administration of pentobarbital sodium (100 mg/kg body wt) and anticoagulated with heparin (1,000 U/kg) by intravenous injection. After intubation via a tracheostoma, mice were ventilated with room air (positive-pressure ventilation) with a tidal volume of 250 μl, 90 breaths/min, and 3-cmH2O positive end-expiratory pressure with a Minivent Type 845 ventilator (Hugo Sachs Elektronik, March-Hugstetten, Germany). Midsternal thoracotomy was followed by insertion of catheters into the pulmonary artery and left atrium. With a peristaltic pump (ISM834A V2.10, Ismatec, Glattbrugg, Switzerland), buffer perfusion via the pulmonary artery was started at 4°C and a flow of 0.2 ml/min. In parallel with the onset of artificial perfusion, ventilation was changed from room air to a premixed gas (21% O2, 5.3% CO2, 73.7% N2). For perfusion Krebs-Henseleit buffer (Serag-Wiessner, Naila, Germany) containing (in mM) 120 NaCl, 4.3 KCl, 1.1 KH2PO4, 2.4 CaCl2, 1.3 MgCl2, and 13.32 glucose as well as 5% (wt/vol) hydroxyethylamylopectin (mol wt 200,000) was used. The pH was adjusted to 7.37–7.40 with NaHCO3. The isolated, perfused lung was placed in a temperature-equilibrated housing chamber freely suspended from a force transducer for continuous monitoring of organ weight. After the lungs were rinsed with 20 ml of buffer, the perfusion circuit was closed for recirculation (total system volume 15 ml) and left atrial pressure (LAP) was set at 0.26 cmH2O. Meanwhile, the flow was slowly increased from 0.2 to 2 ml/min, and the entire system was heated to 37°C. Pressures in the pulmonary artery and left atrium were registered via small-diameter catheters. After an initial steady-state period of 20 min, the capillary filtration coefficient (Kfc) was determined (time set at zero). At 40 min LAP was increased to 11.5 cmH2O (vs. 1.5 cmH2O in controls). Repetitive measurements of Kfc were done at 60 and 80 min. In treated lungs IMD (10 nM) was added to the buffer fluid at 25 min. In controls, LAP was held constant at 1.5 cmH2O. Kfc was determined gravimetrically from the slope of the lung weight gain curve induced by a 10-cmH2O step elevation (= hydrostatic challenge).
Macromolecule permeability of endothelial monolayers.
The permeability of Trypan blue-labeled albumin across human lung blood microvascular endothelial cell (HMVEC-L) monolayers was studied in a two-compartment system separated by a filter membrane as described previously (28). Both compartments contained as basal medium modified Tyrode solution (composition in mM: 150 NaCl, 2.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.0 CaCl2, and 30.0 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4, 37°C) supplemented with 2% (vol/vol) normal calf serum. There was no hydrostatic pressure gradient between the compartments. The “luminal” compartment containing the monolayer had a volume of 2.5 ml, and the “abluminal” compartment had a volume of 6.5 ml. The fluid in the abluminal compartment was constantly stirred. Trypan blue-labeled albumin (60 μM) was added to the luminal compartment. The appearance of the labeled albumin in the abluminal compartment was continuously monitored by pumping the liquid through a spectrophotometer (Specord 10, Zeiss). Increases of the concentration of labeled albumin were detected with a time delay of <15 s. The concentration of labeled albumin in the luminal compartment was determined every 10 min of incubation. It did not change significantly in the time frame of the experiments.
The albumin flux [F, expressed as mol/(s × cm2)] across the monolayer with surface S was determined from the rise of albumin concentration (d[A]2) during the time interval (dt) in the abluminal compartment (volume V): F = (d[A2]/dt × V)/S.
To facilitate the comparison of data obtained in the present study with those of other studies, the permeability coefficient (P, expressed as cm/s) of the combined system of monolayer and filter support was calculated from F according to Fick's law of diffusion as follows: P = F/([A]1 − [A]2), where [A]1, and [A]2 denote tracer concentrations in the luminal and abluminal compartments, respectively. Because the driving force ([A]1 − [A]2) remained virtually unchanged in the course of the described experiments, the relative changes in F correspond to similar changes in P.
Cell culture.
Pulmonary microvascular endothelial cells (PMEC) were isolated as described by Lips et al. (26) with Dynabeads coated with Bandeiraea simplicifolia lectin. HL-1 cells were kindly provided by Dr. W. C. Claycomb (Louisiana State University Health Science Center, New Orleans, LA). NIH3T3 fibroblasts were from the German Resource Center for Biological Material (DSMZ, Braunschweig, Germany). NS20Y cells were from PAA (Pasching, Austria) and HMVEC-L from Lonza (Basel, Swizerland). For hypoxia experiments, cells were exposed for 6 h to either normoxia (21% O2, 5% CO2, 74% N2) or hypoxia (1% O2, 5% CO2, 94% N2).
RNA isolation and real-time RT-PCR.
Total RNA was isolated with the RNeasy mini kit (Qiagen, Hilden, Germany), and genomic DNA was removed by treatment with DNase (Invitrogen, Karlsruhe, Germany). Superscript RNase H− reverse transcriptase was used for reverse transcription. Real-time quantitative PCR was performed as described by Pfeil et al. (31). Primer sequences are specified in Table 1. Sequencing of the PCR products was done by MWG Biotech (Ebersberg, Germany).
Table 1.
Primers used for RT-PCR
| Gene | Species | Sequence | Product Length | Accession No. |
|---|---|---|---|---|
| IMD | Mouse | Forward: GGTAACCCTCGGTTGCATCAG | 178 bp | NM182828 |
| Reverse: GGCATGACGACGAGACTTCC | (197–375) | |||
| AM | Mouse | Forward: GAAGCCCACATTCGTGTCA | 140 bp | NM009627 |
| Reverse: TGCCGTCCTTGTCTTTGTC | (434–574) | |||
| β2-MG | Mouse | Forward: ATGGGAAGCCGAACATACCTG | 176 bp | NM009735 |
| Reverse: CAGTCTCAGTGGGGGTGAAT | (154–340) | |||
| eNOS | Mouse | Forward: GTGCCTTGAGCAAGGACATAT | 300 bp | BC052636 |
| Reverse: TCAGGAACCAGGTATTTCTGG | (3315–3615) |
IMD, intermedin; AM, adrenomedullin; β2-MG, β2-microglobulin; eNOS, endothelial nitric oxide synthase.
Analysis of intermedin gene promoter reporter activity.
IMD gene sequences from human and mouse were retrieved from GenBank and analyzed with the TRANSFAC database and Perl-based scripts. A luciferase reporter construct, pGL2–2.7 kb IMD promoter-luc (pGL2-Enhancer luciferase vector, Promega, Mannheim, Germany), which contains a 2.7 kb (−1 ∼ −2650) proximate region of mouse IMD gene promoter, was cotransfected with a HIF-1α expression construct (GeneCopoeia, Germantown, MD) in HEK293T cells. Cells were transfected with 4 μg of pGL2–2.7 kb IMD promoter-luc plasmid, together with 2, 4, 8, or 16 μg of the HIF-1α expression vector plasmid, by the calcium phosphate precipitation method. Five-hundred nanograms of a pCMV-β-galactosidase vector were cotransfected to monitor transfection efficiency. The reporter activity is expressed as the ratio of relative luciferase unit to β-galactosidase activity.
RESULTS
IMD is expressed by pulmonary endothelial cells.
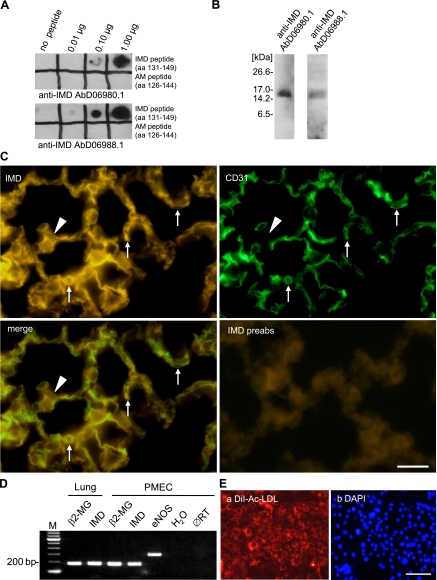
The library screen identified nine antibody clones that recognized IMD, but not AM, in ELISA. Two of these (clones AbD06980.1 and AbD06988.1) were suitable for immunofluorescence labeling of formaldehyde-fixed tissue. Specificity of these anti-IMD antibodies was shown in dot blot and Western blot analysis. Both antibodies recognized their cognate peptides but showed no cross-reactivity to AM (Fig. 1A). In Western blot analysis both peptides labeled a single 16-kDa peptide in mouse lung homogenates, corresponding to prepro-IMD (Fig. 1B). Immunolabeling of lung sections could be abolished by preabsorption of the antibody with mouse IMD(1–47) (Fig. 1C) but not with an AM peptide and with CGRP. When applied in double-labeling experiments, staining intensity obtained with clone AbD06988.1 decreased. Thus clone AbD06980.1 was chosen for further analysis. Double labeling with mouse monoclonal anti-CD31 antibody, an endothelial cell marker, revealed a large overlap with IMD immunoreactivity (Fig. 1C). In addition, some nonendothelial cells of the alveolar septa were also labeled by clone AbD06980.1 antibody (Fig. 1C). This dominant localization of IMD immunoreactivity in endothelial cells in the lung was further supported by RT-PCR showing the expression of IMD mRNA in murine lung homogenates and in PMEC (Fig. 1D). Uptake of fluorescently labeled acetylated low-density lipoprotein (Ac-LDL) indicated that primary isolates of PMEC were quite homogeneous (Fig. 1E). PMEC were further characterized by their ability to express endothelial nitric oxide synthase (eNOS) mRNA (Fig. 1D).
Fig. 1.
Intermedin (IMD) is expressed in lung and pulmonary microvascular endothelium. A: dot blot analysis of synthetic peptides showed specificity of the anti-IMD antibody AbD06980.1 and AbD06988.1 generated against aa 131–149 of mouse IMD. The anti-IMD antibodies recognized mouse IMD peptide but showed no cross-reactivity to adrenomedullin (AM). In Western blot analysis (B) the anti-IMD antibodies labeled a single 16-kDa peptide in mouse lung homogenates, corresponding to prepro-IMD. C: double-labeling immunofluorescence of alveolar region with monoclonal antibodies directed against IMD and CD31 (endothelial marker). Endothelial cells are double-labeled (arrows). In addition, there are also IMD-immunoreactive nonendothelial cells (arrowheads). Preabsorption (preabs) with the IMD antibody with mouse IMD(1–47) resulted in complete absence of labeling. Bar, 20 μm. D: a single band of 178 bp was amplified with IMD-specific primers from cDNA of murine lung and murine pulmonary microvascular endothelial cells (PMEC). β2-Microglobulin (β2-MG) served as a housekeeping gene control. H2O, control run without template; ØRT, control run without reverse transcriptase; M, marker. PMEC are characterized by amplification of an endothelial nitric oxide synthase (eNOS) mRNA-specific band of 300 bp by RT-PCR and by DiI-Ac-LDL (E) uptake as shown by the cytoplasmic fluorescent red staining (E, a). E, b: nuclear counterstaining with DAPI. Bar, 100 μm.
IMD expression is upregulated by hypoxia.
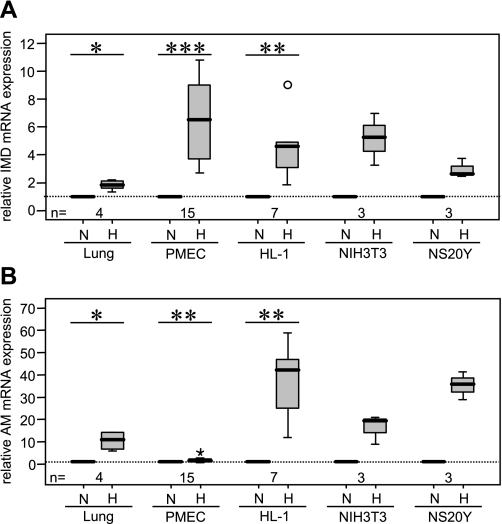
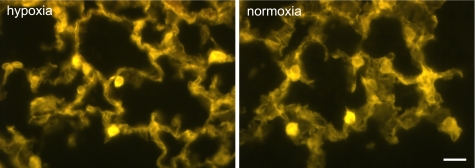
Quantitative RT-PCR showed a 1.9-fold increase in IMD mRNA expression in the lung of mice housed 15 h under hypoxic conditions (10% O2) compared with control animals (Fig. 2A). As judged by immunofluorescence, cellular IMD expression pattern did not change in hypoxia (Fig. 3). Hence we assumed that the general increase in IMD mRNA observed in the whole lung was due to increased expression at the cellular level rather than recruitment of cell types that did not express IMD under normoxic conditions. This was tested in a cell culture model. Exposure of murine PMEC to hypoxia (1% O2, 6 h) caused a 6.3-fold increase in IMD mRNA expression compared with normoxic control (Fig. 2A). A hypoxia-induced increase in IMD mRNA expression was also found in the murine cardiomyocyte cell line HL-1 (4.5-fold), in NIH3T3 fibroblasts (5.2-fold), and in the murine forebrain neuroblastoma cell line NS20Y (2.9-fold) (Fig. 2A). Similar to studies of IMD transcripts, we found that hypoxia increases AM mRNA expression in the lung (10.4-fold), PMEC (1.9-fold), HL-1 cardiomyocytes (42-fold), NIH3T3 fibroblasts (19.7-fold), and NS20Y neuroblastoma cells (36.3-fold) (Fig. 2B).
Fig. 2.
IMD expression is induced by hypoxia. Hypoxia caused an increase in IMD (A) and AM (B) mRNA expression in lungs of mice housed under hypoxic conditions (15 h, 10% O2) and in murine PMEC, HL-1 cardiomyocytes, NIH3T3 fibroblasts, and NS20Y neuroblastoma cells treated with hypoxia (6 h, 1% O2). Percentiles 0, 25, 50, 75, and 100 are presented in box plots. Differences among experimental groups were analyzed first with the Kruskal-Wallis test and, if significant differences were observed, with subsequent Mann-Whitney test. Star and circle represent data beyond 3 SD. N, normoxia; H, hypoxia. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Fig. 3.
Cellular distribution pattern of IMD immunoreactivity persists in hypoxia. The general pattern of IMD immunofluorescence is not altered in lungs of mice housed for 15 h at 10% O2 (hypoxia) compared with normoxic controls. Bar, 20 μm.
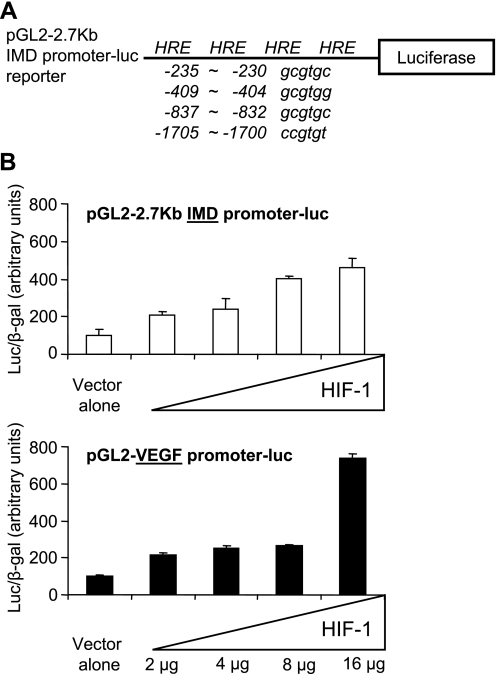
To investigate the molecular mechanisms underlying the regulation of IMD expression under hypoxic conditions, we retrieved IMD gene promoter sequences of human and mouse from the GenBank genome database and searched for the presence of putative hypoxia response elements (HRE) with a consensus 5′-RCGTG-3′ sequence (Fig. 4A). Consistent with the finding that IMD expression was increased in response to hypoxia, we found that the proximate region of the IMD gene promoter from both human and mouse contains multiple HRE sequences. In addition to sites for AP2, SP1, ERE, PR, HFH8, GATA1, and WT1 transcription factors (data not shown), there are at least four HRE sites within a 2.7-kb proximate region of the mouse IMD gene promoter fragment that we had subcloned previously (Fig. 4A) (9). To test our hypothesis that the stimulation of IMD expression by hypoxia is mediated by HIF-1α, we analyzed the effect of HIF-1α on IMD gene promoter activity, using a luciferase reporter construct, pGL2–2.7 kb IMD promoter-luc, in transfected HEK293T cells (Fig. 4A). Cotransfection of the pGL2–2.7 kb reporter with an increasing dose of a HIF-1α expression vector resulted in a dose-dependent increase of the luciferase activity in transfected cells (Fig. 4B, top), reminiscent of the increase of VEGF gene promoter activity by HIF-1α (Fig. 4B, bottom).
Fig. 4.
Transcriptional activation of the IMD gene promoter by hypoxia-inducible factor (HIF)-1α. A: localization of potential hypoxia response element (HRE) sequences present within the 2.7-kb 5′-proximate region of mouse IMD gene promoter. Positions of HRE sites in nucleotides upstream of the luciferase reporter are shown. B: relative luciferase activity of cells cotransfected with the pGL2–2.7 kb IMD-promoter luc reporter (top) or a VEGF-promoter luc reporter (bottom) and an increasing dose of a HIF-1α expression vector at 48 h after transfection. Data are shown as means ± SE of triplicate samples. Similar results were observed in 3 independent transfection experiments.
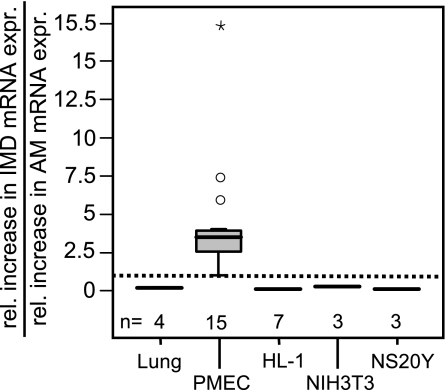
IMD expression exceeds that of AM in PMEC.
Comparison of hypoxia-induced IMD expression with that of AM showed that the increase in IMD expression in PMEC exceeded that of AM by ∼3.3-fold (Fig. 5). In contrast, hypoxia-induced increase in IMD mRNA expression in the lung was only 0.18-fold of that of AM mRNA expression. Likewise, analysis of HL-1 cardiomyocytes, NIH3T3 fibroblasts, and NS20Y neuroblastoma cells showed that hypoxia mainly induced AM expression and that hypoxia-induced increase in IMD mRNA expression was 0.1-fold, 0.26-fold, and 0.08-fold of that of AM mRNA expression, respectively.
Fig. 5.
Hypoxia-induced increase in IMD expression exceeds that of AM in PMEC. Hypoxia-induced IMD expression exceeded that of AM by ∼3.3-fold, whereas in the lung and all other cell types investigated, HL-1 cardiomyocytes, NIH3T3 fibroblasts, and NS20Y neuroblastoma cells, hypoxia-induced AM mRNA expression surpassed that of IMD.
IMD is a stabilizer of endothelial permeability.
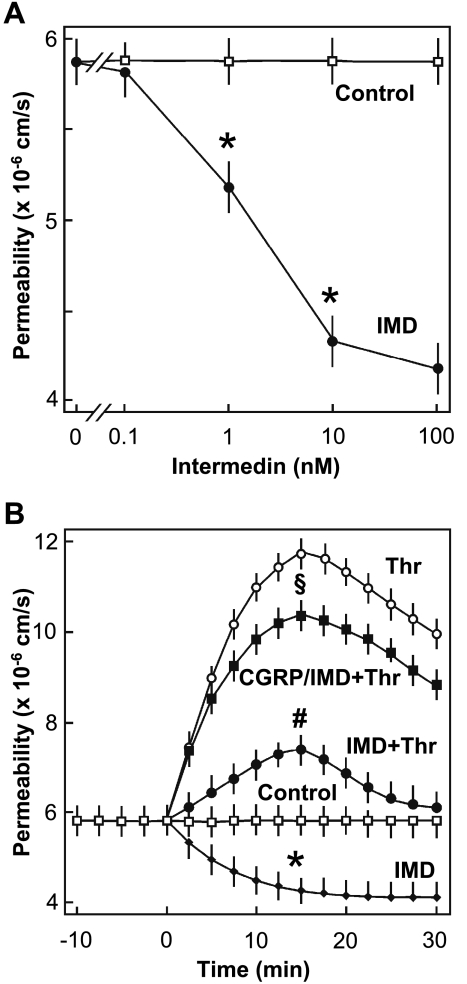
To analyze the IMD effect on endothelial barrier function, flux of Trypan blue-labeled albumin across monolayers of HMVEC-L was determined. As shown in Fig. 6A, IMD reduced macromolecular permeability in a concentration-dependent manner, with half-maximum effect at ∼1 nM and producing maximum effect at 10 nM, which was used for all further experiments. The reduction in macromolecular permeability obtained with 10 nM IMD was comparable with that evoked by forskolin (10 μM), a direct activator of adenylyl cyclase (data not shown). In the next step, we analyzed whether IMD can also antagonize thrombin-induced hyperpermeability. As shown in Fig. 6B, addition of thrombin (0.2 U/ml) caused a rapid increase in macromolecule permeability that peaked at 15 min. Addition of IMD (10 nM) together with thrombin blunted the thrombin-induced hyperpermeability response (Fig. 6B). This barrier-protective effect of IMD was abolished when endothelial cells were pretreated with a CRLR antagonist, α-CGRP8–37 (1 μM) (46), indicating that IMD mediates its barrier-protective effects via CRLR activation. The CRLR antagonist alone had no effect on endothelial macromolecule permeability (data not shown).
Fig. 6.
IMD reduces macromolecule permeability of human lung blood microvascular endothelial cell (HMVEC-L) monolayers. A: concentration-dependent reduction of macromolecule permeability by IMD. *P ≤ 0.05 vs. control. B: effect of IMD on thrombin-induced hyperpermeability. The following additions were made: none (□, Control), 0.2 U/ml thrombin (○, Thr), or 10 nM IMD (♦); cells were coincubated with 10 nM IMD and 0.2 U/ml Thr (●); or cells were preincubated with 1 μM α-calcitonin gene-related peptide (CGRP)8–37 (CGRP) for 10 min and then stimulated with 10 nM IMD and 0.2 U/ml Thr (□). #P ≤ 0.05, IMD + Thr vs. Thr alone; *P ≤ 0.05, IMD vs. control; §P ≤ 0.05, CGRP/IMD + Thr vs. IMD + Thr. Data are means ± SD of n = 3 experiments with 3 independent cell preparations. Differences in peak permeability between groups were analyzed by 1-way analysis of variance (ANOVA) followed by a Student-Newman-Keuls post hoc test.
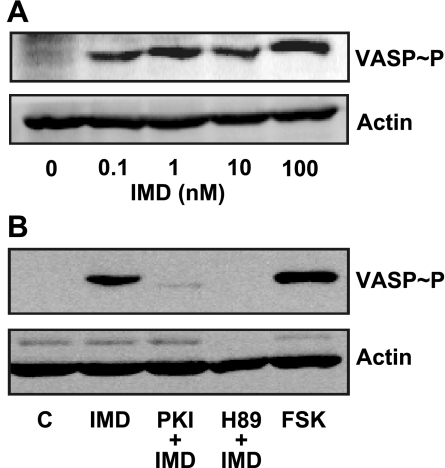
In other cell types IMD mediates most of its effects via activation of PKA pathway (33). Therefore, the activation of PKA in HMVEC-L was analyzed by detection of the phosphorylation state of VASP at Ser157. VASP is a cytoskeleton protein that is an established endogenous substrate for PKA (6, 11). Under basal conditions, the phosphorylation of VASP was below our detection limits in HMVEC-L. However, IMD induced VASP phosphorylation in a concentration-dependent manner, and 10 nM IMD was as effective as 10 μM forskolin (Fig. 7A). IMD-induced VASP phosphorylation was completely blocked by protein kinase inhibitor (PKI) and H-89, two chemically nonrelated PKA inhibitors (Fig. 7B), indicating activation of PKA by IMD in HMVEC-L.
Fig. 7.
IMD induces vasodilator-stimulated phosphoprotein (VASP) phosphorylation at Ser157 in HMVEC-L. Representative Western blots of VASP phosphorylation at Ser157; actin was used as loading control. A: cells were either incubated with increasing concentrations of IMD as indicated for 10 min or vehicle (0). B: cells were treated with 10 nM IMD, 200 μM protein kinase inhibitor (PKI) + IMD, 10 μM H-89 + IMD, forskolin (FSK; 10 μM), or vehicle (C; control).
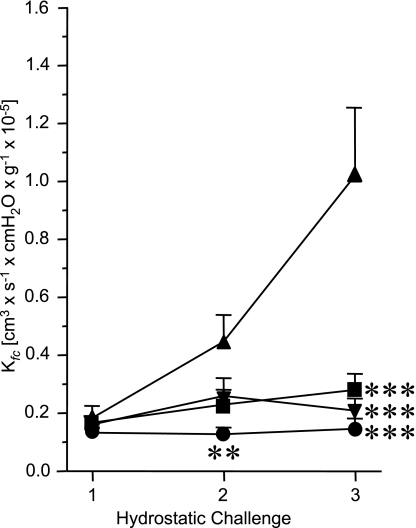
In addition to the in vitro experiments, we further investigated the effect of IMD on endothelial permeability in an isolated lung model (Fig. 8). Here endothelial permeability was increased by hydrostatic challenge, i.e., increase of LAP to 11.5 cmH2O (vs. 1.5 cmH2O in controls). During repetitive hydrostatic challenges, Kfc was measured as an index of endothelial permeability. In the absence of IMD, Kfc increased during two subsequent hydrostatic challenges from 0.2 × 10−5 to 1.0 × 10−5 cm3·s−1·cmH2O·g−1. Addition of IMD at a concentration of 10 nM 15 min before the pressure increase prevented the rise in Kfc. The effect of IMD was comparable to that exerted by dibutyryl cAMP (DBcAMP) at a concentration of 130 nM (Fig. 8).
Fig. 8.
IMD dampens pressure-induced increase in pulmonary endothelial permeability. Repetitive hydrostatic challenges were performed in isolated, perfused, and ventilated mouse lungs to quantify the capillary filtration coefficient (Kfc). Left atrial pressure (LAP) was continuously increased by 10 cmH2O (Control LAP increase, ▴) after the first hydrostatic challenge for Kfc determination. In IMD- and dibutyryl cAMP (DBcAMP)-treated lungs, these agents were added to the buffer fluid 15 min before the LAP increase (■, 10 nM IMD; ▾, 130 nM DBcAMP). For comparison, Kfc measurement from lungs not challenged with an LAP increase are shown (●, Control). Kfc data were analyzed by 1-way ANOVA with the Student-Newman-Keuls post hoc test. Data are means ± SE of n = 5 experiments each. ***P ≤ 0.001 and **P ≤ 0.01, significant difference vs. Control LAP increase.
DISCUSSION
The present study identifies IMD as a hypoxia-regulated pulmonary endothelial peptide stabilizing endothelial barrier function. IMD reduced basal and thrombin-induced endothelial hyperpermeability in vitro, and in isolated, perfused mouse lungs it decreased pressure-induced hyperpermeability and edema formation, pointing to a pivotal role of IMD in the regulation of endothelial cell function in the pulmonary microvasculature.
Our quantitative RT-PCR data demonstrate that hypoxia increases the level of IMD mRNA. Fully in line with these observations are our results from transfection studies in HEK293T cells, in which we show the existence of four potential HRE sequences. Coexpression of HIF-1α in HEK293T cells dose-dependently increases luciferase reporter activity of an IMD-promoter luciferase reporter, reminiscent of that of many HIF-1α-inducible genes (13, 34, 35). IMD shares ∼30% sequence homology to AM (33, 37), and for both peptides similar effects on blood pressure and heart rate and cardioprotective functions are described (15, 18, 39, 43). Even though there are structural and functional homologies between IMD and AM, the extent of hypoxia-induced mRNA expression differed for both peptides. In PMEC, hypoxia-induced IMD expression exceeded that of AM by about threefold, whereas in the other investigated cell types hypoxia-induced increase in AM surpassed that of IMD. This finding suggests that in the pulmonary microvasculature IMD rather than AM might be part of a protective regulatory mechanism involved in the adaptive response to hypoxia.
As shown by Western blot analysis IMD induced phosphorylation of VASP at Ser157, which is a well-established endogenous substrate of PKA in endothelial cells (6). PKA site-specific VASP phosphorylation was abolished by two chemically nonrelated inhibitors of PKA, indicating that IMD can activate PKA signaling pathway in HMVEC-L. Phosphorylation of VASP by PKA has been shown to play a role in the regulation of endothelial barrier function (11). However, analyzing the precise role of VASP phosphorylation in the barrier-protective effects of IMD was beyond the scope of this study. The in vitro macromolecule permeability assay clearly shows that IMD reduced the permeability in a concentration- and time-dependent manner. Accordingly, at a concentration of 10 nM, IMD was as effective as forskolin (10 μM) (21). Moreover, IMD also counteracted the stimulatory effect of thrombin on permeability, much in line with a recent study showing a stabilizing effect of IMD on hydrogen peroxide-induced reduction in transendothelial electrical resistance in cerebral endothelial cells (10). This is consistent with earlier studies showing that agonists activating adenylyl cyclase can antagonize thrombin-induced hyperpermeability (4). IMD-mediated barrier protection was abolished by a peptide antagonist of CRLR, which clearly demonstrates that IMD produces its effects via CRLRs. Together these data suggest that IMD protects endothelial barrier function against imminent failure via activation of PKA pathway involving CRLR.
The barrier-protective effect of IMD is further supported by our data obtained in an isolated, buffer-perfused lung model in which IMD substantially decreased pressure-induced endothelial permeability as measured by the alteration of Kfc. The effect of IMD on endothelial permeability matched that of DBcAMP, consistent with the concept that IMD acts on endothelial cells via the CRLR signaling cascade with stimulation of adenylyl cyclase and cAMP synthesis.
In the lung, hypoxia is closely associated with a fall in intracellular cAMP levels (38) and results in suppression of epi- and endothelial barrier function, leading to pulmonary edema (12, 45). Since IMD is upregulated by hypoxia and exerts profound stabilizing effects on endothelial barrier function both in cell culture and in the isolated, perfused lung, it might be part of a protective regulatory mechanism counteracting hypoxia-induced pulmonary edema.
In conclusion, the present data indicate a pivotal role of IMD in the regulation of endothelial cell function in the pulmonary microvasculature, where IMD is upregulated by hypoxia and serves as a stabilizer of endothelial permeability. This may be critical for improved treatment of patients with disturbances of this vascular segment as they occur in high-altitude pulmonary edema, acute respiratory distress syndrome, and sepsis.
GRANTS
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 547, projects A3, B7, and C1; Excellence Cluster Cardiopulmonary System) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-70652 (S. Y. T. Hsu).
ACKNOWLEDGMENTS
The skillful technical assistance of K. Michael is greatly appreciated.
REFERENCES
- 1.Abdelrahman AM, Pang CC. Vasodilator mechanism of intermedin/adrenomedullin-2 in anesthetized rats. Proc West Pharmacol Soc 50: 43–46, 2007 [PubMed] [Google Scholar]
- 2.Bell D, McDermott BJ. Intermedin (adrenomedullin-2): a novel counter-regulatory peptide in the cardiovascular and renal systems. Br J Pharmacol 153: 247–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg JT, Ramanathan S, Swenson ER. Inhibitors of hypoxic pulmonary vasoconstriction prevent high-altitude pulmonary edema in rats. Wilderness Environ Med 15: 32–37, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bogatcheva NV, Garcia JGN, Verin AD. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochemistry 67: 75–84, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Burak Kandilci H, Gumusel B, Wasermann A, Witriol N, Lippton H. Intermedin/adrenomedullin-2 dilates the rat pulmonary vascular bed: dependence on CGRP receptors and nitric oxide release. Peptides 27: 1390–1396, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem 269: 14509–14517, 1994 [PubMed] [Google Scholar]
- 7.Carpenter TC, Stenmark KR. Hypoxia decreases lung neprilysin expression and increases pulmonary vascular leak. Am J Physiol Lung Cell Mol Physiol 281: L941–L948, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Chakravarty P, Suthar TP, Coppock HA, Nicholl CG, Bloom SR, Legon S, Smith DM. CGRP and adrenomedullin binding correlates with transcript levels for calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPs) in rat tissues. Br J Pharmacol 130: 189–195, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CL, Roh J, Hsu SYT. Intermedin, a novel calcitonin family peptide that exists in teleosts as well as in mammals: a comparison with other calcitonin/intermedin family peptides in vertebrates. Peptides 25: 1633–1642, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Kis B, Hashimoto H, Busija DW, Takei Y, Yamashita H, Ueta Y. Adrenomedullin 2 protects rat cerebral endothelial cells from oxidative damage in vitro. Brain Res 1086: 42–49, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J 16: 583–585, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Dehler M, Zessin E, Bärtsch P, Maierbäuerl H. Hypoxia causes permeability oedema in the constant-pressure perfused rat lung. Eur Respir J 27: 600–606, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Forsythe JA, Bing-hua J, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujisawa Y, Nagai Y, Miyatake A, Miura K, Nishiyama A, Kimura S, Abe Y. Effects of adrenomedullin 2 on regional hemodynamics in conscious rats. Eur J Pharmacol 558: 128–132, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Fujisawa Y, Nagai Y, Miyatake A, Miura K, Shokoji T, Nishiyama A, Kimura S, Abe Y. Roles of adrenomedullin 2 in regulating the cardiovascular and sympathetic nervous system in conscious rats. Am J Physiol Heart Circ Physiol 290: H1120–H1127, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Garayoa M, Martinez A, Lee S, Pio R, An WG, Neckers L, Trepel J, Montuenga LM, Ryan H, Johnson R, Gassamnn M, Cuttitta F. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: a possible promotion mechanism of carcinogenesis. Mol Endocrinol 14: 848–862, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Halstead SB. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis 4: 830–839, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Hamid SA, Baxter GF. Adrenomedullin limits reperfusion injury in experimental myocardial infarction. Basic Res Cardiol 100: 387–396, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hippenstiel S, Witzenrath M, Schmeck B, Hocke A, Krisp M, Krull M, Seybold J, Seeger W, Rascher W, Schutte H, Suttrop N. Adrenomedullin reduces endothelial hyperpermeability. Circ Res 91: 618–625, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Hocke AC, Temmesfeld-Wollbrueck B, Schmeck B, Berger K, Frisch EM, Witzenrath M, Brell B, Suttrop N, Hippenstiel S. Perturbation of endothelial junction proteins by Staphylococcus aureus alpha-toxin: inhibition of endothelial gap formation by adrenomedullin. Histochem Cell Biol 126: 305–316, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol 23: 305–314, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadl A, Leitinger N. The role of endothelial cells in the resolution of acute inflammation. Antioxid Redox Signal 11–12: 1744–1754, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kandilci HB, Gumusel B, Lippton H. Intermedin/adrenomedullin-2 (IMD/AM2) relaxes rat main pulmonary arterial rings via cGMP-dependent pathway: role of nitric oxide and large conductance calcium-activated potassium channels (BKCa). Peptides 29: 1321–1328, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 25.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38: 1949–1971, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lips KS, Pfeil U, Reiners K, Rimasch C, Kuchelmeister K, Braun-Dullaeus RC, Haberberger RV, Schmidt R, Kummer W. Expression of the high-affinity choline transporter CHT1 in rat and human arteries. J Histochem Cytochem 51: 1645–1654, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Mairbäurl H. Role of alveolar epithelial sodium transport in high altitude pulmonary edema (HAPE). Respir Physiol Neurobiol 151: 178–191, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Noll T, Wozniak G, McCarson K, Hajimohammad A, Metzner HJ, Inserte J, Kummer W, Hehrlein FE, Piper HM. Effect of factor XIII on endothelial barrier function. J Exp Med 189: 1373–1382, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogunrinade O, Kameya GT, Truskey GA. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann Biomed Eng 30: 430–446, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Peters CJ, Zaki SR. Role of endothelium in viral hemorrhagic fevers. Crit Care Med 30: 268–273, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Pfeil U, Paddenberg R, Kummer W. Mitochondrial regulation of hypoxia-induced increase of adrenomedullin mRNA in HL-1 cells. Biochem Biophys Res Commun 343: 885–892, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Qing X, Svaren J, Keith IM. mRNA expression of novel CGRP1 receptors and their activity-modifying proteins in hypoxic rat lung. Am J Physiol Lung Cell Mol Physiol 280: L547–L554, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Roh J, Chang CL, Bhalla A, Klein C, Hsu SYT. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem 279: 7264–7274, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 269: 23757–23763, 1994 [PubMed] [Google Scholar]
- 35.Shih SC, Claffey KP. Hypoxia-mediated regulation of gene expression in mammalian cells. Int J Exp Pathol 79: 347–357, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szekanecz Z, Koch AE. Vascular endothelium and immune responses: implications for inflammation and angiogenesis. Rheum Dis Clin North Am 30: 97–114, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett 556: 53–58, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Taylor AE. Pulmonary edema: ischemia reperfusion endothelial injury and its reversal by c-AMP. Proc Natl Sci Counc Repub China B 15: 191–195, 1991 [PubMed] [Google Scholar]
- 39.Taylor MM, Bagley SL, Samson WK. Intermedin/adrenomedullin-2 acts within central nervous system to elevate blood pressure and inhibit food and water intake. Am J Physiol Regul Integr Comp Physiol 288: R919–R927, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Ten VS, Pinsky DJ. Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction. Curr Opin Crit Care 8: 242–250, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Weissmann N, Manz D, Buchspies D, Keller S, Mehling T, Voswinckel R, Quanz K, Ghofrani HA, Schermuly RT, Fink L. Congenital erythropoietin over-expression causes “anti-pulmonary hypertensive” structural and functional changes in mice, both in normoxia and hypoxia. Thromb Haemost 94: 630–638, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Weissmann N, Quanz K, Schermuly RT, Ghofrani HA, Fink L, Hänze J, Rose F, Seeger W, Grimminger F. Basic features of hypoxic pulmonary vasoconstriction in mice. Respir Physiol Neurobiol 139: 191–202, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Yang JH, Jia YX, Pan CS, Zhao J, Ouyang M, Yang J, Chang JK, Tang CS, Qi YF. Effects of intermedin(1–53) on cardiac function and ischemia/reperfusion injury in isolated rat hearts. Biochem Biophys Res Commun 327: 713–719, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Yang BC, Lippton H, Gumusel B, Hyman A, Metha JL. Adrenomedullin dilates rat pulmonary artery rings during hypoxia: role of nitric oxide and vasodilator prostaglandins. J Cardiovasc Pharmacol 28: 458–462, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Zhang SX, Miller JJ, Stolz DB, Serpero LD, Zhao W, Gozal D, Wang Y. Type I epithelial cells are the main target of whole-body hypoxic preconditioning in the lung. Am J Respir Cell Mol Biol 40: 332–339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmermann U, Fischer JA, Muff R. Adrenomedullin and calcitonin gene-related peptide interact with the same receptor in cultured human neuroblastoma SK-N-MC cells. Peptides 16: 421–424, 1995 [DOI] [PubMed] [Google Scholar]