Abstract
Selecting what is important to remember, attending to this information, and then later recalling it can be thought of in terms of the strategic control of attention and the efficient use of memory. In order to examine whether aging and Alzheimer's disease (AD) influenced this ability, the present study used a selectivity task, where studied items were worth various point values and participants were asked to maximize the value of the items they recalled. Relative to younger adults (N=35) and healthy older adults (N=109), individuals with very mild AD (N=41) and mild AD (N=13) showed impairments in the strategic and efficient encoding and recall of high value items. Although individuals with AD recalled more high value items than low value items, they did not efficiently maximize memory performance (as measured by a selectivity index) relative to healthy older adults. Performance on complex working memory span tasks was related to the recall of the high value items but not low value items. This pattern suggests that relative to healthy aging, AD leads to impairments in strategic control at encoding and value-directed remembering.
Keywords: memory, Alzheimer's disease, aging, efficiency, value and strategic encoding
The ability to attend to important information is critical in order to later recall this information. Selecting what is important to remember, attending to this information, and then recalling it can be thought of in terms of the strategic control of attention, and can lead to the efficient use of memory (Castel, 2007). Although Alzheimer's disease (AD) is most often characterized in terms of loss of memory function, there is accumulating evidence that suggests that part of the initial impairment lies in attentional control (see Balota & Faust, 2001, and Perry & Hodges, 1999, for reviews). Impairments in attentional control can lead to impairments in encoding and maintaining relevant information in working memory (Hasher & Zacks, 1988), inhibitory control (Amieva, Phillips, Della Sella, & Henry, 2004; Belleville, Chertkow, & Gauthier, 2007), as well as retrieval and response control (Castel, Balota, Hutchison, Yap, & Logan, 2007). The present study examines how the ability to selectively attend to information that differs in value is influenced by aging and Alzheimer's disease. This approach not only allows one to examine attentional control and memory, but also the efficient use of memory in the context of paying attention to, and encoding, high value information.
In addition to changes in attention and memory in Alzheimer's disease, there is also considerable evidence that healthy older adults perform significantly less well than healthy younger adults on a wide range of cognitive tasks (Balota, Dolan, & Duchek, 2000). Age group differences are greatest on tasks that involve executive processes, working memory, and frontal lobe function, perhaps leading to difficulties on other attention and memory tasks (West, 1996). This has lead to various theories that attempt to describe and account for the changes in cognitive function in old age, centering on reductions in available processing resources (Craik, 2002), general slowing of processing (Salthouse, 1996), declines in working memory capacity (McCabe, Smith, & Parks, 2007; Park et al., 1996) and reductions in inhibitory control (Hasher & Zacks, 1988). The notion that there are impairments in the ability to control partially activated, but incorrect, information has also been quite useful in terms of accounting for some of the cognitive deficits that are associated with AD (e.g. Balota & Ferraro, 1996; Spieler, Balota, & Faust, 1996; Perry & Hodges, 1999). Hence, tests that examine the impairments in the control of attention, and the development of behavioral measures that can detect early changes and declines in these areas, may also serve as useful measures for the early diagnosis and treatment of AD.
The role of attention in memory performance has been a central theme in many lines of research, with the main finding being that distraction or divided attention lead to reductions in overall memory (e.g., Craik, Govoni, Naveh-Benjamin & Anderson, 1996). However, one method of reducing memory deficits that result from a lack of available attentional resources is to use some form of strategic control to focus attention on the necessary “to be remembered” information, and thereby encouraging selectivity about which information is processed (Logan, Sanders, Snyder, Morris, & Buckner, 2002). This process of strategic control likely relies on a form of attentional control that has been examined using many techniques, and has often shown robust individual differences (e.g., Engle & Kane, 2004). Furthermore, control of attention and working memory has also revealed somewhat striking differences between individuals and various groups and populations in terms of age differences and AD (e.g. Balota & Faust, 2001; Belleville, Chertkow, & Gauthier, 2007). However, in many of these studies, the critical measure is overall memory quantity, whereas very few studies have examined memory efficiency, which may be more related to frontal lobe function and executive control systems. In the present context, memory efficiency can be conceptualized as the strategic use of memory in light of limited capacity (e.g., a way to optimize or maximize performance given a set of resources), and provides an additional measure of memory efficiency in addition to (or even above and beyond) more standard measures of quantity and accuracy. The present study seeks to determine how attentional control can lead to efficient encoding of high value information, by comparing measures of both memory quantity (number of items recalled) and memory efficiency (the average value of recalled items). Of primary interest is whether memory quantity and efficiency are both impaired in AD relative to healthy younger and older adults.
In order to examine how one can selectively encode information using strategic control, in the present study we used a paradigm in which different values (e.g., points) were assigned to to-be-remembered information (see Castel, Benjamin, Craik & Watkins, 2002; Castel, Farb, & Craik, 2007; Hanten, Li, Chapman, Swank, Gamino, Roberson, & Levin, 2007; Watkins & Bloom, 1999). This procedure allows one to examine the extent to which people use this value-based information to guide the efficient use of memory (e.g., by recalling the high point value items). The point value assigned to each item during encoding indicates how important it is to remember each item. This task differs from traditional episodic measures of memory (e.g., a typical free recall test) in that it examines how working memory contributes to the strategic control of encoding high value information.
In the selectivity paradigm, participants are presented with lists of words, with each word in the list having a distinct value ranging from 1 point to 12 points. Participants are told to remember as many words as they can, and that their goal is to maximize their score, which is the sum of the point values of each recalled word. After recall, participants are told their score, and then are given a new list, with instructions to try to achieve as high a score as possible. In addition to simply measuring the overall total point score achieved, a selectivity index (SI) can be calculated, which is the participant's score relative to an ideal score based on the number of words recalled. For example, if you were a participant and you recalled three words (the 8, 10, and 12 point words), your score would be 8+10+12 = 30 points. An ideal score (if you recalled 3 words) would be 10+11+12 = 33 points (i.e., recalling the top three value words). Your efficiency index would be your actual score divided by the ideal score, actual/ideal = 30/33 = .91 (see Castel et al., 2002, for more details about the selectivity index). Thus, the SI provides a selectivity, or efficiency, index based on one's actual score, relative to an ideal score, taking into account the number of words recalled. In previous work, although healthy older adults recalled few words than younger adults, they were able to enhance their selectivity score (to levels similar to younger adults) by recalling high value items. Thus, the selectivity index provides a useful measure of memory efficiency, one that goes beyond simply measuring the overall quantity of recalled items.
The selectivity task can also provide a measure of how people learn which items to attend to across lists. Specifically, participants are presented with several lists or trials, and after each list they are given feedback about their score, which is the sum of the point values of the words that they recalled. The number of items presented in each list (12) is greater than the typical memory span of an individual, so participants soon realize they cannot remember all of the items. Participants typically learn to modulate which items to attend to, as reflected by the finding that the selectivity index begins to increase across successive lists. Thus, in order to achieve an optimal score (i.e., efficient use of memory), participants need to focus or attend to the high value items, and recall them on the immediate memory test. This ability (a form of strategic and adaptive control over memory) has been examined with children and begins to emerge as early as the age of six (Hanten et al., 2004) and is impaired in children with traumatic brain injury (Hanten et al., 2007). In addition, Castel (2007) has shown that healthy older adults begin to develop a strategy (after several lists) of focusing on the higher value items, in order to maximize their score, despite recalling fewer items relative to younger adults. Thus, although healthy older adults recall fewer words than younger adults, they are efficient in terms of focusing on high value words in order to maximize their overall score.
In addition to examining how selective encoding is affected by healthy aging and AD, we were also interested in examining how age-related and AD-related changes in executive attention, as reflected by performance on complex working memory span tasks, might influence selective encoding. According to many models of working memory, attention is allocated to task demands in working memory by a limited capacity central executive (Baddeley, 2000; Engle, Tuholski, Laughlin, & Conway, 1999). Individual differences in the efficiency of the central executive, or working memory capacity, have been shown to predict many higher-level cognitive tasks (see Engle & Kane, 2004 for a review) and have typically been measured using complex span tasks. Because selective encoding in the selectivity task involves strategically allocating limited attentional resources to ongoing processing, we were interested in examining whether individual differences in working memory capacity would be related to efficient selection (as measured by the selectivity index). Because age and AD have both been found to reduce the efficiency of the working memory system (Logie, Cocchini, Della Sala, & Baddeley, 2004; McCabe, Robertson & Smith, 2005), we hypothesized that age and AD related changes in working memory capacity would mediate, to some degree, any changes seen in selective encoding. Specifically, because encoding high value information in the selectivity task requires allocation of attention to high value items while concurrently ignoring or inhibiting low value items, we predicted that working memory capacity should be related to selective encoding of high value items.
Methods
Participants
Participants were recruited from the Washington University Alzheimer's Disease Research Center (ADRC), and consisted of 109 healthy older adults (68 females) and 54 individuals (22 females) with early stage AD. The healthy older adults (range of ages 57–96) had a mean age of 74.8 years (n =109, SD = 8.6), the individuals with very mild AD (ages 56-88) had a mean age of 75.9 years (n = 41, SD = 7.3), and the individuals with mild AD (ages 61–86) had a mean age of 76.8 years (n = 13, SD = 7.1). Healthy older adults reported 15.2 mean years of formal education, and individuals with AD reported 14.7 years of formal education. There were no significant differences among the groups of older adults in terms of mean age or education (all ps > .41). In addition, 35 younger adults (age 25 or younger) were recruited from the Washington University student community and participated for course credit or were paid $10. The younger adults had a mean age of 19.5 years (n = 35, SD = 1.5).
The healthy older adults and the individuals with AD were seen by a physician and completed a battery of psychometric tests approximately once a year, and were screened by a physician for neurological, psychiatric, or medical disorders with the potential to cause dementia. The inclusion and exclusion criteria for a diagnosis of AD have been described in detail elsewhere (e.g., Morris, McKeel, Fulling, Torack, & Berg, 1988; Morris, 1993) and conform to those outlined in the criteria of the National Institute of Neurological and Communications Disorders and Stroke—Alzheimer's Disease and Related Disorders Association (McKhann et al., 1984). Dementia severity for each individual with AD recruited from the Washington University Medical School Alzheimer's Disease Research Center (ADRC) was staged in accordance with the Washington University Clinical Dementia Rating Scale (Hughes, Berg, Danziger, Coben, & Martin, 1982; Morris, 1993). According to this scale, a score of 0 indicates no cognitive impairment, a score of 0.5 indicates very mild dementia, a score of 1.0 indicates mild dementia, and a score of 2.0 indicates moderate dementia. At the Washington University Medical School ADRC, a Clinical Dementia Rating Scale (CDR) score of 0.5 has been found to accurately indicate the earliest stages of AD (Morris et al., 1988). Both the reliability of the CDR and the validation of the diagnosis (based upon autopsy) by the research team have been excellent (93% diagnostic accuracy) and well documented (e.g., Berg et al., 1998). Thus, individuals given an AD diagnosis of CDR 0.5 are very likely in the earliest detectable form of AD, and are referred to as very mild AD. We feel it is important to present data separately for the two AD groups as differentiation between these groups is critical for theories of early detection of AD, although for some analyses (noted below) we combine the groups to increase power.
Psychometric Test Information
In addition to participating in the experimental task, all of the healthy older adults and those with AD who were recruited from the ADRC participated in a two hour battery of psychometric tests as part of a larger longitudinal study of cognitive performance in healthy aging and AD. The results from complete set of Psychometric tests are available by contacting the first author, while results from tests pertinent to the present study are presented in Table 1. Mini-mental state exam scores, or MMSE, (Folstein, Folstein, McHugh, 1975) indicate that the healthy older adults (M=29.1) and the very mild (CDR 0.5) AD group (M=27.2) performed well within what is considered the normal range (24-30) on this test, while the mild (CDR 1.0) AD group's mean score (M=22.8) was significantly lower (post-hoc tests showed that all three groups were significantly different from one another on this measure, p < .001). Thus, the very mild CDR 0.5 group could be considered fairly high functioning.
Table 1.
Means (and standard deviations) of scores on tests for healthy older adults and individuals with AD. F and p values reflect one-way ANOVAs.
| Test | Healthy Older Adults (N=109) |
Very mild AD (N=41) |
Mild AD* (N=10) |
F value p value |
|---|---|---|---|---|
| Mini Mental State Exam (MMSE) | 29.1 (1.15) |
27.2 (2.37) |
22.8 (4.43) |
66.72** p<.0001 |
| Reading Span | 7.27 (1.60) |
5.59 (1.90) |
4.00 (1.41) |
35.51** (p<.0001) |
| Computation Span | 8.42 (3.78) |
6.28 (3.47) |
4.33 (2.67) |
21.81** (p<.0001) |
complete psychometric data only available from 10 of the 13 mild AD participants
all group means differed significantly from one another based on post-hoc tests, p < .001.
Participants also completed the reading span and computation span tasks, tests of working memory capacity (WMC, see Engle & Kane, 2004). The reading span task required participants to read sentences (e.g., The four-legged animal that barks is the mouse) on a computer screen, decide whether the sentences were statements that were true or false, and commit the final word in each sentence to memory. One to four sentences were presented, three at each length, and participants attempted to recall the final word of each sentence auditorily, in order, immediately after the last sentence was presented. Sentence sets began with set size one and increased to the next longer set size, provided recall was correct for two of the three trials at a given length. The reading span score was the number of trials correctly recalled through the largest set size at which they recalled most of the trials correctly. Computation span was identical to reading span except that rather than reading sentences and recalling words, subjects were asked to complete simple addition and subtraction problems (e.g., 6 + 4 = 9), decide whether they were correct or not, and remember the middle number from the problem (e.g., 4). As shown in Table 1, the AD groups performed more poorly than the healthy older group on most tests. Since the younger adults were not recruited by the ADRC, they did not receive the psychometric battery, but they did complete the WMC tasks. Because younger adult participants did not receive the full psychometric battery, we simply note here that, as expected, they outperformed healthy older adults (and therefore all groups) on reading span (M = 8.61, SD = 1.94), F(1, 138) = 15.55, MSE = 43.78, η2p = .10, p < .0001, and computation span (M = 12.58, SD = 4.06), F(1, 131) = 27.76, MSE = 411.25, η2p = .18, p < .0001.
Procedure
In the selectivity task, participants were told that they would be studying lists of words, and each word would be paired with a number (a point value) ranging from 1 to 12. The words were visually presented one at a time on the center of a computer screen at a rate of one word every two seconds, and each list contained 12 words with each word paired with a unique number between 1 and 12. Participants were told that each word and number would appear on the screen for two seconds, followed by another word and number. They were told that the number that was paired with each word was a point value and that the point value indicates how important it is to remember the word (e.g., much like a game in which the words are worth different amounts of money). They were told that their task was to try to get as many points as possible, and that this could be accomplished by remembering as many of the high point value words as they could, although recalling any word would increase their score. Participants were told they just needed to verbally recall the word, and not the value of the word, and that the experimenter would record their response. Examples of the scoring procedure were given, such that participants were made aware that their score would be composed of the point values of the words they recalled (e.g., if you recall three words, table, donkey, apple, and these words were paired with the 8, 10, and 12 point values, then your score would be 8 + 10 + 12, which is 30). Participants were told that after they had seen the list words, they will see the word “RECALL” on the screen, and that they should immediately recall as many words as they could remember, and would then be told their point value total for that list. Participants were given up to 30 seconds to recall the words, and were then given feedback regarding their score. They were then given another list of new words, and would repeat this for seven more lists. They were told that their task for each list was to maximize their total point score. They were also told that they should pay as much attention to the words and the numbers, and that although it would be difficult to remember all of the words, they should try to keep their score as high as possible. After being invited to ask any questions they had about the procedure, participants were presented with the first list and recall session, after which they were once again prompted to ask any questions about the procedure.
Materials and Design
The words were presented on the center of a computer screen in white Times New Roman 48-point font, on a black background. The words in each list were concrete nouns that contained between four and five letters. The mean hyperspace analog to language (or HAL, a model of semantics which derives representations for words from analysis of text, Burgess & Lund, 1997) frequency of the words was 33,374, (Log HAL = 9.03) as obtained from the elexicon.wustl.edu Web site (see Balota et al., 2007). The words were randomly sorted into eight lists of 12 words. For each list, each word was assigned a unique number between 1 and 12, such that a different value was present in each serial position for each list (to ensure that the higher and lower value words were well distributed across serial positions). To ensure that this was the case, the mean value of each word for each serial position ranged from 6.2 to 6.8. Finally, three different versions of the order of the eight lists were created, and participants were assigned to one of the three versions.
Results
The selectivity task affords several measures of memory performance, including memory capacity (mean number of words recalled), sensitivity to value (how well one successfully recalls words based on the point value of the words), as well as memory efficiency (the selectivity index, or SI). The results will be presented in terms of (a) overall recall performance and measures from the selectivity index, (b) the degree to which various groups were sensitive to point value, (c) selectivity and recall performance as a function of list, and finally (d) the relationship between selectivity, recall and measures of working memory.
Recall and Selectivity Index
The results for overall recall performance and the mean selectivity index for each group are displayed in Table 2. The younger adults recalled more words than the other groups, and a one-way ANOVA showed a main effect of group on recall performance, F(3, 194) = 73.99, MSE = .952, η2p = .53, p < .0001. Post-hoc (Tukey) tests showed that all groups differed significantly from one another in terms of overall recall (p < .001).
Table 2.
The mean number of words recalled, and mean selectivity index, for healthy younger adults, healthy older adults and individuals with very mild and mild Alzheimer's disease (AD). Standard error is presented below each mean. (Coefficient alpha for recall was .88 and for SI was.72).
| Words Recalled | Selectivity Index | |
|---|---|---|
| Young adults (N=35) | 5.68 (0.24) |
.59 (.04) |
| Older adults (N=109) | 3.54 (0.08) |
.57 (.02) |
| Very mild AD (N=41) | 2.83 (0.14) |
.40 (.05) |
| Mild AD (N=13) | 1.95 (0.23) |
.29 (.12) |
In terms of the selectivity index (SI), a one-way ANOVA showed a significant effect of group on SI, F(3, 194) = 7.01, MSE = .080, η2p = .10, p < .0001. Post-hoc (Tukey) tests showed that younger and older adults did not differ from one another (p = .81) in terms of the mean SI, but these two groups did differ from the two AD groups (all ps<.001). Interestingly, while there was a difference in magnitude of the SI between mild and very mild AD, this difference did not reach significance, p = .21, possibly due to the smaller sample size of the mild AD group. In order to demonstrate that the impairment in value-directed remembering was independent from overall recall performance for the individuals with AD, we compared the selectivity index as a function of group (younger, older, very mild, mild AD) while controlling for recall performance in an ANCOVA. The results were the same as the original ANOVA in which recall performance was not controlled, supporting the idea that the deficit in value-directed remembering was independent of group differences in recall performance. Namely, there was still a group difference in the SI, F(3, 194) = 4.65, MSE = .32, η2p = .07, p < .01. Moreover, there was no difference in the SI for younger and older adult groups, or for the two AD groups, but both of the AD groups showed lower SI than both the younger and older adults groups (p's < .02).
In summary, although younger adults recalled more words, healthy older adults were just as selective even though they did not recall as many words, as shown in other studies using the selectivity index (replicating Castel et al., 2002, 2007). In contrast, the individuals with early stage AD had poorer recall, as well as significantly lower SI, suggesting an impairment in being able to selectivity encode high value items.
The Overall Effect of Value on Recall
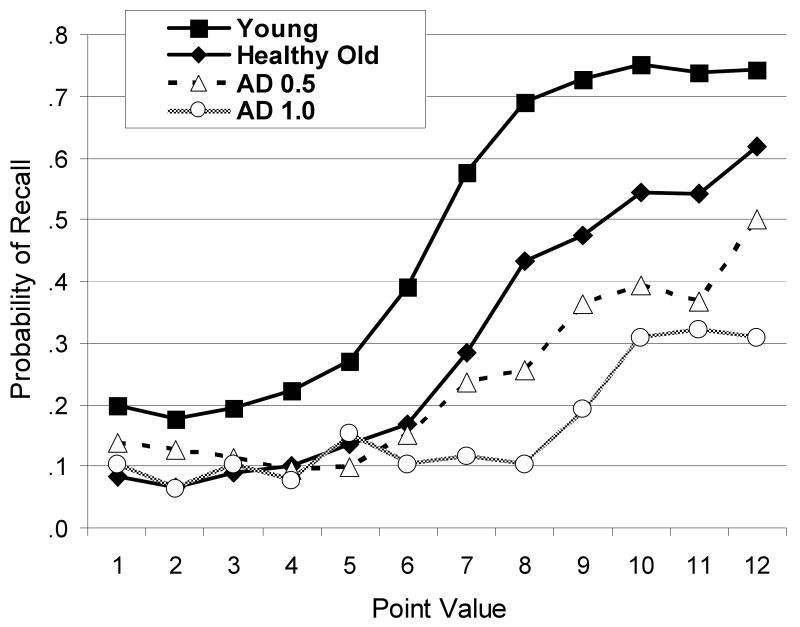
Figure 1 displays the probability of recalling a word based on the point value of each word. Overall, participants' recall was sensitive to value, with higher value words being recalled more often than lower value words. The shape or slopes of these functions for each group provide insight regarding the degree of selectivity, or sensitivity to value. A 4(group: young, healthy old, very mild AD and mild AD) × 12 (point value) mixed model ANOVA was conducted, and yielded a main effect of group F(3, 194) = 73.65, MSE = .079, η2p = .53 p < .0001, indicating that the groups differed in overall memory performance (see Table 2 for overall recall collapsed across value for each group). There was a main effect of point value, F(11, 2134) = 92.38, MSE = .035, η2p = .32 p < .0001, indicating that overall, memory performance was influence by point value. There was also a significant interaction of group and point value, F(33, 194) = 5.85, MSE = .079, η2p = .083, p < .0001, suggesting that the groups differed in terms of the degree to which point value influenced recall. As shown, there were large differences in recall for the high value words, but virtually no difference for the low value words (although floor effects may be present for some of the groups, making it somewhat difficult to detect differences in recall for low value items).
Figure 1.
The mean probability of recall as a function of point value averaged across alls lists for healthy younger adults, healthy older adults and individuals with very mild Alzheimer's disease (AD 0.5) and mild Alzheimer's disease (AD 1.0).
Selectivity Index and Recall across Lists
Participants were presented with eight different lists in succession, and it is likely that over the course of the experiment, performance changed as participants adaptively learned effective strategies to enhance recall (i.e., remembering high value words to maximize their total point score). Hence, one might ask if there are different levels of improvement across groups across the lists. In order to address this, we examined whether recall and SI changed over the course of the task, and whether these measures changed at different rates for different groups of participants.
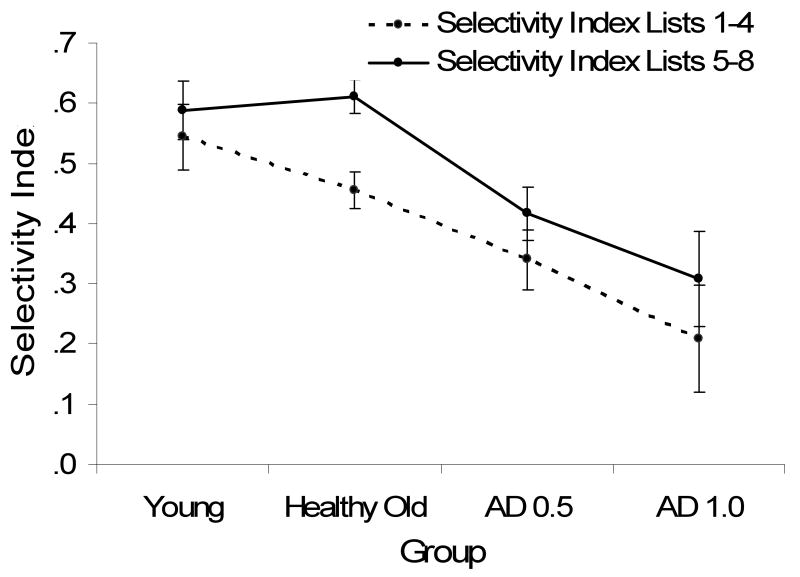
In order to examine whether participants learned to be more selective across lists, the average selectivity index was calculated separately for List 1-4 and List 5-8, and these were examined as a function of group, and are presented in Figure 2A. A 4 (group: young, healthy old, very mild AD and mild AD) × 2 (list position: Lists 1-4, Lists 5-8) mixed model ANOVA was conducted, which yielded a main effect of group F(3, 194) = 7.78, MSE = 1.07, η2p = .11 p < .001, indicating that the groups differed in overall SI performance. There was also a main effect of list order, F(1, 194) = 9.92, MSE = .50, η2p = .049 p < .01, indicating that SI increased for lists 5-8, as compared to lists 1-4. Most importantly, however, there was no significant group × list order interaction, F < 1, indicating that the increase in selectivity during the task was independent of group membership. There was a nominally greater increase in SI for older adults as compared to the other groups, but there were no significant interactions of list position and group when comparing healthy older adults with any of the other groups (p's > .09).
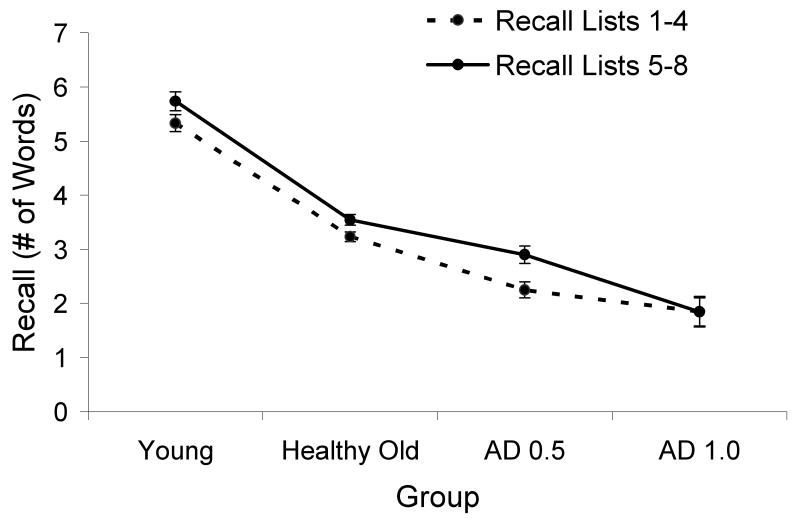
Figure 2.
Figure 2A (top panel): Selectivity Index (SI) in Lists 1-4 versus Lists 5-8
Figure 2B (bottom panel): Mean Recall in Lists 1-4 versus Lists 5-8.
In order to examine whether overall recall changed over the course of the task we also calculated average recall for Lists 1-4 and Lists 5-8 separately, and this is displayed in Figure 2B. A 4 (group: young, healthy old, very mild AD, mild AD) × 2 (list order: Lists 1-4, Lists 5-8) mixed model ANOVA was conducted, which yielded a main effect of group F(3, 194) = 88.13, MSE = 144.31, η2p = .58, p < .0001, indicating that the groups differed in overall recall performance. There was also main effect of list order, F(1, 194) = 23.21, MSE = 6.72, η2p = .11, p < .0001, indicating that SI increased for lists 5-8 of the task, as compared to lists 1-4. Finally, there was a significant group × list order interaction, F(1, 227) = 3.18, MSE = .92, η2p = .047, p < .05. We examined this interaction further by comparing recall as a function of list order for each group separately. This analysis revealed an increase in recall from the first-four to the last-four lists for young, F(1, 65) = 6.89, MSE = 2.90, η2p = .16, p < .05, healthy old, F(1, 108) = 19.19, MSE = 5.15, η2p = .18, p < .0001, and very mild AD, F(1, 40) = 31.22, MSE = 8.73, η2p = .12, p < .0001, but not for the mild AD group, F < 1. Thus, the mild AD group did not improve their recall performance across the first and second halves of the task, despite an increase in SI on par with the other groups. This indicates that in terms of learning during the task, SI and recall were dissociated, providing additional evidence that these two measures yield distinct indices of performance.
Analysis of Primacy and Recency Effects
Given that the selectivity task involved immediate free recall, we suspected that some primacy and recency effects may be present, such that recall was better for the first few items and the last few items, relative to items in the middle of the list, regardless of value, and that AD might show strong recency effects (Bayley et al., 2000; Capitani, Della Sala, Logie, & Spinnler, 1992). To examine this for the three groups (data for the two AD groups was combined to increase power), the recall data was broken down into primacy items (recall of words in serial position 1-4) middle items (serial position 5-8) and recency items (serial position 9-12), regardless of the item's value. These data are presented in Table 3, and were analyzed using a 3 (group: young, healthy old, and AD) by 3 (position: primacy, middle, recency) ANOVA. There was a main effect of group, F(2, 195) = 132.78, p < .0001, η2p = .94, main effect of position F(2, 390) = 26.06, p < .0001, η2p = .12, as well as an interaction, F(4, 390) = 5.27, p < .001, η2p = .051. Follow-up t-tests (Tukey) showed that for younger adults, primacy was greater than middle and recency effects (p <.001), and middle did not differ from recency (p = .48). For older adults, all comparisons yielded significant differences (ps < .043). For the AD group, primacy did not differ from recency (p > .23), but both were greater than recall of middle items (ps < .001). Thus, while all three groups showed strong primacy effects, the younger adults did not show recency, and both the older adults and AD group showed a recency effect (see also Bayley, 2000). One possibility is that these individuals have greater difficulty avoiding the recall of recently presented items, which might preclude them from recalling only high value items. If this were the case, then individuals with AD would not show any primacy effect. However, as noted, it is clear that both the healthy older adults and the early stage AD individuals also produced a strong primary effect. Hence, the present decreased efficiency in the AD individuals cannot be attributed to their only recalling recency items, and not being able to recall items at other positions, i.e., primacy items. It should also be noted that because the point value of each item was randomly assigned to serial position, in some situations it would be beneficial to recall items that were of high value rather than simply the first few and last few items—and this would then be a demonstration of value-directed remembering. Watkins and Bloom (1999) suggest that differentially weighting the value of primacy and recency items could lead to a reduction in the standard U-shaped curve that illustrates primacy and recency effects in younger adults, and future research that examines this in the context of Alzheimer's disease would be useful (e.g., Buschke et al., 2006), in light of value and serial position effects.
Table 3.
The average probability of recall (and standard deviation) of items based on their grouped serial position within the list for the three groups.
| Group | Primacy items (serial positions 1-4) |
Middle items (serial positions 5-8) |
Recency items (serial positions 9-12) |
|---|---|---|---|
| Younger adults | .54 (.11) |
.41 (.12) |
.45 (.17) |
| Healthy older adults | .32 (.14) |
.23 (.09) |
.28 (.12) |
| AD groups | .21 (.13) |
.14 (.10) |
.23 (.11) |
Factor Analysis of Recall as a Function of Point Value
According to the value-directed remembering framework, participants prioritize encoding according to an item's value, and selectively attend to items of higher value. If this is indeed the case, it may be possible that low- and high-value items would comprise distinct factors. In addition, recent models of working memory capacity and attention (e.g., Cowan, 2001), suggest that people can maintain a fixed number of items (e.g., four, plus or minus one unit) in a short term activated working memory store. To examine this possibility in the present context, the average recall level of items of each value, 1-12, were submitted to an exploratory factor analysis. Exploratory factor analysis (or EFA) provides a method for reducing a large number of variables, or items, to a smaller number of factors based on similarities among those items. In the present context, factor analysis can be used to examine whether the 12 items of differing values in each list could be reduced to a smaller number of factors, and furthermore, whether low- and high-value items created separate factors that could be distinguished from one another.
The EFA was conducted using a principal components analysis, keeping factors with eigen values greater than one, and then submitting the factors to a varimax rotation. As shown in Table 4, the EFA distinguished two factors that accounted for a total of 54% of the variance in recall performance. The stronger of the two factor loadings for each item is highlighted in the Table. The factor loadings can be interpreted as the strength of the relationship between that item and the factor, in much the same way that a correlation coefficient would be interpreted. As shown in Table 4, the first factor clearly included items of Values 1-7, and the second factor included items of Values 8-12. Thus, based on the pattern of recall across individuals, there was a clear division between the five highest value items and the seven lowest value items, which is somewhat consistent with fixed capacity models of working memory. This factor structure is revealed to some extent by a visual inspection of Figure 1, which shows a relatively flat recall function for low value items for all groups, with a sharp increase in recall somewhere between items of values 6-8.
Table 4.
Factor Analysis of Recall as a Function of Point Value, Showing the Two Primary Factors Underlying Recall Performance for Low-Value Items (1-7) and High-Value Items (8-12). In All Groups Items of Values 1-7 and Items of Values 8-12 Loaded on Separate Factors
| All Groups | Young | Old | AD | |||||
|---|---|---|---|---|---|---|---|---|
| V1-7 | V8-12 | V1-7 | V8-12 | V1-7 | V8-12 | V1-7 | V8-12 | |
| Value 1 | .73 | -.14 | .79 | .20 | .43 | -.44 | .83 | -.15 |
| Value 2 | .72 | -.14 | .87 | -.13 | .50 | -.38 | .41 | -.33 |
| Value 3 | .78 | -.04 | .84 | -.14 | .73 | -.12 | .72 | -.09 |
| Value 4 | .71 | .10 | .79 | -.11 | .69 | -.03 | .67 | .21 |
| Value 5 | .66 | .04 | .65 | .10 | .62 | -.09 | .35 | -.54 |
| Value 6 | .63 | .23 | .53 | .52 | .55 | .04 | .66 | -.23 |
| Value 7 | .50 | .44 | .50 | .17 | .45 | .15 | .33 | .21 |
| Value 8 | .25 | .72 | .19 | .61 | .23 | .61 | .30 | .64 |
| Value 9 | .16 | .76 | .01 | .77 | .12 | .69 | .24 | .67 |
| Value 10 | -.05 | .80 | -.14 | .46 | -.10 | .71 | -.14 | .81 |
| Value 11 | -.10 | .78 | .11 | .74 | -.19 | .69 | -.54 | .63 |
| Value 12 | -.20 | .71 | -.01 | .31 | -.44 | .48 | -.26 | .78 |
| % Variance | 28% | 26% | 31% | 18% | 22% | 20% | 25% | 26% |
Based on Figure 1, and the factor structure revealed by the EFA, it appears that age and AD have differential effects on recall of high-value and low-value information. In order to better understand the pattern of data, separate one-way ANOVAs examining recall of the high-value items (values 8-12) and low-value items (values 1-7) as a function of group (younger, older, AD) were conducted. The ANOVA examining the high-value items revealed a main effect of group, F(2, 195) = 62.72, MSE = 1.60, η2p = .39, p < .0001, which resulted from greater recall for younger adults compared to older adults, F(1, 142) = 51.60, MSE = 1.15, η2p = .27, p < .0001, as well as greater recall by older adults compared to individuals with AD, F(1, 161) = 41.18, MSE = 1.14, η2p = .20, p < .0001. The ANOVA examining the low-value items revealed a main effect of group, F(2, 195) = 31.97, MSE = 0.37, η2p = .25, p < .0001, which resulted from greater recall for younger adults compared to older adults, F(1, 142) = 51.89, MSE = 0.66, η2p = .27, p < .0001, but there was no difference in recall for older adults compared to individuals with AD, F < 1. In summary, aging affected recall regardless of value, but AD specifically affected recall of high-value items, rather than low-value items (although a potential floor effect may have affected recall of low value items).
It is also worthwhile to address whether the factor structure that was apparent in the entire sample was similar within the young, old, and AD participant groups separately. If it was, it would suggest that each group is using similar strategies in terms of selectively encoding the few highest value items and ignoring the majority of lower value items. We addressed this issue by calculating a two-factor solution for each of groups separately. The very mild and mild AD participants were combined in to one group (AD) in order to increase power (as there were only were only 13 participants in the original mild AD group). Although the sample sizes for the younger adult and AD groups were small, the factor structure in the overall sample replicated for all groups. Indeed, the data tend to support the contention that the five highest value items comprise one factor, and the seven lowest value items comprise another factor, for all three groups in the study (although there were some factor loadings that were lower for the AD group on the V1-7 factor). This factor analysis is both novel and powerful in terms of providing converging validity for the notion that all participants are selectively attending to only the highest value items, but the AD group is simply less efficient at doing so.
The Role of Working Memory Capacity in Selectivity
The selectivity task would appear to require efficient WMC because participants are asked to maintain the goal of discriminating high from low value items, while quickly allocating attentional resources to the encoding of the higher value items at the expense of the low value items. Hence, we examined the relationship between the WMC measures and both the recall of low-value and high-value items, and the selectivity index. Past research has shown that performance on complex span tasks is related to recall, which is consistent with the idea that recall depends on executive attention for effective encoding and retrieval of items from long-term memory (McCabe, Smith, & Parks, 2007; Park et al., 1996). Of greater interest in the current context is the extent to which WMC, or the efficiency of the central executive, is differentially related to encoding and recall of low-value and high-value items. If WMC is related to recall of high-value items (but not low value items), it would provide converging evidence for the role of attentional resources in value-directed remembering. Moreover, to the extent that WMC is related to value-directed remembering in AD patients, it would provide converging evidence that differences in attention are an important factor in the overall level of memory impairment seen in these individuals.
The role of WMC in the recall of low-value and high-value items, as well as overall selectivity, as measured by the selectivity index (SI), was examined by first calculating z-scores for both the reading span and computation span tasks, and then combining the z-scores for both tasks to create a single, WMC factor score. Note that scores for the computation span task were missing for 4 of the 35 young adults, 7 of the 108 healthy older adult controls, and 6 of the 54 individuals with AD. Because some of these missing scores were due to difficulty with the arithmetic portion of the task, and therefore they were not missing at random, these participants' data for all WMC tasks were removed from the regression analyses.
The correlations between WMC and recall are presented in Table 5. There were no significant correlations between WMC and recall in young adults, likely owing to a small sample size and a restriction of range of general intellectual ability (i.e., they were undergraduate students at a highly selective university). However, for healthy older adults and individuals with AD, WMC was positively correlated with recall of high-value items, but not with recall of low-value items, as shown in Table 5. This supports the hypothesis that selectively attending to higher value items depends on the ability to control attention, and furthermore, that within a sample of individuals diagnosed with AD, having a greater ability to control attention is related to more efficient control of memory. Moreover, there was a significant negative correlation between recall of low-value and high-value items in healthy older aduls and individuals with AD, indicating that when participants recalled more of the higher value items they also recalled fewer lower value items. One interpretation of these data is that when limited attentional resources were allocated to encoding of higher value items, there were less resources available for selection of lower value items, leading to an adaptive pattern of selection and recall, precisely what an efficient executive control system should do.
Table 5.
Correlations between Working Memory Capacity and Recall of Low-Value Items (Recall 1-7), High-Values Items (Recall 8-12), and Selectivity Index.
| WMC | Recall 1-7 | Recall 8-12 | SI | |
|---|---|---|---|---|
| Young Adults (N = 35) | ||||
| WMC | - | |||
| Recall 1-7 | .05 | - | ||
| Recall 8-12 | .16 | .14 | - | |
| Selectivity | .00 | -.61 | .44 | - |
| Healthy Older Adults (N = 102) | ||||
| WMC | - | |||
| Recall 1-7 | -.08 | - | ||
| Recall 8-12 | .30 | -.28 | - | |
| Selectivity | .22 | -.70 | .64 | - |
| Alzheimer's Disease (N = 48) | ||||
| WMC | - | |||
| Recall 1-7 | .17 | - | ||
| Recall 8-12 | .57 | -.30 | - | |
| Selectivity | .28 | -.69 | .78 | - |
Correlation coefficients in bold type are significant at p < .05
General Discussion
The present study investigated selective learning in healthy younger and older adults, and those with very mild and mild AD. Although previous research has widely documented impairments in memory in old age and AD, the present study shows that AD is also associated with a specific deficit in being selective and strategic about encoding operations, which likely contributes to their poorer memory efficiency. This builds on research that examines how attentional control and working memory are affected by AD, and extends this work to control over encoding operations in light of prioritizing items in memory. Older adults, who recalled fewer words than younger adults, were still able to selectively encode and recall high value words (see also Castel et al., 2002, 2007), whereas AD lead to an impairment in both recall and selectivity. This ability to selectively encode information is likely dependent on several possibly interrelated abilities, including inhibitory control, working memory capacity, monitoring, and metacognitive control related to using performance on previous trials to update resource allocation strategies.
We also investigated how executive control, as reflected by working memory capacity, was related to selective encoding. In the older adult and AD groups the results clearly showed that working memory capacity was related to recall of high value items, but not with recall of low value items, indicating that those with more efficient central executive functioning were more effective at directing their attention to encoding of higher value items. The correlation between working memory capacity and recall of high value items was not simply due to general memory ability, because the correlations between working memory capacity and recall of low value items were not significant. Moreover, the correlation between working memory capacity and the selectivity index was significant in the older adult group, and was marginally so in the AD group (p = .054), suggesting that the efficiency of memory encoding is related to the efficiency of the central executive component of the working memory system. However, the finding that the correlation between working memory capacity and the selectivity index was fairly weak, suggesting that there are strategic processes involved in selective encoding that are not shared with complex span tasks. These strategic processes may be more metacognitive in nature, and possibly involve monitoring and control functions that are not necessarily attention dependent (e.g., using feedback to decide how many items one should attempt to encode on the next list).
Another noteworthy finding is that an exploratory factor analysis revealed two distinct factors related to recall of high and low value items. The five highest value items loaded on one factor and the seven lowest value items loaded on another. This factor structure was the same when all groups were combined into one analysis, and also when separate analyses were conducted on each group. This suggests that all participants were selectively attending to the few highest value items, and these items were psychologically distinct from the lower value items. Thus, despite age and AD related declines in recall, there were no declines in the number of high value items that individuals were attempting to selectively rehearse. Nonetheless, there were important differences between groups in recall of low and high value items. First, older adults were better able to encode and recall the high value items than were individuals with AD (see Figure 1), which was confirmed by comparing the factor scores for high-value and low-value items. The factor analysis also showed that older adults and individuals with AD appeared to show a tradeoff in recall of high and low value items. This was revealed by the negative correlation between the high and low value factors within these groups. This result was not found in the younger adult group, which suggests that older adults and AD groups were using their limited resource in a selective manner. In other words, those who were able to effectively encode the higher value items did so at a cost to being able to recall lower value items.
The ability to effectively ‘use memory’ (see Benjamin, 2007), in light of memory impairment and to judge how important it is to remember certain information, is a critical function for older adults (e.g., Castel, 2007). Thus, the examination of memory efficiency provides additional measures of cognitive function that are important to consider as older adults attempt to adaptively optimize memory. Being able to focus on important information has implications in day to day functioning, such as remembering grocery prices and items (e.g., Castel, 2005). For example, imagine you have made a grocery list with 12 items to buy at the grocery store, but when you get to the store you have forgotten to bring the list. If you can only remember some of the items, it would be advantageous to remember to buy the most important items. The present study examined this form of prioritizing in the context of immediate recall, and additional research is necessary to determine the relationship between immediate memory efficiency and longer term memory, and if these two processes are related.
Although the selectivity task could be compared to the directed forgetting paradigm in which participants are told explicitly to remember or forget certain items (Bjork, Bjork, & Anderson, 1998), the selectivity task involves the use of strategic control and choice regarding which items to rehearse and try to recall, and unlike the directed forgetting paradigm, all items are useful to remember, but the reward is graded based on point value. Thus, the present findings provide some insight regarding the strategic control of remembering and forgetting, and how this relates to value, in older adults and those with AD.
The selectivity task can also be considered a task with prominent goal maintenance demands (e.g., Kane & Engle, 2003), as one must maintain the task goal of selectively attending to high-value items during the task. Because participants are trying to maintain items in working memory while strategically allocating attention to the encoding of more items, maintenance of the goal of attending to high-value items may be very difficult. Thus, the selectivity task shares some processing overlap with complex working memory span tasks (e.g., reading span), but unlike memory span tasks the selectivity task does not simply involve the use of a covert rehearsal strategies to maintain and recall short lists of items in serial order (e.g., McCabe, 2008). Indeed, although recall of high-value items was related to performance on complex span tasks in the current study, particularly for the AD group, selectivity was still largely independent of working memory capacity.
Primacy and recency effects that are typically found in free recall can influence selectivity, such that one must overcome these effects in order to remember high value words that might be presented in the middle of the list (see Watkins and Bloom, 1999), and that recall by AD individuals is strongly influenced by recency (Bayley et al., 2000). In the present study, AD individuals did display a robust recency effect, but a primacy effect was also present, suggesting that the AD group was not simply recalling the last few items. Additional research that examines how AD individuals can prevent recency in light of value will be useful (e.g., Buschke et al., 2006), and measures like the selectivity index that go beyond simply assessing how much information can be retained are important because they provide insight regarding how higher-level strategy use can influence memory efficiency.
In the present study, the AD individuals did show some degree of selectivity, and were well above chance in terms of selecting high value items relative to lower value items, and were not simply recalling a random selection of items, or simply the last few items that were presented. Thus, each group demonstrated some proficiency with the task, and was able to direct attention to high value information, but individuals with AD still recalled proportionally more of the lower value information than the other groups. This could also be interpreted in terms of poor metacognitive skills in AD, or less awareness (and execution) of the need to focus on high value information in order to optimize one's score (although the AD group was similar to other groups in terms of recalling more higher value items relative to lower values). Although the distinction between WMC and the selectivity index was partially established in the present analyses, it may be the case AD individuals were not able to efficiently and adaptively focus on fewer items with higher values in order to achieve an optimal score. Future research could examine whether AD individuals could enhance selectivity by focusing on fewer items, via training or explicit instructions about the nature of the selectivity index. Furthermore, in more everyday settings, learning to be selective about encoding high value information could allow one to remember important information, possibly at the expense of less important information.
Acknowledgments
We would like to thank the Clinical and Psychometric Cores of the Washington University Alzheimer's Disease Research Center (ADRC) for their diagnostic and testing assistance, Martha Storandt for providing the psychometric performance data, Jan Duchek for useful comments, and Jeff Templeton and Brian Webber for help developing the stimulus materials and testing the participants. This work was presented at the 12th Biennial Cognitive Aging Conference, Atlanta, GA, and was supported by National Institute on Aging Grants AG10145, AG03991, and AG05681.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/neu.
References
- Amieva H, Phillips LH, Della Sella S, Henry JD. Inhibitory functioning in Alzheimer's disease. Brain. 2004;127:949–967. doi: 10.1093/brain/awh045. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. The episodic buffer: A new component of working memory? Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy young and older adults. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. Oxford University Press; 2000. pp. 395–410. [Google Scholar]
- Balota DA, Faust ME. Attention in dementia of the Alzheimer's type. In: Bolla F, Cappa S, editors. Handbook of neuropsychology: Vol 6 Aging and dementia. 2nd. New York: Elsevier Science; 2001. pp. 51–80. [Google Scholar]
- Balota DA, Ferraro FR. Lexical, sublexical, and implicit memory processes in healthy young and healthy older adults and in individuals with dementia of the Alzheimer type. Neuropsychology. 1996;10:82–95. [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KI, Kessler B, Loftis B, et al. The English lexicon project. Behavior Research and Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Salmon DP, Bondi MW, Bui BK, Olichney J, Delis DC, Thomas RG, Thal LJ. Comparison of the serial position effect in very mild Alzheimer's disease, mild Alzheimer's disease, and amnesia associated with electroconvulsive therapy. Journal of the International Neuropsychological Society. 2000;6:290–298. doi: 10.1017/s1355617700633040. [DOI] [PubMed] [Google Scholar]
- Belleville S, Chertkow H, Gauthier S. Working memory and control of attention in persons with Alzheimer's disease and mild cognitive impairment. Neuropsychology. 2007;21:458–459. doi: 10.1037/0894-4105.21.4.458. [DOI] [PubMed] [Google Scholar]
- Benjamin AS. Memory is more than just remembering: Strategic control of encoding, accessing memory, and making decisions. In: Benjamin AS, Ross BH, editors. The psychology of learning and motivation. Vol. 48. London: Academic Press; 2007. [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: Relation of histologic markers to dementia severity. Archives of Neurology. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Bjork EL, Bjork RA, Anderson MC. Varieties of goal-directed forgetting. In: Golding JM, MacLeod C, editors. Intentional forgetting: Interdisciplinary approaches. Hillsdale, NJ: Erlbaum; 1998. pp. 103–137. [Google Scholar]
- Burgess C, Lund K. Modeling parsing constraints with high dimensional context space. Language and Cognitive Processes. 1997;12:177–210. [Google Scholar]
- Buschke H, Sliwinski MJ, Kuslansky G, Katz M, Verghese J, Lipton RB. Retention weighted recall improves discrimination of Alzheimer's disease. Journal of the International Neuropsychological Society. 2006;12:436–440. doi: 10.1017/s135561770606053x. [DOI] [PubMed] [Google Scholar]
- Capitani E, Della Sala S, Logie RH, Spinnler H. Recency, primacy, and memory: reappraising and standardising the serial position curve. Cortex. 1992;28:315–342. doi: 10.1016/s0010-9452(13)80143-8. [DOI] [PubMed] [Google Scholar]
- Castel AD. Memory for grocery prices in younger and older adults: The role of schematic support. Psychology and Aging. 2005;20:718–721. doi: 10.1037/0882-7974.20.4.718. [DOI] [PubMed] [Google Scholar]
- Castel AD. The adaptive and strategic use of memory by older adults: Evaluative processing and value-directed remembering. In: Benjamin AS, Ross BH, editors. The psychology of learning and motivation. Vol. 48. London: Academic Press; 2007. pp. 225–270. [Google Scholar]
- Castel AD, Benjamin AS, Craik FIM, Watkins MJ. The effects of aging on selectivity and control in short-term recall. Memory & Cognition. 2002;30:1078–1085. doi: 10.3758/bf03194325. [DOI] [PubMed] [Google Scholar]
- Castel AD, Farb N, Craik FIM. Memory for general and specific value information in younger and older adults: Measuring the limits of strategic control. Memory & Cognition. 2007;35:689–700. doi: 10.3758/bf03193307. [DOI] [PubMed] [Google Scholar]
- Castel AD, Balota DA, Hutchison KA, Logan JM, Yap MJ. Spatial attention and response control in healthy younger and older adults and individuals with Alzheimer's disease: Evidence for disproportionate selection breakdowns in the Simon task. Neuropsychology. 2007;21:170–182. doi: 10.1037/0894-4105.21.2.170. [DOI] [PubMed] [Google Scholar]
- Craik FIM. Human memory and aging. In: Bäckman L, von Hofsten C, editors. Psychology at the turn of the millennium. Hove, UK: Psychology Press; 2002. pp. 261–280. [Google Scholar]
- Craik FIM, Govoni R, Naveh-Benjamin M, Anderson ND. The effects of divided attention on encoding and retrieval processes in human memory. Journal of Experimental Psychology: General. 1996;125:159–180. doi: 10.1037//0096-3445.125.2.159. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Engle RW, Kane MJ. Executive attention, working memory capacity, and a two-factor theory of cognitive control. In: Ross B, editor. The Psychology of Learning and Motivation. Vol. 44. NY: Elsevier; 2004. pp. 145–195. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hanten G, Chapman SB, Gamino JF, Zhang L, Benton SB, Stallings-Roberson G, Hunter JV, Levin HS. Verbal selective learning after traumatic brain injury in children. Annals of Neurology. 2004;56:847–853. doi: 10.1002/ana.20298. [DOI] [PubMed] [Google Scholar]
- Hanten G, Li X, Chapman SB, Swank P, Gamino JF, Roberson G, Levin HS. Development of verbal selective learning. Developmental Neuropsychology. 2007;32:585–596. doi: 10.1080/87565640701361112. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology learning and motivation. Vol. 2. San Diego, CA: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal maintenance, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and non-selective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Logie R, Cocchini G, Della Sala S, Baddeley A. Is there a specific executive capacity for task co-ordination? Evidence from Alzheimer's disease. Neuropsychology. 2004;18:504–513. doi: 10.1037/0894-4105.18.3.504. [DOI] [PubMed] [Google Scholar]
- McCabe DP. The role of covert retrieval in working memory span tasks: Evidence from delayed recall tests. Journal of Memory and Language. 2008;58:480–494. doi: 10.1016/j.jml.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe DP, Smith AD, Parks CP. Inadvertent plagiarism in young and older adults. Memory & Cognition. 2007;35:231–241. doi: 10.3758/bf03193444. [DOI] [PubMed] [Google Scholar]
- McCabe DP, Robertson CL, Smith AD. Age differences in Stroop interference in working memory. Journal of Clinical and Experimental Neuropsychology. 2005;27:633–644. doi: 10.1080/13803390490919218. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Jr, Storandt M, Rubin EH, Price JL, Grant EA, Ball MJ, Berg L. Very mild Alzheimer's disease: Informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991;41:469–478. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Jr, Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer's disease. Annals of Neurology. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, Earles J, Frieske D, Zwahr M, Gaines C. Mediators of long-term memory performance across the life span. Psychology and Aging. 1996;11:621–637. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease: A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer type. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Watkins MJ, Bloom LC. Selectivity in memory: An exploration of willful control over the remembering process. 1999 Unpublished manuscript. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]