Abstract
Excessive inflammation in cystic fibrosis (CF) lung disease is a contributor to progressive pulmonary decline. Effective and well-tolerated anti-inflammatory therapy may preserve lung function, thereby improving quality and length of life. In this paper, we assess the anti-inflammatory effects of the synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) in preclinical models of CF airway inflammation. In our experiments, mice carrying the R117H Cftr mutation have significantly reduced airway inflammatory responses to both LPS and flagellin when treated with CDDO before inflammatory challenge. Anti-inflammatory effects observed include reduced airway neutrophilia, reduced concentrations of proinflammatory cytokines and chemokines, and reduced weight loss. Our findings with the synthetic triterpenoids in multiple cell culture models of CF human airway epithelia agree with effects previously described in other disease models (e.g., neoplastic cells). These include the ability to reduce NF-κB activation while increasing nuclear factor erythroid-related factor 2 (Nrf2) activity. As these two signaling pathways appear to be pivotal in regulating the net inflammatory response in the CF airway, these compounds are a promising potential anti-inflammatory therapy for CF lung disease.
Keywords: anti-inflammatory therapy, antioxidant, nuclear factor-κB, nuclear factor erythroid-related factor 2, synthetic triterpenoid
in cystic fibrosis (CF), the abnormal airway surface environment causes retention of inhaled bacteria and initiates a perpetual cycle of airway obstruction, endobronchial infection, and exuberant inflammation. Pulmonary manifestations are the major cause of morbidity and mortality of CF (12) and are a primary focus of therapeutic intervention. Airway inflammation in CF begins early in life and is disproportionate to the inflammatory stimuli (e.g., infectious microbes; Refs. 2, 3, 5, 7, 8, 11, 18, 19, 21, 24, 30, 31). This inflammation, whether inherent to the CFTR defect (18, 34) or in response to early infections (11), becomes self-sustaining and maintains an acute neutrophil-dominated phenotype rather than converting to the mononuclear cell pattern exhibited in many chronic inflammatory conditions (18, 21). Neutrophilic activity is typically sequestered in the airways and prevents systemic spread of bacteria, but it also causes significant collateral damage to the normal tissue. The high concentrations of proteases and oxidants in the inflammatory milieu of the CF airway overcome the natural defenses, help perpetuate the neutrophilic immune response (7, 10, 16), and, over time, cause lung destruction, bronchiectasis, and progressive loss of pulmonary function, ultimately leading to an early death.
Current therapies for lung disease largely focus on bronchodilation, airway clearance, and combating infection. In addition to retained airway secretions and chronic endobronchial infection, most, but not all, studies of CF lung disease find excessive inflammatory responses in the airway. Both prospective and retrospective studies in humans demonstrate that anti-inflammatory therapies reduce the rate of decline in lung function in patients with CF (20, 22, 23, 26, 38), a measure closely correlated with survival. Therefore, judicious anti-inflammatory treatment that would mitigate the inflammatory response without impairing innate antimicrobial ability would likely benefit the majority of patients with CF. Concern over adverse effects has limited the use of current anti-inflammatory medications (e.g., corticosteroids, NSAIDs) and new treatment options are desired (32). The synthetic triterpenoids are highly potent derivatives of naturally occurring anti-inflammatory and anticarcinogenic compounds. These novel derivatives have been shown, in non-CF tissues, to limit activation of NF-κB, a pivotal transcriptional regulator for CF-related pulmonary inflammation (1, 39). Reduction in NF-κB activation is achieved by direct binding and inhibition of the IKK, thereby limiting the nuclear translocation of NF-κB (1, 39, 48). Interestingly, IKK activity is significantly higher in CF cells, compared with non-CF cells, which partially explains the mechanism of NF-κB overactivation (27, 44). Further activation of NF-κB is also driven by the increased oxidant load present in the CF lung (7, 37, 41, 42, 46). Reactive oxygen species (ROS) participate in normal intracellular signaling, including the activation of NF-κB (4, 28). This is pertinent since, besides directly reducing activation of NF-κB, synthetic triterpenoids also activate the nuclear factor erythroid-related factor 2 (Nrf2), a master transcriptional regulator of numerous antioxidant and cytoprotective genes (14, 47). This is achieved by direct binding and inhibition of KEAP1, the cytoplasmic inhibitor of Nrf2 nuclear translocation (47). Recently, we (9) have demonstrated impairment in Nrf2 activity in CF cells despite increases in steady-state intracellular peroxide, which normally increase Nrf2 activity. This work demonstrated that Nrf2 impairment in CF is linked to increased levels of intracellular peroxide and the production of proinflammatory mediators by a number of in vitro and in vivo epithelial cell models that lack CFTR function (9). Further studies revealed that increasing Nrf2 activity in CF cells returned elevated peroxide and cytokine levels to that seen in cells with functional CFTR (9).
It is well-established that synthetic triterpenoids reduce NF-κB activation while inducing Nrf2, two signaling events that are pivotal to the net inflammatory response in the CF airway. However, the biological significance of synthetic triterpenoid treatment in CF airway inflammation is not known. In this paper, we test the hypothesis that the synthetic triterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) can effectively suppress the inflammatory response in preclinical models of CF airway inflammation using human airway epithelial cells lacking CFTR and mice carrying the Cftr mutation R117H. Our data demonstrate, for the first time in models of CF, that these compounds impact both NF-κB and Nrf2 signaling with a net effect of reducing excessive cytokine production in cell and animal models and neutrophilic airway inflammation in animals.
MATERIALS AND METHODS
Reagents.
CDDO was the gift of the manufacturer (Reata Pharmaceuticals, Dallas, TX) in lyophilized powder and was dissolved in DMSO and diluted in PBS. Cells were transfected with two plasmids containing NF-κB-firefly luciferase and Renilla luciferase using the Lipofectamine 2000 kit (11668-027; Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Opti-MEM (Invitrogen) was the carrier fluid for drug exposure. Nrf2-antioxidant response element (ARE)-luciferase plasmid (Panomics, Fremont, CA) transfection used FuGENE HD (Roche Diagnostics, Indianapolis, IN). Recombinant human TNFα and IL-1β were purchased from the manufacturer R&D Systems (Minneapolis, MN). CFTRinh-172 (Sigma-Aldrich, St. Louis, MO) was used for chemical CFTR channel inhibition. LPS derived from Pseudomonas aeruginosa and purified by phenol extraction (L9143; Sigma-Aldrich) or flagellin purified from Bacillus subtilis (InvivoGen, San Diego, CA) was used as the inflammatory stimulus in animal studies. Dual-Luciferase Reporter assay systems (E1910; Promega, Madison, WI) were performed according to manufacturer's recommendations. IL-8 concentration released from cultured cells was measured using an ELISA kit (R&D Systems). The peroxide-sensitive fluorescent dye 10-acetyl-3,7-dihydroxyphenoxazine (Amplex UltraRed; A36006) was purchased from Invitrogen and used according to manufacturer's instructions. Cytokine concentrations from murine lung lavage samples were determined using LINCOplex Multiplex immunoassay kits (Linco Research, St. Charles, MO) and reagents from R&D Systems to generate standard curves. IL-1β from these samples was measured by ELISA, according to the manufacturer's recommendations (R&D Systems).
Cells.
Immortalized human bronchial epithelial (HBE) cells transfected with CFTR nucleotides 1–131 in the sense (16HBE14o−S) or antisense (16HBE14o−AS) configuration were grown as previously described (36) and were used for transfection studies. CFBE41o− human airway epithelial cells homozygous for the ΔF508 CFTR mutation as previously characterized (25) were grown as previously described (36). Primary human tracheal epithelial cells (HTE) recovered from necropsy specimens with approval from the University Hospitals of Cleveland Institutional Review Board (IRB exemption EM-03-01) were grown in an air-liquid interface (ALI) on semipermeable membranes (Costar; Corning, Corning, NY) as previously described (9, 17) for 3–4 wk before use to allow for tight junction formation and differentiation. For HTE cultures with CFTR inhibition, cells were grown at ALI as above and then switched to submerged culture (liquid-liquid interface; LLI) and treated with either 1:1,000 DMSO (vehicle control) or 20 μM CFTRinh-172, as described (33) and characterized (34) previously. CFTRinh-172 is a chemical inhibitor of the CFTR ion channel leading to rapid, reversible, and voltage-independent inhibition of ion transport. Stock solution was prepared in DMSO and diluted 1:1,000. CFTRinh-172 was added to both the basolateral and apical side and replenished in media every 24 h. Vehicle, 150 nM CDDO, or 300 nM CDDO was added to the apical surface during the last 24 h of CFTR inhibition before stimulation with TNFα. After 1-h TNFα exposure, cells were washed three times with HBSS, and growth media was replaced for 24 h, at which time, media from the basolateral surface was collected and frozen at −80°C until IL-8 was measured using an ELISA kit (R&D Systems).
NF-κB-luciferase and IL-8 promoter-luciferase reporter expression.
Cells were plated on multiwell plates to 95–100% confluence. Cells were cotransfected with NF-κB-firefly luciferase and a constitutive herpes simplex virus (HSV)-Renilla luciferase (controls for transfection efficiency). In separate experiments, an IL-8 promoter-luciferase reporter possessing a κB-responsive element was tested. Cells were exposed to variable concentrations of study drug (or vehicle) in Opti-MEM overnight and stimulated with recombinant TNFα for 30 min the following day to activate NF-κB. In initial experiments, 100 ng/ml TNFα was used for stimulation. However, we determined that little further stimulation is achieved by concentrations of TNFα above 10 ng/ml, so later experiments used 10 ng/ml. Cells were harvested and lysed, and luciferase activity was quantified using a microplate automated injection luminometer (Berthold Detection Systems, Oak Ridge, TN). Results are reported as the ratio of firefly luciferase (induced by NF-κB or IL-8 promoter activation) to Renilla luciferase to control for transfection efficiency between wells. Data are also corrected for protein concentrations. Experiments were performed at least three separate times, and each experiment involved multiple wells per treatment group.
Western blot for NF-κB-p65.
16HBE14o−AS cells were grown and treated with CDDO as above. One day after stimulation with TNFα-IL-1β for 1 h, nuclear and cytoplasmic extracts were prepared with a nuclear extraction kit (Panomics). Whole cell lysates were made with Complete Lysis Buffer-M supplemented with complete protease inhibitor cocktail minitablets (Roche Diagnostics). The extracts were run on 10% SDS-PAGE and blotted to nitrocellulose. Phosphorylated (phospho-) NF-κB p65 (Cell Signaling) were detected using the Cell Signaling Western blot protocol. Blots were blocked in 5% milk made in TBS-0.1% Tween 20 (TBS-T). The primary antibodies (1:1,000) were incubated in 5% BSA at 4°C overnight. The blots were washed in TBS-T and probed with anti-rabbit horseradish peroxidase (HRP) (Jackson ImmunoResearch) for phospho-NF-κB p65 (Jackson ImmunoResearch) at 1:20,000. Chemiluminescence was detected using the SuperSignal West Pico Chemiluminescent substrate (Thermo Scientific Pierce).
Nrf2-ARE-luciferase reporter expression.
Nrf2 activation was measured by firefly luciferase expression driven by the ARE promoter for glutathione S-transferase (GST; 5′-GCT CTT CCG GTG CTC TTC CGG TGC TCT TCC GGT GCT CTT CCG GT-3′). Cultured cells were grown to 60% confluence in 24-well plates and transfected as above, using Renilla-luciferase to control for transfection efficiency. One day before transfection, cells were treated with either 300 nM CDDO or vehicle control. All cells were washed, and normal growth media ± 300 nM CDDO was replaced overnight. Twenty-four hours after transfection, cells were harvested and lysed as above. Luciferase expression was quantified from 20-μl cell lysate samples and 100-μl luciferase assay reagent in a 96-well plate using a luminometer with 10-s measurement per well. Protein concentration was determined using the Bio-Rad DC protein assay per manufacturer's instructions. Data are expressed as the ratio of Nrf2 relative light units (RLU) to HSV-Renilla RLU per milligram of protein normalized to vehicle-treated cells.
NQO1 assay.
CFBE41o− cells were grown as above and treated with 150 nM CDDO every 24 h for 3 days. After removing media, cells were washed twice with PBS and scraped into 0.5-ml ice-cold sonication buffer (25 mM Tris·HCl, pH 7.4, 250 mM sucrose, 5 μM FAD) and sonicated for 10 s. Cells were then centrifuged at 4°C for 10 min at 10,000 rpm, and supernatants were collected and stored on ice. NAD(P)H:quinone oxidoreductase 1 (NQO1) measurement was performed as described by Ernster (15) and modified by Benson et al. (6) using 2,6-dichlorophenolindophenol (DCPIP) as a substrate. The reaction mix contained 25 mM Tris (pH 7.4), 40 μM DCPIP, 200 μM NADH, and 0.07 mg/ml BSA, with or without 20 μM dicumarol. NQO1 activity measured by spectrophotometry is represented by the dicumarol-inhibitable decrease in absorbance at 600 nm with DCPIP as a substrate. Measurement was recorded over 30 s. To ensure linear values, the first 5 s of each assay were ignored, and data are expressed in nanomoles of DCPIP reduced per minute per milligram of protein.
IL-8 secretion in primary cultures.
HTE cells grown at ALI as above were exposed to vehicle (n = 6 wells), 300 nM CDDO (n = 6 wells), or 1,000 nM CDDO (n = 3 wells) for 6 h, followed by 1-h stimulation with 100 ng/ml both TNFα and IL-1β on the apical surface. Cells were then washed with HBSS, and growth media was replaced for 6 h, at which time the basolateral media was collected and frozen at −80°C until quantified. To determine the need for preincubation with CDDO before TNFα/IL-1β exposure, HTE cells (n = 6 wells per group) were treated as follows: vehicle without TNFα/IL-1β, vehicle plus TNFα/IL-1β (100 ng/ml), 300 nM CDDO placed 15 h before stimulation with TNFα/IL-1β, 300 nM CDDO coadministered with TNFα/IL-1β, and 300 nM CDDO administered after stimulation with TNFα/IL-1β. TNFα/IL-1β was placed on the apical surface for 1 h, after which the apical surface was washed with HBSS, and growth media was replaced for 24 h before collecting the apical media for IL-8 assay as above.
Intracellular H2O2 measurement.
For assay of intracellular H2O2 content, HTE cells were grown and treated with CFTRinh-172 as above. Cells were treated with either 300 nM CDDO or vehicle control on both the apical and basolateral surfaces for 48 h before analysis. Sixteen hours before analysis, all cells were treated with 100 ng/ml TNFα and 50 ng/ml IL-1β on the apical surface. At time of analysis, cells were washed three times with HBSS and lysed with 1% MP20 detergent in HBSS for 1 h. For H2O2 quantification, 50 μl of cell lysate was mixed with 50 μl of working solution of 100 μM Amplex UltraRed reagent and 0.4 U/ml HRP and incubated at room temperature for 30 min protected from light. Standards and samples were analyzed for fluorescence at 544-nm excitation and 590-nm emission with 20-ms capture time.
Animals.
Thirty-two mice with the R117H CFTR mutation (B6.129S6-Cftrtm2Mrc) and 18 wild-type (WT) mice on the C57BL/6 genetic background were bred and housed in our Animal Research Core facility under specific pathogen-free conditions. R117H mice were selected as this is a human CF mutation and may avoid additional NF-κB activation caused by protein misfolding and the buildup of dysfunctional proteins in the endoplasmic reticulum (ER) that can occur with other mutations (e.g., ΔF508) (45). ER stress confounds measures of inflammatory signaling and oxidative stress attributable to CFTR dysfunction, which was our specific interest for these studies. Electrophysiological phenotype (i.e., nasal potential difference) and inflammatory responses in the lung to P. aeruginosa are very similar in R117H mice and multiple other CF transgenic mice, including mice bearing the ΔF508 or S489X mutation (40). After weaning, mice were fed a normal diet (P3000) until they were euthanized. Mice were ∼12-wk-old and weighed 20–30 g at the time of the experiment and were sex-matched for each experiment. The research protocol was approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
Intratracheal CDDO administration and inflammatory stimulation.
Three separate experiments were performed using a total of 32 CF mice and 18 WT mice, 12 wk of age, separated into CDDO and vehicle (control) groups. Daily weights were recorded. Mice were sedated on experimental days 1-4 using isoflurane inhalation. Once sedated, mice were placed on a tilting workstation (a3467; Hallowell, Pittsfield, MA), and the vocal cords were visualized with a small animal laryngoscope (22820, Welch Allyn, Skaneateles Falls, NY; LS-1, PennCentury, Philadelphia, PA) fitted with a magnifying loupe to improve visualization. The trachea was intubated with a MicroSprayer (PennCentury), and 25 μl of CDDO (10 μM) or vehicle (3% DMSO in PBS) was administered. The MicroSprayer is designed to administer quantifiable volumes of atomized liquid into the airways of rodents, simulating aerosol delivery. Mice were placed in microisolator cages for recovery. On experimental day 4, all mice were given an inflammatory stimulus intratracheally along with CDDO or vehicle. In separate experiments, the stimulus consisted of 1-μg LPS or 1-μg flagellin per mouse. Approximately 20 h after LPS or flagellin challenge, mice were killed using >70% carbon dioxide in air followed by exsanguination by direct cardiac puncture, in accordance with 2000 American Veterinary Medical Association Panel on Euthanasia Guidelines.
Specimen collection.
After mice were killed, bronchoalveolar lavage (BAL) was performed by intubating the trachea and instilling and withdrawing 2 ml of sterile pyrogen-free PBS divided into three aliquots. The BAL fluid was centrifuged, and the sterile filtered supernatant was processed for cytokine and chemokine quantification as described elsewhere (40). The cell pellets from BAL specimens were resuspended in sterile PBS and cytospun onto glass slides for microscopy to determine leukocyte differential as described elsewhere (40). Serum was obtained from separated blood samples for urea analysis.
Proteomic analysis of murine lung tissue and HBE.
Immediately after euthanasia and BAL sampling, lung tissue was separated, incubated in lysis buffer containing protease inhibitors, and frozen at −80°C. On thawing, the lungs were homogenized, and whole lung protein was extracted. One milligram of lung protein from each mouse was separated by two-dimensional gel electrophoresis (isoelectric point and size). Gels were imaged by a Bio-Rad GS-800 Calibrated Densitometer and analyzed with PDQuest software to identify protein bands differentially expressed in drug-treated mice compared with controls, focusing on the region of gels known to contain several proteins involved in redox control. Proteins bands were excised from the gels, and trypsin was digested and analyzed by liquid chromatography-tandem mass spectrometry for sequencing and identification. Three separate comparisons were made between CF mice treated with CDDO and vehicle. A list of proteins identified as being significantly different on at least two of three comparisons and known to be involved in control of oxidative stress was compiled. Separately, primary HTE were grown at an ALI and allowed to differentiate on semipermeable membranes. The apical surface of cells was exposed to either 300 nM CDDO or vehicle (3% DMSO in PBS) for 24 h, after which 5 ng/ml TNFα was added to the apical surface and cultures were incubated an additional 24 h. The medium was then removed, and 100-μl cell lysis buffer containing protease inhibitors was added. Cells were placed on an orbital rocker for 1 h and immediately frozen at −80°C. On thawing, cell protein was processed and analyzed as above, comparing four CDDO-treated cell groups with two vehicle-treated cell groups. Proteins for which expression increased with CDDO exposure in multiple comparisons were compiled.
Statistics.
Results from cell cultures and mice given CDDO or vehicle (control) were analyzed using unpaired t-tests in the SigmaStat 2.03 software, and graphs were generated with SigmaPlot 10.0. Cell culture results that failed normality tests were compared by Mann-Whitney rank sum. Results are shown as means ± SE. For cytokine data and H2O2 assay, values that fell below the limits of detection for the assay were assigned a value equal to the lower limit of detection. Statistical significance was defined as P < 0.05.
RESULTS
CDDO reduces NF-κB activation in human airway epithelia with and without CFTR function.
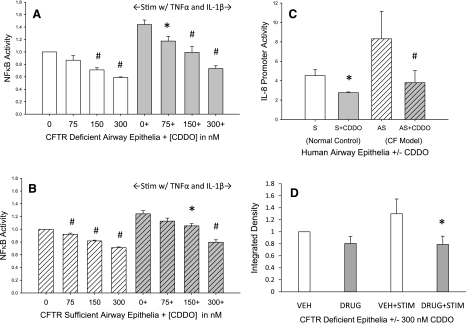
Increasing nanomolar concentrations of CDDO reduced NF-κB activation in both CF-like (Fig. 1A) and non-CF (Fig. 1B) human airway epithelial cells. This was also seen when using a separate luminescent-based reporter specific for IL-8 promoter activation, which contains a κB-responsive element (Fig. 1C). Immunoblotting for phospho-NF-κB p65 subunit in the nuclear fraction of epithelial cells demonstrated that CDDO reduces nuclear translocation in CF epithelial cells, particularly when challenged with TNFα/IL-1β. This is consistent with decreased NF-κB nuclear translocation and a subsequent decrease of transcriptional activation.
Fig. 1.
NF-κB activation in human airway epithelia. A: relative NF-κB activation in human airway epithelial cells lacking CFTR (16HBE14o−AS). B: relative NF-κB activation in human airway epithelial cells with functional CFTR (16HBE14o−S). C: IL-8 promoter activity in human airway epithelial cell with (S) and without (AS) CFTR. D: nuclear protein content of NF-κB phosphorylated p65 subunit in cystic fibrosis (CF)-like human airway epithelial cells (16HBE14o−AS). *P < 0.05; #P < 0.01. Performed in 3 separate experiments, n = 2–4 replicates per experiment. Stim, stimulation; CDDO, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid; VEH, vehicle.
Inhibition of NF-κB was not significantly greater with 1,000 nM CDDO, so 300 nM was used in subsequent experiments (Supplemental Fig. S1 available in the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site). Preincubation with CDDO was significantly more effective than either coadministration with TNFα/IL-1β (P < 0.05) or administration of drug after stimulation (P < 0.01) (Supplemental Fig. S2). Therefore, preincubation was used to test the anti-inflammatory effects of CDDO in our CF models.
CDDO increases Nrf2 activation and antioxidative capacity in airway epithelia.
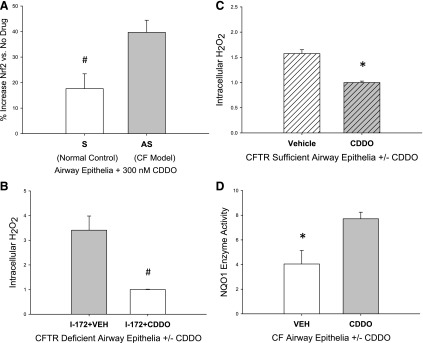
CDDO significantly increased Nrf2 transcription factor binding in both CF-like and non-CF human airway epithelial cell culture. This effect was greater in CF (40% increase at 300 nM CDDO) than in non-CF cells (20% increase) (Fig. 2A). Consistent with an antioxidative effect, primary HBE cells grown at ALI and treated with a CFTR channel inhibitor had significantly less intracellular H2O2 if also treated with 300 nM CDDO (Fig. 2B). This was also observed in cells not treated with the CFTR inhibitor (Fig. 2C). These data are consistent with an increase in Nrf2 transcription of antioxidant proteins for which functions include catalysis of peroxide to water and thus would decrease steady-state H2O2 levels. To test the activation of Nrf2 by an alternative approach, we examined the expression of NQO1, which is an antioxidant enzyme synthesized in response to Nrf2 activation. Using human airway epithelial cells homozygous for the ΔF508 CFTR mutation (CFBE41o−), we observe that 300 nM CDDO nearly doubles NQO1 enzymatic activity in these cells (Fig. 2D).
Fig. 2.
Nuclear factor erythroid-related factor 2 (Nrf2) activation in human airway epithelia. A: increased Nrf2 transcriptional activation above baseline (vehicle treatment) achieved by 300 nM CDDO exposure in human airway epithelial cells with (S) and without (AS) functional CFTR. Intracellular H2O2 concentration in human airway epithelia cells at air-liquid interface (ALI) with chemically inhibited CFTR (B) or functional CFTR (C). D: NAD(P)H:quinone oxidoreductase 1 (NQO1) activity in human airway epithelial cells homozygous for the ΔF508 CFTR mutation (CFBE41o−). *P < 0.05; #P < 0.01. Nrf2 reporter assays performed with 2 separate experiments, n = 6 replicates per experiment. H2O2 assay, n = 6 per condition. NQO1 assay, n = 4 per condition. I-172, CFTRinh-172.
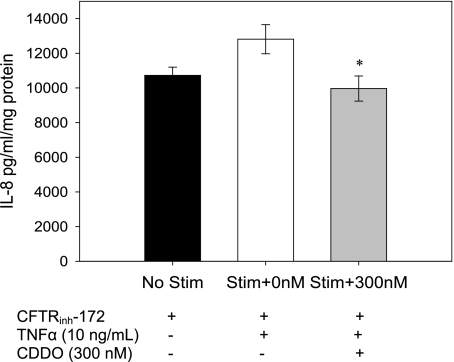
CDDO decreases IL-8 secretion in primary HTE cells grown at ALI with CFTR inhibition.
To further test the anti-inflammatory effect of CDDO on primary cell culture models of CF, CDDO was tested in HBE cells grown at ALI with CFTRinh-172, an inhibitor of the CFTR channel that has been shown to increase inflammatory markers in the cells (14). CDDO (300 nM) significantly reduced the secretion of IL-8 in response to TNFα (P < 0.05; Fig. 3), which is consistent with an ability to decrease activation of NF-κB in these cells.
Fig. 3.
IL-8 secretion in primary human airway epithelia IL-8 secretion from primary human airway epithelial cells at ALI treated with chemical CFTR inhibitor, with and without exposure to 300 nM CDDO before stimulation with TNFα. *P < 0.05. Performed in 3 separate experiments, total n = 7–9 replicates per condition.
Airway administration of CDDO reduces the inflammatory response to LPS in CF and WT mice.
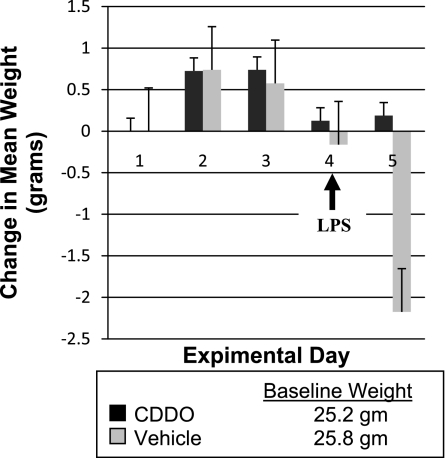
To test the effects of CDDO in vivo, WT and CF mice were treated with CDDO or vehicle for 4 days and then challenged with LPS. Our first outcome measure was whole body weight, a global measure of health. Average body weight did not significantly change during treatment with either CDDO or vehicle. After intratracheal LPS administration, mice treated with CDDO maintained their weight, whereas CF mice treated with vehicle lost 4–8% body wt and WT mice lost 5% body wt within 24 h (Fig. 4). These differences were statistically significant: CF mice (+0.2 vs. −7.9%; P < 0.001); WT mice (+0.7 vs. −4.9%; P = 0.005).
Fig. 4.
Mean daily weight of CF mice treated with CDDO or vehicle control before and after LPS challenge.
Neutrophil content of lung lavage after stimulation.
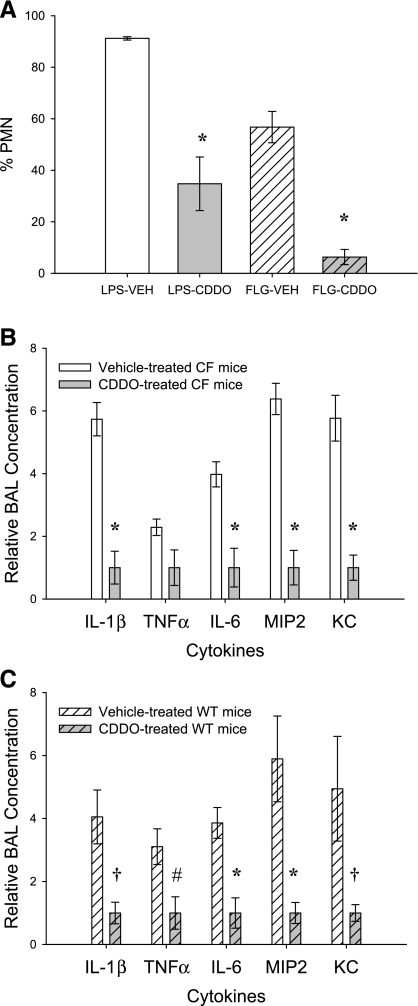
Next, we examined a hallmark of CF airway inflammation, increased neutrophil infiltration. CF and WT mice treated with intratracheal CDDO had a lower percentage of neutrophils in BAL after LPS or flagellin stimulation. CF mice treated with CDDO and stimulated with intratracheal LPS had 35% neutrophils compared with 91% in vehicle-treated animals (P < 0.001; Fig. 5). WT mice treated with CDDO and stimulated with intratracheal LPS had 47% neutrophils compared with 89% in vehicle-treated animals (P < 0.01).
Fig. 5.
Bronchoalveolar lavage (BAL) neutrophilia and proinflammatory mediators. A: neutrophil percentage of leukocytes in response to intratracheal LPS or flagellin in CF mice. *P < 0.001. Fold difference in the concentration of cytokines and chemokines in BAL of CF mice (B) and wild-type (WT) mice (C) in response to LPS stimulation are shown. *P < 0.001; †P < 0.01; #P < 0.05. PMN, polymorphonuclear neutrophils; FLG, flagellin; MIP-2, macrophage inflammatory protein-2; KC, keratinocyte-derived chemokine.
CDDO reduces the concentration of proinflammatory mediators in BAL.
To further examine the effect of CDDO on inflammatory signaling in vivo, we measured cytokines in BAL. IL-1β, IL-6, TNFα, and the murine analogs of IL-8 (MIP-2 and KC) were reduced by 56–83% in CF and WT mice given CDDO before intratracheal challenge with LPS compared with mice given vehicle and then LPS (Fig. 5, B and C). All cytokines tested were reduced with CDDO treatment, and all comparisons reached statistical significance except for TNFα in the CF mice (P = 0.058). These data are consistent with a decrease in the activation of proinflammatory transcription factors, such as NF-κB.
Effect of CDDO on pulmonary response to flagellin in CF mice.
Multiple stimuli play a role in CF pulmonary inflammation. Therefore, to determine whether CDDO affected other modes of stimulation, we challenged CF mice with intratracheal flagellin, a Toll-like receptor 5 (TLR-5) stimulus for epithelial cells. Weight loss was not observed in mice receiving CDDO or vehicle control during the 4 days of intratracheal administration, including the 20-h period after challenge with 1-μg flagellin. Total leukocyte counts in BAL fluid were modestly reduced in mice given CDDO compared with vehicle, but this was not statistically significant. However, the percentage of neutrophils and total neutrophils per milliliter of BAL fluid were significantly reduced with CDDO administration (57 vs. 6%, P < 0.001; 21.5 × 103 vs. 2.0 × 103, P < 0.001; Fig. 5). For flagellin-challenged mice, many cytokine and chemokine measures fell at or near the lower limits of detection for the assays in both groups, limiting statistical comparisons between CDDO and vehicle treatment. Measurable values for IL-1β were obtained for all mice and demonstrated a 74% reduction in CF mice treated with CDDO compared with mice treated with vehicle (P < 0.001). Therefore, flagellin was a considerably weaker inflammatory stimulus than LPS. Nevertheless, with both detectable markers of inflammation in this experiment (neutrophil content and IL-1β production), CDDO exhibited an anti-inflammatory effect when challenged with a TLR-5 ligand.
Proteomic analysis of CDDO effect on redox control.
To analyze the mode of action of CDDO in vivo and in vitro, we examined the activation of Nrf2, a previously reported effect of many synthetic triterpenoids. For a global analysis, we tested for the expression of a number of proteins transcribed by Nrf2. Purified protein from whole lung homogenates were compared from CF mice treated with CDDO or vehicle control. Two-dimensional SDS-PAGE analysis revealed an increase in the expression, by two or more fold, of a number of proteins promoted by Nrf2 activation in CF mice that received CDDO vs. those that received vehicle (Table 1). These data are consistent with the activation of Nrf2 following CDDO treatment. Identified proteins play a role in regulating the processing of H2O2 and were repeatedly identified in multiple separate comparisons. Identifications were made with a high degree of certainty based on protein coverage (the numbers of peptides for each protein sequenced by mass spectrometry, at least P < 0.001). Parallel experiments were performed with primary HBE cells grown at ALI after CDDO exposure and stimulation with TNFα (Table 1). Findings in human airway cell culture are consistent with the proteomic changes observed in vivo, indicating a specific effect for CDDO that occurs in humans as well as mice.
Table 1.
Redox proteins increased with CDDO exposure
| Protein Name | Fold Increase with CDDO Exposure | Average Sequence Coverage, % | Average Number of Peptide Ions |
|---|---|---|---|
| CF Mouse Lung | |||
| CuZn-superoxide dismutase | 2.47±0.14 | 54±9.3 | 21±2.3 |
| Glutathione S-transferase (μ1, μ2) | 3.56±0.35 | 64±11.0 | 19±1.7 |
| Peroxiredoxin-3 | 4.31±0.18 | 62±6.4 | 14±1.2 |
| Peroxiredoxin-5 | 2.41±0.36 | 34±6.9 | 9±1.7 |
| Peroxiredoxin-6 | 2.71±0.24 | 28±9.3 | 12±2.3 |
| Enolase 1 (α, nonneuron) | 2.47±0.12 | 43±7.5 | 14±4.1 |
| α1-Antiproteinase | 2.21±0.25 | 22±9.3 | 7±2.3 |
| Apolipoprotein A1 | 2.15±0.21 | 33±10.4 | 8±1.7 |
| Primary HBE cells | |||
| CuZn-superoxide dismutase | 3.14±0.26 | 68±4.6 | 23±1.2 |
| Glutathione S-transferase (π) | 3.74±0.20 | 52±6.4 | 17±2.3 |
| Peroxiredoxin-1 | 5.48±0.21 | 26±12.1 | 7±1.7 |
| Peroxiredoxin-6 | 5.24±0.37 | 56±11.0 | 15±2.3 |
| Transaldolase 1 | 3.84±0.16 | 10±95.5 | 3±1.2 |
| Dihydrodiol dehydrogenase type 1 | 3.13±0.33 | 13±3.5 | 4±0.6 |
Proteins involved in the control of oxidative stress that were identified on at least 2 of 3 separate comparisons of mice or primary human bronchial epithelial (HBE) cells treated with 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) (vs. vehicle) are shown. Confidence of protein identification, based on number of peptides and % sequence coverage in mass spectrometry analysis, is high for all listed (P < 0.00001). CF, cystic fibrosis.
DISCUSSION
We find that the synthetic triterpenoid CDDO has potent anti-inflammatory effects in preclinical models of CF airway inflammation. Although not specific for CF, these effects are prominent in systems with impaired CFTR function, indicating that this drug may have therapeutic utility in CF lung disease. Research has demonstrated that NF-κB and Nrf2 activation are primary targets of CDDO (1, 14, 39, 47, 48). Work by our group and others indicates that CF airway epithelial cells have a signaling imbalance, with increased proinflammatory (i.e., NF-κB) and reduced anti-inflammatory/antioxidative (i.e., Nrf2) signaling. This imbalance would be predicted to play a central role in the perpetual neutrophilic airway inflammation observed clinically. Indeed, correcting the Nrf2 impairment present in CF cells normalizes protective gene expression and components of the hyperinflammatory phenotype associated with CFTR dysfunction (9). Therefore, it is encouraging that, in our studies, CDDO reduced NF-κB and increased Nrf2 activity in multiple cell models of CF. CDDO decreased IL-8 release from primary HBE cells after inflammatory stimulus, limited NF-κB activation and IL-8 promoter activation in HBE cell lines, and reduced nuclear content of NF-κB p65. CDDO also increased Nrf2 promoter activation in CF-like and non-CF cells, increased the downstream product NQO1 in ΔF508 homozygous cells, and reduced intracellular H2O2 content in primary HBE cells with impaired CFTR.
In addition to characterizing the effect of CDDO in CF models, a primary aim of this research was to test the biological relevance of this synthetic triterpenoid in an in vivo CF model using mice carrying the R117H Cftr mutation. We find significant anti-inflammatory effects of CDDO in these mice when administered directly into the airway. These effects manifest as decreased airway neutrophilia, decreased airway concentrations of proinflammatory cytokines, and decreased weight loss-all markers relevant to human CF airway disease. Taken together with our studies in vitro, these findings serve as supportive evidence for future evaluation of synthetic triterpenoids in CF patients.
Closely related derivatives of CDDO have been formulated that are orally bioavailable. However, it is presently unknown whether systemic delivery, which might be more broadly acceptable to patients, would achieve similar anti-inflammatory effects. Also, the multiple derivatives of CDDO that have been developed will likely have variable effects on NF-κB and Nrf2 signaling, and preclinical studies in CF models of specific compounds proposed for clinical trials should be considered. Although there appears to be a general class effect of the triterpenoids on these two signaling pathways (NF-κB and Nrf2), other effects have been reported for certain derivatives of CDDO (e.g., inhibition of STAT signaling and promoting cell survival through the Akt pathway; Refs. 29, 35). Mechanisms not considered in our investigation make it possible that unanticipated effects could be observed in clinical trials. Likewise, the current mouse models of CF lung disease do not fully recapitulate the human condition and cannot always predict the response to novel therapeutics in clinical trials. However, the safety profile of these compounds in non-CF human trials has been excellent, and, based on our findings and the work of others studying CF-related inflammatory lung disease, we believe that this group of compounds is worthy of further evaluation as a potential new therapeutic approach (9, 13, 43).
GRANTS
Support from the National Institutes of Health Grants P30-DK27651 and 5R0173870 and the Cystic Fibrosis Foundation, including a Leroy Matthews Physician/Scientist Award for D. Nichols, is gratefully acknowledged. We also thank Reata Pharmaceuticals for departmental financial support through a contracted research agreement with Case Western Reserve University.
Supplementary Material
ACKNOWLEDGMENTS
We thank Raymond Rancourt and Shama Ahmad for advice and technical assistance with NQO1 in vitro assay.
Portions of this work appeared in poster and abstract form as follows: Nichols DP, Davis PB. Anti-inflammatory effects of synthetic triterpenoids in models of CF lung disease. ATS International Conference 2007, p. A933. Nichols D, Ziady AG, Shank S, Davis P. Proteomic analysis of the anti-inflammatory effects of CDDO in models of CF pulmonary disease. NACFC 2007, abstract 259. Pediatr Pulmonol Suppl 30: 293, 2007. Nichols DP, Ziady AG, Davis P. The anti-inflammatory effect of the synthetic triterpenoid CDDO is partly achieved through antioxidative mechanisms. NACFC 2008 abstract 320. Pediatr Pulmonol Suppl 31: 314, 2008.
REFERENCES
- 1.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem 281: 35764–35769, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DS, Grimwood K, Carlin JB, Carzino R, Gutièrrez JP, Hull J, Olinsky A, Phelan EM, Robertson CF, Phelan PD. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 156: 1197–1204, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DS, Grimwood K, Carzino R, Carlin JB, Olinsky A, Phelan PD. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 310: 1571–1572, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in toll-like receptor 4-dependent activation of NFκB. J Immunol 172: 2522–2529, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Balough K, McCubbin M, Weinberger M, Smits W, Ahrens R, Fick R. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol 20: 63–70, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Benson AM, Hunkler MJ, Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci USA 77: 5216–5220, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birrer P, McElvaney NG, Rüdeberg A, Sommer CW, Liechti-Gallati S, Kraemer R, Hubbard R, Crystal RG. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med 150: 207–213, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol 104: 72–78, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Kinter M, Shank S, Cotton C, Kelley TJ, Ziady AG. Dysfunction of Nrf-2 in CF epithelia leads to excess intracellular H2O2 and inflammatory cytokine production. PLoS One 3: e3367, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chmiel JF, Konstan MW. Anti-inflammatory medications for cystic fibrosis lung disease: selecting the most appropriate agent. Treat Respir Med 4: 255–273, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chmiel JF, Konstan MW, Berger M. The role of inflammation in the pathophysiology of CF lung disease. Clin Rev Allergy Immunol 23: 5–27, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Davis PB, Drumm M, Konstan MW. Cystic fibrosis: state of the art. Am J Respir Crit Care Med 154: 1229–1256, 1996 [DOI] [PubMed] [Google Scholar]
- 13.DiMango E, Ratner AJ, Bryan R, Tabibi S, Prince A. Activation of NF-κB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J Clin Invest 101: 2598–2605, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA 102: 4584–4589, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernster L. DT-diaphorase. Methods Enzymol 10: 309–317, 1967 [Google Scholar]
- 16.Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol 180: 5662–5669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol 188: 15–37, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 151: 1075–1082, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol 24: 137–142, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med 332: 848–854, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med 150: 448–454, 1994. [Erratum. Am J Respir Crit Care Med 151: 260, 1995.] [DOI] [PubMed] [Google Scholar]
- 22.Konstan MW, Schluchter MD, Storfer-Isser A, Davis PB. Use of ibuprofen for the treatment of airway inflammation in CF: an update. Pediatr Pulmonol Suppl 24: 164–165, 2002 [Google Scholar]
- 23.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med 176: 1084–1089, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konstan MW, Walenga RW, Hilliard KA, Hilliard JB. Leukotriene B4 is markedly elevated in the epithelial lining fluid of patients with cystic fibrosis. Am Rev Respir Dis 148: 896–901, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Kunzelmann K, Schwiebert EM, Zeitlin PL, Kuo WL, Stanton BA, Gruenert DC. An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the delta F508 CFTR mutation. Am J Respir Cell Mol Biol 8: 522–529, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Lands LC, Milner R, Cantin AM, Manson D, Corey M. High-dose ibuprofen in cystic fibrosis: Canadian safety and effectiveness trial. J Pediatr 151: 228–230, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Li J, Johnson XD, Iazvovskaia S, Tan A, Lin A, Hershenson MB. Signaling intermediates required for NF-κB activation and IL-8 expression in CF bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 284: L307–L315, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Engelhardt JF. Interleukin-1β induction of NFκB is partially regulated by H2O2-mediated activation of NFκB-inducing kinase. J Biol Chem 281: 1495–1505, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Liby K, Voong N, Williams CR, Risingsong R, Royce DB, Honda T, Gribble GW, Sport MB, Letterio JJ. The synthetic triterpenoid CDDO-Imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin Cancer Res 12: 4288–4293, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory response to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med 160: 186–191, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Noah TL, Black HR, Cheng PW, Wood RE, Leigh MW. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis 175: 638–647, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Oermann CM, Sockrider MM, Konstan MW. The use of anti-inflammatory medications in cystic fibrosis: trends and physician attitudes. Chest 115: 1053–1058, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, Verkman AS. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest 115: 2564–2571, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez A, Issler AC, Cotton CU, Kelley TJ, Verkman AS, Davis PB. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am J Physiol Lung Cell Mol Physiol 292: L383–L395, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Pitha-Rowe I, Liby KT, Royce D, Sporn MB. Synthetic triterpenoids attenuate cytotoxic retinal injury: crosstalk between Nrf2 and PI3K/AKT signaling through inhibition of the lipid phosphatase PTEN. Invest Ophthalmol Vis Sci . In press. [DOI] [PubMed] [Google Scholar]
- 36.Rajan S, Cacalano G, Bryan R, Ratner AJ, Sontich CU, van Heeckeren A, Davis PB, Prince A. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am J Respir Cell Mol Biol 23: 304–312, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Reid DW, Misso N, Aggarwal S, Thompson PJ, Walter EH. Oxidative stress and lipid-derived inflammatory mediators during acute exacerbations of cystic fibrosis. Respirology 12: 63–69, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Schluchter MD, Konstan MW, Xue L, Davis PB. Relationship between high-dose ibuprofen use and rate of decline in FEV1 among young patients with mild lung disease in the CFF Registry (Abstract). Pediatr Pulmonol Suppl 27: A385, 322, 2004 [Google Scholar]
- 39.Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res 12: 1828–1838, 2006 [DOI] [PubMed] [Google Scholar]
- 40.van Heeckeren AM, Schluchter MD, Drumm ML, Davis PB. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 287: L944–L952, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Velsor LW, Kariya C, Kachadourian R, Day BJ. Mitochondrial oxidative stress in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Respir Cell Mol Biol 35: 579–586, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velsor LW, van Heeckeren A, Day BJ. Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Physiol Lung Cell Mol Physiol 281: L31–L38, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Venkatakrishnan A, Stecenko A, King G, Blackwell TR, Brigham KL, Christman JW, Blackwell TS. Exaggerated activation of nuclear factor-κB and altered IκB-β processing in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol 23: 396–403, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Verhaeghe C, Remouchamps C, Hennuy B, Vanderplasschen A, Chariot A, Tabruyn SP, Oury C, Bours V. Role of IKK and ERK pathways in intrinsic inflammation of cystic fibrosis airways. Biochem Pharmacol 73: 1982–1994, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-κB in airway epithelial cells is dependent on CFTR trafficking and Cl− channel function. Am J Physiol Lung Cell Mol Physiol 281: L71–L78, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, Liu C, Clark JC, Whitsett JA. Functional genomic responses to cystic fibrosis transmembrane conductance regulator (CFTR) and CFTR(delta508) in the lung. J Biol Chem 281: 11279–11291, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, Gribble GW, Johnson DA, Johnson JA, Burton NC, Guilarte TR, Yamamoto M, Sport MB, Kensler TW. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther 6: 154–162, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl] imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther 5: 3232–3239, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.