Abstract
Inner ear damage may lead to structural changes in the central auditory system. In rat and chinchilla, cochlear ablation and noise trauma result in fiber growth and synaptogenesis in the ventral cochlear nucleus (VCN). In this study, we documented the relationship between carboplatin induced hair cell degeneration and VCN plasticity in the chinchilla. Unilateral application of carboplatin (5 mg/ml) on the round window membrane resulted in massive hair cell loss. Outer hair cell degeneration showed a pronounced basal-to-apical gradient while inner hair cell loss was more equally distributed throughout the cochlea. Expression of the growth associated protein GAP-43, a well-established marker for synaptic plasticity, was up-regulated in the ipsilateral VCN at 15 and 31 days post-carboplatin, but not at 3 and 7 days. In contrast, the dorsal cochlear nucleus showed only little change. In VCN, the high-frequency area dorsally showed slightly yet significantly stronger GAP-43 up-regulation than the low-frequency area ventrally, possibly reflecting the high-to-low frequency gradient of hair cell degeneration. Synaptic modification or formation of new synapses may be a homeostatic process to re-adjust mismatched inputs from two ears. Alternatively, massive fiber growth may represent a deleterious process causing central hyperactivity that leads to loudness recruitment or tinnitus.
Keywords: carboplatin, GAP-43, ventral cochlear nucleus, chinchilla, hair cells
Introduction
Cochlear ablation or auditory nerve transection leads to degeneration of the auditory nerve (Wenthold and Gulley, 1977; Hoeffding and Feldman, 1988) and deafferentation of neurons in the cochlear nucleus. In addition to this degenerative response, potentially important, but less well understood regenerative processes have been observed in the cochlear nucleus after cochlear damage. Several studies have shown that cochlear ablation or noise trauma results in pronounced growth of fibers and presynaptic endings in the ventral cochlear nucleus (VCN) of the rat (Illing et al., 1997; Michler and Illing, 2002) and chinchilla (Bilak et al., 1997; Benson et al., 1997; Muly et al., 2002; Kim et al., 2004). The regenerative processes generally emerged after the degenerative process had subsided, in some cases 6-8 months following cochlear damage (Kim et al., 2004;).
The growth associated protein 43 (GAP-43) is a well-established marker for axonal outgrowth, synaptogenesis and synaptic remodeling (Benowitz and Routtenberg, 1997). It is a membrane-associated phospho-protein located in axonal growth cones (de Graan et al., 1985) and is associated with growth cone function (Baetge and Hammang, 1991; Meiri et al., 1998). During neurite outgrowth and early stages of synaptogenesis, GAP-43 is produced at high levels (Skene and Willard, 1981; Mahalik et al., 1992). It is widely expressed in early ontogeny but is down-regulated with maturation in most neurons (Skene, 1989; Benowitz and Perrone-Bizzozero, 1991). Axotomy reinduces GAP-43 synthesis in several neuronal systems of the adult mammalian brain (Woolf et al., 1990; Linda et al., 1992; Verhaagen et al., 1993; Palacios et al., 1994; Schaden et al., 1994; Liabotis and Schreyer, 1995; Elliott et al., 1997). In rats, hearing loss caused by cochlear ablation or noise trauma results in an up-regulation of GAP-43 in fibers and presynaptic endings in VCN (Illing et al., 1997; Michler and Illing, 2002). Additionally, expression of GAP-43 emerged in cell bodies of lateral olivocochlear neurons (LOC neurons) in the lateral superior olive (LSO) after cochleotomy (Illing et al., 1997; Illing et al., 1999) as well as after noise trauma (Michler and Illing, 2002).
The platinum based anticancer drug carboplatin has been shown to be cytotoxic to hair cells. In the chinchilla, IHC are more vulnerable to carboplatin than outer hair cells (OHC). Systemic injections of carboplatin lead to destruction of hair cells in a dose dependent manner (Wake et al., 1993, 1994; Takeno et al., 1994a, b; Trautwein et al., 1996; Hofstetter et al., 1997a, b; Ding et al., 1999). High dose, systemic injections (200 mg/kg) of carboplatin cause degeneration of both inner hair cells (IHC) and outer hair cells (OHC). OHC degeneration follows a base-to-apex gradient while IHC degenerate more uniformly throughout the cochlea. In contrast, a low to moderate dose of carboplatin (75-150 mg/kg) results in selective IHC loss with little or no damage to OHC. Carboplatin is also cytotoxic to type I spiral ganglion neurons (SGN) resulting in loss of nerve fibers to the cochlear nucleus (Wang et al., 2003; Ding et al., 1999; Ding et al., 1998). Carboplatin induced nerve fiber loss did not alter cochlear nucleus volume (Li et al., 2002), but significantly reduced glutamate, aspartate and gamma amino butyric acid (GABA) concentrations in the ventral cochlear nucleus and GABA levels in subdivisions of the dorsal cochlear nucleus (Godfrey et al., 2005). The loss of nerve fibers and reduction in amino acids involved in excitatory and inhibitory neurotransmission will undoubtedly lead to reorganization; however, the time course and location of these changes are largely unknown. To begin to address these issues, we examined the relationship between carboplatin-induced hair cell loss in the chinchilla and the spatio-temporal pattern of GAP-43 immunolabeling, a marker for growth of nerve fibers and presynaptic endings.
Material and methods
Animals
14 adult chinchillas of either gender were used in this study. Experimental animals received carboplatin on the right round window for induction of unilateral hair cell degeneration. Animals were allowed to survive for 3 (n=2), 7 (n=3), 15 (n=5) or 31 days (n=3). One animal remained untreated for a control. All animal procedures were approved by the University of Buffalo Institutional Animal Care and Use Committee (Protocol #HER05080Y).
Carboplatin application
Animals were anesthetized with isofluorane (5% induction and 2% maintenance) and positioned in a standard head holder. Before surgery, animals were given 10 ml 0.9% saline, atropine (0.15mg/kg, s.q.), and analgesic (buprenorphine; 0.05 mg/kg i.m.). On the right side, a 3 mm hole was made in the posterior bulla to provide access to the round window. With a microsyringe a drop of carboplatin (50 μl, 5 mg/ml in saline) was gently placed on the round window. The hole was closed with dental cement and the wound sutured. Post surgery, animals received buprenorphine (0.05 mg/kg i.m) and carprofen (4 mg/kg i.m.).
Tissue preparation
On the day of sacrifice, animals received a lethal dose of pentobarbital and were transcardially perfused with phosphate buffered saline (PBS, 0.1M, pH 7.4) for 5 minutes and then with 10% phosphate buffered formalin (containing 4% paraformaldehyde) at room temperature for 15 minutes. The brain and the bulla containing the cochleae were removed from the skull, and the brains were post-fixated in 10% phosphate buffered formalin for one week. The bullas from experimental animals were carefully examined to rule out the possibility of middle ear pathologies caused by the surgery, infection or mechanical damage.
Cochleograms
Our procedures for preparing cytocochleograms have been described previously (Ding et al., 1998; 1999). Briefly, the organ of Corti was carefully dissected out of the cochlea as a flat surface preparation and stained with Harris’ hematoxylin solution. The basal, middle and apical turns of the organ of Corti were mounted on glass slides in glycerin and coverslipped. Cochleograms were prepared showing percent missing hair cells as a function of percent distance from apex of the cochlea using lab norms. At high magnification (400x), hair cells were counted in 0.24 mm intervals along the entire length of the cochlea. Hair cells were counted as present when cell body and cuticular plate were intact.
Immunolabeling of cochlear nucleus and superior olivary complex
Brainstems containing the cochlear nuclei and superior olive were treated with 30% sucrose in 0.1M PBS pH 7.4 overnight at 4°C for cryoprotection. The following day brainstems were cut into 30 μm thin frontal sections at -30 °C. Free floating sections were collected and washed in 0.1M PBS, pH 7.4, pretreated with H2O2 for peroxidase deactivation, then pre-incubated in blocking buffer containing 10% normal horse serum and 0.05% Triton X-100 in 0.1M PBS, pH 7.4 for 30 minutes at room temperature. Subsequently, sections were exposed for 2 h at room temperature to an antibody against GAP-43 (MAB347; made in mouse; clone 9-1E12; Millipore), which has been shown to specifically detect and bind GAP-43 (Goslin et al., 1991; Schreyer and Skene, 1991). GAP-43 antibody was added at a concentration of 0.2 μg/ml in 0.1M PBS pH 7.4 with 1.0% normal horse serum and 0.05% Triton. GAP-43 was visualized through the indirect staining method utilizing a secondary antibody (anti-mouse IgG, Vector Laboratories, Burlingame, CA), the avidin-biotin-peroxidase complex technique (Elite-ABC, Vector Laboratories), and diaminobenzidine tetrahydrochloride (DAB, 0.05%, Sigma) with nickel ammoniumsulfate (0.3%) and H2O2 (0.0015%) in 0.1 M Tris buffer, pH 7.2 (Trizma-base, Sigma). The secondary antibody and ABC incubation steps lasted for 1 hour each at room temperature. Between all incubation steps, sections were washed in 0.1M PBS, pH 7.4. Care was taken to keep staining at a comparable intensity level in all animals, and not to understain or overstain tissue in order to obtain reliable staining ratios. After staining, sections were washed in PBS and mounted on gelatin-coated slides (Fisherbrand SuperFrost plus; Fisher Scientific) and dried overnight. Then sections were dehydrated in increasing concentrations of ethanol, cleared in xylene and sealed with DPX.
Photomicrographs, quantification and statistical analysis
Images visualized under bright field illumination (Axioskop, Carl Zeiss Inc) were photographed using a digital camera (SPOT Insight, Diagnostic Instruments Inc) and processed with imaging software (SPOT Software, version 4.6) into 8-bit grey tone scale. Evaluation and assembly of images were done with Adobe Photoshop 5.5. Statistical evaluations were done with GraphPad Prism (Version 5.01; GraphPad Software Inc).
For evaluation of GAP-43 expression in the cochlear nucleus, images of 6 VCN sections and 6 DCN sections ipsilaterally, and 6 VCN sections and 6 DCN sections contralaterally were taken under identical illumination conditions. Ipsilateral and contralateral DCN and VCN were matching in respect to location on the rostral-caudal axis. From each image of VCN or DCN, mean grey tone values were obtained from one representative field in the dorsal high-frequency area and one in the ventral low-frequency area. The size of the field was adjusted to fit within the area to be analyzed: a 0.6 × 0.6 mm square in posterior VCN, 0.5×0.5 mm in anterior VCN, 0.3×0.3 mm in posterior DCN and 0.2×0.2 mm in anterior DCN. Regions at the margin of VCN, areas containing axon bundles or larger blood vessels were excluded from measurement. In DCN, the area of interest was limited to the fusiform layer. Mean grey tone values of unstained tissue plus background grey tone were subtracted from GAP-43 immunolabeled sections in order to obtain net GAP-43 immunostaining values. These data were collected and processed by a person blinded to pattern of hair cell loss in the respective animals.
Statistical comparisons were made to determine significant changes of GAP-43 expression in the VCN and DCN. First, we determined if there was a significant difference in staining between the treated side and control side for each individual animal (Student’s t-test, paired, where matching ipsilateral and contralateral regions were paired). Then dorsal-to-ventral ratios ipsilaterally and contralaterally were compared in survival times where changes in GAP-43 expression was observed (Student’s t-test, paired, where ipsilateral and contralateral results from each individual animal were paired). Finally, the correlation between pattern of hair cell lesion and GAP-43 up-regulation was analyzed (non-parametric test, Spearman). All average values are presented as means ± standard error of mean (SEM). Significance level was set to P < 0.05 in all tests.
Results
General observations
Application of carboplatin (5 mg/ml) on the round window caused severe degeneration of both IHC and OHC in the cochlea (Fig. 1). In the normal hearing control animal, the growth associated protein GAP-43 was expressed in fibers and presynaptic endings, but not in cell bodies, in a pattern observed in rat in earlier studies (Illing et al., 1997): In VCN, GAP-43 was equally or almost equally expressed throughout the nucleus. In DCN, GAP-43 expression was most prominent in the fusiform layer and more pronounced than in VCN. There was only little expression in the molecular layer. After carboplatin application to the round window, expression of GAP-43 was increased in the VCN ipsilateral to the lesion at 15 and 31 days after treatment (Fig. 2), in fibers as well as presynaptic endings (Fig. 3). In contrast, there was no or only little changes in GAP-43 expression in DCN (Fig. 4). We could not find any cell bodies positive for GAP-43 in the superior olivary complex (SOC), including LSO, at any survival time from 3 to 31 days.
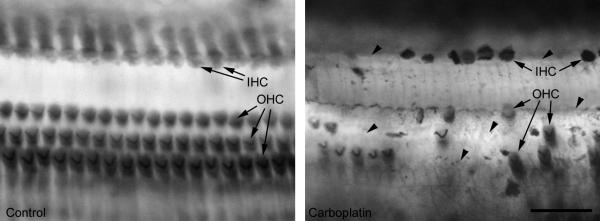
Figure 1.
Inner and outer hair cells (IHC and OHC, arrows), in an untreated control ear (left) and after application of carboplatin (5mg/ml) on the round window (right): After carboplatin treatment a great amount of IHC as well as OHC are missing. Arrow heads point to empty spaces between few remaining hair cells. Scale bar = 50μm
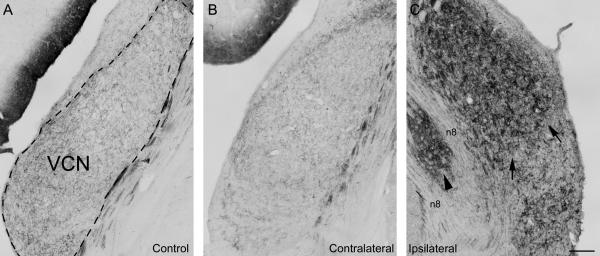
Figure 2.
Expression of the growth-associated protein GAP-43 in the ventral cochlear nucleus (VCN) in a normal hearing control animal and in an experimental animal with unilateral carboplatin application on the round window 31 days after treatment (#8608): In the control animal (A), GAP-43 is expressed at a modest level in fibers and presynaptic endings. In the experimental animal, the VCN GAP-43 expression on the untreated control side (B) remained at a moderate level as seen in the control. In contrast, VCN on the treated side (C) showed a strong increase of GAP-43 expression, which resulted in a darker staining. Staining dorsally was darker than ventrally (arrows), showing a high-to-low frequency gradient of expression. A small medial area (arrowhead) which is located within the 8th nerve (n8) belongs to VCN but appears isolated due to bifurcation of the fiber bundle and showed similar GAP-43 expression as the rest of the nucleus. Comparing with anatomical observations done in rat (Paxinos and Watson, 2004) this may be the region where olivocochlear fibers pass by or enter VCN. Dashed line in (A) indicates outlines of the VCN. Scale bar = 200μm
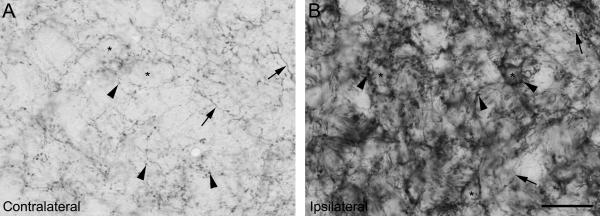
Figure 3.
Expression of GAP-43 in VCN in one experimental animal surviving for 31 days (#8608) at high magnificantion: Contralaterally (A) as well as ipislaterally (B), GAP-43 is located in presynaptic endings (arrowheads point to some examples) as well as in fibers (arrows). Asterisks indicate unstained cell bodies. 31 days after carboplatin treatment, expression ipislaterally is strongly increased as compared to the contralateral side. Scale bar = 100μm
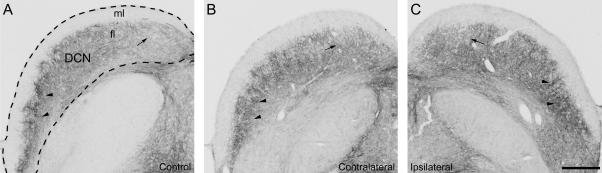
Figure 4.
Expression in GAP-43 in the fusiform layer (fl) in the dorsal cochlear nucleus (DCN) in a normal hearing control (A) and in an experimental animal (B, C) with unilateral application of carboplatin on the round window, 31 days after treatment (#8333): GAP-43 expression showed only little or no difference between control and experimental animal on either of the sides. In the experimental animal, expression on the ipsilateral DCN (C) showed a slightly stronger expression than contralaterally (B). Control as well as experimental DCNs showed stronger expression ventrally (arrowheads) than dorsally (arrow). Dashed line in (A) indicates outlines of DCN. fl: fusiform layer; ml: molecular layer. Scale bar = 500μm
Hair cell degeneration
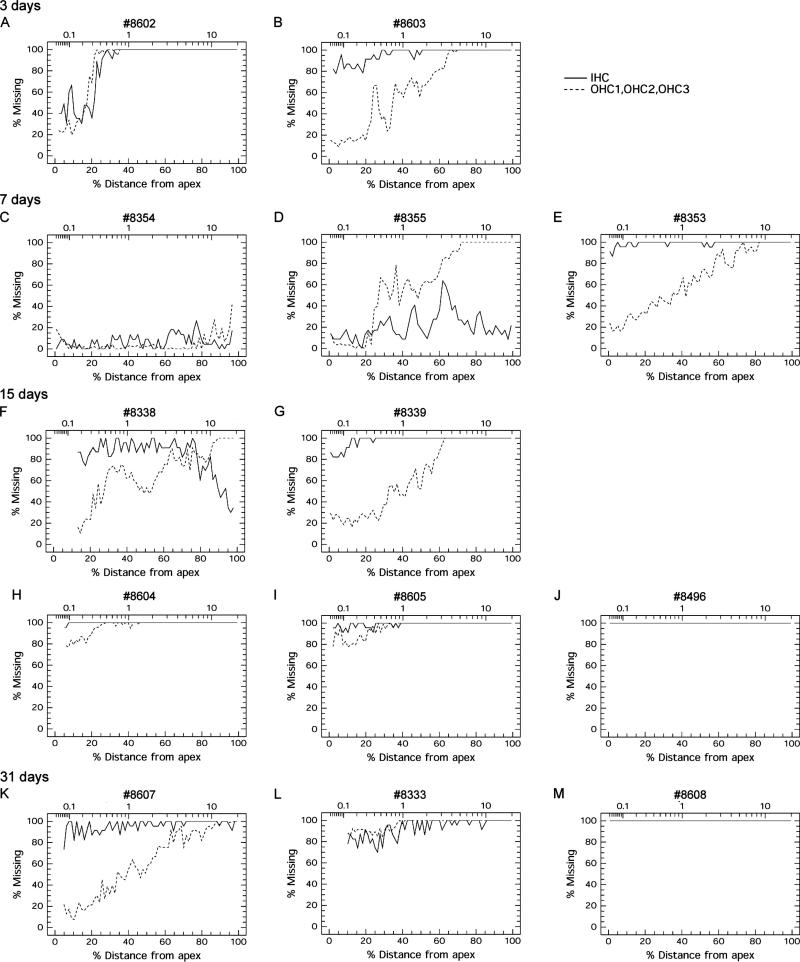
In all animals, IHC as well as OHC were affected after treatment with carboplatin (Fig. 6). Losses ranged from a weak loss of less than 1/4 of all hair cells (Fig. 6 C) to complete degeneration where all hair cells were missing (Fig. 6 J,M). IHC degeneration was more severe than OHC loss in many (Fig. 6 B,E,G,H,I,K) but not all animals (Fig. 6 D,L). Degeneration of OHC tended to be considerably greater in the basal, high frequency region than in the apical, low frequency area. In contrast, IHC degeneration tended to be equally distributed throughout the cochlea although strongest in the middle or basal turn in some cases. Overall, hair cell degeneration was always more pronounced in the base than the apex, except in animals where all hair cells were gone. The most typical patterns of hair cell degeneration were ones with almost complete IHC loss coupled with OHC loss with a strong base-to-apex gradient (Fig. 6 B,E,G,K) or others with almost complete loss of both IHC and OHC with only a few cells remaining in the apex (Fig. 6 H,I,L). As early as 3 days after carboplatin, a severe loss of hair cells could be observed, suggesting that most hair cell degeneration occurs during the first few days after treatment. But additionally, since hair cell loss at 15 and 31 days tended to be more pronounced than after 3 or 7 days, it appears that some hair cell degeneration continues at later time points
Figure 6.
Cochleograms from carboplatin treated ears at different survival times, showing degree of hair cell degeneration along the apical-to-basal axis in percent. Solid line: inner hair cells (IHC); dashed line: outer hair cells from all three rows (OHC1, OHC2, OHC3). A, B: As early as after 3 days, severe hair cell degeneration could be observed. Basally more hair cells were missing than apically. C, D, E: Three animals surviving for 7 days showed varying degrees and patterns of hair cell degeneration. One animal had only little hair cell loss throughout the cochlea (C). A second animal showed more severe hair cell loss, where OHC degeneration was complete basally but only low apically, while IHC degeneration was more equally pronounced throughout the cochlea with strongest degeneration in the middle turn (D). A third animal also showed severe OHC loss following a strong basal to apical gradient, and almost all IHC were missing (E). F, G, H, I, J: At 15 days, hair cell loss was severe, almost complete or complete, and IHC loss was more pronounced than OHC loss: In two animals, OHC degeneration again showed a strong basal-to-apical gradient (F, G). Almost all IHC had disappeared, with remaining cells predominantly in the basal turn (F) or only apically (G). In two other animals, almost all hair cells were gone, with only few IHC and OHC remaining apically (H, I). In the fifth animal, all hair cells had disappeared (J). K, L, M: At 31 days post-carboplatin, pattern and variability in hair cell degeneration resembled observations at 15 days: one animal showed OHC degeneration following basal-to-apical gradient and an almost complete IHC degeneration (K). In a second animal, degeneration was almost complete for both IHC and OHC (L). In the third animal all hair cells were missing (M).
Expression of GAP-43 in the cochlear nucleus
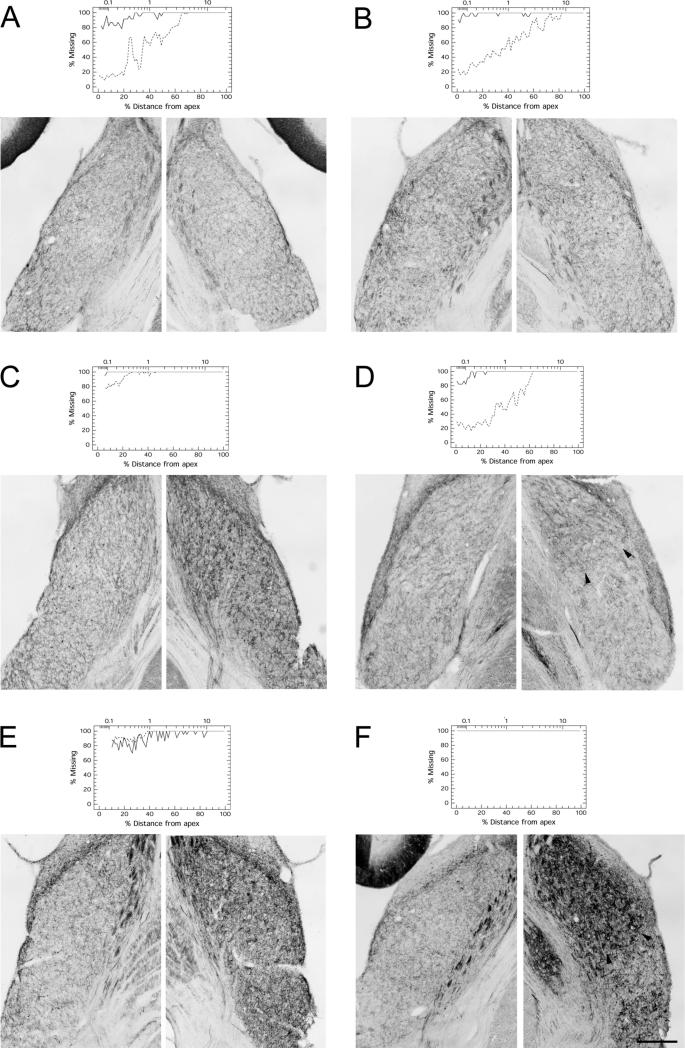
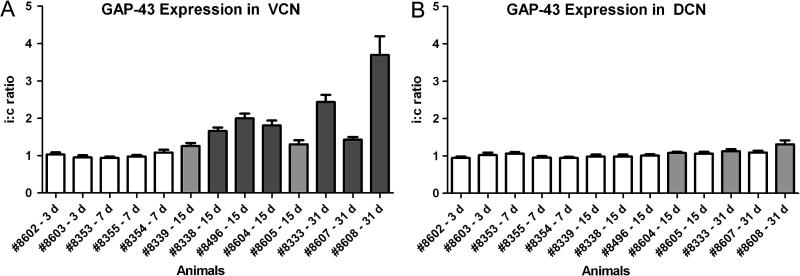
In the normal untreated chinchilla, GAP-43 is expressed at moderate level in fibers and presynaptic endings in VCN (Fig. 2 A). After carboplatin-induced hair cell loss, GAP-43 expression was strongly increased in the VCN ipsilateral to the hair cell lesion (Fig. 2 C; Fig. 7). Contralaterally, there was little or no change in GAP-43 expression (Fig. 2 B). This is in accordance with cochlear ablation studies done on rat, with increased GAP-43 expression in VCN only ipsilaterally, but no apparent changes contralaterally (Illing et al., 1997, Kraus and Illing, 2004). Thus, the contralateral VCN served as an in-animal control for quantification of ipsilateral up-regulation. Up-regulation of GAP-43 in the ipsilateral VCN could be observed in animals surviving for 15 or 31 days post-carboplatin (Fig. 7 C-F): staining intensity in the VCN ipsilateral to the carboplatin-treated ear was significantly higher than in the contralateral, control VCN (paired Student’s t-test; N=12 for each animal; Fig. 8 A) in all animals at 15 and 31 day survival. The ratio between sides, and thus GAP-43 up-regulation ipsilaterally, varied from modest (1.26 ± 0.78) to high (3.70 ± 0.51). Animals with shorter survival times (3 or 7 days), including those with pronounced hair cell lesions, showed no increase in GAP-43 expression (Fig. 7 A, B). In these animals, the ipsi-to-contralateral ratio of GAP-43 staining intensity was close to 1.0 (Fig. 8), and there were no significant differences between the sides. In DCN, there were no or only little changes. Three animals, one at day 15 and two at day 31, showed a significant difference between sides, with more expression of GAP-43 ipsilaterally (Fig. 8 B). However, the ipsi-to-contralateral ratio in these animals was small (1.08 ± 0.029, 1.13 ± 0.057 and 1.31 ± 0.102 respectively).
Figure 7.
Varying patterns and extent of GAP-43 immunoreactivity in the VCN at different survival times after unilateral carboplatin treatment of the ear; Contralateral (c) VCN to the left, VCN ipsilateral (i) to treatment to the right: Corresponding cochleograms from same animals are added for comparison. A, B: Animals surviving for 3 (A) or 7 days (B) showed no up-regulation of GAP-43 expression. There was no difference between the left and right VCN in these animals. C, D, E, F: Significant increase in GAP-43 expression ipsilaterally could be seen at 15 days (C, D) and 31 days (E, F) post-carboplatin. Some animals also showed a dorsal-to-ventral gradient of GAP-43 expression (D, F), possibly reflecting the basal-to-apical gradient of hair cell degeneration in the cochlea at present (D) or in the case of 31 days (F) possibly at an earlier time point. Arrow heads indicate region with higher expression dorsally. Scale bar = 400μm
Figure 8.
Expression of GAP-43 in VCN (A) and DCN (B) after unilateral carboplatin treatment, as shown by the ipsilateral-to-contralateral (i:c) ratio of GAP-43 immunoreactivity. Animals surviving for 3 or 7 days showed ratios close to 1.0 in VCN as well as DCN and thus no difference between sides. At 15 and 31 days the ratio increased in VCN in all animals, demonstrating an increase of ipislateral GAP-43 expression. The difference in immuno-reactivity between sides were statistically analyzed and was found significant in all animals surviving for 15 or 31 days (grey bars; P < 0.05, paired t-test where matching ipsilateral and contralateral regions were paired) and highly significant (dark grey bars; P < 0.0001) in most of these animals. In DCN, the ratio between sides was close to 1.0 in all animals. Three animals, surviving for either 15 days or 31 days showed significant difference in GAP-43 expression (grey bars; P <0.05).
Tonotopic response
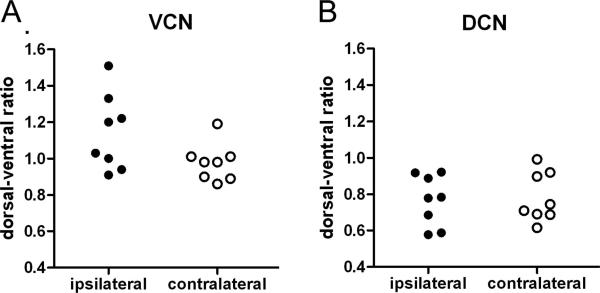
We also calculated the dorsal-to-ventral ratio of GAP-43 expression in the VCN and DCN (Fig. 9). In animals with significant GAP-43 up-regulation (i.e. all animals surviving for 15 or 31 days; Fig. 9 A) the average dorsal-to-ventral ratio in the ipsilateral VCN (1.143 ± 0.0741) was higher than in the VCN on the contralateral side (0.978 ± 0.0365; Table 2). This tendency was small but significant (P = 0.021; Student’s t-test, paired, N=8). For animals surviving for 3 or 7 days, the ipsilateral VCN showed no significant tendency towards an increased dorsal-to-ventral ratio in GAP-43 expression. In contrast, GAP-43 expression in DCN showed no difference between sides (P = 0.63). The average dorsal-to-ventral ratio was 0.768 ±0.140 ipsilaterally and 0.782 ± 0.135 contralaterally, showing more expression ventrally than dorsally on both sides. However, this pattern could also be observed in the untreated control with a ratio of 0.710 ± 0.180.
Figure 9.
Dorsal-to-ventral (d:v) ratios of GAP-43 expression in VCN (A) and DCN (B) on the treated side (filled symbols) and the untreated control side (open symbols) in chinchillas surviving for 15 or 31 days after carboplatin treatment: In VCN, the average d:v ratio on control side was 0.978 ± 0.036 and close to the theoretical value of 1.0 with no difference between dorsal and ventral expression. The ratio on the lesioned side was 1.143 ± 0.074, showing a tendency towards a stronger up-regulation dorsally than ventrally. A paired t-test where the d:v-ratios on lesioned and control side from individual animals were coupled showed that the difference between lesioned side and control side was significant (P = 0.021). In DCN, the d:v ratio showed no difference between sides (P = 0.63). Ipsilaterally as well as contralaterally, GAP-43 expression was higher ventrally than dorsally, a pattern also observed in the untreated control.
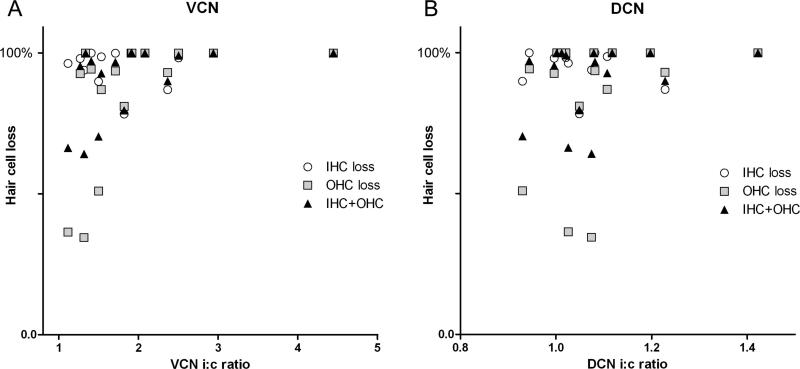
In figure 10, extent of ipsilateral GAP-43 up-regulation is plotted against degree of hair cell loss, at 15 days and 31 days after carboplatin treatment. Values were obtained from the high frequency regions (hair cell loss in % in the basal half of the cochlea coupled with the GAP-43 up-regulation in the dorsal area of VCN) and from low frequency regions (hair cell loss from the apical half of the cochlea coupled with GAP-43 up-regulation in the ventral area of VCN). Analysis of the plots show that the extent of GAP-43 up-regulation significantly correlated with the extent OHC loss or total hair cell loss, but not with IHC loss alone (OHC: P=0.0051; OHC+IHC: P=0.019; IHC: P=0.2424, nonparametric correlation test; Spearman). In DCN, there was no correlation between GAP-43 expression and hair cell loss (Fig. 10 B).
Figure 10.
Scattergram where hair cell loss (IHC and OHC) is plotted against up-regulation of GAP-43 expression (ipsi-to-contralateral ratio) in VCN (A) and DCN (B) at 15 and 31 days after carboplatin treatment: Values were obtained separately from low-frequency regions and from high-frequency regions. A nonparametric correlation test (Spearman) showed that GAP-43 expression in VCN correlated significantly with OHC loss (P=0.0051) and total hair cell loss (OHC+IHC; P=0.019), but not with IHC loss (P=0.2424). In DCN, the pattern of GAP-43 expression did not show any correlation with hair cell loss (OHC: P=0.39; OHC+IHC: P=0.41; IHC: P=0.63).
Discussion
Unilateral round window application of carboplatin (50 μl, 5 mg/ml) resulted in degeneration of IHC and OHC. The main new finding of this study was that carboplatin-induced cochlear lesions resulted in a significant up-regulation of GAP-43 expression in fibers and presynaptic endings in the ipsilateral VCN in animals surviving for 15 or 31 days post-carboplatin. GAP-43 showed little or no change at the 3 or 7 day time points. At 15 and 31 day survival times, the increase in GAP-43 expression in VCN was positively associated with the degree of hair cell loss with the strongest extent in the high frequency region of the cochlea and VCN. We could not detect any prominent changes of GAP-43 expression in the DCN. There were no cell bodies containing GAP-43 in the LSO.
Hair cell degeneration pattern
Although the extent and pattern of IHC and OHC degeneration varied across animals, there were some general trends. IHC lesions were generally larger than OHC lesions consistent with earlier reports (Hofstetter et al., 1997a, b; Reyes et al., 2001). The pattern of OHC degeneration tended to be greater in the base than the apex raising the possibility that the gradient of OHC loss might be due to a drug diffusion gradient from the round window. However, the diffusion hypothesis seems unlikely given that IHC degeneration tended to be relatively uniform along the length of the cochlea. Moreover, OHC showed a strong base-to-apex gradient also after systemic injections of carboplatin (200 mg/kg) (Hofstetter et al., 1997a, b) where a strong diffusion gradient appears unlikely. Collectively, the pattern of hair cell death after carboplatin treatment appears to be related to differential hair cell vulnerability
Expression of GAP-43 in the cochlear nucleus after hearing loss
After carboplatin-induced hair cell loss, expression of GAP-43 in the ipsilateral VCN was increased. This up-regulation could be seen at 15 and 31 days post-treatment, but not at 3 and 7 days. Up-regulation was significantly more pronounced in the dorsal, high frequency region than the ventral, low-frequency region of the VCN.
Up-regulation of GAP-43 expression in the rat VCN is well documented after cochlear ablation and noise trauma (Illing et al., 1997; Michler and Illing, 2002). However, there are considerable differences in spatial and temporal pattern of GAP-43 expression between the damaging paradigms. After cochlear ablation, where all hair cells and SGN are removed immediately, auditory nerve fibers degenerate within a few days (Illing et al., 2005) and the first signs of GAP-43 up-regulation in the ipsilateral VCN could be seen as early as 4 days (Illing et al, 1997). One week after lesion induction, GAP-43 expression reached a peak that was approximately three-fold greater than normal (Kraus and Illing, 2004; Meidinger et al., 2006). Unilateral noise trauma, which resulted in a permanent threshold shift of 80 dB (Michler and Illing, 2002) resulted in a less uniform pattern of GAP-43 up-regulation among individual animals: Some animals showed increased expression ipsilaterally, others contralaterally, and some bilaterally. At survival times of three weeks or more, the ipsilateral increase in the VCN was the predominant response. In contrast to cochleotomy, the noise exposure did not lead to a complete hearing loss, and onset of auditory nerve fiber degeneration began as late as three weeks post-trauma. Carboplatin-induced hair cell loss in chinchilla resulted in a similar pattern of GAP-43 up-regulation as cochlear ablation in the rat, with a significant up-regulation in VCN ipsilaterally and no apparent up-regulation contralaterally. The main difference between the effects of unilateral carboplatin treatment in chinchilla and cochleotomy in rats was that the onset of carboplatin-induced GAP-43 expression appeared approximately 7 days later than after cocheotomy. Cochlear ablation also results in auditory nerve fiber degeneration within few days, and GAP-43 is up-regulated in VCN regions where fiber degeneration occurs (Illing et al., 2005). Also carboplatin treatment cell loss may induce SGN loss and auditory nerve fiber degeneration. Studies with systemic injection of carboplatin cause SGN loss additionally to hair cell loss, possibly through excitotoxicity caused by increased glutamate release from malfunctioning hair cells as early as 24 hours post-treatment (Wang et al., 2003). Alternatively, GAP-43 expression may be triggered by reduced sensory input or auditory nerve malfunction after hair cell loss. Further research is required to determine the fate of SGN after local carboplatin treatment and their role in central VCN plasticity.
In contrast to VCN, there were only little or no changes in GAP-43 expression in the chinchilla DCN after carboplatin treatment. In the rat, Illing et al (1997) could observe a moderate increase in DCN ipsilaterally 4 days after cochlear ablation. This huge difference in response of VCN and DCN show that changes in activity or degeneration of the auditory nerve, which projects DCN as well as VCN, cannot be the only trigger for GAP-43 up-regulation. One additional factor may be specific neurons projecting to VCN but not DCN. Medial olivocochlear neurons (MOC neurons), which project to the cochlea and thus are directly affected by hair cell loss, may be one source for fiber sprouting in VCN (Kraus and Illing, 2004). MOC neurons as a possible source are discussed further below.
Absence of GAP-43 in cell bodies of olivocochlear neurons
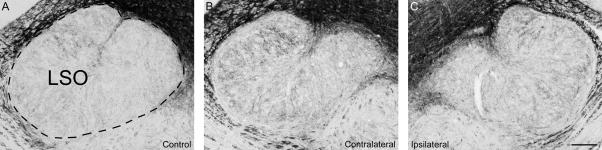
In contrast to observation done in rat after cochlear ablation or noise trauma (Illing et al., 1997; Michler and Illing, 2002), we could not detect any cell bodies expressing GAP-43 in the chinchilla SOC after carboplatin treatment. Observations in the rat have shown that cell bodies of many but not all LOC neurons show increased expression of GAP-43 after cochlear ablation (Kraus and Illing, 2005). After cochlear ablation, these neurons lose all their synaptic targets and increased GAP-43 expression may be an attempt of regeneration and re-innervation of new potential target cells. However, this has been shown to fail and the neurons degenerate instead (Kraus and Illing, 2005). Neurons with axonal collaterals to other regions in the brain which remain undamaged after lesion do not accumulate GAP-43 protein in their cell bodies. Thus, these observations suggest that all lateral and medial olivocochlear neurons in the chinchilla may have axonal collaterals projecting to regions other than the cochlea, like inferior colliculus or VCN.
Tonotopic response and possible triggers for increased GAP-43 expression
Evidence suggests that the pattern of GAP-43 expression in the VCN is related to location of the lesion with respect to the tonotopic organization of the cochlea. Carboplatin-induced hair cell loss was generally stronger in the high frequency basal region than in the apical, low frequency area. Centrally, there was a significant tendency for a stronger up-regulation in GAP-43 in VCN in the high frequency area dorsally than the low frequency region ventrally, shown by an increased dorsal-to-ventral gradient of GAP-43 expression 15 and 31 days after carboplatin treatment. Thus, the increase in the dorsal-ventral ratio of GAP-43 expression is presumably linked to the base-to-apex gradient in hair cell loss. Similar observations have been made in rats 7 days after cochlear ablation (Illing et al., 2005): When only the low-frequency, apical end of the cochlea was surgically removed, GAP-43 expression in VCN was increased only in the low-frequency, ventral portion of the VCN, not in the dorsal, high-frequency part of VCN.
In contrast to cochlear ablation where all IHC and OHC are uniformly removed, carboplatin treatment results in IHC and OHC loss with different patterns. A correlation analysis showed that extent and localization of GAP-43 up-regulation was significantly correlated with OHC loss or OHC plus IHC loss, but not with IHC loss alone. OHC loss may induce GAP-43 up-regulation through two different kinds of mechanisms: Impairment of the cochlear amplifier (Dallos and Evans, 1995) results in reduced signal input to the cochlear nucleus, which may trigger compensatory growth of excitatory synapses from other sources. Alternatively or additionally, medial olivocochlear neurons (MOC neurons) which project from the SOC to the cochlea and synapse on OHC (Warr, 1992) lose their postsynaptic targets, which may result in compensatory sprouting of MOC collateral fibers projecting to the VCN. In cochlear ablation studies on the rat, MOC neurons do not show expression of GAP-43 protein in their cell bodies, but in situ hybridization showed increased amounts of GAP-43 mRNA (Kraus and Illing, 2004), suggesting that GAP-43 protein is synthesized but immediately transported to their presynaptic endings in VCN. Sprouting synapses in VCN expressing GAP-43 after cochlear ablation have been shown to belong to MOC neurons (Kraus and Illing, 2004). Thus, MOC neurons may be origin of synaptic plasticity in VCN after carboplatin in the chinchilla, even though we could not detect any GAP-43 positive cell bodies in the SOC.
Since IHC loss varied very little among animals (most were complete lesions or almost complete), we cannot rule out the possibility that IHC loss may also contribute to GAP-43 up-regulation in addition to OHC loss. To determine if or to which extent IHC also may contribute to GAP-43 up-regulation, greater variations in IHC lesions will be needed. A selective IHC lesion leaving all OHC intact would show if IHC loss alone is sufficient to induce fiber growth in VCN. IHC damage is connected with either degeneration of type I spiral ganglion fibers or loss of their functionality, and the cell bodies of their postsynaptic targets in VCN may create a demand for formation of new synaptic inputs and possibly for outgrowth of new synapses. Current research is underway to address these issues.
Functional significance
Fiber growth and formation of new synapses in VCN after hearing loss has been observed in several paradigms, such as cochlear ablation, noise trauma or application of carboplatin. However, the functional correlates to this plasticity are still unknown. Previous studies have shown that hearing loss caused by systemic injection of carboplatin leads to a reduction of the compound action potential (CAP) amplitude in the auditory nerve (Trautwein et al., 1996); the amplitude reduction is proportional to the extent of IHC loss (Qiu et al., 2000). Also the amplitude of the evoked response in colliculus inferior (IC) was reduced, but to a smaller extent than the CAP amplitude (Qiu et al., 2000; Salvi et al., 2000a, b). In the auditory cortex (AC), the response amplitude remained unchanged or surprisingly even increased in some cases (Qiu et al., 2000; Salvi et al., 2000 a,b). The increase in signal gain in the IC and AC was seen 2 and 5 weeks after carboplatin treatment, but at earlier time points such as 3 days there was little or no change in gain (Qiu et al., 2000). These findings show that the central auditory system is able to undergo compensatory changes as a result of reduced neural output from the cochlea.
The synaptic growth in the VCN seen at 15 and 31 days post-carboplatin, but not at 3 or 7 days, may represent the neuroanatomical correlates for the increase in central signal gain. A number of studies have shown that growing fibers in VCN after hearing loss express excitatory neurotransmitters. In the case of cochlear ablation-induced synaptic growth in VCN, the cells of origin of the fibers were cholinergic MOC neurons (Kraus and Illing, 2004; Meidinger et al., 2006) and thus excitatory (Benson and Brown, 1990; Warr 1992). The number of cholinergic presynaptic endings in VCN after cochlear ablation increased by approximately three-fold 7 days post-lesion (Meidinger et al., 2006). Cochleotomy has also been shown to increase choline acetyltransferase activity (Jin et al., 2005) and muscarinic acetylcholine receptor binding (Jin and Godfrey, 2006) in VCN. Kim et al. (2004) found that new synapses in the VCN after cochleotomy or noise trauma in the chinchilla are excitatory. Noise exposure leads to increased expression of choline acetyltransferase in the hamster (Jin et al., 2006). In VCN, acetylcholine receptors exhibit excitatory transmission, both for nicotinic acetylcholine receptors (Happe and Morley, 1998; Yao and Godfrey, 1999; Fujino and Oertel, 2001) as well as muscarinic acetylcholine receptors (Fujino and Oertel, 2001). Moreover, presynaptic endings with increased GAP-43 expression contact large numbers of glutamatergic neurons, but only a few glycinergic neurons and no GABAergic neurons (Illing et al., 1997, 2005). Thus, given that most postsynaptic neurons are excitatory, the increase in excitatory synapses following cochlear damage will likely increase excitatory VCN output rather than inhibitory output. If so, this could result in increased signal gain in higher regions such as IC or AC.
Balanced input from the two ears is important for binaural processing such as sound localization in the horizontal plane, where signals from both sides are compared in order to compute location of the sound source (for review, see Yin 2002). After unilateral hearing loss, where the level of sensory input from one side is strongly reduced and there is a mismatch in signal strength between ears, strengthening of excitatory neuronal activity in VCN may compensate for the decreased input and readjust the signal balance from the two ears. Horizontal sound localization has been shown to recover after hearing loss in humans (Florentine, 1976; Moore, 1993) as well as in animals (King et al., 2001). Alternatively, an abnormal increase of excitatory synapses in VCN may be detrimental for the individual. An abnormal increase of excitatory synaptic activity in VCN may result in central hyperactivity, which may cause loudness recruitment or central tinnitus in case of spontaneous activity. The VCN fiber growth seen in the present study may be relevant to auditory malfunctions such as loudness recruitment or tinnitus.
Figure 5.
Expression of GAP-43 in the lateral superior olive (LSO) in a normal hearing control animal (A) and in an experimental animal (B, C) with unilateral carboplatin application on the round window, 31 days after treatment (#8608). For comparison, a previous study on the rat has shown that cochlear ablation results in accumulation of GAP-43 in cell bodies of lateral olivocochlear neurons in the LSO ipsilateral to lesion (Illing et al., 1999). However, in the chinchilla, we could not detect any immunopositive cell bodies in the ipsilateral LSO (C) at any survival times including 31 days. Dashed line in (A) indicates outlines of the LSO. Scale bar = 200μm
Acknowledgements
Supported in part by NIH grant R01 DC06630 and NOHR grant 1068911
List of Abbreviations
- AC
auditory cortex
- CAP
compound action potential
- DCN
dorsal cochlear nucleus
- GAP-43
Growth associated protein-43
- IC
inferior colliculus
- IHC
inner hair cells
- LOC neurons
lateral olivocochlear neurons
- LSO
lateral superior olive
- MOC neurons
medial olivocochlear neurons
- OHC
outer hair cells
- SGN
spiral ganglion neurons
- SOC
superior olivary complex
- VCN
ventral cochlear nucleus
References
- Baetge EE, Hammang JP. Neurite outgrowth in PC12 cells deficient in GAP-43. Neuron. 1991;6:21–30. doi: 10.1016/0896-6273(91)90118-j. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Perrone-Bizzozero NI. The expression of GAP-43 in relation to neuronal growth and plasticity: when, where, how, and why? Prog. Brain Res. 1991;89:69–87. doi: 10.1016/s0079-6123(08)61716-1. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. TINS. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Benson CG, Gross JS, Suneja SK, Potashner SJ. Synaptophysin immunoreactivity in the cochlear nucleus after unilateral cochlear or ossicular removal. Synapse. 1997;25:243–257. doi: 10.1002/(SICI)1098-2396(199703)25:3<243::AID-SYN3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Benson TE, Brown MC. Synapses formed by olivocochlear axon branches in the mouse cochlear nucleus. J. Comp. Neurol. 1990;295:52–70. doi: 10.1002/cne.902950106. [DOI] [PubMed] [Google Scholar]
- Bilak M, Kim J, Potashner SJ, Bohne BA, Morest DK. New growth of axons in the cochlear nucleus of adult chinchillas after acoustic trauma. Exp. Neurol. 1997;147:256–268. doi: 10.1006/exnr.1997.6636. [DOI] [PubMed] [Google Scholar]
- Dallos P, Evans BN. High-frequency motility of outer hair cells and the cochlear amplifier. Science. 1995;267:2006–2009. doi: 10.1126/science.7701325. [DOI] [PubMed] [Google Scholar]
- de Graan PN, van Hooff CO, Tilly BC, Oestreicher AB, Schotman P, Gispen WH. Phosphoprotein B-50 in nerve growth cones from fetal rat brain. Neurosci. Lett. 1985;61:235–241. doi: 10.1016/0304-3940(85)90470-7. [DOI] [PubMed] [Google Scholar]
- Ding D, Wang J, Salvi R, Henderson D, Hu BH, McFadden SL. Selective loss of inner hair cells and type-I spiral ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann. N. Y. Acad. Sci. 1999;884:152–170. doi: 10.1111/j.1749-6632.1999.tb08640.x. [DOI] [PubMed] [Google Scholar]
- Ding D, Wang J, Zheng X-Y, Salvi RJ. Early damage of spiral ganglion caused by carboplatin in chinchilla. Journal of Audiology and Speech Pathology. 1998;6:65–67. [Google Scholar]
- Elliot EJ, Parks DA, Fishman PS. Effect of proximal axotomy on GAP-43 expression in cortical neurons in the mouse. Brain Res. 1997;755:221–228. doi: 10.1016/s0006-8993(97)00100-5. [DOI] [PubMed] [Google Scholar]
- Florentine M. Relation between lateralization and loudness in asymmetrical hearing loss. J. Am. Aud. Soc. 1976;1:243–251. [PubMed] [Google Scholar]
- Fujino K, Oertel D. Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J. Neurosci. 2001;21:7372–7383. doi: 10.1523/JNEUROSCI.21-18-07372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DA, Godfrey MA, Ding D, Chen K, Salvi RJ. Amino acid concentrations in chinchilla cochlear nucleus at different times after carboplatin treatment. Hear. Res. 2005;206:64–73. doi: 10.1016/j.heares.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Goslin K, Schreyer DJ, Skene JH, Banker G. Changes in distribution of GAP-43 during the development of neuronal polarity. J. Neurosci. 1991;10:588–602. doi: 10.1523/JNEUROSCI.10-02-00588.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe HK, Morley BJ. Nicotinic acetylcholine receptors in rat cochlear nucleus: [125]-alpha-bungarotoxin receptor autoradiography and in situ hybridization of alpha 7 nAChR subunit mRNA. J. Comp. Neurol. 1998;397:163–180. doi: 10.1002/(sici)1096-9861(19980727)397:2<163::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hoeffding V, Feldman ML. Degeneration in the cochlear nerve of the rat following cochlear lesions. Brain Res. 1988;449:104–115. doi: 10.1016/0006-8993(88)91029-3. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Salvi RJ. Magnitude and pattern of inner and outer hair cell loss in chinchilla as function of carboplatin dose. Audiology. 1997a;36:301–311. doi: 10.3109/00206099709071981. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Powers N, Salvi RJ. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hear. Res. 1997b;112:199–215. doi: 10.1016/s0378-5955(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Illing RB, Cao QL, Forster CR, Laszig R. Auditory brainstem: development and plasticity of GAP-43 mRNA expression in the rat. J. Comp. Neurol. 1999;412:353–372. doi: 10.1002/(sici)1096-9861(19990920)412:2<353::aid-cne12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Illing RB, Horvath M, Laszig R. Plasticity of the auditory brainstem: effects of cochlear ablation on GAP-43 immunoreactivity in the rat. J. Comp. Neurol. 1997;382:116–138. doi: 10.1002/(sici)1096-9861(19970526)382:1<116::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Illing RB, Kraus KS, Meidinger MA. Reconnecting neuronal networks in the auditory brainstem following unilateral deafening. Hear. Res. 2005;206:185–199. doi: 10.1016/j.heares.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Jin YM, Godfrey DA. Effects of cochlear ablation on muscarinic acetylcholine receptor binding in the rat cochlear nucleus. J. Neurosci. Res. 2006;83:157–166. doi: 10.1002/jnr.20706. [DOI] [PubMed] [Google Scholar]
- Jin YM, Godfrey DA, Sun Y. Effects of cochlear ablation on choline acetyltransferase activity in the rat cochlear nucleus and superior olive. J. Neurosci. Res. 2005;81:91–101. doi: 10.1002/jnr.20536. [DOI] [PubMed] [Google Scholar]
- Jin YM, Godfrey DA, Wang J, Kaltenbach JA. Effects of intense tone exposure on choline acetyltransferase activity in the hamster cochlear nucleus. Hear. Res. 2006;216-217:168–175. doi: 10.1016/j.heares.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Gross J, Morest DK, Potashner SJ. Quantitative study of degeneration and new growth of axons and synaptic endings in the chinchilla cochlear nucleus after acoustic overstimulation. J. Neurosci. Res. 2004;77:829–842. doi: 10.1002/jnr.20211. [DOI] [PubMed] [Google Scholar]
- King AJ, Kacelnik O, Mrsic-Flogel TD, Schnupp JWH, Parsons CH, Moore DR. How Plastic Is Spatial Hearing? Audiol. Neuro Otol. 2001;6:182–186. doi: 10.1159/000046829. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Illing RB. Superior olivary contributions to auditory system plasticity: medial but not lateral olivocochlear neurons are the source of cochleotomy-induced GAP-43 expression in the ventral cochlear nucleus. J. Comp. Neurol. 2004;475:169–191. doi: 10.1002/cne.20180. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Illing RB. Cell death or survival: molecular and connectional conditions for olivocochlear neurons after axotomy. Neurosci. 2005;134:467–481. doi: 10.1016/j.neuroscience.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Li Y, Godfrey DA, Godfrey MA, Ding D, Salvi R. Effects of carboplatin on amino acid chemistry in chinchilla cochlear nucleus. Hear. Res. 2002;165:19–29. doi: 10.1016/s0378-5955(01)00389-6. [DOI] [PubMed] [Google Scholar]
- Liabotis S, Schreyer DJ. Magnitude of GAP-43 induction following peripheral axotomy of adult dorsal root ganglion neurons is dependent on lesion distance. Exp. Neurol. 1995;135:28–35. doi: 10.1006/exnr.1995.1063. [DOI] [PubMed] [Google Scholar]
- Linda H, Piehl F, Dagerlind A, Verge VM, Arvidsson U, Cullheim S, Risling M, Ulfhake B, Hokfelt T. Expression of GAP-43 mRNA in the adult mammalian spinal cord under normal conditions and after different types of lesions, with special reference to motoneurons. Exp. Brain Res. 1992;91:284–295. doi: 10.1007/BF00231661. [DOI] [PubMed] [Google Scholar]
- Mahalik TJ, Carrier A, Owens GP, Clayton G. The expression of GAP-43 mRNA during late embryonic and early postnatal development of the CNS of the rat: an in situ hybridization study. Dev. Brain. Res. 1992;67:75–83. doi: 10.1016/0165-3806(92)90027-t. [DOI] [PubMed] [Google Scholar]
- Meiri KF, Saffell JL, Walsh FS, Doherty P. Neurite outgrowth stimulated by neural cell adhesion molecules requires growth associated protein-43 (GAP-43) function and is associated with GAP-43 phosphorylation in growth cones. J. Neurosci. 1998;18:10429–10437. doi: 10.1523/JNEUROSCI.18-24-10429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meidinger MA, Hildebrandt.Schoenfeld H, Illing RB. Cochlear damage induces GAP-43 expression in cholinergic synapses of the cochlear nucleus in the adult rat: a light and electron microscopy study. Eur. J. Neurosci. 2006;23:3187–3199. doi: 10.1111/j.1460-9568.2006.04853.x. [DOI] [PubMed] [Google Scholar]
- Michler SA, Illing RB. Acoustic trauma induces reemergence of the growth- and plasticity-associated protein GAP-43 in the rat auditory brainstem. J. Comp. Neurol. 2002;451:250–266. doi: 10.1002/cne.10348. [DOI] [PubMed] [Google Scholar]
- Moore DR. Plasticity of binaural hearing and some possible mechanisms following late-onset deprivation. J. Am. Acad. Adiol. 1993;4:277–283. [PubMed] [Google Scholar]
- Muly SM, Gross JS, Morest DK, Potashner SJ. Synaptophysin in the cochlear nucles following acoustic trauma. Exp. Neurol. 2002;177:2002–221. doi: 10.1006/exnr.2002.7963. [DOI] [PubMed] [Google Scholar]
- Palacios G, Mengod G, Sarasa M, Baudier J, Palacios JM. De novo synthesis of GAP-43: in situ hybridization histochemistry and light microscopy immunohistochemical studies in regenerating motor neurons of cranial nerve nuclei in the rat brain. Mol. Brain Res. 1994;24 doi: 10.1016/0169-328x(94)90122-8. 107.117. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson G. The rat brain in stereotaxic coordinates. Elsevier Academic Press; 2004. [Google Scholar]
- Qiu CX, Salvi R, Ding D, Burkard R. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hear. Res. 2000;139:153–71. doi: 10.1016/s0378-5955(99)00171-9. [DOI] [PubMed] [Google Scholar]
- Reyes S, Ding D, Sun W, Salvi R. Effect of inner and outer hair cell lesions on electrically evoked otoacoustic emissions. Hear. Res. 2001;158:139–150. doi: 10.1016/s0378-5955(01)00309-4. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Ding D, Wang J, Jiang HY. A review of the effects of selective inner hair cell lesions on distortion product otoacoustic emissions, cochlear function and auditory evoked potentials. Noise Health. 2000a;2:9–26. [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear. Res. 2000b;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Schaden H, Stuermer CAO, Bähr M. GAP-43 immunoreactivity and axon regeneration in retinal ganglion cells of rat. J. Neurobiol. 1994;25:1570–1578. doi: 10.1002/neu.480251209. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Fate of GAP-43 in ascending spinal axons of DRG neurons after peripheral nerve injury: delayed accumulation and correlation with regenerative potential. J. Neurosci. 1991;11:3738–3751. doi: 10.1523/JNEUROSCI.11-12-03738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annu. Rev. Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Skene JH, Willard M. Changes in axonally transported proteins during axonal regeneration in toad retinal ganglion cells. J. Cell. Biol. 1981;89:86–95. doi: 10.1083/jcb.89.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeno S, Harrison RV, Ibrahim D, Wake M, Mount RJ. Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear. Res. 1994a;75:93–102. doi: 10.1016/0378-5955(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Takeno S, Harrison RV, Mount RJ, Wake M, Harada Y. Induction of selective hair cell damage by carboplatin. Scanning Microsc. 1994b;8:97–106. [PubMed] [Google Scholar]
- Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear. Res. 1996;96:71–82. doi: 10.1016/0378-5955(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Verhaagen J, Zhang Y, Hamers FP, Gispen WH. Elevated expression of B-50 (GAP-43)-mRNA in a subpopulation of olfactory bulb mitral cells following axotomy. J. Neurosci. Res. 1993;35:162–169. doi: 10.1002/jnr.490350206. [DOI] [PubMed] [Google Scholar]
- Wake M, Takeno S, Ibrahim D, Harrison R. Selective inner hair cell ototoxicity induced by carboplatin. Laryngoscope. 1994;104:488–493. doi: 10.1288/00005537-199404000-00016. [DOI] [PubMed] [Google Scholar]
- Wake M, Takeno S, Ibrahim D, Harrison R, Mount R. Carboplatin ototoxicity: an animal model. J. Laryngol. Otol. 1993;107:585–589. doi: 10.1017/s0022215100123771. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Carboplatin-induced early cochlear lesion in chinchillas. Hear. Res. 2003;181:65–72. doi: 10.1016/s0378-5955(03)00176-x. [DOI] [PubMed] [Google Scholar]
- Warr WB. Organization of olivocochlear efferent systems in mammals. In: Webster DB, Popper AN, Fay RR, editors. The mammalian auditory pathway: Neuroanatomy. Springer Verlag; New York: 1992. pp. 410–448. [Google Scholar]
- Wenthold RJ, Gulley RL. Aspartic acid and glutamic acid levels in the cochlear nucleus after auditory nerve lesion. Brain Res. 1977;138:111–123. doi: 10.1016/0006-8993(77)90787-9. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Reynolds ML, Molander C, O’Brian C, Lindsay RM, Benowitz LI. The growth-associated protein GAP-43 appears in dorsal root ganglion cells and in the dorsal horn of the rat spinal cord following peripheral nerve injury. Neuroscience. 1990;34:465–478. doi: 10.1016/0306-4522(90)90155-w. [DOI] [PubMed] [Google Scholar]
- Yao W, Godfrey DA. Immunolocalization of alpha4 and alpha7 subunits of nicotinic receptor in rat cochlear nucleus. Hear. Res. 1999;128:97–102. doi: 10.1016/s0378-5955(98)00199-3. [DOI] [PubMed] [Google Scholar]
- Yin TCT. Neuronal mechanics of encoding binaural sound localization cues in the auditory brain stem. In: Oertel D, Fay RR, Popper AN, editors. Integrative functions in the mammalian auditory pathway. Springer Verlag; New York: 2002. pp. 99–159. [Google Scholar]