Abstract
Background
Guidelines recommend warfarin use in patients with atrial fibrillation solely on the basis of risk for ischemic stroke without antithrombotic therapy. These guidelines rely on ischemic stroke rates observed in older trials and do not explicitly account for increased risk for hemorrhage.
Objective
To quantify the net clinical benefit of warfarin therapy in a cohort of patients with atrial fibrillation.
Design
Mixed retrospective and prospective cohort study of patients with atrial fibrillation between 1996 and 2003.
Setting
An integrated health care delivery system.
Patients
13559 adults with nonvalvular atrial fibrillation.
Measurements
Warfarin exposure, patient characteristics, and outcome events were ascertained from health plan records and databases. Outcome events were validated by formal physician review. Net clinical benefit was defined as the annual rate of (ischemic strokes and systemic emboli prevented by warfarin) minus (intracranial hemorrhages attributable to warfarin multiplied by an impact weight). For the base case, the impact weight was 1.5, reflecting the greater clinical impact of intracranial hemorrhage versus thromboembolism.
Results
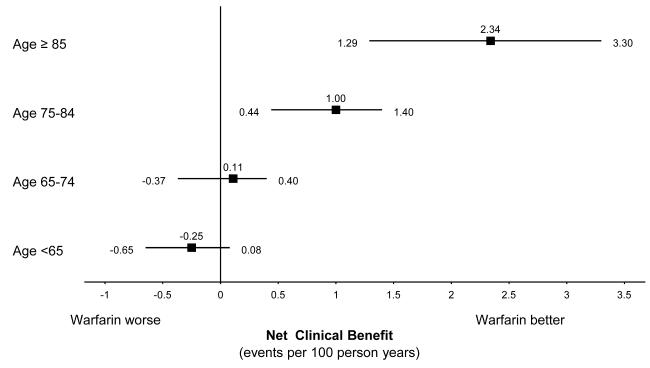
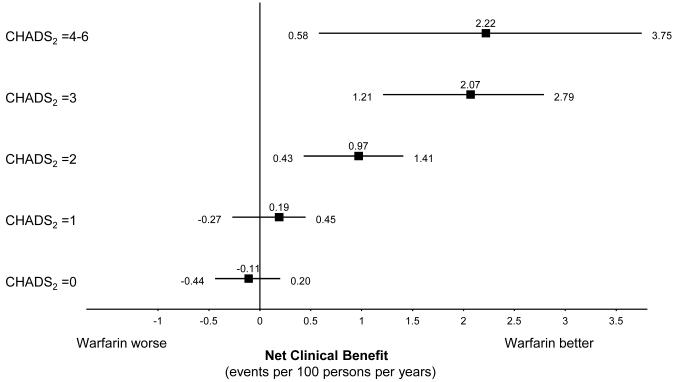
Patients accumulated more than 66000 person-years of follow-up. The adjusted net clinical benefit of warfarin for the cohort overall was 0.68% per year (95% CI, 0.34% to 0.87%). Adjusted net clinical benefit was greatest for patients with a history of ischemic stroke (2.48% per year [CI, 0.75% to 4.22%]) and for those 85 years or older (2.34% per year [CI, 1.29% to 3.30%]). The net clinical benefit of warfarin increased from essentially zero in CHADS2 (congestive heart failure/hypertension/age/diabetes/prior stroke2) stroke risk categories 0 and 1, to 2.22% per year (CI, 0.58% to 3.75%) in CHADS2 categories 4 to 6. The patterns of results were preserved using weighting factors for intracranial hemorrhage of 1.0 and 2.0.
Limitations
Residual confounding is a possibility. Some outcome events were probably missed by the screening algorithm or when medical records were unavailable.
Conclusion
Expected net clinical benefit of warfarin therapy is highest among patients with the highest untreated risk for stroke, which includes the oldest age category. Risk assessment that incorporates both risk for thromboembolism and risk for intracranial hemorrhage provides a more quantitatively informed basis for the decision on antithrombotic therapy in patients with atrial fibrillation.
Primary Funding Source
National Institute on Aging; National Heart, Lung, and Blood Institute; and Massachusetts General Hospital.
Warfarin anticoagulation is very efficacious in preventing thromboembolism (primarily ischemic stroke but also systemic thromboembolism) in patients with atrial fibrillation and is highly effective in practice (1, 2). Nonetheless, warfarin increases the risk for major hemorrhage and is burdensome for both physicians and patients. Therefore, clinical guidelines (3, 4) recommend warfarin only for patients with atrial fibrillation considered to be at intermediate or high risk for ischemic stroke. However, these risk-based anticoagulation guidelines have substantial limitations. First, they do not explicitly account for the risk for fatal and disabling hemorrhage due to warfarin. Second, they are primarily based on studies conducted more than 15 years ago. Recent studies (2, 5, 6) of patients with atrial fibrillation suggest a lower absolute risk for stroke while not receiving warfarin therapy, perhaps reflecting better treatment of hypertension or other risk factors. Finally, the net clinical benefit of risk-based recommendations for anticoagulation in patients with atrial fibrillation has not been demonstrated in large populations followed for long periods of time. In our observational study, we quantify the net clinical benefit of warfarin anticoagulation, defined as the estimated reduction in rate of thromboembolism, mainly ischemic stroke, minus 1.5 times the estimated increase in rate of intracranial hemorrhage attributable to warfarin therapy in standard stroke risk subgroups in the community-based cohort of patients in the ATRIA (AnTicoagulation and Risk Factors In Atrial Fibrillation)Study.
Methods
Study Population
The ATRIA cohort consists of 13559 adults with diagnosed nonvalvular atrial fibrillation who received care within Kaiser Permanente of Northern California, a large integrated health care delivery system. Cohort assembly has been described in detail elsewhere (2, 7). In brief, we identified patients with a diagnosis of atrial fibrillation between 1 July 1996 and 31 December 1997 by searching outpatient databases for internal medicine or cardiology visits in which an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), diagnosis of atrial fibrillation (427.31) was assigned and by searching electrocardiographic databases for a diagnosis of atrial fibrillation. The date of the first diagnosis of atrial fibrillation during the period of cohort assembly was considered the patient’s index date. Cohort assembly was finalized by November 1998, and the cohort was followed through 30 September 2003 with a combination of retrospective (back to July 1996) and prospective (through September 2003) review of health plan databases and medical records. Patients who died or withdrew from the health plan during follow-up were censored at their date of death or on the last day of the month in which they terminated their membership. Date of death was ascertained from health plan clinical or administrative files, from the California Automated Mortality Linkage System, or from the Social Security Administration Death Master File. The institutional review boards at Massachusetts General Hospital and the Kaiser Foundation Research Institute approved the research.
Patient Characteristics
We searched databases of clinical inpatient and ambulatory visits (outpatient clinic and emergency department) during the 5 years before each patient’s index date to identify previously diagnosed ischemic stroke, heart failure, coronary heart disease, and hypertension by using relevant ICD-9-CM codes (2). We used a validated, comprehensive health plan diabetes registry to identify patients with diabetes mellitus. Ascertainment of these stroke risk factors was validated against review of samples of outpatient medical records; crude agreement was high (78% to 96%), and corresponding κ statistics ranged from 0.51 to 0.89. Of note, stroke risk prediction based on risk factors ascertained in the ATRIA study was in good agreement with risk schemes generated by pooled randomized trial and chart-based cohort study data (2, 8–10). Information from medical care received at both health plan and non—health plan facilities was obtained through these databases. Data on age and sex were obtained from administrative databases.
Warfarin Exposure
We determined warfarin exposure based on information from automated clinical, pharmacy, and laboratory databases by using methods described elsewhere (2). We validated this computerized approach in 1207 patients by comparing it with warfarin status documented in their medical record at the time of an outcome event; the resulting κ statistic was 0.84. Nearly all discrepancies were due to transient discontinuation of warfarin treatment. If medical chart review of an outcome event showed that the patient stopped receiving warfarin within 5 days before the event, the event was considered to take place while the patient was receiving warfarin. For patients considered to be receiving warfarin, an adapted linear interpolation method was used to determine the person-time spent in the therapeutic international normalized ratio (INR) range of 2.0 to 3.0 (11). If the interval between consecutive INR measurements was greater than 8 weeks, the INR person-time for this period was considered “not available.”
Outcome Assessment
Thromboembolic Events
We searched hospitalization and billing claims databases through 30 September 2003 for primary ICD-9-CM discharge diagnoses that indicated potential thromboembolism, including ischemic stroke and other systemic embolism (ICD-9-CM codes available on request) (2). For both potential thromboembolic events and hemorrhagic events, medical record analysts obtained the relevant medical records by using a structured protocol. We did not investigate hospitalizations with the selected discharge diagnosis codes in a secondary position, because such hospitalizations rarely were prompted by new thromboembolic events. We estimate that our rates of ischemic stroke would have been increased relatively by 6% had we reviewed all such hospitalizations (2). We defined a validated ischemic stroke as a sudden neurologic deficit lasting more than 24 hours, corresponding to a vascular territory in the absence of primary hemorrhage, that was not explained by other causes (for example, trauma or infection). We defined a valid peripheral embolism as being identified by radiographic imaging, intraoperative examination, or pathologic findings (with no atherosclerotic disease in the affected artery). Thromboembolic events occurring after hospital admission, generally as a complication of medical care, were not included as outcome events. Two members of the physician outcomes review committee reviewed all potential thromboembolic events by using a formal protocol. Disagreements were resolved by a third committee member or by a consulting neurologist, if needed.
Intracranial Hemorrhage
We searched hospitalization and billing databases for primary and secondary discharge diagnoses of intracranial hemorrhage, including intraparenchymal, subdural, subarachnoid, and other hemorrhage (ICD-9-CM codes available on request). We excluded intracranial hemorrhage associated with a concomitant discharge diagnosis of major trauma (ICD-9-CM codes 852.1, 852.3, 852.5, and 853.1) and events not leading to hospitalization or those that occurred as a complication of a hospitalization for another problem.
Net Clinical Benefit
We defined the core net clinical benefit of warfarin therapy in atrial fibrillation as the annualized rate of thromboembolic events (TE rate) prevented minus the annualized rate of intracranial hemorrhages (ICH rate) induced multiplied by a weighting factor. The following equation illustrates this definition:
The weighting factor reflects the relative impact, in terms of death and disability, of an intracranial hemorrhage (including intracerebral, subdural, subarachnoid, and other hemorrhages) while receiving warfarin versus experiencing an ischemic stroke while not receiving warfarin (12). We assigned our base case a weighting factor of 1.5, reflecting outcomes in the ATRIA cohort (13, 14). We also provide additional sensitivity analyses by using weighting factors of 1.0 and 2.0.
For 10% of potential thromboembolic events and intracranial hemorrhages identified by ICD-9-CM codes, the patients’ medical records were unavailable or contained insufficient information to determine whether a valid event had occurred. We did not include these events. For events with missing clinical information, the ratio of patients receiving warfarin to those not receiving warfarin was similar to the ratio for validated events. Making the improbable assumption that all such potential events met our validity criteria increased our estimate of unadjusted overall net clinical benefit by only 0.07% per year.
Statistical Analysis
We calculated incidence rates of thromboembolism and intracranial hemorrhage as the number of events per 100 person-years of follow-up. Because individual participants in the ATRIA cohort could have alternating periods of receiving and not receiving warfarin, we used Poisson regression models with generalized estimating equations to estimate the net clinical benefit adjusted for stroke and intracranial hemorrhage risk factors. Our models treated risk factors as time-varying covariates. The overall model included warfarin use and risk factors. For the analysis of subgroups, we included an interaction term between warfarin use and the risk factor defining the subgroup. For estimation of overall net clinical benefit the model did not include an interaction term with warfarin. The risk factors used as control variables included age (4 categories: < 65 years, 65 to 74 years, 75 to 84 years, and ≥85 years), sex, previous ischemic stroke, hypertension, diabetes, heart failure, and coronary disease. We used the marginal prediction approach to obtain the adjusted absolute rates of thromboembolism and intracranial hemorrhage in patients who were receiving and not receiving warfarin (15, 16). To obtain the marginal adjusted means, we averaged the predicted rates generated from the models by first assuming all observations were in patients receiving warfarin and then assuming all observations were in patients not receiving warfarin. To obtain the 95% CIs of adjusted rates, differences between groups who were receiving and not receiving warfarin, and net clinical benefit, we used a bootstrap sample of 1000 replications (17). The analyses stratified by the CHADS2 (congestive heart failure/hypertension/age/diabetes/prior stroke2) risk score were not further adjusted, because CHADS2 is itself a risk adjustment scheme (Appendix Table). We performed all analyses by using SAS software, version 9.1.3 (SAS Institute, Cary, North Carolina).
Role of the Funding Source
The National Institute on Aging; the National Heart, Lung, and Blood Institute; and the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital provided funding for the study. The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
At baseline, the median age of our cohort of 13559 persons with atrial fibrillation was 73 years, with an interquartile range of 66 to 80 years. Overall, 20.2% had no major risk factors for ischemic stroke with atrial fibrillation, including age 75 years or older, previous ischemic stroke, diabetes mellitus, hypertension, or congestive heart failure (8). We recorded the events of the patients’ clinical course over a median of 6 years, accumulating more than 66000 person-years of observation in aggregate. During follow-up, 30% of patients developed at least 1 more stroke risk factor.
At entry to the cohort, 53% of patients were receiving warfarin treatment. At baseline, compared with those patients not taking warfarin, patients taking warfarin were less likely to be aged 75 years or older or to be women (Table 1). Overall, patients receiving warfarin tended to have a higher prevalence of other stroke risk factors and were less likely to have had a previous bleeding event (Table 1). During the study period, an additional 15.5% received warfarin for at least 30 days. Fourteen percent of INR tests for specific patients were separated by more than 8 weeks, thereby precluding interpolations to estimate time in various INR ranges for that 14% of tests. Excluding these noninterpolatable INR test intervals, the percentage of time in the therapeutic INR range (INR, 2.0 to 3.0) in patients receiving warfarin was 65.4%, with 24.2% below an INR of 2.0 and 10.4% above an INR of 3.0.
Table 1.
ATRIA cohort baseline characteristics stratified by warfarin status
| Baseline Warfarin Status |
||||
|---|---|---|---|---|
| Off Warfarin (n=6353) | On Warfarin (n=7206) | |||
| Characteristic | n | % | n | % |
| Age ≥ 75 years | 3090 | 48.6 | 3051 | 42.3 |
| Female | 2857 | 45.0 | 2938 | 40.8 |
| Prior ischemic stroke | 366 | 5.8 | 886 | 12.3 |
| Congestive heart failure | 1658 | 26.1 | 2494 | 34.6 |
| Hypertension | 3147 | 49.5 | 3760 | 52.2 |
| Diabetes mellitus | 980 | 15.4 | 1359 | 18.9 |
| Coronary artery disease | 1716 | 27.0 | 2210 | 30.7 |
| Prior intracranial bleed | 57 | 0.9 | 34 | 0.5 |
| Prior gastrointestinal bleed | 381 | 6.0 | 222 | 3.1 |
| Prior hematuria | 125 | 2.0 | 129 | 1.8 |
| Prior other bleed | 64 | 1.0 | 77 | 1.1 |
During follow-up, we identified 1092 valid thromboembolic events in the ATRIA cohort (1017 ischemic strokes and 75 systemic emboli), 407 in patients receiving warfarin and 685 in patients not receiving warfarin at the time of their event. We also identified 299 valid intracranial hemorrhagic events, including 139 intracerebral hemorrhages, 101 subdural hemorrhages, 45 subarachnoid or other hemorrhages, and 14 intracranial hemorrhages of unknown type. Among patients taking warfarin, 193 intracranial hemorrhage events occurred (98 intracerebral, 63 subdural, 24 subarachnoid or other intracranial, and 8 of unknown type).
The overall unadjusted annual rate of ischemic stroke or systemic embolism was 2.10% (95% CI, 1.96% to 2.28%) in patients not receiving warfarin therapy versus 1.27% (CI, 1.19% 1.44%) in patients receiving warfarin therapy. The overall unadjusted annual rate of intracranial hemorrhage was 0.58% (CI, 0.51% to 0.68%) in patients receiving warfarin therapy versus 0.32% (CI, 0.27% to 0.39%) in patients not receiving warfarin therapy. After adjustment for age, sex, previous stroke, diagnosed hypertension, diagnosed heart failure, diagnosed diabetes, and coronary artery disease, and incorporation of a weighting factor of 1.5 for intracranial hemorrhages (our base case), the overall adjusted net clinical benefit of warfarin was 0.68 (CI, 0.34 to 0.87) adverse events prevented per 100 patients per year (Tables 2 and 3).
Table 2.
Adjusted rates* and rate differences for thromboembolism (TE) and intracranial hemorrhage (ICH) by warfarin status
| Risk Factor |
Risk Factor Level |
TE | ICH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Off Warfarin | On Warfarin | Difference in Rate of TE† |
Off Warfarin | On Warfarin | Difference in Rate of ICH† |
||||||
| PY | Annual Rate/100 |
PY | Annual Rate/100 |
PY | Annual Rate/100 |
PY | Annual Rate/100 |
||||
| All Patients | All Patients | 32611 | 2.29 | 32130 | 1.25 | 1.04 | 32994 | 0.33 | 33415 | 0.57 | 0.24 |
| Age Group | <65 | 7752 | 0.64 | 5006 | 0.59 | 0.05 | 7762 | 0.10 | 5122 | 0.30 | 0.20 |
| 65-74 | 8421 | 1.57 | 10131 | 0.98 | 0.59 | 8482 | 0.12 | 10379 | 0.44 | 0.32 | |
| 75-84 | 11116 | 2.80 | 13651 | 1.54 | 1.26 | 11317 | 0.43 | 14327 | 0.60 | 0.17 | |
| ≥ 85 | 5322 | 4.70 | 3342 | 1.84 | 2.86 | 5433 | 0.79 | 3587 | 1.14 | 0.35 | |
| Sex | Male | 18649 | 1.70 | 18809 | 1.13 | 0.57 | 18781 | 0.32 | 19408 | 0.61 | 0.29 |
| Female | 13962 | 3.09 | 13320 | 1.39 | 1.70 | 14214 | 0.34 | 14007 | 0.51 | 0.17 | |
| Prior Stroke |
N | 31133 | 1.98 | 29232 | 1.09 | 0.89 | 30923 | 0.29 | 29286 | 0.51 | 0.22 |
| Y | 1478 | 6.57 | 2897 | 3.46 | 3.11 | 2072 | 0.75 | 4128 | 1.16 | 0.42 | |
| Hyper- tension |
N | 14307 | 1.73 | 12583 | 0.78 | 0.95 | 14406 | 0.30 | 12896 | 0.44 | 0.14 |
| Y | 18304 | 2.68 | 19547 | 1.57 | 1.11 | 18589 | 0.35 | 20519 | 0.66 | 0.31 | |
| CHF | N | 24090 | 1.84 | 20088 | 1.10 | 0.74 | 24320 | 0.29 | 20872 | 0.51 | 0.22 |
| Y | 8521 | 3.28 | 12041 | 1.58 | 1.70 | 8674 | 0.42 | 12543 | 0.70 | 0.28 | |
| Diabetes | N | 27395 | 2.06 | 25500 | 1.08 | 0.98 | 27625 | 0.32 | 26453 | 0.58 | 0.26 |
| Y | 5216 | 3.29 | 6629 | 1.98 | 1.31 | 5369 | 0.38 | 6962 | 0.53 | 0.16 | |
| CHADS2 Score* |
0 | 6143 | 0.37 | 3414 | 0.38 | (0.01) | 6133 | 0.05 | 3413 | 0.12 | 0.07 |
| 1 | 10262 | 1.19 | 9083 | 0.59 | 0.59 | 10191 | 0.22 | 9082 | 0.48 | 0.27 | |
| 2 | 9741 | 2.54 | 10810 | 1.26 | 1.28 | 9677 | 0.40 | 10897 | 0.61 | 0.20 | |
| 3 | 4761 | 3.89 | 5965 | 1.86 | 2.03 | 4835 | 0.64 | 6236 | 0.61 | (0.03) | |
| 4-6 | 1704 | 6.34 | 2857 | 3.25 | 3.08 | 2158 | 0.51 | 3787 | 1.08 | 0.57 | |
All rates and rate differences, except those for CHADS2, strata are adjusted to account for the effects of the remaining risk factors listed in this table plus coronary artery disease (see METHODS). The rates presented for CHADS2 are stratum-specific crude rates, i.e., not adjusted further since these strata themselves are based on multivariable models.
Difference in Rate of TE = (TE rate off warfarin−TE rate on warfarin);
Difference in Rate of ICH= (ICH rate on warfarin −ICH rate off warfarin)
TE=thromboembolic event; ICH=intracranial hemorrhage; PY=person-years; CHF=congestive heart failure; CHADS2 is the acronym for the stroke risk scoring system for patients with atrial fibrillation where C=congestive heart failure, H=hypertension, A=age ≥75 years, D=diabetes, and S=prior stroke (risk factors C, H, A and D are given 1 point each, S is doubly weighted, hence S2).
Table 3.
The net clinical benefit of warfarin therapy overall and by individual risk factors using different weights for ICH
| ICH weight = 1.5 (base case) | ICH weight = 1 | ICH weight = 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk Factor | Risk Factor Level |
Net Clinical Benefit |
95% CI | Net Clinical Benefit |
95% CI | Net Clinical Benefit |
95% CI | |||
| LCL | UCL | LCL | UCL | LCL | UCL | |||||
| All Patients | All Patients | 0.68 | 0.34 | 0.87 | 0.80 | 0.51 | 0.96 | 0.56 | 0.18 | 0.77 |
| Age Group | <65 | (0.25) | (0.65) | 0.08 | (0.15) | (0.48) | 0.14 | (0.35) | (0.81) | 0.05 |
| 65-74 | 0.11 | (0.37) | 0.40 | 0.27 | (0.16) | 0.55 | (0.05) | (0.58) | 0.27 | |
| 75-84 | 1.00 | 0.44 | 1.40 | 1.09 | 0.59 | 1.44 | 0.92 | 0.29 | 1.34 | |
| ≥ 85 | 2.34 | 1.29 | 3.30 | 2.51 | 1.62 | 3.37 | 2.17 | 0.93 | 3.18 | |
| Sex | Male | 0.13 | (0.23) | 0.37 | 0.28 | (0.03) | 0.49 | (0.02) | (0.43) | 0.25 |
| Female | 1.44 | 0.90 | 1.75 | 1.52 | 1.02 | 1.79 | 1.35 | 0.75 | 1.72 | |
| Prior Stroke | N | 0.56 | 0.22 | 0.72 | 0.67 | 0.37 | 0.81 | 0.45 | 0.06 | 0.64 |
| Y | 2.48 | 0.75 | 4.22 | 2.69 | 1.12 | 4.42 | 2.27 | 0.36 | 4.09 | |
| Hypertension | N | 0.74 | 0.35 | 1.02 | 0.81 | 0.47 | 1.06 | 0.66 | 0.22 | 0.99 |
| Y | 0.65 | 0.20 | 0.90 | 0.80 | 0.40 | 1.04 | 0.49 | (0.01) | 0.77 | |
| CHF | N | 0.41 | 0.09 | 0.66 | 0.52 | 0.24 | 0.75 | 0.30 | (0.08) | 0.58 |
| Y | 1.28 | 0.56 | 1.68 | 1.42 | 0.76 | 1.78 | 1.14 | 0.35 | 1.58 | |
| Diabetes | N | 0.59 | 0.25 | 0.82 | 0.72 | 0.42 | 0.92 | 0.46 | 0.06 | 0.72 |
| Y | 1.07 | 0.15 | 1.59 | 1.15 | 0.32 | 1.63 | 0.99 | (0.03) | 1.56 | |
| CHADS2 Score |
0 | (0.11) | (0.44) | 0.20 | (0.07) | (0.38) | 0.20 | (0.14) | (0.53) | 0.21 |
| 1 | 0.19 | (0.27) | 0.45 | 0.33 | (0.06) | 0.57 | 0.06 | (0.50) | 0.34 | |
| 2 | 0.97 | 0.43 | 1.41 | 1.07 | 0.61 | 1.45 | 0.87 | 0.26 | 1.35 | |
| 3 | 2.07 | 1.21 | 2.79 | 2.06 | 1.26 | 2.72 | 2.09 | 1.12 | 2.85 | |
| 4-6 | 2.22 | 0.58 | 3.75 | 2.51 | 1.04 | 4.01 | 1.94 | 0.19 | 3.52 | |
CHF=congestive heart failure; CI=confidence interval; LCL=lower confidence limit; UCL=upper confidence limit; CHADS2 is the acronym for the stroke risk scoring system for patients with atrial fibrillation where C=congestive heart failure, H=hypertension, A=age ≥75 years, D=diabetes, and S=prior stroke (risk factors C, H, A and D are given 1 point each, S is doubly weighted, hence S2)
For ICH weighted as 1, Net Clinical Benefit=(TE rateoff warfarin − TE rateon warfarin) − 1.0 × (ICH rateon warfarin − ICH rateoff warfarin), derived from adjusted rate differences shown in Table 2A
For ICH weighted as 1.5, Net Clinical Benefit=(TE rateoff warfarin − TE rateon warfarin) − 1.5 × (ICH rateon warfarin − ICH rateoff warfarin), derived from adjusted rate differences shown in Table 2A
For ICH weighted as 2, Net Clinical Benefit=(TE rateoff warfarin − TE rateon warfarin) − 2.0 × (ICH rateon warfarin − ICH rateoff warfarin), derived from adjusted rate differences shown in Table 2A
The benefits of warfarin increased as the absolute risk for thromboembolism increased, whereas the harms increased only moderately. Rates of thromboembolism in patients not receiving warfarin were clearly higher in the presence of any risk factor, with age 85 years or older and previous stroke conferring the greatest risk for subsequent ischemic stroke (Table 2). Also, the rates of thromboembolism were lower in patients receiving warfarin than in patients not receiving warfarin in the presence of any risk factor. However, the absolute reduction in rates of thromboembolism attributable to warfarin therapy in any risk factor category decreased as the absolute risk while not receiving warfarin therapy decreased. Although the adjusted rate of intracranial hemorrhage while not receiving warfarin was substantially higher among older persons and among those with a history of ischemic stroke, the absolute increase in the rate of intracranial hemorrhage associated with warfarin use stayed within a narrow range across the subgroups.
A history of ischemic stroke was the strongest risk factor for future ischemic stroke and also a strong risk factor for future intracranial hemorrhage in patients receiving warfarin. Nonetheless, using the base-case weighting factor of 1.5 for intracranial hemorrhage, the impact of warfarin on thromboembolism risk was much larger than its effect on intracranial hemorrhage risk, such that the adjusted net clinical benefit was 2.48% per year (CI, 0.75% to 4.22%) among patients with a history of stroke as opposed to 0.56% per year (CI, 0.22% to 0.72%) among those without a history of stroke (Table 3). The adjusted point estimates of net clinical benefit of warfarin therapy were higher in all other risk factor—positive categories (that is, older age, female sex, diagnosed heart failure, and diagnosed diabetes) except for diagnosed hypertension, in which the net clinical benefit was similar for patients with and without hypertension. These differences in estimates of net clinical benefit largely reflect the increased absolute benefit of warfarin therapy in reducing the rate of thromboembolism in the risk factor—positive groups.
Net clinical benefit increased monotonically with age, regardless of the weighting factor for intracranial hemorrhage (Table 3 and Figure, top). The benefit was near zero for patients younger than 75 years. Indeed, the point estimate suggests a small net harm in patients younger than 65 years, although the accompanying CI includes zero effect. The net clinical benefit of warfarin also increased substantially as the CHADS2 risk score increased (Table 3 and Figure, bottom). Using impact weights of 1.0 or 2.0 for the difference in rates of intracranial hemorrhage between patients receiving and not receiving warfarin, our estimates of net clinical benefit were changed in the expected direction. The overall pattern of results was largely preserved, reflecting the greater importance of the effect of warfarin on thromboembolism rates relative to its effect on intracranial hemorrhage rates (Table 3).
Figure.
The net clinical benefit of warfarin by age group (top) and CHADS2 score (bottom). Net clinical benefit is plotted as the adjusted, weighted adverse events prevented per 100 person-years by warfarin treatment, according to age group and CHADS2 score, respectively. Intracranial hemorrhage is weighted as 1.5 times the value of thromboembolism (see Methods section). Stratified rates for CHADS2 score are not further adjusted. CHADS2 = Congestive Heart Failure/Hypertension/Age/Diabetes/Prior Stroke2.
Discussion
To our knowledge, we provide the first quantitative, empirically based description of the net clinical benefit of warfarin treatment for patients with atrial fibrillation according to standard risk strata. We observed the clinical care of the large ATRIA cohort, which provided more than 66000 person-years of follow-up, with about half the person-years observed during warfarin treatment. We observed 1092 thromboembolic and 299 intracranial hemorrhagic events, all validated by physician review, allowing analyses of both the preventive benefit and the toxicity of warfarin treatment. In general, our assessment of net clinical benefit supports previous risk analyses, because risk for thromboembolism drives net clinical benefit (4, 8). However, our estimates of clinical benefit are generally smaller than those of previous studies for 2 reasons: First, and most important, the absolute risk for thromboembolism among patients in the ATRIA cohort not receiving warfarin was lower than older estimates of absolute risk, primarily derived from previous randomized clinical trials. Second, we explicitly subtracted an estimate of harm due to warfarin, here estimated as 1.5 times the net increase in rate of intracranial hemorrhage. Our findings emphasize the sizable benefit of warfarin therapy expected in patients at higher risk for thromboembolism because the absolute increase in risk for intracranial hemorrhage due to warfarin therapy remains fairly stable across thromboembolic risk categories. Patients with a history of ischemic stroke and those in the highest CHADS2 categories benefit most from anticoagulation. By contrast, patients in the lowest risk categories seem to gain little. In particular, our results validate recent guidelines (3, 4) restricting strong recommendations for anticoagulant therapy to patients with a CHADS2 score of 2 or more.
A notable finding was that the average net benefit of warfarin was greater the older the patient. Indeed, the point estimate for the net clinical benefit for the oldest age group (≥85 years) was substantially greater than the benefit estimated for the group between the ages of 75 and 84 years. This finding suggests that age 85 years or older may be an additional risk indication for anticoagulation. These age effects largely reflect the increased absolute risk for stroke with atrial fibrillation in patients not receiving warfarin as age increases and the relatively constant absolute net increase in risk for intracranial hemorrhage with increasing age. These results are consistent with the recent BAFTA (Birmingham Atrial Fibrillation Treatment of the Aged Study) trial (18), in which patients with atrial fibrillation whose average age was 82 years benefited substantially from warfarin therapy. Although patients in the ATRIA cohort are probably less highly selected than those in the BAFTA trial, they were undoubtedly considered reasonably safe candidates for anticoagulation by their treating physicians, and our results should be interpreted in that context.Our results reflect the clinical care provided to ATRIA cohort members. The quality of anticoagulation was very good with 65% of time on warfarin in the therapeutic range of INR 2-3 (excluding the 14% of time when TTR results could not be calculated).(19) While we don’t have explicit information on the use of aspirin among cohort members not taking warfarin, a previous case-control study nested within the ATRIA cohort revealed that approximately half were using aspirin regularly.(2) Our estimate of overall adjusted stroke-preventive effectiveness was 45%, modestly lower than that reported for the meta-analysis of pooled trials testing warfarin versus aspirin among patients with atrial fibrillation(20).
The overall unadjusted rate of ischemic stroke we observed in patients not receiving warfarin therapy, 2.10% per year, is substantially lower than the overall rate of 4.5% per year observed in the pooled results of the first 5 randomized trials of warfarin for patients with atrial fibrillation. These trials were conducted up to 2 decades ago (1). Likewise, the risk-stratified stroke rates in patients who were not receiving warfarin in the ATRIA cohort are substantially lower than those in the pooled trial data and also lower than those in the original hospitalized cohort in which the CHADS2 stroke risk score was validated. (1, 8) Several recent trials of antithrombotic therapy for atrial fibrillation (6, 21–23) have reported lower-than-expected ischemic stroke rates. These findings suggest that the risk for ischemic stroke among patients with atrial fibrillation has been decreasing in recent years, perhaps reflecting better control of hypertension and other risk factors (5). Decreasing stroke rates lessen the net clinical benefit of anticoagulation, thereby increasing the importance of high-quality anticoagulation (19). These decreasing rates also highlight the pressing need for research on more accurate prediction of stroke risk in patients with atrial fibrillation to allow more discriminating use of anticoagulants (9).
Our definition of net clinical benefit balances the preventive impact of warfarin on risk for thromboembolism, mainly ischemic stroke, versus the impact of increased risk for intracranial hemorrhage. The outcome of intracranial hemorrhage while receiving warfarin is generally worse than the outcome of atrial fibrillation—related ischemic stroke while not receiving warfarin, although both result in death or disability in most cases (13, 14, 24). Although intracerebral hemorrhage is particularly fatal, many of our cases of intracranial hemorrhage were subdural hematomas, whose outcomes are similar to those of ischemic stroke (14, 25). As a consequence, we weighted intracranial hemorrhage 50% worse than ischemic stroke. Although such a weighting is somewhat arbitrary, we provide estimates of net clinical benefit for weights of 1.0 and 2.0 as well, and the general pattern of results persists. Unlike other studies (26), ours did not include extracranial hemorrhages in the definition of net clinical benefit, because intracranial hemorrhages account for most fatal and disabling adverse events attributable to vitamin K antagonist therapy.(14) Our estimates of net clinical benefit can provide a useful anchoring point for discussions about antithrombotic therapy in patients with atrial fibrillation. The ATRIA cohort study has the advantages of distinctly long-term follow-up and many outcome events, allowing assessment of stratum-specific risks for thromboembolism and risks for intracranial hemorrhage in patients receiving and not receiving warfarin therapy. Similar analyses are not available from individual or pooled randomized trials. However, our nonrandomized observational assessment of the effect of warfarin is susceptible to confounding, particularly by indication and by contraindication, which will lead to underestimates of efficacy and toxicity, respectively (27). We feel these concerns are lessened by the fact that only a limited number of clinical risk factors for stroke or intracranial hemorrhage in patients with atrial fibrillation have been identified and that we had good measurements of them. (1, 4, 9, 28). Although our use of health plan databases may lead to some misclassification of comorbid conditions, our validation studies using medical chart review have been reassuring. In addition, these risk factors, as assessed in our cohort, did confer increased risk for thromboembolism, as in previous studies (2). The results we report, other than those for CHADS2 strata, are adjusted for these risk factors. But, as with all observational analyses of medical intervention, residual confounding is a concern.
We generated adjusted stratum-specific estimates of net clinical benefit by averaging rates of events over patient profiles within strata, which complicates the interpretation of comparisons across strata because the distribution of patient profiles will differ. However, when we used a uniform patient profile to generate adjusted rate estimates for all strata, the results were very similar to those reported in Table 2 (data not shown). We also may have missed some valid events either because they did not meet our ICD-9-CM codes screening criteria (2) or because clinical information was missing for a potential event. However, sensitivity analyses demonstrate that missing events would not have markedly altered our results.
In sum, we provide quantitative assessments of the net clinical benefit of warfarin anticoagulation within a usual care setting among patients with atrial fibrillation according to stroke risk strata. The absolute reduction in stroke risk by warfarin increased in higher stroke risk categories, whereas the absolute increase in risk for intracranial hemorrhage due to warfarin treatment across strata of clinical characteristics was fairly consistent. Our estimates of stroke risk reduction due to warfarin are lower than reductions seen in older clinical trials. Nonetheless, our findings highlight the potential sizable net clinical benefit of warfarin anticoagulation for patients with atrial fibrillation at higher risk for ischemic strokes.
Supplementary Material
Acknowledgments
Grant Support: By the National Institute on Aging (R01 AG15478); the National Heart, Lung, and Blood Institute (U19 HL091179); and the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital. Dr. Fang’s efforts were also partially supported by the National Institute on Aging (K23 AG28978).
Anticoagulation in Atrial Fibrillation:The ATRIA Study
CHADS2 stroke risk classification scheme for patients with atrial fibrillation1.
CHADS2 is an acronym for the risk factors and their scoring.
| Risk Factor | Letter Assigned | Points Assigned |
|---|---|---|
| CHF | C | 1 |
| Hypertension | H | 1 |
| Age ≥ 75 | A | 1 |
| Diabetes mellitus | D | 1 |
| History of ischemic stroke or transient ischemic attack |
S | 2 |
A patient’s CHADS2 score is the sum of the points assigned for each risk factor. For example, an 85 year old patient (+1) with hypertension (+1) and prior stroke (+2) but none of the other risk factors would have a CHADS2 score of 4. Possible scores range from 0 to 6. The higher the score, the higher the patient’s risk of stroke.
- 1.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001 Jun 13;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
Footnotes
Potential Financial Conflicts of Interest: Consultancies: D.E. Singer (Boehringer Ingelheim, Bayer, AstraZeneca, Sanofi-Aventis, Daiichi Sankyo, Johnson & Johnson). Honoraria: D.E. Singer (Bristol-Myers Squibb, Pfizer). Grants received: D.E. Singer (Daiichi Sankyo), A.S. Go (Johnson & Johnson).
Reproducible Research Statement: Study protocol and statistical code: Available from Dr. Singer (dsinger@partners.org). Data set: Not available.
Publisher's Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
References
- 1.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–57. [PMID: 8018000] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–92. doi: 10.1001/jama.290.20.2685. [PMID: 14645310] [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. American College of Cardiology ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:651–745. doi: 10.1093/europace/eul097. [PMID: 16987906] [DOI] [PubMed] [Google Scholar]
- 4.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, et al. American College of Chest Physicians Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:546S–592S. doi: 10.1378/chest.08-0678. [PMID: 18574273] [DOI] [PubMed] [Google Scholar]
- 5.Arima H, Hart RG, Colman S, Chalmers J, Anderson C, Rodgers A, et al. PROGRESS Collaborative Group Perindopril-based blood pressure-lowering reduces major vascular events in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke. 2005;36:2164–9. doi: 10.1161/01.STR.0000181115.59173.42. [PMID: 16141420] [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA. Current status of stroke risk stratification in patients with atrial fibrillation. Stroke. 2009 doi: 10.1161/STROKEAHA.109.549428. [PMID: 19461020] [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–34. doi: 10.7326/0003-4819-131-12-199912210-00004. [PMID: 10610643] [DOI] [PubMed] [Google Scholar]
- 8.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [PMID: 11401607] [DOI] [PubMed] [Google Scholar]
- 9.Fang MC, Go AS, Chang Y, Borowsky L, Pomernacki NK, Singer DE, ATRIA Study Group Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2008;51:810–5. doi: 10.1016/j.jacc.2007.09.065. [PMID: 18294564] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Boode BS, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–92. doi: 10.1161/01.CIR.0000145172.55640.93. [PMID: 15477396] [DOI] [PubMed] [Google Scholar]
- 11.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–9. [PMID: 8470047] [PubMed] [Google Scholar]
- 12.Glasziou PP, Irwig LM. An evidence based approach to individualising treatment. BMJ. 1995;311:1356–9. doi: 10.1136/bmj.311.7016.1356. [PMID: 7496291] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–26. doi: 10.1056/NEJMoa022913. [PMID: 12968085] [DOI] [PubMed] [Google Scholar]
- 14.Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120:700–5. doi: 10.1016/j.amjmed.2006.07.034. [PMID: 17679129] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korn EL, Graubard BI. Analysis of Health Surveys. J Wiley; Hoboken, NJ: 1999. [Google Scholar]
- 16.Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System. 2nd edition SAS Institute; Cary, NC: 2000. [Google Scholar]
- 17.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman and Hall/CRC; Boca Raton, FL: 1993. [Google Scholar]
- 18.Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, et al. BAFTA investigators Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [PMID: 17693178] [DOI] [PubMed] [Google Scholar]
- 19.Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, et al. ACTIVE W Investigators Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–37. doi: 10.1161/CIRCULATIONAHA.107.750000. [PMID: 18955670] [DOI] [PubMed] [Google Scholar]
- 20.van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441–8. doi: 10.1001/jama.288.19.2441. [PMID: 12435257] [DOI] [PubMed] [Google Scholar]
- 21.Healey JS, Hart RG, Pogue J, Pfeffer MA, Hohnloser SH, De Caterina R, et al. Risks and benefits of oral anticoagulation compared with clopidogrel plus aspirin in patients with atrial fibrillation according to stroke risk: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE-W) Stroke. 2008;39:1482–6. doi: 10.1161/STROKEAHA.107.500199. [PMID: 18323500] [DOI] [PubMed] [Google Scholar]
- 22.Olsson SB, Executive Steering Committee of the SPORTIF III Investigators Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362:1691–8. doi: 10.1016/s0140-6736(03)14841-6. [PMID: 14643116] [DOI] [PubMed] [Google Scholar]
- 23.Bousser MG, Bouthier J, Büller HR, Cohen AT, Crijns H, Davidson BL, et al. Amadeus Investigators Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open-label, non-inferiority trial. Lancet. 2008;371:315–21. doi: 10.1016/S0140-6736(08)60168-3. [PMID: 18294998] [DOI] [PubMed] [Google Scholar]
- 24.Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. Stroke. Lancet. 2003;362:1211–24. doi: 10.1016/S0140-6736(03)14544-8. [PMID: 14568745] [DOI] [PubMed] [Google Scholar]
- 25.Gladstone DJ, Bui E, Fang J, Laupacis A, Lindsay MP, Tu JV, et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40:235–40. doi: 10.1161/STROKEAHA.108.516344. [PMID: 18757287] [DOI] [PubMed] [Google Scholar]
- 26.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, et al. ACTIVE Writing Group of the ACTIVE Investigators Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–12. doi: 10.1016/S0140-6736(06)68845-4. [PMID: 16765759] [DOI] [PubMed] [Google Scholar]
- 27.Miettinen OS. The need for randomization in the study of intended effects. Stat Med. 1983;2:267–71. doi: 10.1002/sim.4780020222. [PMID: 6648141] [DOI] [PubMed] [Google Scholar]
- 28.Fang MC, Singer DE, Chang Y, Hylek EM, Henault LE, Jensvold NG, et al. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation. 2005;112:1687–91. doi: 10.1161/CIRCULATIONAHA.105.553438. [PMID: 16157766] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.