Abstract
Ephrin family receptor tyrosine kinases are mediators of angiogenesis that may also regulate endothelial barrier function in the lung. Previous work has demonstrated that stimulation of EphA ephrin receptors causes increased vascular leak in the intact lung and increased permeability in cultured endothelial cells. Whether EphA receptors are involved in the permeability changes associated with lung injury is unknown. We studied this question in young rats exposed to viral respiratory infection combined with exposure to moderate hypoxia, a previously described lung injury model. We found that the EphA2 receptor is expressed in normal lung and that EphA2 expression is markedly upregulated in the lungs of hypoxic infected (HV) rats compared with normal control animals. Immunohistochemistry showed increased EphA2 expression principally in areas of edematous alveolar septae. In HV rats, EphA2 antagonism with either the soluble decoy receptor EphA2/Fc or with monoclonal anti-EphA2 antibody reduced albumin extravasation and histological evidence of edema formation (P < 0.01). Vascular leak in HV rats is mediated in large part by increased lung endothelin (ET) levels. In HV rats, ET receptor antagonism with bosentan resulted in reduced EphA2 mRNA and protein expression (P < 0.01). Experiments with cultured rat lung microvascular endothelial cells demonstrated that ET increases endothelial EphA2 expression. These results suggest that EphA2 expression is increased in lung injury, contributes to vascular leak in the injured lung, and is regulated in endothelial cells by ET. EphA2 may be a previously unrecognized contributor to the pathophysiology of lung injury.
Keywords: pulmonary edema, endothelin
pulmonary edema formation is a key feature of many respiratory illnesses. Alterations in pulmonary vascular permeability are a critical factor in the formation of pulmonary edema in the injured lung, but the mechanisms regulating permeability remain incompletely understood. Interestingly, of the three major families of receptor tyrosine kinases (RTKs) and ligands that have been described to control angiogenesis, members of two of these families, VEGFs and angiopoietins, have been implicated both in the control of vascular permeability and in the pathophysiology of human and experimental lung injuries, suggesting a possible link between angiogenic mediators and edema formation (14, 17).
The third major family of proangiogenic RTKs is the ephrin family. Ephrin receptors and ligands are divided into two broad classes, A and B, and ligands bind preferentially to receptors of the same class. Of these molecules, the EphA2 receptor is the most thoroughly studied with regard to its role in angiogenesis, and it has been implicated in responses such as endothelial cell migration and vascular assembly as well as regulation of epithelial cell junctions (2, 10). We (19) have recently reported that the activation of EphA receptors by soluble ephrin-a1 ligand can act both in vivo and in vitro to loosen pulmonary vascular endothelial junctions and to promote vascular protein extravasation in the lung. These considerations suggested to us that A class EphA receptors, and in particular EphA2, could modulate vascular permeability in the injured lung and thus might contribute to the pathophysiology of vascular leak in lung injury.
The combination of hypoxia and inflammation has been shown to promote lung injury and pulmonary edema formation. For example, we (6–8) have shown that exposure to hypoxia following a mild viral respiratory infection leads to increases in lung water and vascular protein leak triggered in large part by increased levels of endothelin (ET) and VEGF in the lung. Little is known about the regulation of EphA2 expression in the lung, although EphA2 is upregulated by hypoxia in skin, and ephrin-a1, the principal ligand for EphA2, is upregulated in endothelial cells by inflammatory stimuli such as TNF-α (21, 29). Given these previous findings, we hypothesized that EphA2 expression could be increased in the lung injured by viral infection and hypoxia and that ET might contribute to the change in EphA2 expression. We studied this hypothesis using both intact rats and cultured rat lung endothelial cells.
METHODS
Experimental animals.
Experimental lung injury due to recent viral respiratory infection combined with exposure to moderate hypoxia was generated as previously described (6, 8). In brief, pathogen-free weanling male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) were lightly anesthetized with halothane, and 50 μl of Sendai virus stock solution [5 × 105 50% tissue culture infective dose (TCID50)] was then instilled intranasally. Successful viral infection was confirmed by RT-PCR as follows (data not shown). Total lung RNA was isolated using a commercial kit (RNeasy Mini Kit; Qiagen). PCR amplification was done using a one-tube RT-PCR system (Invitrogen, Carlsbad, CA) with gene-specific primers designed from the published sequences of the Sendai virus HN and NP genes. Animals were considered positive for Sendai virus infection if either HN or NP RNA was detected in the lung. No animal in the uninfected test groups tested positive for either Sendai virus gene.
Animals were studied in two experimental groups: normal controls (C) and viral infection combined with hypoxic exposure (HV). Animals in the HV group were exposed to normobaric hypoxia [fraction of inspired oxygen (FiO2) = 0.1] for 24 h, beginning 6 days after viral infection. The effects of EphA2 in the lungs of both control and HV animals were antagonized using two approaches. One cohort of HV animals was injected subcutaneously with 300 μg/kg EphA2/Fc (R&D Systems, Minneapolis, MN) 2 h before exposure to hypoxia. As an alternate approach, another cohort of HV animals was injected subcutaneously with 100 μg/kg antibody directed against EphA2 (R&D Systems), also 2 h before hypoxic exposure. Control animals were injected with either EphA2/Fc or anti-EphA2 antibody 24 h before measurement of lung albumin extravasation. Additional studies of the effects of ET receptor antagonism using the dual ET receptor antagonist bosentan were performed as previously described (8). Studies of the effects of VEGF antagonism using the VEGF-Trap decoy receptor were also performed as previously described (7).
All animals were allowed free access to food and water and were subjected to a similar day and night light cycle. Animals were housed at Denver, CO, altitude (1,600 m) at all times. The University of Colorado Health Sciences Center Institutional Animal Care and Use Committee approved all procedures and animal use.
Measurement of vascular protein leak.
Leakage of albumin out of the vasculature into the distal lung was measured using Evans blue dye as previously described (8).
Immunohistochemical studies.
For immunohistochemical studies, lungs from normoxic control or HV animals were inflation-fixed via the trachea with zinc formalin at 20 cmH2O pressure for 18 h before paraffin-embedding and sectioning. Sections were probed with a rabbit polyclonal antibody for EphA2 (Santa Cruz Biotechnology, Santa Cruz, CA) and a commercial ABC-AP kit (Vector Laboratories, Burlingame, CA). For histological studies of edema formation, lungs were fixed by microwave treatment as previously described (5) and then paraffin-embedded and sectioned for standard hematoxylin and eosin staining.
Western blotting studies.
To determine whether the changes in lung albumin extravasation were associated with changes in lung EphA2 protein expression, lung homogenates were studied by standard Western blotting techniques using commercially available antibodies (EphA2, α-tubulin, Santa Cruz Biotechnology; β-actin, Sigma, St. Louis, MO). Membranes were reprobed for α-tubulin or β-actin to confirm equal protein loading and transfer. The resulting images were scanned into a computer and analyzed by densitometry using NIH ImageJ software. Results were expressed as arbitrary units, representing the ratio of target to α-tubulin or β-actin in each lane.
Real-time RT-PCR studies.
For studies of lung mRNA expression, frozen lung tissue was homogenized in TRI reagent (Sigma), and total RNA was isolated following the manufacturer's instructions. Reverse transcription to cDNA was accomplished by priming 5 μg of total RNA per sample with oligo(dT) and then using the SuperScript II Reverse Transcriptase kit (Invitrogen). Real-time PCR was performed using a Bio-Rad thermal cycler and PCR reactions including SYBR Green dye. Preliminary gradient optimization experiments (data not shown) for each primer set determined the ideal annealing temperature for future experiments. Results for target genes were normalized to α-tubulin mRNA expression for each sample, and relative expression was calculated using the comparative threshold cycle (ΔΔCT) method.
Cultured endothelial cells.
Rat lung microvascular endothelial cells (RLMVEC) were generously provided by the Center for Lung Biology at the University of South Alabama and propagated per their instructions. RLMVEC were serum-starved in media with 0.1% serum for 4 h before experimental exposures.
Statistical analysis.
Statistical analysis was performed using Prism 4.0 (GraphPad Software, Durham, NC). Multiple-group comparisons were made using a one-way ANOVA with Tukey posttesting. Two-group comparisons were made using an unpaired Student's t-test. Differences were considered statistically significant for P < 0.05. Results are expressed as means ± SE unless otherwise noted.
RESULTS
EphA2 is upregulated in the hypoxic infected lung.
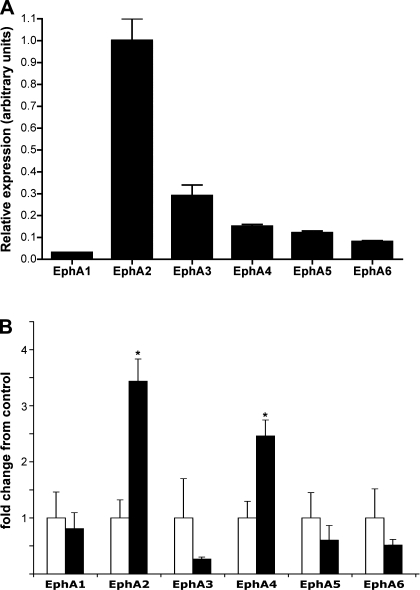
The expression of EphA receptors in the rat lung has not been extensively studied. To determine which EphA receptors are expressed in the normal rat lung, we performed real-time RT-PCR analysis of EphA receptor mRNA expression for those EphA receptors previously shown to be expressed in normal bovine lung tissue (19). As shown in Fig. 1A, mRNA for EphA1, EphA2, EphA3, EphA4, EphA5, and EphA6 were all detected in the normal rat lung. Normalized to the expression of α-tubulin mRNA, EphA2 was the most abundant EphA mRNA, exceeding the others by factors of 3- to 30-fold. We next used the same approach to determine whether the lung injury seen with combined exposure to viral respiratory infection and moderate hypoxia is associated with changes in lung EphA receptor mRNA expression. As seen in Fig. 1B, animals exposed to hypoxia and virus (HV) demonstrated significant increases in EphA2 and EphA4 mRNA expression compared with normal control animals (P = 0.003 and 0.01, respectively). EphA3, EphA5, and EphA6 mRNA expression all showed a trend toward reduced expression in the HV animals that did not achieve statistical significance.
Fig. 1.
A: relative mRNA expression of EphA receptors in normal rat lung. n = 4. B: changes in EphA receptor mRNA expression in hypoxic infected (HV) rat lung, expressed as fold change from normal control lung. Open bars = normal control, black bars = HV; n = 4. *P < 0.05 vs. normal control.
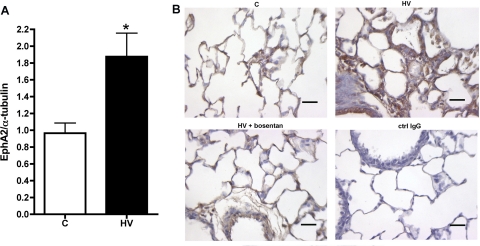
As EphA2 was highly expressed in the lung and showed large changes between the control and HV groups, we performed further studies of EphA2 protein expression. As shown in Fig. 2A, Western blotting studies also showed a significant increase in EphA2 protein expression in the lungs of HV animals (P = 0.01). To identify the anatomic compartment within the lung in which EphA2 expression increased in the HV animals, we performed immunohistochemical studies of lung tissue from control and HV animals. Lungs from control animals (Fig. 2B, bottom, left) showed generally uniform EphA2 protein expression in the airway epithelium as well as at lower levels in alveolar corners and around small pulmonary vessels. In the lungs of HV animals, patchy but marked increases in EphA2 immunoreactivity were found, most notably in areas characterized by thickened edematous-appearing alveolar septae (Fig. 2B, top, right). Taken together, these findings demonstrate a substantial increase in EphA2 expression in the distal lung parenchyma of HV animals.
Fig. 2.
A: increased EphA2 protein expression in HV lungs compared with normal control (C), shown by Western blot and densitometry of EphA2 bands normalized to α-tubulin. n = 5 per group; *P < 0.01 vs. normal controls. B: increased EphA2 immunostaining (brown) in distal lung of HV animals (top, right) compared with control (top, left). Scale bar = 50 μm. ctrl, Control.
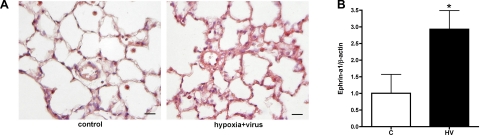
Ephrin-a1 is the principal ligand for EphA2 and is expressed in lung endothelium. To determine whether the expression of ephrin-a1 changes in the injured lung, we assessed ephrin-a1 protein expression by immunohistochemistry and Western blotting. Immunostaining for ephrin-a1 in normoxic control animals showed weakly detectable expression in distal lung parenchyma with somewhat brighter staining of alveolar macrophages. In contrast, HV animals showed heterogeneous staining for ephrin-a1 with areas of markedly increased expression most notable in those areas with thickened septae and evidence of inflammation (Fig. 3A). Western blotting of lung homogenates showed a similar pattern, with a statistically significant increase in lung ephrin-a1 protein in the HV animals (Fig. 3B). Thus protein levels of both EphA2 and its ligand ephrin-a1 are increased in the injured lung.
Fig. 3.
A: increased ephrin-a1 immunostaining (red) in distal lung of HV animals compared with normal controls (C). Scale bar = 50 μm. B: increased ephrin-a1 protein in lungs of HV animals compared with normal controls (C), shown by densitometry of Western blot. n = 5 per group; *P < 0.05 vs. normal controls.
EphA2 antagonism reduces vascular leak in the hypoxic infected lung.
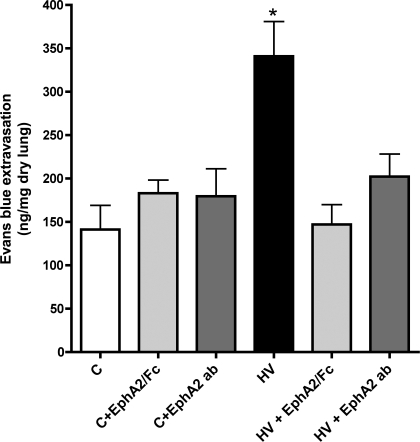
To determine whether the increased EphA2 expression in the HV animals contributes to the vascular leak seen in those animals, we injected HV animals before exposure to hypoxia with EphA2/Fc, a soluble chimeric molecule that acts as a decoy receptor and blocks activation of EphA receptors (1). Lung albumin extravasation was then measured as leak of Evans blue-labeled albumin into the distal lung. As shown in Fig. 4, and as we have previously reported, HV animals leak significantly more albumin into the lung than control animals (P = 0.001). HV animals receiving EphA2/Fc, however, demonstrated a marked reduction in albumin extravasation into the distal lung compared with untreated HV animals (P < 0.01). As a second and more specific method of antagonizing EphA2, additional HV animals were injected subcutaneously with a monoclonal antibody directed against EphA2 before exposure to hypoxia. As with EphA2/Fc, the anti-EphA2 antibody also resulted in a significant reduction in albumin extravasation compared with untreated HV animals (P < 0.05). To determine whether EphA2 antagonism has effects in the uninjured lung, we also injected normal control animals with EphA2/Fc or anti-EphA2 antibody (n = 4 each) and measured lung albumin extravasation. As shown in Fig. 4, neither method of EphA2 antagonism significantly altered lung albumin extravasation in the normal animals.
Fig. 4.
EphA2 antagonism with either EphA2/Fc or anti-EphA2 antibody (EphA2 ab) does not change lung Evans blue-labeled albumin extravasation in normal control rats (C) but significantly reduces albumin leak in HV rats. *P < 0.05 vs. all other groups.
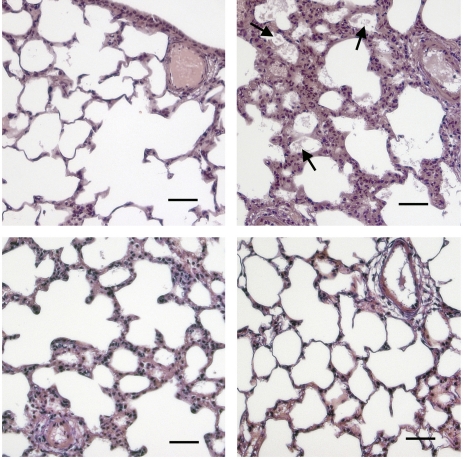
To determine whether these reductions in albumin extravasation correlated with histological evidence of pulmonary edema, lungs from HV animals receiving each method of EphA2 antagonism were fixed using a microwave fixation technique to better preserve edema fluid (5, 28). As shown in Fig. 5, lungs from HV animals showed patchy areas of alveolar flooding and septal thickening, which were not found in control animals. HV animals given EphA2/Fc or anti-EphA2 antibody showed no alveolar flooding and much reduced septal thickening compared with HV animals. Taken together, these results suggest not only that EphA2 expression is increased in the injured lung, but also that EphA2 is an important mediator of the vascular leak and edema formation seen.
Fig. 5.
Formation of alveolar edema in HV animals (top, right) compared with normal controls (top, left) is blocked by EphA2/Fc (bottom, left) or anti-EphA2 antibody (bottom, right), shown by hematoxylin and eosin staining of microwave-fixed lung tissue. Note proteinaceous flooding of alveoli in hypoxic infected lung (arrows). Scale bar = 50 μm.
ET upregulates EphA2 expression.
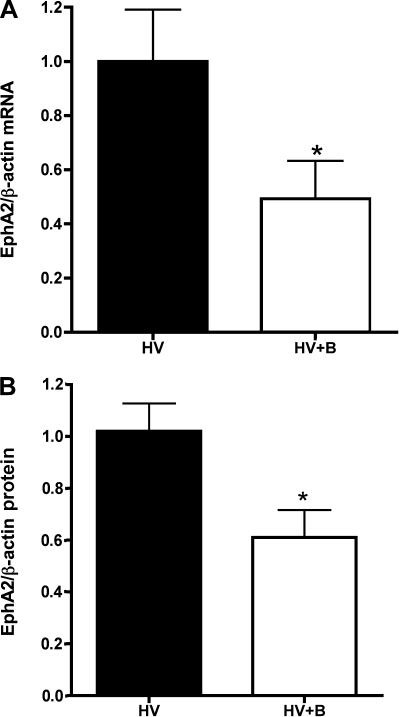
We (8) have previously demonstrated that ET contributes to the vascular leak seen in this model of lung injury and that ET antagonism with the dual ET receptor antagonist bosentan can reduce that leak. The finding that EphA2 also mediates leak in this model raises the question of whether ET regulates EphA2 expression in the hypoxic infected lung. To answer this question, we measured EphA2 expression in the lungs of HV animals given bosentan compared with the lungs of untreated HV animals. As shown in Fig. 6, both lung EphA2 mRNA expression and lung EphA2 protein expression were significantly lower in HV animals given bosentan than in untreated HV animals. Similarly, ET receptor blockade with bosentan reduced EphA2 immunostaining in the lungs of HV animals (Fig. 2, bottom, left). These results strongly suggest that ET regulates EphA2 expression in the injured lung.
Fig. 6.
A: reduced lung EphA2 mRNA expression in HV animals given bosentan (HV+B) compared with hypoxia and virus alone (HV). n = 4; P < 0.05. B: reduced lung EphA2 protein expression in HV animals given bosentan (HV+B) compared with hypoxia and virus alone (HV). n = 5; P < 0.03.
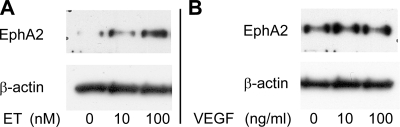
Given the near-ubiquitous expression of ET in the lung, EphA2 expression in many cell types in the lung could be regulated by ET. Changes in endothelial cell EphA2 expression, however, would be most likely to contribute to changes in vascular permeability, and we (19) have previously demonstrated that stimulation of lung endothelial EphA receptors increases endothelial permeability. To determine whether ET can directly alter endothelial cell EphA2 expression, cultured RLMVEC were treated with exogenous ET-1 at concentrations of 0, 10, and 100 nM for 24 h. As shown in Fig. 7A, ET-1 increased EphA2 expression in a dose-dependent manner in these cells.
Fig. 7.
In rat lung microvascular endothelial cells, exposure to endothelin (ET) for 24 h increases EphA2 protein (A), and exposure to VEGF for 24 h does not alter EphA2 expression (B). Blots are representative of 3 similar experiments.
Effect of VEGF on EphA2 expression.
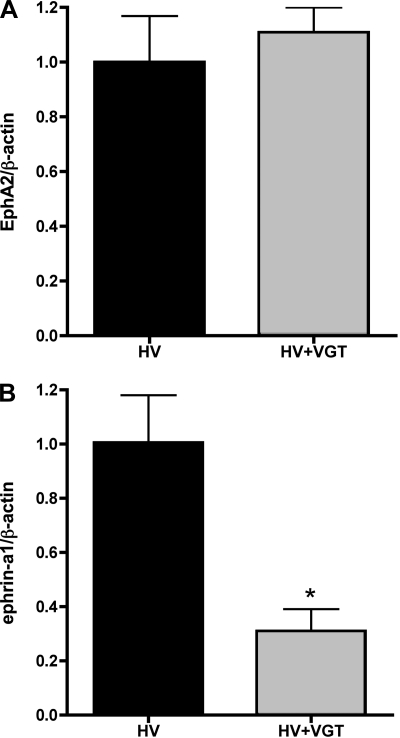
We (7) have also previously reported that ET acts in part via upregulation of VEGF to promote leak in this model, raising the question of whether VEGF regulates EphA2 expression as well. VEGF has not been previously described to regulate EphA2 expression, although VEGF has been shown to upregulate ephrin-a1 expression in endothelial cells (10). To determine whether VEGF contributes to the upregulation of EphA2 and ephrin-a1 in the HV rat lung, we compared EphA2 and ephrin-a1 expression in the lungs of HV animals with HV animals given VEGF-Trap, a soluble decoy VEGF receptor. Interestingly, lung EphA2 protein expression was not significantly changed in the HV/VEGF-Trap animals compared with HV alone (n = 6; P = 0.56), although ephrin-a1 protein was significantly reduced in the lungs of the animals that received VEGF-Trap (Fig. 8; P < 0.01). We also investigated the effect of VEGF on endothelial cell EphA2 expression (Fig. 7B). Cultured RLMVEC were exposed to exogenous VEGF at 0, 10, or 100 ng/ml for 24 h. Exposure to exogenous VEGF did not cause any change in endothelial cell EphA2 expression.
Fig. 8.
A: lung EphA2 protein expression does not change in HV animals given VEGF-Trap (HV+VGT) compared with HV alone (n = 6; P = 0.56). B: lung ephrin-a1 protein expression is reduced in HV animals given VEGF-Trap (HV+VGT) compared with HV alone (n = 6; *P < 0.01).
DISCUSSION
The major finding of these studies is that EphA2 receptor expression in the lung is upregulated in the setting of lung injury caused by viral infection combined with exposure to hypoxia. Importantly, we found that strategies to block EphA2 activation reduce markers of lung injury, including protein leak into the distal lung and histological evidence of edema formation. In addition, both in vivo and in vitro data suggest that elevated ET levels in the injured lung are a major regulator of EphA2 expression in the lung endothelium. These results are the first evidence that ephrin receptors in general and specifically EphA2 may be important mediators of vascular permeability in the injured lung and may represent viable targets for efforts to reduce the effects of injury in the lung.
Ephrins are a large class of RTKs and ligands with clearly demonstrated roles in neural and vascular development. EphA2, in particular, is known to be expressed in pulmonary vascular endothelial cells and has been implicated in postnatal angiogenic responses in the lung (2, 3, 10). We (19) have recently reported that ligand stimulation of lung endothelial EphA receptors leads to disruption of endothelial adherens and tight junctions and to increases in endothelial permeability in vitro and to increases in lung albumin extravasation in vivo. Although no previous data exist linking ephrins to lung injury, the finding that lung endothelial EphA receptors modulate permeability suggested to us that EphA2, in particular, as the most highly expressed EphA receptor in the lung vasculature, could be involved in the pathogenesis of lung injury.
We (6, 8) investigated that hypothesis using a previously described animal model of mild viral respiratory infection combined with exposure to moderate hypoxia. In lungs injured by exposure to viral infection and hypoxia, we found marked increases in both mRNA and protein expression of EphA2. As judged by immunohistochemistry, these increases occurred most prominently in edematous appearing areas of lung characterized by thickened alveolar septae. More importantly, our finding that antagonism of EphA2 activation in HV animals led to reduced lung albumin leak and reduced histological evidence of edema formation is the first evidence that EphA2 contributes to the pathophysiology of lung injury. Given previous findings that EphA2 is expressed on lung microvascular endothelial cells and that stimulation with ephrin-a1 ligand leads to alterations of endothelial tight and adherens junctions, alterations in endothelial permeability are one likely mechanism for the effects of EphA2 in the injured lung, although other mechanisms are certainly possible. For instance, type 2 alveolar epithelial cells reportedly express EphA2 in culture, and the role of EphA2 in those cells is not known (23). EphA2 is expressed, however, by other epithelial cell types and regulates cell-cell junctions in those cells (13), raising the possibility that EphA2 might also regulate epithelial permeability in the injured lung.
To determine whether the increase in lung EphA2 expression contributed to the vascular leak seen in our experimental lung injury, we used two strategies to antagonize EphA2 signaling. Antagonism of ephrin signaling is complicated by the known signaling mechanisms and promiscuous binding of ephrin receptors and ligands. For example, the effect of EphA2/Fc as an antagonist has several possible mechanisms of action. By acting as a decoy receptor and binding available ephrin-a ligands, EphA2/Fc would be expected to block activation and forward signaling not just through EphA2 but also through other EphA receptors. In addition, ephrin-a ligands can exhibit “reverse signaling,” a phenomenon in which a signal is transduced into the ligand-bearing cell following Eph receptor binding. A soluble decoy receptor compound such as EphA2/Fc could conceivably then activate reverse signaling via ephrin ligands in the injured lung, although the extent and effects of such signals in endothelial cells have not been described to date. To address this uncertainty, we used a second mechanism of EphA2 antagonism, a monoclonal antibody directed against EphA2 itself. The fact that both methods resulted in a significant reduction in albumin extravasation in the HV animals suggests strongly that EphA2 forward signaling leads to increased endothelial permeability and that blockade of that signaling reduces edema formation in the injured lung.
The regulation of EphA2 expression remains poorly understood, although some data suggest that EphA2 may be upregulated during inflammatory responses (15), and both Hox and HIF family transcription factors can regulate EphA2 expression at a transcriptional level in tissues outside the lung (9, 29). ET is a potent vasoactive mediator that has been implicated in lung injury and pulmonary edema formation in many settings, including in the hypoxic infected lung (12, 18, 20). Recent evidence also demonstrates that ET can be an important mediator of postnatal angiogenesis (11, 16, 24). We found that ET antagonism reduces EphA2 expression in the injured lung and that ET directly regulates EphA2 expression in cultured lung microvascular endothelial cells. These results are the first evidence that ET regulates EphA2 expression. Although the exact signaling and transcriptional mechanisms underlying these effects remain to be determined in future studies, these findings suggest that ET-mediated regulation of ephrin expression may be an important component of the angiogenic effects of ET, including increases in vascular permeability in the injured lung.
Previous work in a variety of settings, again including the viral infection and hypoxia lung injury model, has also demonstrated that ET can upregulate VEGF expression (7, 22, 27), raising the question of whether ET acts via VEGF to upregulate EphA2. VEGF has not been previously reported to regulate EphA2, however, and we were not able to demonstrate any effect of VEGF on EphA2 expression in cultured RLMVEC. Consistent with those results, in HV rats, VEGF antagonism did not significantly change lung EphA2 protein expression. In contrast, we did find that VEGF antagonism reduced lung ephrin-a1 expression, which is consistent with previous reports that VEGF upregulates ephrin-a1 in endothelial cells (10). These results suggest that ET increases both VEGF and EphA2 expression in the lung and that VEGF (and likely other mediators), in turn, increases ephrin-a1 expression, leading to upregulation of both EphA2 and its ligand in settings characterized by excess ET and VEGF. The source of the increased ephrin-a1 in the injured lung remains uncertain, although ephrin-a1 can be expressed not only by endothelial cells, but also by monocytes, lymphocytes, and platelets (4, 25, 26).
This study has several important limitations. For clinically significant alveolar flooding to occur, both the endothelial and epithelial barriers must be breached. In these experiments, we have focused on the effects of EphA2 on the endothelial barrier. As discussed above, these studies do not rule out possible effects of EphA2 antagonism on the alveolar epithelial barrier. In addition, changes in vascular pressures can generate hydrostatic forces favoring edema formation. Although these studies have certainly not ruled out an effect of EphA2 antagonism on pulmonary hemodynamics, the combination of previous results showing that ephrin-a1 stimulation can increase endothelial permeability (19) and our present findings is highly suggestive that EphA2 antagonism does have a protective effect on the endothelial barrier in the injured lung.
In summary then, we have demonstrated that EphA2 is upregulated in lung injury caused by viral infection combined with hypoxia and that antagonism of EphA2 ameliorates pulmonary edema formation in that model. Intriguingly, the upregulation of EphA2 appears to be mediated in large part by the effects of ET, and thus prevention of increases in EphA2 expression may partially account for the benefit of ET receptor antagonists in this model of lung injury. The role of EphA receptors and ligands in the injured lung is a promising area deserving of further investigation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01-HL-077743 (T. C. Carpenter).
REFERENCES
- 1.Brantley DM, Cheng N, Thompson EJ, Lin Q, Brekken RA, Thorpe PE, Muraoka RS, Cerretti DP, Pozzi A, Jackson D, Lin C, Chen J. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene 21: 7011–7026, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci 117: 2037–2049, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Brantley-Sieders DM, Fang WB, Hicks DJ, Zhuang G, Shyr Y, Chen J. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J 19: 1884–1886, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Brass LF, Zhu L, Stalker TJ. Novel therapeutic targets at the platelet vascular interface. Arterioscler Thromb Vasc Biol 28: s43–s50, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Carpenter T, Schomberg S, Steudel W, Ozimek J, Colvin K, Stenmark K, Ivy DD. Endothelin B receptor deficiency predisposes to pulmonary edema formation via increased lung vascular endothelial cell growth factor expression. Circ Res 93: 456–463, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Carpenter TC, Reeves JT, Durmowicz AG. Viral respiratory infection increases susceptibility of young rats to hypoxia-induced pulmonary edema. J Appl Physiol 84: 1048–1054, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Carpenter TC, Schomberg S, Stenmark KR. Endothelin-mediated increases in lung VEGF content promote vascular leak in young rats exposed to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol 289: L1075–L1082, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Carpenter TC, Stenmark KR. Endothelin receptor blockade decreases lung water in young rats exposed to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol 279: L547–L554, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Ruley HE. An enhancer element in the EphA2 (Eck) gene sufficient for rhombomere-specific expression is activated by HOXA1 and HOXB1 homeobox proteins. J Biol Chem 273: 24670–24675, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Cheng N, Brantley DM, Liu H, Lin Q, Enriquez M, Gale N, Yancopoulos G, Cerretti DP, Daniel TO, Chen J. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol Cancer Res 1: 2–11, 2002 [PubMed] [Google Scholar]
- 11.Daher Z, Noël J, Claing A. Endothelin-1 promotes migration of endothelial cells through the activation of ARF6 and the regulation of FAK activity. Cell Signal 20: 2256–2265, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Donaubauer B, Busch T, Lachmann R, Deja M, Petersen B, Francis R, Träger A, Ebsen M, Boemke W, Kaisers U. Low-dose inhalation of an endothelin-A receptor antagonist in experimental acute lung injury: ET-1 plasma concentration and pulmonary inflammation. Exp Biol Med (Maywood) 231: 960–966, 2006 [PubMed] [Google Scholar]
- 13.Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, Chen J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci 121: 358–368, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto T, Pittet JF. Angiopoietin-2: modulator of vascular permeability in acute lung injury? PLoS Med 3: e113, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov AI, Steiner AA, Scheck AC, Romanovsky AA. Expression of Eph receptors and their ligands, ephrins, during lipopolysaccharide fever in rats. Physiol Genomics 21: 152–160, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Knowles J, Loizidou M, Taylor I. Endothelin-1 and angiogenesis in cancer. Curr Vasc Pharmacol 3: 309–314, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kosmidou I, Karmpaliotis D, Kirtane AJ, Barron HV, Gibson CM. Vascular endothelial growth factors in pulmonary edema: an update. J Thromb Thrombolysis 25: 259–264, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kuzkov VV, Kirov MY, Sovershaev MA, Kuklin VN, Suborov EV, Waerhaug K, Bjertnaes LJ. Extravascular lung water determined with single transpulmonary thermodilution correlates with the severity of sepsis-induced acute lung injury. Crit Care Med 34: 1647–1653, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Larson J, Schomberg S, Schroeder W, Carpenter TC. Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol 295: L431–L439, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano Y, Tasaka S, Saito F, Yamada W, Shiraishi Y, Ogawa Y, Koh H, Hasegawa N, Fujishima S, Hashimoto S, Ishizaka A. Endothelin-1 level in epithelial lining fluid of patients with acute respiratory distress syndrome. Respirology 12: 740–743, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science 268: 567–569, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Pedram A, Razandi M, Hu RM, Levin ER. Vasoactive peptides modulate vascular endothelial cell growth factor production and endothelial cell proliferation and invasion. J Biol Chem 272: 17097–17103, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Reddy NM, Kleeberger SR, Yamamoto M, Kensler TW, Scollick C, Biswal S, Reddy SP. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol Genomics 32: 74–81, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Ribatti D, Conconi MT, Nussdorfer GG. Nonclassic endogenous novel [corrected] regulators of angiogenesis. Pharmacol Rev 59: 185–205, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Sharfe N, Nikolic M, Cimpeon L, Van De Kratts A, Freywald A, Roifman CM. EphA and ephrin-A proteins regulate integrin-mediated T lymphocyte interactions. Mol Immunol 45: 1208–1220, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Sobel RA. Ephrin A receptors and ligands in lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol 15: 35–45, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spinella F, Rosanò L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem 277: 27850–27855, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Turner CR, Zuczek S, Knudsen DJ, Wheeldon EB. Microwave fixation of the lung. Stain Technol 65: 95–101, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Vihanto MM, Plock J, Erni D, Frey BM, Frey FJ, Huynh-Do U. Hypoxia up-regulates expression of Eph receptors and ephrins in mouse skin. FASEB J 19: 1689–1691, 2005 [DOI] [PubMed] [Google Scholar]