Abstract
Eosinophils are implicated in the pathophysiology of respiratory virus infection, most typically in negative roles, such as promoting wheezing and bronchoconstriction in conjunction with virus-induced exacerbations of reactive airways disease and in association with aberrant hypersensitivity responses to antiviral vaccines. However, experiments carried out in vitro and in vivo suggest positive roles for eosinophils, as they have been shown to reduce virus infectivity in tissue culture and promote clearance of the human pathogen, respiratory syncytial virus (RSV) in a mouse challenge model. The related natural rodent pathogen, pneumonia virus of mice (PVM) is highly virulent in mice, and is not readily cleared by eosinophils in vivo. Interestingly, PVM replicates in eosinophils and promotes cytokine release. The molecular basis of virus infection in eosinophils and its relationship to disease outcome is currently under study.
Keywords: Eosinophils, Inflammation, Virus, Ribonuclease, Cytokine
Enigmatic Eosinophils
Eosinophils are among the most enigmatic of all cells of the immune system. In the recent past, much about their basic biology, the nature of their effector functions, and roles played in various disease states has come under renewed scrutiny [1, 2]. For example, the tacit assumption that eosinophils provide protection against infection with helminthic parasites is now a subject of profound disagreement [reviewed in 3]. Likewise, eosinophils have long been perceived as major pathophysiologic mediators in respiratory allergy and asthma, yet therapeutic trials with humanized anti-interleukin-5 monoclonal antibodies [4, 5] and studies with eosinophil-ablated mouse models [6] have created substantial controversy. Finally, even the most treasured belief, that eosinophils mediate all significant effector functions via degranulation of cationic proteins has been called into question [7]. At the same time, the role of eosinophils in respiratory virus infection, a connection that had more or less gone unnoticed, has emerged as one of the many new and exciting areas of study linking infectious pathogens and allergic responses [8–11].
Eosinophils, Asthma and Antiviral Host Defense – a Double-Edged Sword?
The double-edged sword is a concept that reflects the competing beneficial and detrimental features of a given physiologic/pathophysiologic response, and has been used to formulate our current understanding of the physiology of the neutrophil and diseases involving dysregulation of neutrophil recruitment and activation [12]. For example, although neutrophils are clearly effective at promoting host defense against bacterial pathogens via degranulation, phagocytosis, and production of reactive oxygen species, the dysregulation of these essential, beneficial responses can leadto reperfusion injury and adult respiratory distress syndrome(reviewed in 13).
We would like to present the possibility of a clear and definitive parallel when considering eosinophils and the pathogenesis of reactive airways disease. There are now several studies demonstrating a striking correlation between severe respiratory syncytial virus (RSV) infection in infancy and the development of reactive airways disease in later childhood years (recently reviewed in 14, 15). Respiratory virus infections, including RSV, are a universal affliction of infancy, childhood and beyond. Once reactive airways disease is established, subsequent respiratory virus infection is clearly accepted as among the most common causes of disease exacerbation [14, 15]. We propose the possibility that asthma and reactive airways disease represent a dysregulation of what are otherwise essential and beneficial responses. Whatever causes this dysregulation to be established -whether it is infection with a specific respiratory virus at a specific developmental stage in a genetically and/or environmentally susceptible individual or some subset of the aforementioned - it is intriguing to consider the possibility that the responses characteristic of the asthmatic state represent the dysregulation of responses primarily directed toward a beneficial role in promoting innate antiviral host defense.
Eosinophils, Evolution and Structure
While all mammals have eosinophils, easily recognized on the basis of nuclear morphology and cytoplasmic granule staining [Figure 1], there are striking species-specific differences. For example, human eosinophils express the high affinity IgE receptor, FcεRI, while mouse eosinophils do not. Furthermore, the Charcot-Leyden crystal (CLC) protein, a major component of human eosinophils, has no functional correlate in mouse eosinophils, nor any identifiable ortholog in the mouse genome. Mouse eosinophils express Siglec F, a sialic acid-binding Ig-like lectin that is a functional but highly divergent ortholog of the human eosinophil protein, Siglec 8 [18]. Vis à vis respiratory inflammation, mouse eosinophils have distinctly different responses to chemotactic cytokines than human eosinophils [19]. Equally remarkable, and the subject of much of our group’s research, are the eosinophil secretory ribonucleases, EDN and ECP, which are present in high concentration in the eosinophil granules. EDN and ECP are among the most rapidly evolving functional coding sequences known among primates [20, 21] so much so that, as shown in Figure 2A, orthologous sequences in non-primate mammals cannot be detected by hybridization methods; the mouse eosinophil-associated RNases, a large cluster of equally rapidly-evolving non-overlapping sequences that have emerged via rapid duplication and gene sorting, are distant orthologs of their human counterparts [22, 23; Figure 2B]. Despite rapid evolution, all coding sequences identified have elements necessary to maintain the canonical RNase A tertiary structure and catalytic elements necessary to support enzymatic activity.
Figure 1. Eosinophils.
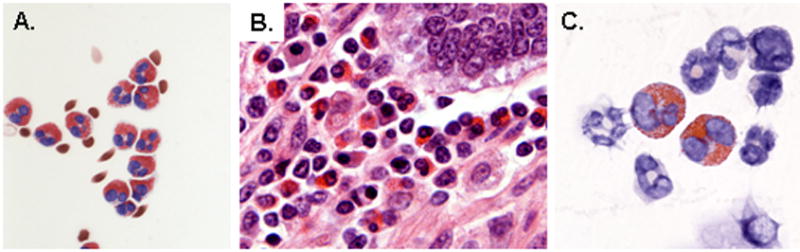
(A) Human eosinophils isolated from peripheral blood by negative selection (B) Eosinophilic infiltrates in the intestinal wall of an Owl monkey, A. trivirgatus (image courtesy of Dr. Alfonso Gozalo, Comparative Medicine Branch, NIAID) (C) Eosinophils in mouse bone marrow cytospin. Eosinophils can be found in all mammalian species, and are easily recognized by their large, prominently stained cytoplasmic granules. Panels (A) and (C) reprinted with permission from reference [36].
Figure 2. Evolution of eosinophil secretory ribonucleases.
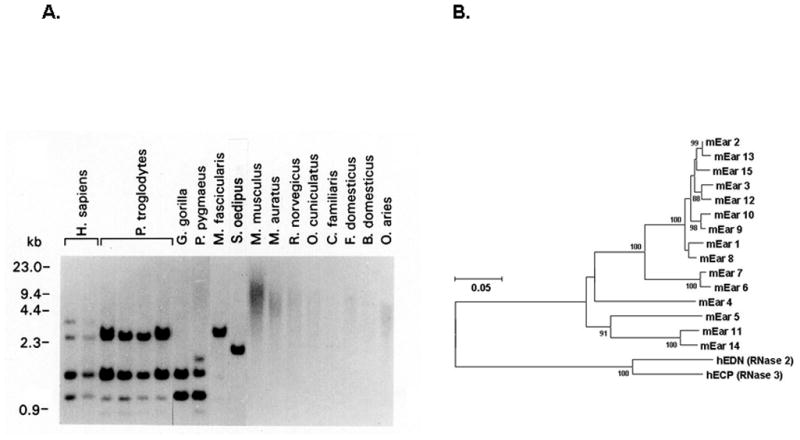
(A) Genomic DNA probed with the coding sequence of the eosinophil-derived neurotoxin (EDN/RNase 2), demonstrating variant genomic structure among closely-related primates, and no detectable hybridizing sequence among non-primate mammals. EDN and eosinophil cationic protein (ECP) are among the most rapidly evolving functional coding sequences identified among primate species. (B) Neighbor-joining tree documenting relationships among mouse eosinophil-associated ribonucleases and human EDN and ECP. Panel (A) reprinted with permission from reference [20].
Initial Hypothesis
Although eosinophils are typically perceived as contributing to respiratory dysfunction, including tissue damage and bronchoconstriction and are associated with asthmatic disease or hypersensitivity responses, either alone or in association with respiratory virus infection, from an evolutionary standpoint, a cell type will not persist in nature if all or most of its major functions are deleterious to the host. As such, we considered the possibility that there are “yin-yang” aspects to eosinophilic inflammation, much like the dual nature of neutrophilic inflammation in the setting of bacterial infection. Initially, we proposed that eosinophils, relying all or in part on their array of powerful and rapidly evolving secretory ribonucleases, could play a role in promoting host defense by direct targeting of ribonucleolytically-vulnerable single-stranded RNA genome of respiratory virus pathogens such as RSV.
Eosinophils Reduce Virus Infectivity In Vitro
To begin, we asked very simple questions. What was the impact of adding isolated human eosinophils to respiratory syncytial virus virions prior to infecting target cells in culture? What we found was fairly straightforward [Figure 3A]. When eosinophils were added in increasing concentration to a fixed number of viruses, a dose-dependent inhibition of virus infectivity was observed [24]. This inhibition could be reversed if ribonuclease inhibitor was added prior to addition of the eosinophils, suggesting that the secretory ribonucleases were promoting this effect, and were necessary, if not wholly sufficient. We were also able to demonstrate a reduction in infectivity with recombinant EDN alone, and that this effect was dependent on EDN’s ribonucleolytic activity. We are continuing to explore the mechanism of the antiviral activity, which is far from straightforward.
Figure 3. Eosinophils and antiviral interactions with respiratory syncytial virus (RSV).
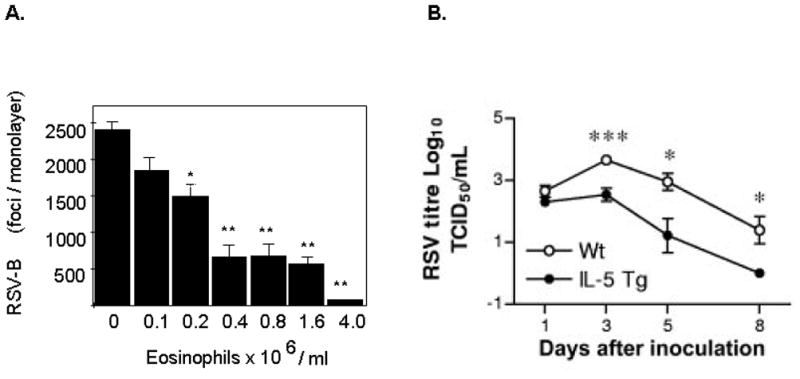
(A) Eosinophils reduce the infectivity of respiratory syncytial virus (RSV) in tissue culture. Increasing concentrations of eosinophils function in a dose dependent fashion to reduce the number of infectious virions as shown. (B) Eosinophils promote clearance of RSV in a mouse challenge model. Virus is cleared more rapidly from the lungs of eosinophil-enriched interleukin-5 (IL-5) transgenic mice when compared to wild type. Panels (A) and (B) reprinted with permission from references [24] and [26], respectively.
Eosinophils and Antiviral Host Defense In vivo
The first study to address this question was that of Adamko and colleagues [25] who were performing a study focused on M2 muscarinic receptor function and allergic responses. However, as part of this work, they explored infection with Sendai virus (family Paramyxoviridae, genus Respirovirus) in guinea pigs initially sensitized with ovalbumin. The authors found that reducing the number of eosinophils in bronchoalveolar lavage fluid by systemic administration of anti-IL-5 antibody resulted in a pronounced increase in virus titer in lung tissue, providing the first evidence in vivo for a role for eosinophils in promoting antiviral host defense.
A second study, by Phipps and colleagues [26], addressed the question directly, exploring clearance of the human pathogen respiratory syncytial virus (RSV; family Paramyxoviridae, genus Pneumovirus) in wild type and eosinophil enriched IL-5 transgenic mice [Figure 3B]. As shown, RSV clearance proceeded more efficiently in the IL-5 transgenic mice and, even more interesting, the authors demonstrated that the full antiviral effects were directly dependent on intact signaling via MyD88, the adaptor protein utilized by TLRs, including TLR 7 to promote signal transduction. Thus, not only is this another clear demonstration of antiviral effects of mouse eosinophils in vivo, but the first suggestion of a role for TLRs – specifically TLR7, in promoting eosinophil-mediated antiviral activity.
Interestingly, initial studies suggest that eosinophils may not be as effective at combating infection with pneumonia virus of mice (PVM), a natural rodent pathogen that undergoes rapid and robust replication in mouse lung tissue. This will be discussed below after this pathogen model has been introduced.
Pneumonia Virus of Mice and Eosinophils
As we mentioned earlier, all mammals have eosinophils, but eosinophil structure and contents differ dramatically among species. Given these evolutionary considerations, we have focused much of our attention on developing a species-matched pathogen model. Pneumonia virus of mice (PVM; family Paramyxoviridae, genus Pneumovirus) is closely related to the human and bovine respiratory syncytial viruses [Figure 4; reviewed in 27–29]. Robust replication of PVM takes place in bronchial epithelial cells in response to a minimal intranasal inoculum. Virus replication in situ results in local production of proinflammatory cytokines (MIP-1α, MIP-2, MCP-1 and IFNγ) and profound granulocyte recruitment to the lung. If left untreated, PVM infection and the ensuing inflammatory response ultimately lead to pulmonary edema, respiratory compromise and death, and as such, the infection resembles the more severe forms of human respiratory syncytial virus infection as experienced by human infants. Using this model, we have presented several combined antiviral/immunomodulatory strategies that have successfully reduced morbidity and mortality when administered to infected, symptomatic mice, and thus hold promise as realistic therapeutic strategies for virus infections in human subjects [30–32].
Figure 4. Pneumonia virus of mice (PVM) is a natural pathogen of rodent species.
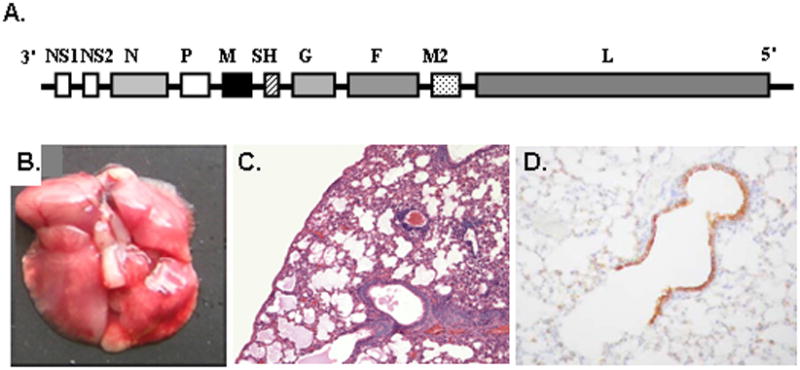
(A) PVM is a negative sense non-segmented single stranded RNA virus, family Paramyxoviridae, genus Pneumovirus, gene order as shown. (B) Challenge with a minimal inoculum (<100 pfu) results in a severe respiratory infection, with macroscopic evidence of hemorrhage and (C) microscopic pathology including profound granulocytic inflammation and edema. (D) Immunoreactive virus can be detected in bronchiolar epithelial cells. Panels (A), (B), (C), and (D) reprinted with permission from references [29], [37], [38], and [33], respectively.
We have recently determined a means to study mice that have survived PVM infection. With a reduced inoculum size and volume, we observe virus clearance by day 9, while clinical symptoms and respiratory dysfunction persist through days 14 and 17 post-inoculation, respectively, even without subsequent provocation [Figure 5]. Via gene microarray and ELISA, we identified expression profiles of proinflammatory mediators that correlate with persistent respiratory dysfunction, including several eosinophil chemoattractants. This promises to be an extremely useful model for studying the role of eosinophils in post-infectious respiratory dysfunction in vivo [33]
Figure 5. Virus replication and prolonged respiratory dysfunction.
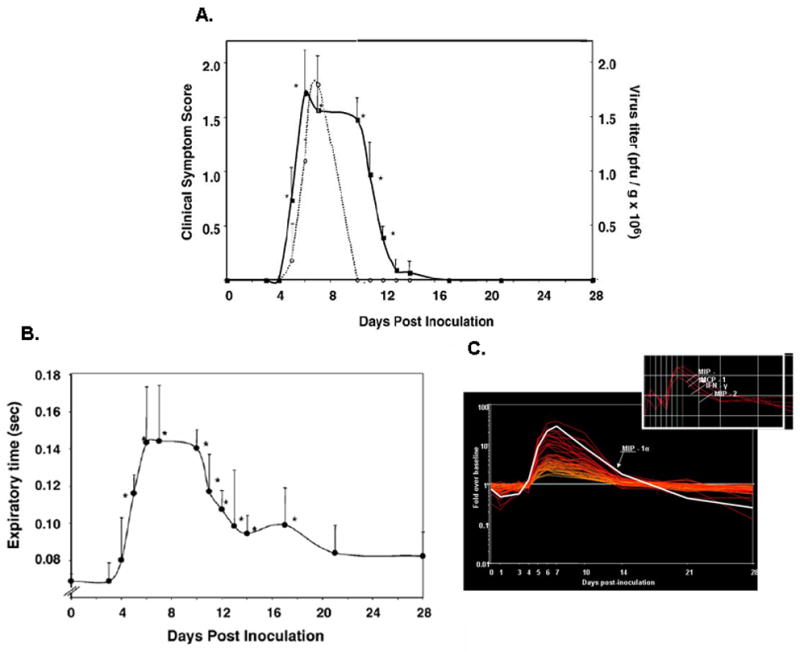
(A) As shown, virus replication (dotted line) peaks at day 7 in response to a minimal inoculum, and infectious virus can no longer be detected after day 10. In contrast, clinical symptom scores (full line; based on 6-point objective criteria) remain elevated through day 14, and (B) respiratory dysfunction, documented here as expiratory time, remains statistically above baseline through day 16. (C) Prolonged respiratory dysfunction correlates directly with persistent production of proinflammatory chemokines, despite resolution of the infection per se. Reprinted with permission from reference [33].
Interestingly, initial studies suggest that eosinophils may not be as effective at clearing PVM from lungs of infected mice as they are against other virus pathogens. These results emerged from studies of mice vaccinated with formalin-fixed PVM antigens, designed to replicate the “enhanced disease” paradigm seen in response to formalin-fixed RSV vaccination (recently reviewed in 34). In wild type mice vaccinated with formalin-fixed PVM antigens, we observed profound pulmonary eosinophilia in response to challenge with active PVM infection. As anticipated, no eosinophilia is observed in response to PVM challenge of eosinophil-ablated ΔdblGATA mice vaccinated with the same formalin-fixed PVM antigens, yet lung virus titers from the PVM challenge are indistinguishable, suggesting that eosinophils are without impact (manuscript in review). Among the reasons for the differences among these models, PVM may have some means of disabling TLR7-mediated signaling, a feature underlying the efficient clearance of RSV. Similarly (and perhaps related to this?), PVM is more virulent in vivo than is RSV or even Sendai virus, and, as discussed below, PVM actually infects and replicates within mouse eosinophils. We are in the process of elucidating this finding and exploring the responses of infected vs. uninfected eosinophils.
Eosinophils are Targets of Virus Infection
As part of a separate initiative in our laboratory, we have recently described an ex vivo culture system which generates large numbers of eosinophils at high purity from unselected mouse bone marrow progenitors [Figure 6A, B]. In addition to expressing the appropriate mouse eosinophil granule proteins and cell surface antigens, we demonstrated that these cells produce characteristic cytokines and undergo chemotaxis toward mouse eotaxin-1 [35].
Figure 6. Eosinophils are targets of virus infection.
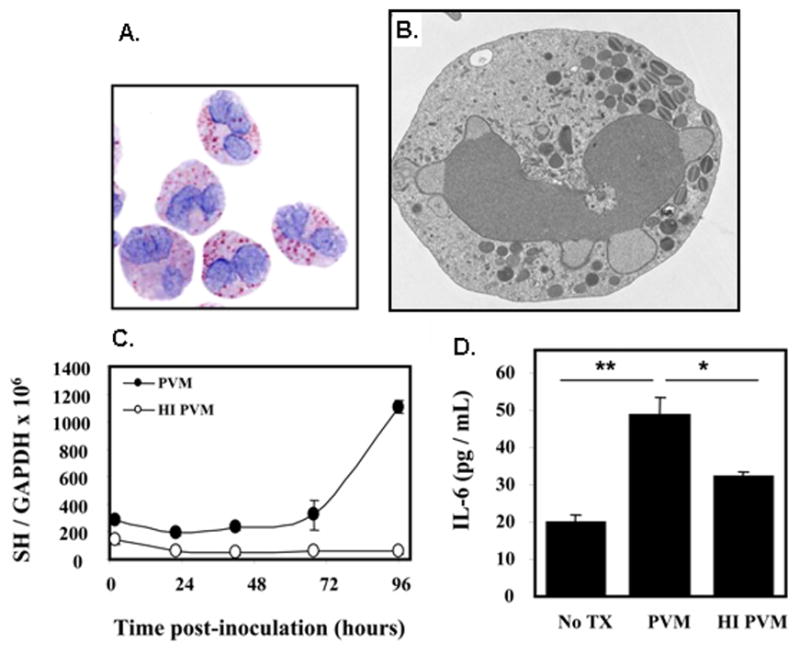
(A) Mouse eosinophils grown in culture from unselected normal bone marrow, (B) Electron micrograph of cultured mouse eosinophil shown in (A) depicting normal nuclear, cytoplasmic and granule morphology; image courtesy of Dr. Elizabeth Fischer, Research Technologies Section, Rocky Mountain Laboratories, NIAID; (C) Replication of PVM in mouse eosinophils as determined by quantitative RT-PCR detection of the virus SH gene; no replication of heat-inactivated (HI) virus is observed. (D) Active replication is accompanied by release of interleukin-6. Panels (A), (C) and (D) are reprinted with permission from reference [35].
As part of our ongoing efforts toward understanding the cellular basis of PVM infection, we found that PVM replication was evident within these eosinophil cultures; clear evidence of virus replication emerges via quantitative RT-PCR detection of the virus SH gene as shown [Figure 6C]. Virus replication is accompanied by release of the cytokine, interleukin-6 [Figure 6D]. We have obtained similar results with mouse eosinophils isolated from IL-5 transgenic mice and we are examining eosinophils isolated from lung of mice undergoing PVM infection in vivo for evidence of active replication and cytokine release.
This finding elicits many critical questions. We are interested in determining precisely how the virus infects an eosinophil and in what subcellular compartment virus replication takes place. To answer this question, we are actively attempting to discern the ultrastructure of PVM-infected mouse eosinophils. Furthermore, we would like to know whether or not replication disables the cell, diminishing, or perhaps augmenting its capacity to undergo chemotaxis, degranulation, and/or antigen presentation, and whether or not an infected eosinophil releases virions (ie… undergoes productive infection) and/or yields to apoptosis. Finally, it will be important to have a full accounting of the mediators released specifically by eosinophils in the lungs of PVM-infected mice in vivo, and to determine how and when these mediators contribute to the active infection and to the post-infectious recovery period.
Conclusions
We continue to explore the role of eosinophils and their interactions with respiratory viruses in vivo and in vitro. Human eosinophils reduce the infectivity of RSV in tissue culture and promote clearance of RSV from mouse lungs in vivo, the latter dependent on TLR7-MyD88 signaling. The rodent pathogen, PVM, is highly virulent in mice, and not readily cleared by eosinophils. We have determined that PVM replicates within eosinophils, and we are currently exploring the impact of this finding on the pathogenesis of the respiratory virus infection in vivo. We are intrigued by the possibility that replication within eosinophils may play a role in the pathophysiology of virus-induced asthma exacerbations.
Acknowledgments
Ongoing studies in the Eosinophil Biology Section of the Laboratory of Allergic Diseases, NIAID are supported by funding from the NIAID Division of Intramural Research.
References
- 1.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 3.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 4.Menzies-Gow A, Robinson DS. Eosinophils, eosinophilic cytokines (interleukin-5), and antieosinophilic therapy in asthma. Curr Opin Pulm Med. 2002;8:33–38. doi: 10.1097/00063198-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Leckie MJ. Anti-interleukin-5 monoclonal antibodies: preclinical and clinical evidence in asthma models. Am J Respir Med. 2003;2:245–259. doi: 10.1007/BF03256653. [DOI] [PubMed] [Google Scholar]
- 6.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 7.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin Exp Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 8.Jacoby DB. Pathophysiology of airway viral infections. Pulm Pharmacol Ther. 2004;17:333–336. doi: 10.1016/j.pupt.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–8. [PubMed] [Google Scholar]
- 11.Welliver RC. Immunology of respiratory syncytial virus infection: eosinophils, cytokines, chemokines and asthma. Pediatr Infect Dis J. 2000;19:780–783. doi: 10.1097/00006454-200008000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Yarza EG, Moreno A, Làzaro P, Mejías A, Ramilo O. The association between respiratory syncytial virus infection and the development of childhood asthma: a systematic review of the literature. Pediatr Infect Dis J. 2007;26:733–739. doi: 10.1097/INF.0b013e3180618c42. [DOI] [PubMed] [Google Scholar]
- 15.Mohapatra SS, Boyapalle S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin Microbiol Rev. 2008;21:495–504. doi: 10.1128/CMR.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukacs NW, Smit J, Lindell D, Schaller M. Respiratory syncytial virus-induced pulmonary disease and exacerbation of allergic asthma. Contrib Microbiol. 2007;14:68–82. doi: 10.1159/000107055. [DOI] [PubMed] [Google Scholar]
- 17.Hansbro NG, Horvat JC, Wark PA, Hansbro PM. Understanding the mechanisms of viral induced asthma: new therapeutic directions. Pharmacol Ther. 2008;117:313–353. doi: 10.1016/j.pharmthera.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–1135. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 19.Borchers MT, Ansay T, DeSalle R, Daugherty BL, Shen H, Metzger M, Lee NA, Lee JJ. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J Leukoc Biol. 2002;71:1033–1041. [PubMed] [Google Scholar]
- 20.Rosenberg HF, Dyer KD, Tiffany HL, Gonzalez M. Rapid evolution of a unique family of primate ribonuclease genes. Nat Genet. 1995;10:219–223. doi: 10.1038/ng0695-219. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg HF, Dyer KD. Eosinophil cationic protein and eosinophil-derived neurotoxin. Evolution of novel function in a primate ribonuclease gene family. J Biol Chem. 1995;270:21539–21544. doi: 10.1074/jbc.270.37.21539. [DOI] [PubMed] [Google Scholar]
- 22.Larson KA, Olson EV, Madden BJ, Gleich GJ, Lee NA, Lee JJ. Two highly homologous ribonuclease genes expressed in mouse eosinophils identify a larger subgroup of the mammalian ribonuclease superfamily. Proc Natl Acad Sci U S A. 1996;93:12370–12375. doi: 10.1073/pnas.93.22.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Dyer KD, Rosenberg HF. Evolution of the rodent eosinophil-associated RNase gene family by rapid gene sorting and positive selection. Proc Natl Acad Sci U S A. 2000;97:4701–4706. doi: 10.1073/pnas.080071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 25.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999 Nov 15;190(10):1465–78. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg HF, Domachowske JB. Pneumonia virus of mice: severe respiratory infection in a natural host. Immunol Lett. 2008;118:6–12. doi: 10.1016/j.imlet.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Easton AJ, Domachowske JB, Rosenberg HF. Pneumonia virus of mice. In: Cane P, editor. Perspectives in Medical Virology. Vol. 12. 2006. pp. 299–319. [Google Scholar]
- 29.Rosenberg HF, Bonville CA, Easton AJ, Domachowske JB. The pneumonia virus of mice infection model for severe respiratory syncytial virus infection: identifying novel targets for therapeutic intervention. Pharmacol Ther. 2005;105:1–6. doi: 10.1016/j.pharmthera.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Bonville CA, Rosenberg HF, Domachowske JB. Ribavirin and cysteinyl leukotriene-1 receptor blockade as treatment for severe bronchiolitis. Antiviral Res. 2006;69:53–59. doi: 10.1016/j.antiviral.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Bonville CA, Lau VK, DeLeon JM, Gao JL, Easton AJ, Rosenberg HF, Domachowske JB. Functional antagonism of chemokine receptor CCR1 reduces mortality in acute pneumovirus infection in vivo. J Virol. 2004;78:7984–7989. doi: 10.1128/JVI.78.15.7984-7989.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonville CA, Easton AJ, Rosenberg HF, Domachowske JB. Altered pathogenesis of severe pneumovirus infection in response to combined antiviral and specific immunomodulatory agents. J Virol. 2003;77:1237–1244. doi: 10.1128/JVI.77.2.1237-1244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonville CA, Bennett NJ, Koehnlein M, Haines DM, Ellis JA, DelVecchio AM, Rosenberg HF, Domachowske JB. Respiratory dysfunction and proinflammatory chemokines in the pneumonia virus of mice (PVM) model of viral bronchiolitis. Virology. 2006;349:87–95. doi: 10.1016/j.virol.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res. 2007;39:225–239. doi: 10.1007/s12026-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 35.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008 doi: 10.4049/jimmunol.181.6.4004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 37.Ellis JA, Martin BV, Waldner C, Dyer KD, Domachowske JB, Rosenberg HF. Mucosal inoculation with an attenuated mouse pneumovirus strain protects against virulent challenge in wild type and interferon-gamma receptor deficient mice. Vaccine. 2007;25:1085–1095. doi: 10.1016/j.vaccine.2006.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garvey TL, Dyer KD, Ellis JA, Bonville CA, Foster B, Prussin C, Easton AJ, Domachowske JB, Rosenberg HF. Inflammatory responses to pneumovirus infection in IFN-alpha beta R gene-deleted mice. J Immunol. 2005;175:4735–4744. doi: 10.4049/jimmunol.175.7.4735. [DOI] [PubMed] [Google Scholar]


